Abstract
BACKGROUND:
Irradiation has been a standard treatment for testicular relapse but is associated with severe hypogonadism. Because CD19-specific CAR-T cells can eradicate leukemic blasts in cerebrospinal fluid, a pharmacologic sanctuary site, we tested the efficacy of this therapy in seven boys with isolated testicular relapse of B-acute lymphoblastic leukemia.
METHODS:
CD19 specific CAR T cells were generated with the use of autologous T cells transduced with a lentiviral vector to express a CAR molecule containing anti-CD19 scFv derived from the HI19α murine monoclonal antibody, human CD8α hinge, and human 4-1BB (CD137) and CD3ζ costimulatory signaling transmembrane domains. After the conditioning regimen consisted of intravenous fludarabine and intravenous cyclophosphamide, seven patients with a median age of nine years (range, two to ten years) with isolated testicular relapse received a single infusion of CD19 CAR-T cells at a total dose of 5×106 all T cells per kilogram.
RESULTS:
All seven patients achieved complete remission with normal testes. Six patients remained in second remission for 5 to 23 months (median, 14 months), and one patient subsequently relapsed in the bone marrow. The probability of event-free survival for all patients at 12 months of follow-up was 83.3%±15.2% (SE). The treatment was well tolerated with grade 1 cytokine-release syndrome developing in five patients.
CONCLUSION:
These results suggest that CAR-T cell therapy is a treatment option for patients with testicular relapse.
Keywords: acute lymphoblastic leukemia, testicular relapse, CD19 CAR-T cell therapy
Introduction
The testes have long been considered a pharmacologic sanctuary site in the treatment of acute lymphoblastic leukemia (ALL). Contemporary treatment including high-dose methotrexate has not only substantially reduced the risk of testicular relapse1 but can also eradicate overt testicular leukemia at diagnosis, without the need for local irradiation.2 Nonetheless, irradiation remains a component of standard treatment for testicular relapse at many centers. Because of the significant late effects associated with testicular irradiation, including sterility, and the requirement for testosterone replacement treatment for most patients of pubertal age or older,3 the Dutch Study Group used intensive chemotherapy only to treat patients with off-therapy isolated testicular relapse, with all five patients attaining second remissions of 1 to 15 years (median, 4 years).4 Encouraged by this finding, the Children’s Oncology Group conducted a larger study using intensive chemotherapy alone to treat 28 B-ALL patients with late-onset (initial remission duration ≥18 months) isolated testicular relapse who had normal testicular size or a negative biopsy for testicular leukemia at the end of remission induction.5 However, of these 28 patients, six subsequently developed a testicular recurrence, two hematological relapse, one a second neoplasm and one death in remission, resulting in a 5-year event-free survival rate of only 60.7%. Hence, the evaluation of other treatment modalities is needed to identify alternative treatments for testicular relapse. Given the ability of CD19-specific chimeric antigen receptor modified T (CAR-T) cells to eradicate leukemic blasts in cerebrospinal fluid of some patients with relapsed CD19+ B-ALL,6,7 we undertook a study to assess the safety and efficacy of CD19-specific CAR-T cell therapy for B-ALL patients with isolated testicular relapse.
Patients and Methods
Between September 2017 and March 2019, seven children less than 18 years of age with CD19+ B-ALL, who developed isolated testicular relapse after treatment including high-dose methotrexate on the CCLG-2008 and CCCG-2015 protocols,8,9 were enrolled in the XH-CAR-T-003 study (Chinese Clinical Trial Registry ChiCTR1900025419) for patients with relapsed or refractory hematologic malignancies. The study design and methods were in compliance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee and Institutional Review Board of the Chinese Academy of Medical Sciences & Peking Union Medical College. Written informed parental consent was obtained for all patients.
CD19-specific CAR-T preparations were generated with the use of autologous T cells transduced with a lentiviral vector to express a CAR molecule containing anti-CD19 scFv derived from the HI19α murine monoclonal antibody, human CD8α hinge, and human 4-1BB (CD137) and CD3ζ costimulatory signaling transmembrane domains (CNCT19, Juventas Cell Therapy).10 The conditioning regimen consisted of intravenous fludarabine 25 to 30 mg/m2 per day on days −4, −3, and −2 and intravenous cyclophosphamide 350 mg/m2 per day on days −4 and −2. All patients received a single infusion of CD19 CAR-T cells at a total dose of 5×106 all T cells per kilogram. No additional antileukemic treatment was given after CAR T-cell therapy.
After infusion, CAR-T cell levels in peripheral blood were determined at regular intervals. Serum immunoglobulin and cytokines including interleukin-1 beta, interleukin-2 receptor, interleukin-6, interleukin-8, interleukin-10 and tumor necrosis factor were also measured monthly. Testicular volume was measured by the b-mode ultrasonography at relapse and monthly after CAR-T cell therapy and was calculated as π / 6 × length × height × width.11 Cytokine-release syndrome was graded according to the revised grading system of Lee et al,12 and other toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03. For the time-to-event analysis, the Kaplan-Meier method was used to estimate the event-free distribution after CAR-T cell infusion. The statistical test was performed with SAS software (version 9.4).
Results
The seven boys in this study had a median age of 9 years (range, 2 to 10). They developed testicular relapse at a median time of 48 months (range, 7 to 60) after the initial diagnosis of ALL (Table 1). At relapse, one patient (no. 6) had minimal residual disease of 0.01% in the bone marrow. Before CD19 CAR-T cell therapy, five patients received two to four courses of high-dose methotrexate (5 g/m2). Testicular size decreased in four of the five patients after high-dose methotrexate treatment and returned to normal in all seven patients after CAR-T therapy (Table 1). However, patient 7, who had a slight increase in testicular size after 2 courses of high-dose methotrexate and a normal size one month after CAR-T cell infusion, developed hematologic relapse 6 months after CAR-T cell therapy with 82.9% blasts in the bone marrow that expressed CD19, CD38, CD123, cCD79a, CD34, CD117, CD10, CD33, and cCD22. This patient had normal testicular size at the time of hematologic relapse. The other six patients remained in second remission for 5 to 23 months (median, 14 months). The estimated 12-month event-free survival rate for the entire cohort was 83.3%±15.2% (SE) (Figure 1).
Table 1.
Patient characteristics and treatment outcome
| Case No | Age at relapse | Time from initial diagnosis to testicular relapse | Site of relapse | Treatment before CAR-T cells | Testicular size (cm3) | Outcome | ||
|---|---|---|---|---|---|---|---|---|
| (year) | ( month ) | At relapse | After HDMTX | After CAR-T cells | (month) | |||
| 1 | 10 | 48 | left testis | HDMTX×4 | 14.88 | 9.96 | 6.85 | CR (23+) |
| 2 | 10 | 48 | left testis | HDMTX×4 | 31.62 | 10.72 | 6.62 | CR (22+) |
| 3 | 10 | 51 | left testis | None | 29.17 | None | 3.23 | CR (15+) |
| 4 | 5 | 39 | left testis | None | 29.72 | None | 3.4 | CR (13+) |
| 5 | 9 | 60 | left testis | HDMTX×2 | 6.55 | 5.62 | 4.86 | CR (5+) |
| 6* | 2 | 7 | left testis | HDMTX×4 | 15.55 | 5.53 | 1.88 | CR (5+) |
| 7 | 5 | 39 | left testis | HDMTX×2 | 6.72 | 8.28 | 2.41 | marrow relapse (6) |
Minimal residual disease of 0.01% in bone marrow at relapse
HDMTX, high-dose methotrexate; CR, complete remission
Figure 1.
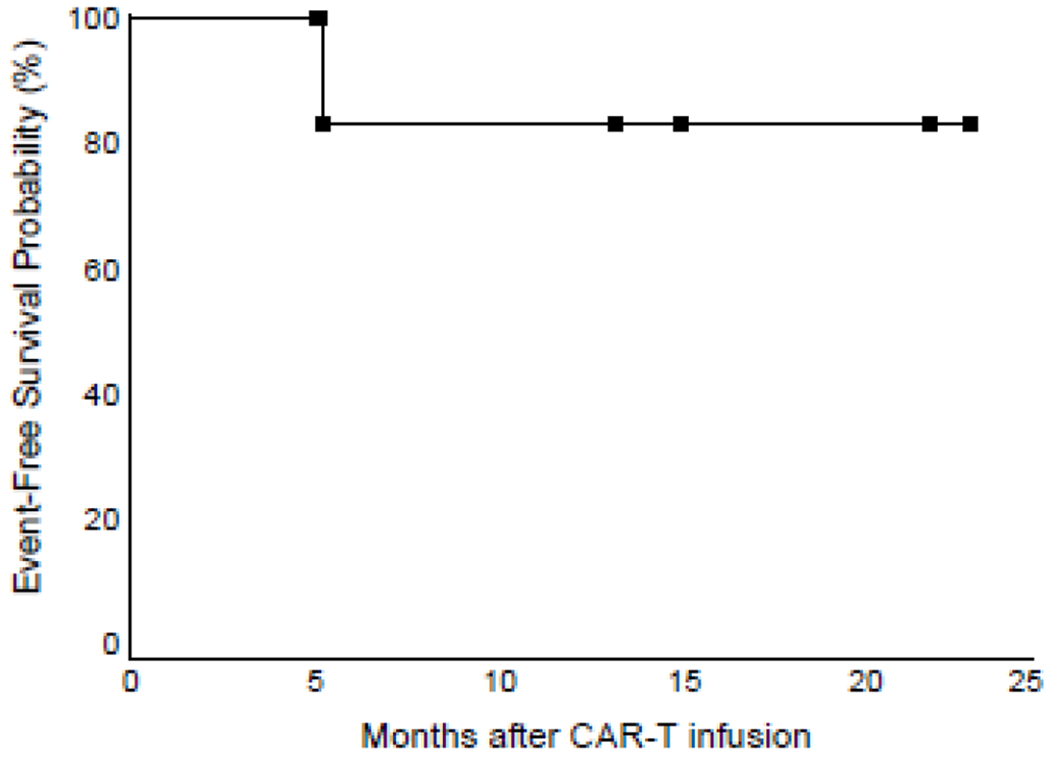
Event-free survival probability after CAR-T cell therapy. Black squares indicate patients still at risk of relapse.
After infusion, CD19 CAR-T cells were readily detected in peripheral blood by flow cytometry. The transduction efficiency was 33%, 25%, 39%, 69.6%, 60.5%, 74.5% and 78.3% for patients 1 to 7, respectively. The highest ratios of CAR-T cells to CD3+ T cells in patients 1 to 7 after infusion were 20%, 43.9%, 29.4%, 40.7%, 10.1%, 19.8%, and 20.7%, respectively (median, 20.7%). This ratio generally peaked between days 14 and 21, with the exception of patient 1, whose peak occurred on day 4 (Figure 2).
Figure 2.
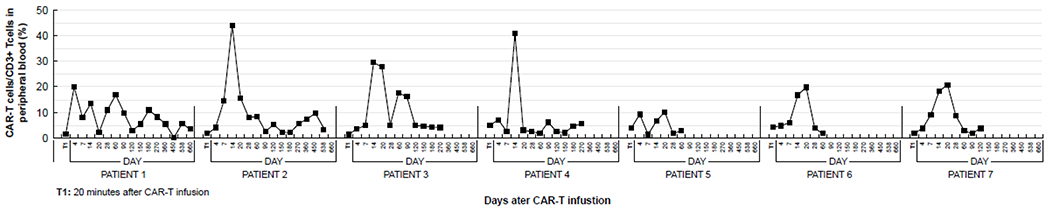
The ratio of CAR-T cells to CD3+ T cells in peripheral blood before and after CAR T infusion according to time.
Levels of the interleukin-2 receptor and interleukin-6 increased in six and five patients, respectively, while changes in the levels of interleukin-1-beta, interleukin-8, interleukin-10 and tumor necrosis factor-α were unremarkable (Table 2). None of patients had serious side effects after CAR-T cell infusion nor required blood transfusions. Grade 1 cytokine-release syndrome developed in five patients between days 8 and 12 after infusion, but not in patients 2 and 7.
Table 2.
Sequential levels of cytokines before and after CAR-T-cell therapy
| Time point | D0 | D7 | D14 | D21 | D28 | D0 | D7 | D14 | D21 | D28 | D0 | D7 | D14 | D21 | D28 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1ß (U/ml) | IL-2R (U/ml) | IL-6 (pg/ml) | |||||||||||||
| Case1 | <5 | <5 | <5 | ND | ND | 710 | 1111 | 1104 | ND | ND | <5.9 | 9.79 | <2 | ND | ND |
| Case2 | <5 | <5 | <5 | ND | ND | 1153 | 1277 | 1563 | ND | ND | 2.56 | 3.13 | 2.72 | ND | ND |
| Case3 | <5 | <5 | <5 | 6.28 | 12.6 | 722 | 1059 | 1724 | 950 | 509 | 4.08 | 4.42 | 3.38 | 26 | 106 |
| Case4 | <5 | <5 | <5 | <5 | <5 | 439 | 587 | 1406 | 839 | 517 | 2.81 | 2.14 | 2.81 | 20.5 | 6.49 |
| Case5 | <5 | <5 | <5 | <5 | <5 | 918 | 1028 | 1847 | 685 | 597 | 6.09 | 3.45 | <2 | <2 | <2 |
| Case6 | ND | ND | ND | ND | ND | 930 | 238 | 1500 | 4970 | 2596 | 11.6 | <2 | 2.98 | 20.3 | <2 |
| Case7 | <5 | <5 | <5 | 8.62 | 13.4 | 692 | 756 | 727 | 804 | 903 | 3.18 | 2.88 | 40.9 | 19.1 | <2 |
| IL-8 (pg/ml) | IL-10 (pg/ml) | TNF-α (pg/ml) | |||||||||||||
| Case1 | <62 | 11.5 | 10 | ND | ND | <9.1 | 78 | <5 | ND | ND | ND | ND | ND | ND | ND |
| Case2 | 9.87 | 14.3 | 9.05 | ND | ND | <5 | <5 | 10.1 | ND | ND | ND | ND | ND | ND | ND |
| Case3 | 25.8 | 8.64 | 10.7 | 430 | 944 | <5 | 22.5 | 67.1 | 6.7 | <5 | 113 | 46.7 | 14.1 | 111 | 411 |
| Case4 | 13.4 | 6.86 | 5.61 | 438 | 5.27 | <5 | 13 | 32.2 | <5 | 5.31 | 23 | 69.3 | 10.6 | 93.3 | 13.4 |
| Case5 | 23.4 | 53.8 | 26.5 | 71.5 | 15.2 | <5 | <5 | <5 | 19.9 | <5 | <4 | 9.08 | 7.04 | <4 | 12.6 |
| Case6 | 442 | 8.96 | 34.1 | 22.6 | 13.3 | 6.93 | <5 | 94 | 144 | 14.2 | 20.5 | 128 | 11.1 | 25.2 | 12.3 |
| Case7 | 53.5 | 149 | 581 | 619 | 120 | <5 | <5 | 5.69 | 5.33 | 6.62 | ND | ND | ND | ND | ND |
ND, not done
Discussion
CAR-T cell treatment was well tolerated in this study. The cytokine-release syndrome was relatively mild in the five patients who developed this complication, while adverse neurologic events did not occur in any of the patients. Importantly, six patients remained in remission for a median of 14 months. In the patient who relapsed in the bone marrow 6 months after the infusion, CAR-T cells were not measured beyond day 120 post infusion; the expression of CD19 on the blasts at the time of the second relapse suggested that his CAR-T cells might have been lost. However, the size of his previously involved testis remained normal, suggesting a lasting therapeutic effect of CAR T-cell therapy.
Our results substantiate recent anecdotal reports that CAR-T cells can eradicate extramedullary leukemia in patients with ALL. In a case report of a 24-year-old male with isolated testicular relapse after haploidentical hematopoietic cell transplantation for Philadelphia chromosome-positive ALL, allogeneic CD19-specific CAR-T cell therapy eradicated testicular leukemia on day 28 and he remained in second remission 153 days after the treatment.13 In another study of 10 patients who had two to four previous relapses and extramedullary involvement of various sites within 0 to 9 months before CAR-T cell infusion, one had progressive leukemia and three had medullary relapse with CD19+ leukemia after CAR-T cell treatment; the remaining six patients remained alive in remission for 3 to 16 months.14
This study suggests that CD19-specific CAR-T cell therapy is a reasonable therapeutic option for testicular relapse in children with B-ALL by virtue of its safety and efficacy. Additional studies of larger number of patients are needed to confirm our results and to determine if CAR T-cell therapy is also effective for patients with combined relapse. Conceivably, the development of dual CD19/CD22-specific CAR-T therapy would further improve treatment outcome in these patients.
Clinical Practice Points.
CD19-specific CAR-T cell therapy is a reasonable therapeutic option for testicular relapse in patients with B-ALL.
CD19-specific CAR-T cell may replace radiotherapy for the treatment of extramedullary disease in B-ALL.
Acknowledgements
This work was supported in part by U.S. National Cancer Institute grant P30CA021765, the VIVA China Children’s Cancer Foundation, American Lebanese and Syrian Associated Charities (ALSAC), and grants from the National Key Research and Development Program of China (2017YFC0909800), the National Natural Science Foundation of China (81830005), the National Natural Science Foundation of China (81870131) ,and CAMS Innovation Fund for Medical Sciences (CIFMS 2016-I2M-1-001). We thank Dr. Wing Leung for constructive review and comments.
Disclosure
Jianxiang Wang received research funding from Celgene Company. There are no conflicts of interest for other authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brecher ML, Weinberg V, Boyett JM, Sinks LF, Jones B, Glicksman A, et al. Intermediate dose methotrexate in childhood acute lymphoblastic leukemia resulting in decreased incidence of testicular relapse. Cancer. 1986;58(5):1024–1028. [DOI] [PubMed] [Google Scholar]
- 2.Hijiya N, Liu W, Sandlund JT, Jeha S, Razzouk BI, Ribeiro RC, et al. Overt testicular disease at diagnosis of childhood acute lymphoblastic leukemia: lack of therapeutic role of local irradiation. Leukemia. 2005;19(8):1399–403. [DOI] [PubMed] [Google Scholar]
- 3.Grundy RG, Leiper AD, Stanhope R, Chessells JM. Survival and endocrine outcome after testicular relapse in acute lymphoblastic leukaemia. Arch Dis Child. 1997;76(3):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg H, Langeveld NE, Veenhof CH, Behrendt H. Treatment of isolated testicular recurrence of acute lymphoblastic leukemia without radiotherapy. Report from the Dutch Late Effects Study Group. Cancer. 1997;79(11):2257–2262. [DOI] [PubMed] [Google Scholar]
- 5.Barredo JC, Hastings C, Lu X, Devidas M, Chen Y, Armstrong D, et al. Isolated late testicular relapse of B-cell acute lymphoblastic leukemia treated with intensive systemic chemotherapy and response-based testicular radiation: A Children’s Oncology Group study. Pediatr Blood Cancer. 2018; 65(5):e26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui L, Li Z-G, Chai Y-H, Yu J, Gao J, Zhu XF, et al. Outcome of children with newly diagnosed acute lymphoblastic leukemia treated with CCLG-ALL 2008: The first nation-wide prospective multicenter study in China. Am J Hematol. 2018; 93(7):913–920. [DOI] [PubMed] [Google Scholar]
- 9.Cai J, Yu J, Zhu X, Hu S, Zhu Y, Jiang H, et al. Treatment abandonment in childhood acute lymphoblastic leukemia in China: a retrospective cohort of the Chinese Children’s Cancer Group. Arch Dis Child. 2019;104(6):522–529. [DOI] [PubMed] [Google Scholar]
- 10.An N, Tao Z, Li S, Xing H, Tang K, Tian Z, et al. Construction of a new anti-CD19 chimeric antigen receptor and the anti-leukemia function study of the transduced T cells. Oncotarget. 2016;7(9):10638–10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goede J, Hack WW, Sijstermans K, van der Voort-Doedens LM, van der Ploeg T, Meji-de Vries A, et al. Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm Res Paediatr. 2011; 76(1): 56–64. [DOI] [PubMed] [Google Scholar]
- 12.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014; 124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J, Hu Y, Pu C, Liang Z, Cui Q, Zhang H, et al. Successful chimeric Ag receptor modified T cell therapy for isolated testicular relapse after hematopoietic cell transplantation in an acute lymphoblastic leukemia patient. Bone Marrow Transplant. 2017;52(7):1065–1067. [DOI] [PubMed] [Google Scholar]
- 14.Talekar MK, Maude SL, Hucks GE, Motley LS. Effect of chimeric antigen receptor-modified T (CAR-T) cells on responses in children with non-CNS extramedullary relapse of CD19+ acute lymphoblastic leukemia (ALL). J Clin Oncol 35(15_suppl):10507–10507 [Google Scholar]


