Abstract
Background
This study aimed to investigate the work status of clinicians in China and their management strategy alteration for patients with hepatocellular carcinoma (HCC) during the COVID-19 pandemic.
Methods
A nationwide online questionnaire survey was conducted in 42 class-A tertiary hospitals across China. Experienced clinicians of HCC-related specialties responded with their work status and management suggestions for HCC patients during the pandemic.
Results
716 doctors responded effectively with a response rate of 60.1%, and 664 were included in the final analysis. Overall, 51.4% (341/664) of clinicians reported more than a 60% reduction of the regular workload and surgeons declared the highest proportion of workload reduction. 92.5% (614/664) of the respondents have been using online medical consultation to substitute for the “face-to-face” visits. Adaptive adjustment for the treatment strategy for HCC was made, including the recommendations of noninvasive and minimally invasive treatments such as transcatheter arterial chemoembolization for early and intermediate stage. Targeted therapy has been the mainstay for advanced stage and also as a bridge therapy for resectable HCC.
Discussion
During the COVID-19 pandemic, online medical consultation is recommended to avoid social contact. Targeted therapy as a bridge therapy is recommended for resectable HCC considering the possibility of delayed surgery.
Introduction
Since December of 2019, coronavirus disease 2019 (COVID-19) has caused a “one-in-a-century” pandemic in China and the rest of the world, posing a major public health challenge all around the world.1 During the whole year of 2020, China had implemented strict traffic restrictions and social distancing policies, aiming to limit the spread of the contagion.2
Under this circumstance, most hospitals in China had taken actions of limiting outpatient visits, controlling of hospitalization, and reducing operation quantities to prevent nosocomial infection. Moreover, over 30,000 medical personnel across the country joined the fight against COVID-19 in the most threatened Hubei province, leading to the relative insufficiency of medical resource outside Hubei.3 Together, clinical routine of patients with cancer was affected to varying degrees. Due to the immune-compromised status, cancer patients are at a high risk of getting respiratory virus infection, which might lead to a delay in antitumor treatments and a high mortality.4 According to a epidemiologic report, among a total of 44,672 confirmed cases, 107 patients had the comorbidity of cancer, with a crude case fatality rate of 5.6%, much high than the overall fatality rate (2.3%).5 Therefore, more intensive attention should be paid to patients with cancer during the COVID-19 crisis.6
Hepatocellular carcinoma (HCC) is a major disease burden, not only for China but also for the whole world.7 The outbreak is threatening to monopolize the attention and resources of the public health system and several countries are taking actions like suspending non-urgent surgeries and freeing medical beds for accommodating COVID-19 cases.8 The global public health burden from the COVID-19 pandemic has inevitably disrupted the routine clinical management for HCC patients. Although the pandemic crisis in China was gradually alleviate and the clinical practice had mostly returned to normal, we think it's necessary to be caution to “black swan events” like the COVID-19 pandemic with scarce medical resources and review our experiences and lessons to be well-prepared. Therefore, through online questionnaire surveying of experienced HCC clinicians from 42 leading general hospitals and cancer hospitals all over China, we collected and summarized their clinical practice and opinions about adjusting the treatment of HCC patients during the pandemic. The recommendations proposed in our study are believed to reveal the situation of clinical practice for HCC in China, which provides further support the threatened patients with HCC around the world.
Materials and methods
The selection of hospitals and respondents
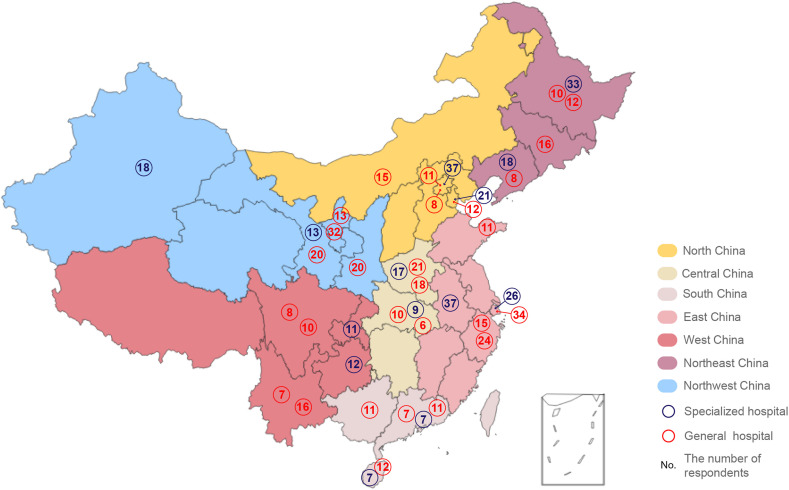
In China, patients with hepatocellular carcinomas are mostly diagnosed and treated in the departments of surgical oncology, medical oncology, interventional oncology, and radiotherapy in tertiary hospitals (the highest level of hospital in China). Therefore, we invited clinicians from these departments in 42 tertiary hospitals all over China, whose professional titles should be senior associate professors or professors, to participate in this survey. The selection method of each hospital is as the following. All 31 provinces of China were divided into seven regions. The provinces that had more confirmed cases of COVID-19 than the median of their corresponding regions were allocated to the severe group, and the rest belonged to the mild group (according to the number of daily reported cases of China CDC in February 29, 2020). Three class-A tertiary hospitals, including one cancer hospital and two general hospitals, were chosen in each group of each region. The distribution of all hospitals is illustrated in Fig. 1 (The nCov2019 R package was used for analysis and visualization9). The institutional ethics committee confirmed that this survey does not require any form of approval, as it was a survey of clinicians and was not based on patient data and individual patient data were not used in this study.
Figure 1.
The distribution of all the selected hospitals
Questionnaire design
The online questionnaire we designed had five parts (eText in the Supplementary material). The first part asked for the general information of the respondent, followed by the second part which consisted of common questions about their workloads and principles of screening, diagnosis and treatment of HCC during the pandemic. The third part asked for their management strategy alteration for each stage based on China liver cancer (CNLC) staging system10 during the COVID-19 outbreak. The decisions about targeted therapy during this special period were in the fourth part. The last part specifically asked for each specialty about their suggestions of reexamination after the treatment during the pandemic. The online questionnaire was sent directly to each respondent through a hyperlink by our coordinators in each hospital. All respondents understood and agreed that their answers to the questionnaires were used for analysis and report. No other personal information was collected using the questionnaire.
Statistical analysis
Statistical analyses were performed with the SPSS software (Ver. 25) and Rstudio software (running environment R 3.6.1). Qualitative data were described as the frequency and percentage. To compare the categorical variables in sub-groups, if any cell number was less than 5 in the frequency table, the result of Fisher's exact test was reported; otherwise the χ2 test was applied. For all the statistical hypothesis, p values < 0.05 were considered significant.
Results
Basic information
In total, the hyperlink of the online questionnaire was opened 1192 times and 716 doctors responded effectively to the online questionnaire with a response rate of 60.1%, while 52 were excluded because they did not meet specialty requirement; thus, 664 were included in the final analysis. The number of effective respondents in each hospital is shown in Fig. 1. General questions of the questionnaire and the response results are shown in eTable 1 in the Supplementary material. Overall, 42 class-A tertiary hospitals across China were included, 33.3% (14/42) of which were specialized hospitals and 66.7% (28/42) were comprehensive hospitals. All of the doctors were either professors (36.7% [244/664]) or associate professors (63.3% [420/664]). Among 664 questionnaires, 288 (43.4%) were answered by surgeons, 83 (12.5%) by interventional oncologists, 163 (24.5%) by medical oncologists, and 130 (19.6%) by radiation oncologists.
Change on the work pattern
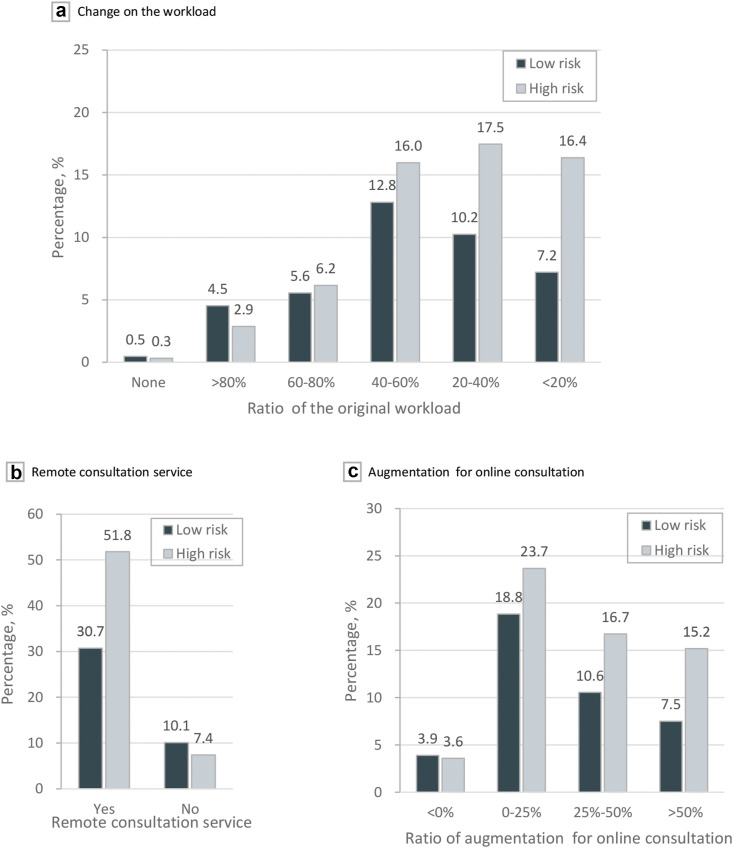
To prevent and control the COVID-19 outbreak, most clinicians had faced a transformation of the “face-to-face” service to the online consultation. During the pandemic, the regular workload was reduced in 99.2% (659/664) of the surveyed hospitals to varying degrees (Fig. 2 a). Moreover, for 51.3% (341/664) of the respondents, the workload dropped to less than 40% of the previous amount. Meanwhile, 82.5% (548/664) of the hospitals in the survey launched a remote consultation service for HCC patients during the COVID-19 outbreak (Fig. 2b), and most respondents (92.5% [614/664]) carried on online out-patient consultation (Fig. 2c).
Figure 2.
The impact of COVID-19 pandemic on the change of workload and online consultation application. (a) Change on the workload. (b) Proportion of hospitals providing remote consultation service. (c) Frequency of online consultation distinguished. The low-risk group included respondents whose provinces had more confirmed cases than the median of the confirmed case number of each province, while the opposites belonged to the high-risk group
We then further investigated the association between the decrease of clinicians’ workloads and epidemic risk in each province. The median of the confirmed case number of each province (296, data from the daily report of China CDC in February 29, 2020) served as the cutoff to assign provinces into high- and low-epidemic risk groups across the nation. Among 23 provinces that were investigated, 12 provinces (confirmed cases ≤ 296) were classified as a low-epidemic risk group, while the other 11 provinces (confirmed cases > 296) were assigned to a high-epidemic risk group. Through the two-sample test for equality of proportions with continuity correction, it was revealed that the change of workload was associated with epidemic risk (χ2 = 12.830, P = 0.0003, eTable 2 in the Supplementary material). Pearson correlation analysis revealed that the decrease of workload was correlated with higher epidemic risk (Pearson = 0.142, P = 0.0002). From the terms of medical specialties, there was a tight correlation between workload changes and medical specialties (χ 2 = 17.362, P = 0.0006, eTable 2 in the Supplementary material). Surgeon hold the greatest proportion (57.6% [166/288]) that declared an extreme workload decrease (over 60%).
Management strategy alteration
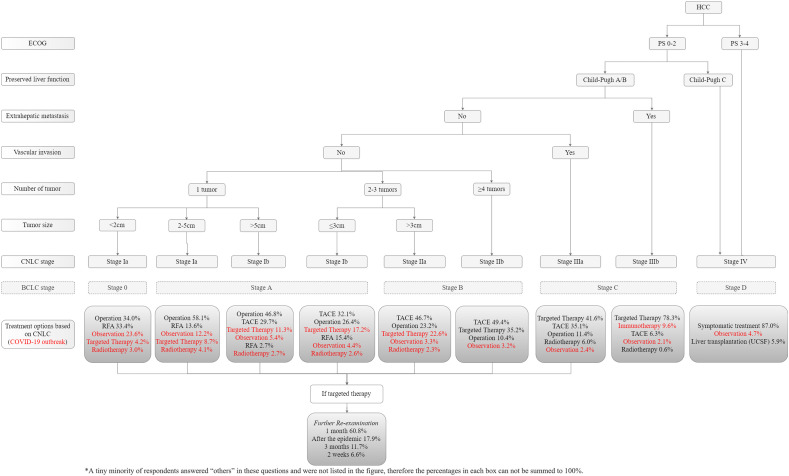
The CNLC staging system was illustrated in the guideline for primary liver cancer of China.10 The detailed treatment guideline and our amendment suggestions during the COVID-19 outbreak are presented in Fig. 3 .
Figure 3.
Management strategy alteration based on CNLC system. The illustration was edited and translated from Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). The red words in “Treatment options” are alternative treatment choices during the COVID-19 outbreak and the percentage of each option indicates the proportion of clinicians who recommended it
For the subgroup of CNLC stage Ia (single tumor, tumor diameter < 2 cm), corresponding to BCLC stage 0, 34.0% (226/664) of the experts recommended liver resection, and a considerable number of the experts recommended non-surgical treatment strategies including radiofrequency ablation (RFA, 33.4% [222/664]) and observation (23.6% [157/664]). For the remaining CNLC stage Ia (single tumor, tumor diameter 2–5 cm), corresponding to BCLC 0 and A, the resection had more support as the first choice (58.1% [386/664]), while RFA and observation declined to 13.6% (90/664) and 12.2% (81/664), respectively. With regard to the single lesion subgroup of CNLC stage Ib (tumor diameter > 5 cm), corresponding to BCLC A1-A3, the resection (46.8% [311/664]) was still preferred to transcatheter arterial chemoembolization (TACE, 29.7% [197/664]). While in the subgroup of CNLC stage Ib (2–3 tumors, tumor diameter ≤ 3 cm), corresponding to BCLC A4, such a trend reversed between resection and TACE (26.4% [175/664] vs. 32.1% [213/664]). TACE appeared to be the dominant choice of CNLC IIa and IIb (46.7% [310/664] and 49.4% [328/664], respectively), corresponding to BCLC B. On the contrary, the choice of resection declined rapidly for CNLC IIa and IIb (23.2% [154/664] and 10.4% [69/664], respectively). For the technically resectable subgroup of CNLC stage IIIa, corresponding to BCLC C, most of the experts (85.1% [565/664]) suggested non-surgical treatment, despite recommended by the CNLC guideline. The majority (78.3% [520/664]) of the experts preferred targeted drugs for HCC with extrahepatic metastasis (CNLC IIIb stage), corresponding to BCLC C. Most (87.0% [578/664]) of the doctors recommended supportive and symptomatic treatment for CNLC Ⅳ stage HCC, corresponding to BCLC D.
Suggestions on targeted therapy
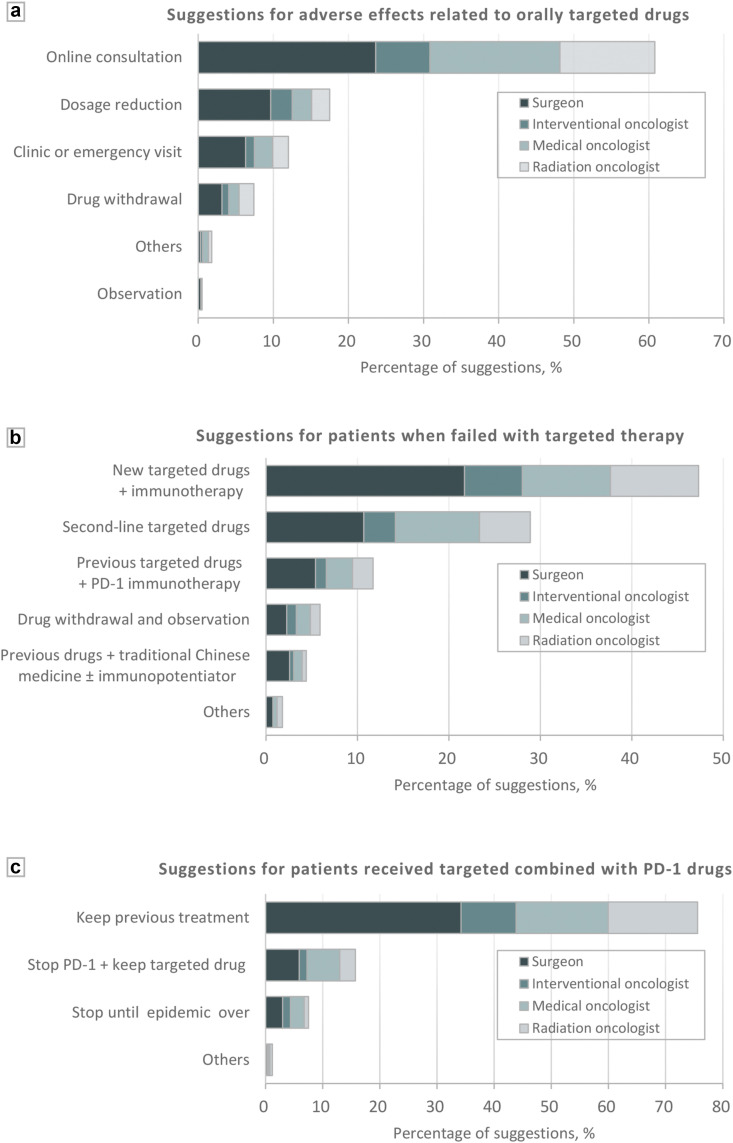
Due to the inconvenience of operations during the outbreak, most experts (68.4% [454/664]) recommended patients with resectable HCC to receive orally targeted therapy, while for patients with advanced HCC, the percentage rose to 92.2% (612/664). When it comes to any adverse effect, 60.8% (404/664) of the experts recommended online consultation and 24.9% (165/664) recommended dosage reduction or withdrawal of the targeted drugs (Fig. 4 a). With the failure of the targeted therapy, most of the clinicians (76.2% [506/664]) suggested replacement of another targeted drug (with or without immunotherapy) (Fig. 4b). For HCC patients who were already receiving the combination therapy of targeted drugs and PD-1 drugs, 75.6% (502/664) of the clinicians supported maintaining the current therapy, while the remaining suggested to pause the treatment or to simply stop using PD-1 mAb (Fig. 4c).
Figure 4.
Suggestions for targeted therapy and immunotherapy. (a) Suggestions for adverse effects related to orally targeted drugs. (b) Suggestions for patients when failed with targeted therapy. (c) Suggestions for patients received targeted combined with PD 1 drugs
Suggestions on follow-up
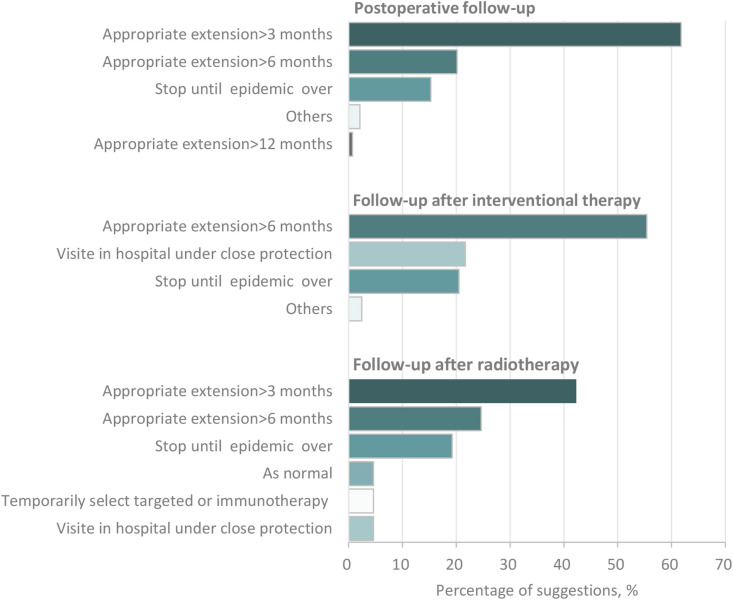
Affected by the pandemic, the follow-up schedules of most patients might need a rearrangement or postponement. For HCC patients underwent routine postoperative follow-up after liver resection, 62.2% (178/286) of the surgeons recommended a follow-up when it is more than three months from the last review, while 15.4% (44/286) suggested a postponement in any case (Fig. 5 ). As for patients who received TACE, 55.4% (46/83) of the interventional oncologists recommended that the patients could appropriately postpone the follow-up, but it should not exceed six months from the previous follow-up, while 20.5% (17/83) did not suggest a follow-up during the pandemic (Fig. 5). Similarly, most clinicians (86.1% [112/130]) also suggested postponement or cancellation of the follow-up for patients who had finished their radiotherapies (patients underwent adjuvant radiotherapy were not included) (Fig. 5). As for patients with advanced HCC receiving systemic therapies, medical oncologists’ most concerns included the antitumor effects and adverse reactions of drugs, quality of life, the sustainability of the treatment, the prevention and control of COVID-19 as well.
Figure 5.
Suggestions for follow-up patients by clinicians of different specialties
Discussion
In this nationwide multicenter survey study, we adopted Chinese guideline to clarify clinical suggestions for HCC patients of different stages because it was followed by and familiar with Chinese doctors in actual practice. While for the convenience of international colleagues, corresponding stages in BCLC staging system were also considered in the questionnaire. Although different guidelines applied in different countries, the questions faced by HCC patients and their oncologists across the world during the pandemic are consistent. Therefore, the recommendations of Chinese experts may provide the overall principle of the management of cancer patients during the COVID-19 pandemic.
Our results showed that their workload decreased by more than 60% for most respondents. These could be attributed to several reasons. Being afraid of the cross infection, patients had themselves reduced their visits to hospitals unless medical emergency occurred.11 The hospitalization and bed rotation were also limited by strict hospital infection control measures.11 These were consistent with our results that the extent of workload decrease was more remarkable in severely affected areas. Of note, among the subspecialties of HCC, the decrease of the workload was more obvious in the surgical department.12 , 13 It could be due to that complex hepatobiliary surgical operations were limited by the insufficient blood supply because of the social distancing policy.13 , 14 In addition, the immunosuppressive status after operations might increase the hazards of infection of COVID-19.
Chinese oncologists have developed several measures to cope with the urgent medical needs of HCC patients. Online medical services have been widely adopted.13 , 15 Similar advices were also provided by American Association For the Study of Liver Diseases (AASLD) in their consensus statement of the practice advice during COVID-19 pandemic.16 For patients who were in the treatment process, the timely responses effectively provided patients with advices on home care, drug selection, adverse reaction management, dose modification, and psychological support. Particularly, authorities have newly enabled online prescription, extended the prescription expiry date, and opened online drugstores during the pandemic, helping patients get sound basic medical supports and reduce the unnecessary hospital visits.13 , 15 Further popularization as well as regulation of the online medical services are encouraged.15
We should also note that the online medical services could not replace all the off-line medical services including interventional therapeutic procedures and surgical operations. Several adjustments were suggested. Briefly, non-invasive procedures, such as orally taken drugs, and minimal invasive procedures, such as interventional therapies, were preferred over the invasive procedures, such as surgical operations, if the medical conditions permitted. Home therapies were more recommended in order to reduce the unnecessary hospital visits. For patients at early and (or) intermediate stages, comprehensive therapies being composed of medication and TACE were more favored.
For the BCLC stage 0 (CNLC Ⅰa: single tumor, tumor diameter < 2 cm) HCC, resection was the first recommended treatment by CNLC guideline. In China, the surgical rate of this stage HCC was about 46% before the pandemic.17 However, our study demonstrated that only 34.0% of the experts recommended resection in this period. In addition, a considerable amount of the experts recommended non-surgical treatment strategies, including RFA (33.4%) and observation (23.6%). A cross-sectional survey in Europe and Africa also reported such need to adopt non-operative treatment strategies for liver cancers.18 Under the pressure of the pandemic, the increased proportion of non-surgical treatment at this stage is understandable and reliable. Some studies have revealed that for solitary tumor within 3 cm, patients underwent RFA had similar overall survival at one year and three years compared with liver resection.19 A retrospective analysis of 175 HCC patients (tumor diameter 1–4.6 cm) without any treatment revealed that the median tumor volume doubling time was 85.7 days.20 Therefore, an appropriate extension of the observation period sparks no apparent progression of HCC.
For HCC patients at BCLC stage A (CNLC Ⅰa: single tumor, tumor diameter 2–5 cm; CNLC Ⅰb: tumor number 2–3, tumor diameter ≤ 3 cm) and subgroup of BCLC stage B (CNLC Ib: single tumor, tumor diameter > 5 cm), liver resection was the first choice by the majority of the surveyed experts. However, our study showed that clinicians inclined to recommend the targeted treatment as bridge therapy for the resectable HCC when waiting for surgical operation. There are evidences supporting the effectiveness of sorafenib in extending the time to progression for the patients waiting for the surgery. Vitale et al. 21 demonstrated that bridge therapy using sorafenib was cost-effective for T2 HCC patients waiting for liver transplantation compared with observation. Likewise, bridging systemic therapy or TACE was recommended by International Liver Cancer Association (ILCA) guidance.22 However, recommending targeted treatment as bridge therapy for HCC patients at an early stage should be cautious. The proportion of targeted therapy as a preferred monotherapy was still low (from 11.3% to 17.2%), and the majority of the experts recommended the resectable HCC patients with targeted therapy should be reviewed every month during this period. Therefore, if surgery or invasive operations cannot be carried out in time during the pandemic, oral targeted drugs under close surveillance is an alternative bridge therapy for this stage HCC patients.
For subgroup of BCLC stage B HCC (CNLC Ⅱa), TACE was the first recommendation in our study. Liver resection could provide an effective treatment for HCC with this stage, and the rate of liver resection was about 46% in Asian HCC patients and about 48.5% in Chinese HCC patients.23 However, our study revealed only 23.2% of the interviewed surgeons recommended the liver resection for this stage patients. For subgroup of BCLC stage B HCC (CNLN Ⅱb), the majority of the surveyed specialists recommended TACE or targeted therapy. TACE was the first choice for this stage HCC by the guideline.10 Some studies revealed that in the absence of a prior TACE, a considerable amount of BCLC stage B HCC patients received sorafenib treatment as a decision of “treatment stage migration” and finally had good survival outcomes,24 which provided the rationale for the temporary use of targeted treatment during this period. Therefore, for HCC patients at this stage, minimal or non-invasive treatment was still more favored.
Most doctors (92.2% [612/664]) in our study preferred to recommend targeted drugs for advanced HCC with extrahepatic metastasis and targeted therapy with sorafenib or lenvatinib was the first-line treatment.25 , 26 To provide support for safety management, internet medicine guidance was widely recommended. This novel way helped resolve some mild to moderate adverse events out of hospital. But patients still need to be guided to the hospital in an emergency for serious adverse reactions, such as malignant hypertension and grade 3/4 diarrhea.25, 26, 27 This pattern for adverse events management can be not only promoted during outbreak periods, but also valuable at ordinary times.
Immunotherapy is a new method for HCC and has just been approved by the state food and drug administration (SFDA) as the second-line treatment after failure with targeted therapy. The combination of treatments containing an immune checkpoint inhibitor was recommended for targeted drugs failed patients and for those who have already received this combination before. The recommendations suggested that Chinese doctors had a positive attitude towards immunotherapy for HCC during the outbreak, especially for the second-line treatment after failure of targeted therapy. Noteworthy, it should be mentioned that immune checkpoint inhibitor may cause 3–7% of immune pneumonia,28 which needs to be distinguished from COVID-19.29 , 30 Therefore, more attention should be paid to the related symptom while using these drugs.
Our results had some limitations. First, instead of being seen as the guideline-level recommendations, the proposed suggestions could only be used as a reference for worldwide oncologists. Second, due to different severity of the pandemic in different regions, the understanding and psychological state of the respondents could affect the choice of treatment strategies, resulting in the bias of the overall results. Moreover, no data of clinical consequences could be provided to prove the effectiveness of these recommendations. Nevertheless, the results objectively represented mutual opinions of sophisticated clinicians in China during the pandemic and were also supported by previous studies. The recommendations will provide the best and available options for HCC patients during the especial period.
China has one of the largest populations of HCC patients, causing an especially major difficulty in clinical management of HCC during the pandemic. As the COVID-19 infection keeping spreading, worldwide clinicians and HCC patients were likely to face similar difficulties that we had experienced. Therefore, this nationwide survey from senior Chinese oncologists would be of great significance. As this pandemic has already caused the shortage of medical resources for all patients with cancers, the overall principles are believed to be of help to more oncologists majoring in other kinds of cancers.
Statement of ethics
The institutional ethics committee confirmed that this survey does not require any form of approval, as it was a survey of clinicians and was not based on patient data and individual patient data were not used in this study.
Author contributions
Conceptualization: HZ; JianguoZ; XBi; JJ; WWei; AZ; JC;
Data curation: JianguoZ; XBi; JJ; JZhao; YH; ZLi; XChe; YT; YS; ZH; YefanZ; XW; YubaoZ; ZLu; XD; TS; CL; PYue; DY; AY; RZhang; SL; JT; XuewenZ; XBai; XuejunZ; MH; YX; WWang; PYang; YY; YaminZ; QL; TP; ZW; YC; CS; YL; BZ; FZ; LW; DL; BL; XZhu; QH; MC; DX; FX; YanqiaoZ; YZeng; YM; XL; YB; TL; FS; LL; XCai; JianZ;
Formal Analysis: SY; QC; ZLuo; XChen;
Funding acquisition: HZ;
Methodology: WWei; RZheng;
Supervision: AZ; JC;
Validation: HZ; JianguoZ; XBi; JJ; WWei;
Visualization: SY; CG; ZLuo;
Writing – original draft: SY; ML; CG; QC; NL; RM; XChen; BC; YH;
Writing – review & editing: All authors.
JianguoZ, XBi, SY; JJ; WWei contributed equally and are joint first authors.
The corresponding authors (HZ, AZ and JC) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Acknowledgements
We thank Prof. Qingyi Wei and Prof. Sheng Luo from Duke University for their insightful comments on the manuscript and helping validating the statistical results. We thank all the clinicians who responded the questionnaire and the institutions for supporting this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hpb.2021.07.002.
Funding sources
The study was funded by the State Key Project on Infection Diseases of China (2017ZX10201021-007-003), CAMS Innovation Fund for Medical Sciences (CIFMS) (2017-12M-4-002), and the Key Laboratory Project of Chinese Academy of Medical Sciences (2019PT310026). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Gates B. Responding to covid-19 — a once-in-a-century pandemic? N Engl J Med. 2020 doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 2.Chen S., Yang J., Yang W., Wang C., Bärnighausen T. COVID-19 control in China during mass population movements at New Year. Lancet. 2020 doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H., Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y.J., Lee E.S., Lee Y.S. High mortality from viral pneumonia in patients with cancer. Inf Disp (Lond.) 2019;51:502–509. doi: 10.1080/23744235.2019.1592217. [DOI] [PubMed] [Google Scholar]
- 5.The Novel Coronavirus Pneumonia Emergency Response Epidemiology T The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Week. 2020;2:113–122. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 8.The New York Times Italy's elderly suffer heavy toll as coronavirus spreads. https://www.nytimes.com/2020/03/04/world/europe/coronavirus-italy-elderly.html
- 9.Wu T., Ge X., Yu G., Hu E. Open-source analytics tools for studying the COVID-19 coronavirus outbreak [published online March 5,2020] medRxiv. 2020 doi: 10.1101/2020.02.25.20027433. [DOI] [Google Scholar]
- 10.Zhou J., Sun H.C., Wang Z., Cong W.M., Wang J.H., Zeng M.S., et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition) Liver Cancer. 2018;7:235–260. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Commission of the People's Republic of China Technical guidelines on prevention and control of novel coronavirus infection in medical institutions (1st ed.) http://www.gov.cn/zhengce/zhengceku/2020-01/23/content_5471857.htm
- 12.Anesthesiologist branch of Chinese Medical Doctor Association, Anesthesiology branch of Chinese Medical Association The routine surgery anesthesia management and prevention and control process recommendations during novel coronavirus pneumonia. Perioper Saf Qual Assur. 2020:9–11. doi: 10.3969/j.issn.2096-2681.2020.01.003. [DOI] [Google Scholar]
- 13.Zhao Y. Hand in hand to overcome difficulties: an initiative for surgeons during the novel coronavirus pneumonia outbreak. Chin J Surg. 2020;58 doi: 10.3760/cma.j.cn112139-20200303-00180. [DOI] [Google Scholar]
- 14.Lei L. Efforts ramp up on blood donation as supply falls. http://www.chinadaily.com.cn/a/202002/19/WS5e4c8a55a310128217278908.html
- 15.The general office of the National Health Commission of the People's Republic of China Notice of the general office of the National Health Commission on the Internet diagnosis and treatment consulting services in epidemic prevention and control. http://www.nhc.gov.cn/yzygj/s7653p/202002/ec5e345814e744398c2adef17b657fb8.shtml
- 16.Fix O.K., Hameed B., Fontana R.J., Kwok R.M., McGuire B.M., Mulligan D.C., et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu P.H., Hsu C.Y., Hsia C.Y., Lee Y.H., Huo T.I. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤2 cm in a propensity score model. Ann Surg. 2016;263:538–545. doi: 10.1097/SLA.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 18.Balakrishnan A., Lesurtel M., Siriwardena A.K., Heinrich S., Serrablo A., Besselink M.G.H., et al. the E-AHPBA Scientific and Research Committee Delivery of hepato-pancreato-biliary surgery during the COVID-19 pandemic: an European-African Hepato-Pancreato-Biliary Association (E-AHPBA) cross-sectional survey. HPB. 2020;22:1128–1134. doi: 10.1016/j.hpb.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X.L., Liu X.D., Liang M., Luo B.M. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287:461–472. doi: 10.1148/radiol.2017162756. [DOI] [PubMed] [Google Scholar]
- 20.An C., Choi Y.A., Choi D., Yong H.P., Park M.S. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clin Mol Hepatol. 2015;21:279–286. doi: 10.3350/cmh.2015.21.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitale A., Volk M.L., Pastorelli D., Lonardi S., Farinati F., Burra P., et al. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: a cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2010;51:165–173. doi: 10.1002/hep.23260. [DOI] [PubMed] [Google Scholar]
- 22.ILCA Education Committee and Executive Committee ILCA Guidance for Management of HCC during COVID-19 Pandemic. https://ilca-online.org/wp-content/uploads/2020/06/ilca-covid-19-.pdf
- 23.Liang L., Xing H., Zhang H., Zhong J., Li C., Lau W.Y., et al. Surgical resection versus transarterial chemoembolization for BCLC intermediate stage hepatocellular carcinoma a systematic review and meta-analysis. HPB. 2018;20:110–119. doi: 10.1016/j.hpb.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Ero F.P., Marciano S., Fernández N., Silva J., Anders M., Zerega A., et al. Intermediate-advanced hepatocellular carcinoma in Argentina: treatment and survival analysis. World J Gastroenterol. 2019;25:3607–3618. doi: 10.3748/wjg.v25.i27.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 26.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 27.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 28.Brahmer J.R., Lacchetti C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.