Abstract
We evaluated if chronic consumption of quercetin (Q) with green tea extract (GTE) enhances the bioavailability of GT polyphenols (GTPs) and reduces methylation activity as previously observed in mouse xenograft tumors. In this prospective, randomized, parallel design, placebo controlled study, thirty-one men with prostate cancer consumed daily 1 gram of GTE (830 mg of GTP) with 800 mg of Q (GT + Q) or placebo (GT + PL) for four weeks before prostatectomy. First morning voided urine was collected at baseline, 3 weeks and the day of surgery, and prostate tissue on the day of surgery. In week 3, plasma concentration of GTPs and Q was measured in blood collected before and 2 hours after the morning dose. Prostate tissue epigallocatechin gallate (EGCG) and epicatechin gallate (ECG) were detected in 67 and 93% of participants in the GT + Q group and 75 and 94% of participants in the GT + PL group. Q was increased 14-fold, 12-fold and 4.5-fold in plasma, urine, and prostate tissue, respectively, in the GT + Q compared to the GT + PL-group. There was a trend for decreased EGC levels in urine collected prior to prostatectomy in the GT + Q compared to GT + PL-group (p = 0.053). Plasma epigallocatechin (EGC) showed a trend to increase (p = 0.066) two hours after capsule intake in the GT + Q vs. the GT + PL-group. There was no significant difference between the groups in GTP content or methylation activity in prostate tissue or RBCs. No liver toxicity was observed. Although our findings are suggestive, further studies are warranted evaluating if Q alters GTP metabolism.
1. Introduction
Natural products from plants are a major source of non-toxic agents for cancer prevention and treatment. In animal models, there is convincing evidence for an anticarcinogenic effect of green tea (GT) and green tea extracts (GTE).1,2 Human epidemiological and intervention studies are less conclusive with regards to chemopreventive and therapeutic effects of GT for men with prostate cancer.3–7 A one-year GT polyphenol (GTP) intervention (600 mg day−1) in men with high-grade prostate intraepithelial neoplasia (HG-PIN) resulted in a significant delay in incidence of adenocarcinoma (3% in the tea group compared to 30% in the placebo group).7 Another study with a similar study design using a daily dose of 400 mg of Polyphenon E (GTE enriched with EGCG) showed a significant decrease in atypical small acinar proliferation (ASAP) but no significant change in progression from PIN to adenocarcinoma incidence.8 In men diagnosed with prostate cancer and scheduled for prostatectomy, consumption of 6 cups of GT daily for 8 weeks was associated with a significant decrease in the prostate tissue inflammatory marker nuclear NFκB, but no change in cancer proliferation (Ki67).9
A major challenge in the field of chemoprevention using tea polyphenols is the limited bioavailability and extensive metabolism during and after absorption in the small intestine. Depending on the chemical structure, polyphenols undergo glucuronidation, sulfation and methylation upon absorption.10,11 Epigallocatechin gallate (EGCG) and epicatechin gallate (ECG) are present in plasma mainly in the nonglucuronidated and non-sulfated forms and are excreted in bile, while the majority of epicatechin (EC) and EGC circulate in glucuronidated and sulfated forms and are excreted in the kidney.12 EGCG and ECG are methylated by catechol-O-methyltransferase (COMT) in the intestine and liver.11 Methylation of EGCG decreases its antiproliferative, proapoptotic and anti-inflammatory effects by about 50%.13,14 Decreasing the methylation of EGCG has the potential to increase the chemopreventive activity of GT.13,14
Quercetin (Q) is a flavonol-type flavonoid found in onions, apples, broccoli, berries, teas, and red wine.15 Q, with a similar catechol structure as tea polyphenols, also has anti-inflammatory activity and is used to treat prostatitis.16–18 In animal models, Q inhibits cancer growth through several mechanisms including induction of apoptosis.19 To date, there are no studies evaluating Q levels in human tissue. Catechol-containing polyphenols such as EGCG and Q inhibit the activity of DNA (cytosine-5) methyltransferases (DNMT).20,21 The inhibition of DNMT1 by EGCG treatment is associated with reversal of DNA hypermethylation and reactivation of several genes including GSTP1 and RARB2 in cell culture.22,23 In addition, in vitro and preclinical studies demonstrate that GTPs and Q inhibit COMT activity.11,20 However, data from a human study showed that ingestion of 750 mg of EGCG increased red blood cell COMT activity by 24% two hours after ingestion.24 We previously demonstrated in cell culture and mouse xenograft models that the combination of Q with GTE enhanced the bioavailability and decreased methylation of EGCG in xenograft tumor tissue compared to GT alone leading to enhanced inhibition of prostate cancer growth.25,26
Based on prior preclinical studies demonstrating that chronic co-ingestion of Q or flavonol-rich food enhances the bioavailabilty and decreases methylation of GTPs, we sought to determine if the same effects are seen in humans.26,27 We used a pre-prostatectomy study design in which blood, urine, and post-intervention prostate tissue are available for analyses. We also sought to determine if combined chronic consumption of GTE with Q for 4 weeks decreases the activity of COMT and DNMT in red blood cells and prostate tissue and if Q is taken up in prostate tissue.
2. Materials and methods
2.1. Participants
Participants were recruited from the urology clinics at the Veterans Administration Greater Los Angeles Healthcare System, UCLA Westwood, and the UCLA Santa Monica Medical Center. Participants (43–74 years) had a diagnosis of clinically localized prostate adenocarcinoma and were scheduled to undergo radical prostatectomy at least three weeks after study entry. Participants were ineligible if they had a history of hepatitis, alcohol abuse and other significant medical or psychiatric condition or took 5-alpha reductase inhibitors, antiandrogens, or luteinizing hormone-releasing hormone agonists. Subjects were instructed to abstain from all teas and tea containing products other than the study tea supplement, and stop nutritional supplements and herbal therapies (i.e., quercetin, lycopene, selenium, vitamin E, fish oil, and saw palmetto). The study was approved by the UCLA and Veterans Administration Institutional Review Boards.
2.2. Clinical trial design and tea and quercetin intervention
This was a prospective randomized, open label, parallel two arm intervention study. The study was carried out in accordance with the guidelines of the Human Subjects Protection Committee of the University of California, Los Angeles. The clinical protocol was approved by the Internal Review Board of the University of California, Los Angeles (12–000886). The trial was registered with ClinicalTrial.gov (NCT01912820). All subjects signed informed consent documents prior to study entry. Men scheduled for prostatectomy, with a minimum of 4 weeks to the surgery date, were randomized to a combination of (1) two capsules of GTE (Tegreen 97) with one capsule of Q (N = 15) or (2) two capsules of GTE and one capsule of placebo (N = 16) for 4 weeks. Men were instructed not to consume tea and dietary/herbal supplements throughout the study. Q and PL capsules were distributed in the same white containers by the UCLA investigative pharmacy. Tegreen97 was distributed in the original bottles by the UCLA investigative pharmacy. Tegreen97 is a hot water GTE.9
Study subjects were randomized according to a permuted block design with 4 blocks of 8 for GT + Q or GT + PL. GT capsules were provided by Pharmanex/NuSkin, Provo, Utah. Q capsules were provided by Nature’s Life (Larkspur, CA). Compliance was determined by capsule count at the end of the intervention and by urine analysis. Intake of 75% of the capsules was the threshold for compliance. At the baseline visit (prior to GT/Q intervention), participants provided a spot urine and fasting blood was collected. After confirming eligibility, participants were randomly assigned to the intervention group and were provided with the capsules. Participants consumed the capsules until the evening prior to surgery. During week 3 participants arrived at the clinic fasting and brought their morning dose of capsules and a morning first voided urine sample. To standardize the procedure, participants received a light breakfast (bagel, 1 oz of cream cheese and 16 oz of water). After consumption of the breakfast time zero (T0) blood was drawn. After breakfast, participants took their morning dose of GTE and Q or placebo. After two hours, a second blood sample (T2) was collected for analyses of GTP, Q, metabolites and liver function. On the day of surgery, participants collected a first voided urine sample and delivered it to the study coordinator in the hospital. At the time of prostatectomy, prostate tissue was processed according to standard protocol of the UCLA and VA pathology departments. Aliquots of fresh prostate tissue obtained after the prostatectomy were stored in cryotubes, frozen in liquid nitrogen, and stored at −70 °C.
Liver function tests (aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase) were measured by the UCLA clinical laboratory. Serum PSA concentration was analyzed by ELISA assay (GenWay Biotech Inc., San Diego, CA) according to the manufacturer’s instruction at the UCLA Center for Human Nutrition. Blood for HPLC analysis was collected in EDTA vacu tubes and for liver function test in serum separation vacutainers (details in ESI†).
2.3. Dosage information/dosage regimen
Participants consumed two capsules of GTE (250 mg per capsule) and one capsule of Q or PL (400 mg per capsule) orally twice daily. The combined GT plus Q doses were chosen based on our previous prostatectomy trial in which consumption of 6 cups of brewed GT with a total of 1010 mg GTP (562 mg EGCG) daily for 3–8 weeks resulted in no liver toxicity.9 The current dose of EGCG in the GTE was 461 mg daily (115 mg per capsule) (Table 1). Q dose was based on a study by Shoskes et al. that demonstrated that 500 mg of Q for 1 month was well tolerated.16 In addition, a dose escalation study by Lu NT et al. showed no adverse events with the consumption of 5 g of Q daily for 4 weeks.28 The GTE dose is equivalent to drinking 4 cups of GT, prepared by steeping about 2 g of tea leaves in 240 ml of boiling water. On average, each cup contains about 150 mg of GTP (∼100 mg EGCG).9 Many fruits, vegetables, nuts and grains contain Q in small amounts and it will not be feasible to consume 800 mg daily from natural food sources.29
Table 1.
Green tea polyphenol content of TeGreen capsules
| EGC | EGCG | EC | ECG | Catechin | Q | |
|---|---|---|---|---|---|---|
| TeGreen capsule (mg) | 32.3 ± 6.8 | 115.2 ± 4.8 | 25.0 ± 8.5 | 29.2 ± 2.3 | 5.9 ± 3.9 | BDL |
Data are mean ± SD. Samples were analyzed in duplicates. BDL, below detection limit.
2.4. Outcomes
The objective of the study was to determine the effect of chronic consumption of Q on prostate, plasma, and urine concentrations of GT polyphenols and polyphenol metabolism (methylation). The primary endpoint of this study was GTP concentration in prostate tissue. Secondary endpoints were GTP concentration in plasma and urine and Q concentration in prostate tissue, plasma, urine, and red blood cells, and prostate enzyme activity of COMT and DNMT, prostate gene and protein expression of COMT and DNMT, COMT polymorphism, and serum prostate-specific antigen (PSA) levels.
2.5. Polyphenol analysis of GT and Q capsules
All HPLC grade solvents were purchased from Fisher Scientific (Pittsburgh, PA). EGCG, EC, ECG, EGC and Q, isorhamnetin were purchased from Sigma-Aldrich (St Louis, MO), and 4′-O-methyl EGC (4′-MeEGC) and 4″-O-methyl EGCG (4″-MeEGCG) were purchased from Nacalai USA Inc. (San Diego, CA).
The decaffeinated GT capsules (Tegreen 97, Pharmanex/NuSkin) were provided by Pharmanex/NuSkin, Provo, UT. Each capsule contained 250 mg of GT leaf extract. The GT polyphenol composition of each GTE capsule was determined by HPLC with CoulArray electrochemical detection (ESA, Chelmsford, MA) (Table 1).9 Q capsules were provided by Nature’s Life (Larkspur, CA). HPLC analysis with photodiode array detection confirmed that each capsule contained 400 mg of Q. Placebo capsules contained inactive excipients (microcrystalline cellulose).
2.6. Tea polyphenol and Q analysis in plasma, urine and prostate tissue
Tea polyphenols (GTPs) in plasma and prostate were analyzed by HPLC with coularray detection.9 Urine GTPs and urine, plasma and prostate quercetin (Q) were analyzed by LC-MS/ MS.30
Total unconjugated tea polyphenols (not glucuronidated or sulfated) were analyzed in prostate tissue, plasma and urine as previously described9 (further details in ESI†). 300 mg of fresh frozen prostate tissue was homogenized and treated with 1000 units of β-glucuronidase from E coli (G8420, Sigma Chemicals, St Louis, MO) and 20 units of sulfatase from abalone entrails (S9754, Sigma Chemicals) at 37 °C for 60 minutes.9 The detection limit was 30 pmol g−1 prostate tissue, 20 nmol L−1 urine and 4 nmol L−1 plasma. 250 μl of urine and 200 μl of plasma were treated with β-glucuronidase from E coli (G8420, Sigma Chemicals, St Louis, MO) and sulfatase from abalone entrails (S9754, Sigma Chemicals) described previously with minor modifications (ESI†).9 Plasma EGCG, ECG, EC, EGC and MeEGC, prostate EGCG, and ECG were analyzed by HPLC coularray detection (method details in ESI†). Urine EGC, EC and MeEGC were quantified by LC-MS/MS. We did not detect 4″-MeEGCG and 4′-MeEGC in the prostate. Urine EGCG, ECG and 4″-MeEGCG concentrations were not quantified due to low concentrations. Prostate, plasma and urine Q concentration was determined using LC-MS/MS as described in ESI†.† 30
2.7. Enzyme activity of COMT and DNMT in red blood cells and prostate tissue
COMT activity was determined in prostate tissue and erythrocyte homogenates by electrochemical detection of the methylated product of dihydroxybenzoic acid (vanillic acid) as described earlier.25,31 In short, 4 mg of RBC protein or 200 μg of prostate homogenate was incubated for 30 min at 37 °C with 0.2 mM S-adenosyl-L-methionine iodide (AdoMet) (Sigma-Aldrich), 5 mM MgCl2, and 200 μM dihydroxybenzoic acid, buffered with 100 mM Na2HPO4 buffer (pH 7.6) in a total volume of 250 μl. After 30 min, the reaction was terminated by adding 50 μl of 4 M perchloric acid. Protein was removed by centrifuge at 16 800g for 15 min, and the supernatant injected in the HPLC-CoulArray detection system to determine vanillic acid at 500 mV. The enzyme activity was expressed as pmol vanillic acid formed per min per mg protein. All assays were performed in duplicate. To measure DNMT enzyme activity in prostate tissue nuclear extracts were prepared from prostate tissue and DNMT enzyme activity was determined using the ELISA kit (Active Motif, Carlsbad, CA) according to manufacturer’s instructions.
2.8. Western blot analysis of COMT and DNMT in prostate tissue
Protein was extracted from prostate tissue using RIPA buffer. 50 μg of protein was separated on a 4–12% NuPAGE® Bis-Tris gel (Life Technologies, Carlsbad, CA), transferred to a nitrocellulose membrane (Amersham, GE Healthcare, Pittsburgh, PA) and incubated with rabbit anti-human primary antibodies from Santa Cruz: COMT (sc-137253), and DNMT1 (sc-135887) followed by goat anti-rabbit IgG-horseradish peroxidase secondary antibody. Protein bands were visualized with ChemiDoc XRS (Bio-Rad Laboratories) chemiluminescent detection and imaging system. β-Actin (Abcam Inc., Cambridge, MA) was used as loading control.
2.9. Gene expression of COMT and DNMT in prostate tissue
mRNA was extracted using RNeasy extraction kit (Qiagen, Germantown, MD) and transcribed to cDNA (details see ESI†). Real-time PCR was performed for quantitative analysis of mRNA transcript number using TaqMan® Gene Expression Assay kits (ID: Hs02511558_s1 for COMT, and ID: Hs00154749_m1 for DNMT1) and Taqman universal PCR master mix (Applied Biosystems, Foster City, CA). PCR amplification was performed by a 7900HT Fast Real-Time System (Applied Biosystems). The 2−(ΔΔCt) method was used to normalize the expression of COMT and DNMT1 in each sample to GAPDH expression and to compare to the mean ΔCt value.
2.10. Statistical analyses
Our power calculation was based on tumor tissue data from our mouse xenograft prostate tumor studies.26 Since we demonstrated that the prostate tissue concentration of EGCG was similar in mouse compared to human prostate, we extrapolated that the effect of combining Q with GTE will lead to a similar effect size (nearly 2-fold) increase in bioavailability and significant decrease in EGCG methylation26 (average of 25 vs. 45 pmol g−1 between groups with a conservative SD estimate of 13, Fig. 2 EGCG GT vs. EGCG GT + 0.4% Q). With a sample size of 14 subjects per group, the study was powered (84%) to detect differences in prostate GTPs between GT + PL and GT + Q groups as small as 15 pmol g−1 (two-sample t-test, α = 0.05). Therefore, our enrollment target was 15 subjects per group to allow for the possibility of a drop-out for each group.
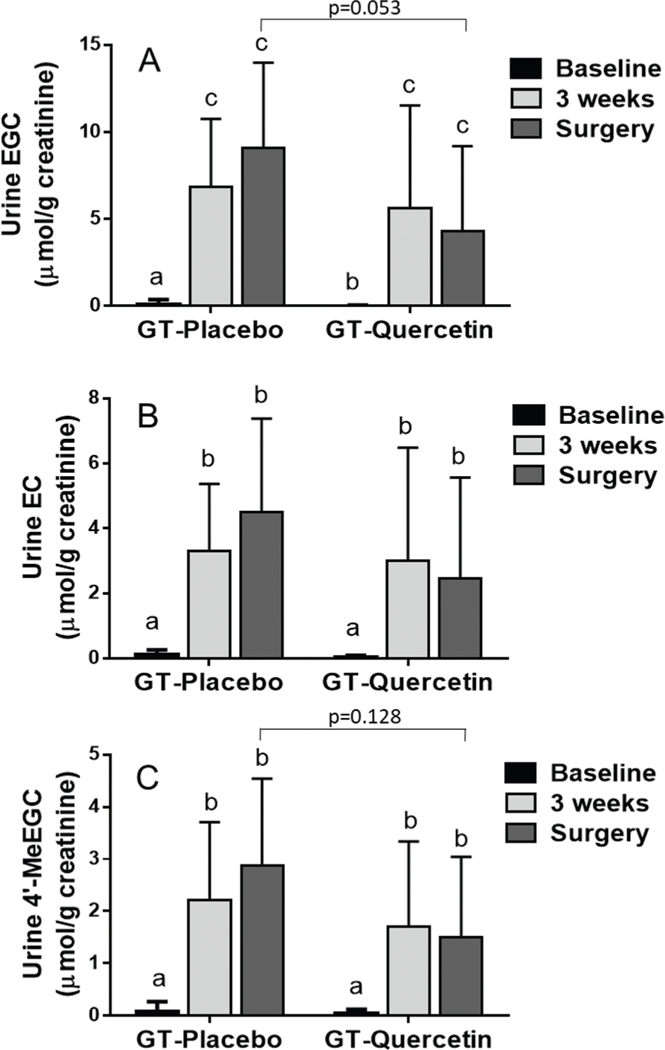
Fig. 2.
Urine concentration of EGC (A), EC (B) and 4’-MeEGC (C) calculated per g of creatinine in urine collected at baseline (prior to GT/Q intervention), 3 weeks and morning of surgery. Data are mean ± SD. A repeated measures ANOVA model was used. When a significant interaction was observed, Tukey/Kramer test was used for post-hoc comparisons across all values. Values not sharing a common letter differ significantly by Tukey/Kramerpost-hoc test. Number of participants: GT + PL = 16, GT + Q = 15. Urinary concentration of EGCG, Me-EGCG and ECG were not quantified due to very low concentration.
The statistical difference between the groups receiving intervention of GT + Q compared to GT + PL was determined using Student’s t-test. For the analyses involving time and treatment dependent assessments a repeated measures, ANOVA model was used with terms for group, time, and the group × time interaction term. When a significant interaction was observed, Tukey/Kramer test was used for post-hoc comparisons. All statistical analyses were performed using SAS software version 9.2 (Institute Inc, Cary, NC, USA). P-Values < 0.05 were considered statistically significant.
3. Results
3.1. Clinical characteristics and compliance
Thirty-three men were screened for the trial. Among these patients, one was found to have hepatitis at baseline and was therefore a screen failure and was not randomized. One participant dropped out after 1-week on the intervention due to noncompliance. Thirty-one subjects completed the study (GT-Q: 15; GT-PL: 16). There were no significant differences in demographics and clinical characteristics between the GT-Q and GT-PL groups in age, body composition, mean biopsy Gleason score and baseline PSA level (Table 2). The mean duration of the intervention in the GT-Q group was 25.5 ± 5 days and 27 ± 5 days in the GT-PL group. There were no serious adverse events related to the interventions, and there was no liver toxicity as measured by pre- vs. 3 weeks post-intervention serum levels of alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase (ESI Table 1†). Both groups were compliant with the intervention with greater than 94% of capsules consumed (Table 2).
Table 2.
Baseline demographics of study participants
| GT + placebo | GT + quercetin | P-Value | |
|---|---|---|---|
| N | 16 | 15 | |
| Age | 62.1 ± 7.1 | 58.2 ± 6.9 | 0.136 |
| Weight (kg) | 84.4 ± 11.8 | 87.4 ± 13.6 | 0.517 |
| Height (cm) | 178.8 ± 7.8 | 178.2 ± 9.2 | 0.843 |
| BMI (kg m−2) | 26.4 ± 3.4 | 27.4 ± 3.2 | 0.388 |
| Intervention days (d) | 25.5 ± 5.1 | 27.3 ± 5.3 | 0.352 |
| Compliance (%) GT | 94.0 ± 8.3 | 94.5 ± 15.4 | 0.899 |
| Compliance (%) Q | 98.7 ± 10.2 | 95.3 ± 17.5 | 0.506 |
| Biopsy Gleason Score | 0.956 | ||
| 6 | 4 (25.0%) | 4 (26.7%) | |
| 7 (3 + 4) | 4 (25.0%) | 5 (33.3%) | |
| 7 (4 + 3) | 2 (12.5%) | 2 (13.3%) | |
| ≥8 | 6 (37.5%) | 4 (26.7%) | |
| Race (%) | 0.210 | ||
| Asian | 0 (0.0%) | 1 (6.7%) | |
| Black | 2 (12.5%) | 5 (33.3%) | |
| White | 13 (81.3%) | 9 (40.0%) | |
| Other | 1 (6.3%) | 0 (0.0%) |
Data are mean±SD. p-Value reflect comparison between GT + Q and GT + PL intervention. Student’s t-test was used to compare parameters from GT + Q with GT + PL groups. Number of participants: GT + PL = 16, GT + Q = 15.
3.2. GT polyphenols and Q concentration in prostate, plasma and urine
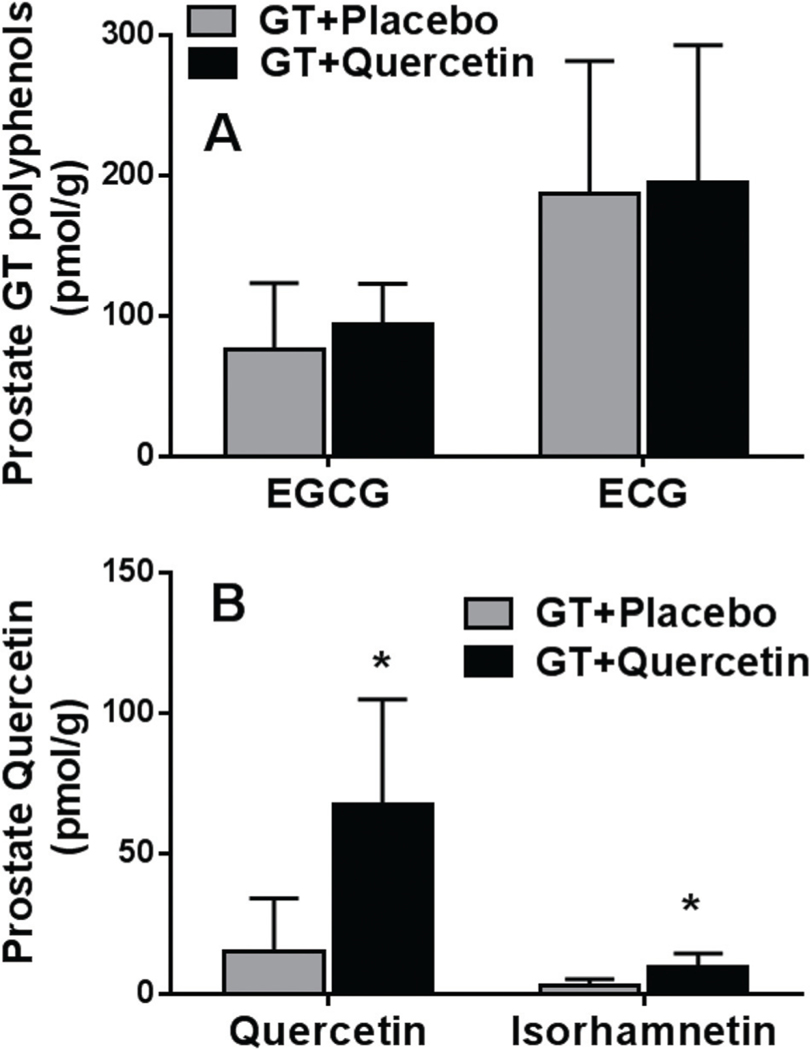
In prostatectomy tissue, EGCG and ECG were found in 67 and 93% of participants consuming GT + Q and 75 and 94% of participants consuming GT + PL, respectively. There was no difference in prostate EGCG or ECG concentration between the GT + Q and GT + PL groups (Fig. 1A). EGC and EC were found in 30 and 2% of participants, respectively, and there was no significant difference between the GT + Q and the GT + PL groups. Q and isorhamnetin concentration was significantly higher in prostate tissue in men consuming GT + Q compared to GT + PL (Fig. 1B).
Fig. 1.
Prostate concentration of tea polyphenols (A) and quercetin/isorhamnetin (B) in prostate collected at time of surgery. Data are mean ± SD. *p-Value (<0.05) reflect comparison of GT + Q with GT + PL intervention. Number of participants: GT + PL = 16, GT + Q = 15. The concentration of EGC and EC was below the detection limit in the majority of prostate samples. We did not detect MeEGCG and MeEGC in the prostate.
In week three, two hours after consumption of the GT + PL or GT + Q supplements (T = 2), plasma concentration of EGC, EC, 4′-MeEGC, EGCG, ECG, and catechin (C)-sum were significantly increased 3.7, 12.3, 16.1, 1.8, 7.5 and 4.2 fold in the GT + PL group and 5.5, 8.2, 6.3, 4.3, 9.1 and 6.2 fold in the GT + Q group relative to T = 0 in both groups (Table 3). Plasma 4″-MeEGCG levels were below detection limit in both groups. Repeated measures ANOVA model was used to assess within group and between group comparisons for plasma concentrations of GTPs and Q (Table 3). Concentrations of GTPs in both groups were increased at T = 2 after capsule intake compared to T0, but only plasma 4′-MeEGC reached statistical significance (p = 0.038) (Table 3). The two hour plasma concentration of EGC showed a trend towards a greater increase from baseline in participants consuming GT + Q compared to GT + PL (interaction p = 0.066). There was no significant effect of Q supplementation for the other GTP plasma concentrations (Table 3).
Table 3.
Plasma concentration of tea polyphenols collected during the third week of GT/Q intervention
| GT − placebo |
GT − quercetin |
Repeated measures ANOVA (interaction) | Repeated measures ANOVA (group) | Repeated measures ANOVA (time) | |||
|---|---|---|---|---|---|---|---|
| T0 nmol L−1 | T2 nmol L−1 | T0 nmol L−1 | T2 nmol L−1 | p-Value | p-Value | p-Value | |
| EGC | 63.5 (114.0)a | 233.7 (141.7)b | 70.5 (121)a | 385.7 (220.9)b | 0.066 | 0.063 | <0.001 |
| EC | 24.5 (57.1)a | 301.4 (203.0)b | 42.5 (65.5)a | 349.5 (183.9)b | 0.650 | 0.396 | <0.001 |
| MeEGC | 3.9 (15.3)a | 62.6 (59.9)b | 16.9 (36.2)a | 106.1 (58.3)b | 0.154 | 0.038 | <0.001 |
| EGCG | 96.7 (189.0)a | 172.2 (103.7)a | 31.2 (48.6)a | 135.1 (111.0)b | 0.533 | 0.182 | 0.001 |
| ECG | 8.4 (17.5)a | 63.3 (16.0)b | 7.4 (13.5)a | 67.1 (28.3)b | 0.620 | 0.771 | <0.001 |
| C-Sum | 197.1 (303)a | 833.2 (311)b | 168.6 (212)a | 1043.5 (477)b | 0.131 | 0.334 | <0.001 |
| Q | 15.1 (15.3)a | 30.4 (24.4)b | 175 (92.1)c | 419.7 (189.7)d | <0.001 | <0.001 | <0.001 |
| IsoR | 9.4 (7.6)a | 10.7 (8.8)a | 43.3 (20.8)b | 57.5 (21.8)c | <0.001 | <0.001 | <0.001 |
Data are mean ± SD. A repeated measures ANOVA model was used. Values not sharing a common letter differ significantly by Tukey/Kramer post-hoc test across all values. Number of participants: GT + PL = 16, GT + Q = 15. Quercetin (Q), isorhamnetin (IsoR), catechin sum (C-sum).
In the GT + Q group, plasma Q concentrations were significantly increased 12 to 14-fold at T = 0 and T = 2 and plasma isorhamnetin (IsoR) 5-fold compared to the GT + PL group (Table 3). Participants in the GT + PL group also had low levels of Q and IsoR, likely because Q is ubiquitous in fruits and vegetables.
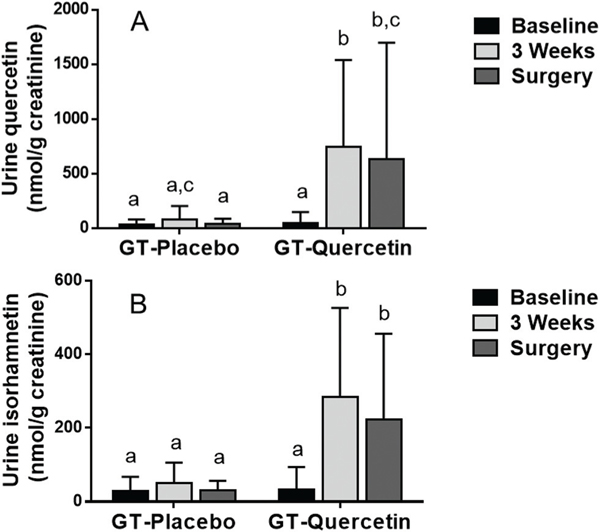
Urine EGC, EC and 4′-MeEGC concentrations were significantly increased at 3-weeks and the day of surgery in both groups (GT + Q and GT + PL) compared to baseline (Fig. 2). In urine collected on the day of surgery there was a trend for lower EGC levels in the GT + Q group compared to GT + PL (p = 0.053; Fig. 2A). Urine Q concentrations were increased significantly at 3 weeks and on the day of surgery compared to baseline in the GT + Q (Fig. 3A). Urine Q at 3 weeks and on the day of surgery was also significantly higher in the GT + Q compared to the GT + PL group (Fig. 3A). Urine IsoR was increased significantly at 3 weeks and on the day of surgery compared to baseline in the GT + Q group and compared to the GT + PL group (Fig. 3B).
Fig. 3.
Urine quercetin (A) and isorhamnetin (B) concentration calculated per g of creatinine in urine collected at baseline (prior to GT/Q intervention), 3 weeks and morning of surgery. Data are mean ± SD. A repeated measures ANOVA model was used. When a significant interaction was observed, Tukey/Kramer test was used for post-hoc comparisons across all values. Values not sharing a common letter differ significantly by Tukey/Kramer post-hoc test. Number of participants: GT + PL = 16, GT + Q = 15.
3.3. COMT and DNMT enzyme activity and protein and gene expression
Supplementation with GT + Q compared to GT + PL did not lead to changes in gene and protein expression or enzyme activity of COMT and DNMT in prostate tissue and red blood cells (Tables 4 and 5). There was no difference in plasma PSA between groups (Table 5).
Table 4.
Effect of combination of GT + Q on methylation biomarkers compared to GT + PL control in prostate collected at surgery
| GT + placebo | GT + quercetin | p-Value | |
|---|---|---|---|
| Prostate COMT activity (pmol mg−1 min−1 vanillic acid) | 43.9 ± 60.5 | 35.1 ± 18.1 | 0.539 |
| Prostate membrane COMT protein expression (FC) | 1.01 ± 0.85 | 0.98 ± 0.70 | 0.916 |
| Prostate soluble COMT protein expression (FC) | 0.5 ± 0.4 | 0.5 ± 0.5 | 0.816 |
| Prostate COMT gene expression (FC) | 1.06 ± 0.40 | 1.08 ± 0.24 | 0.822 |
| Prostate DNMT1 gene expression (FC) | 1.13 ± 0.59 | 1.00 ± 0.64 | 0.552 |
Data are mean ± SD. p-Value reflect comparison of GT + Q with GT + PL intervention using Student’s t-test. Green tea (GT), quercetin (Q), placebo (PL), fold change (FC), catechol-O-methyltransferase (COMT), DNA (cytosine-5) methyltransferase 1 (DNMT1). Number of participants: GT + PL = 16, GT + Q = 15.
Table 5.
Effect of combination of GT + Q on methylation biomarkers and serum PSA compared to GT + PL control
| GT + placebo | GT + quercetin | Repeated measures ANOVA (interaction) p-value | Repeated measures ANOVA (group) p-value | Repeated measures ANOVA (time) p-value | |||
|---|---|---|---|---|---|---|---|
| T0 | T2 | T0 | T2 | ||||
| RBC COMT (pmol mg−1 min−1 vanillic acid) | 2.6 ± 1.5 | 2.3 ± 1.0 | 2.3 ± 1.1 | 2.3 ± 0.8 | 0.561 | 0.657 | 0.270 |
| Baseline | 3-weeks | Baseline | 3-weeks | ||||
| Serum PSA (ng mL−1) | 13.9 ± 9.0 | 12.6 ± 7.1 | 13.1 ± 6.6 | 13.1 ± 7.3 | 0.335 | 0.954 | 0.398 |
RBC COMT activity was determined in RBCs collected during week 3 before (T0) and 2 hours after (T2) capsule intake. Serum PSA was determined in serum collected at baseline (prior to GT/Q intervention) and at T0 during week 3. Data are mean ± SD. A repeated measures ANOVA model was used. Green tea (GT), quercetin (Q), P placebo (PL), fold change (FC), catechol-O-methyltransferase (COMT). Number of participants: GT + PL = 16, GT + Q = 15.
4. Discussion
Based on prior in vitro and mouse studies in our lab demonstrating that chronic consumption of Q increased GTP levels and decreased methylated GTP levels in xenograft prostate cancer tissue, we hypothesized we would see the same effects in prostate tissue in men undergoing radical prostatectomy.26 We did not find this to be the case. Q supplementation (combined with GTE) for 3-weeks did not result in a significant increase in EGCG and ECG concentrations or a decrease in methylated GTPs in prostate tissue. In the prior mouse studies, at higher Q dosing (0.4% of diet), there was a significant effect on GTP and methylated levels in xenograft tissue, whereas at lower Q dosing (0.2% of diet – equivalent to a daily intake of 1.5 g of Q for an adult) combining GT and Q did not change the GTP and GTP methylated levels.26 Possibly higher Q dosing in humans will achieve the hypothesized effects. Given the lack of toxicity of GTE combined with Q in the present study, future trials with higher Q dosing are indicated. To our knowledge this is the first study to measure human prostate Q levels, which were similar in concentration (67 ± 37 pmol g−1) to EGCG (75 ± 94) and lower compared to ECG (187 ± 195 pmol g−1) in the GTE + Q group. Prior studies in pigs supplemented with 50 mg kg−1 Q reported higher Q levels in tissues involved in metabolism and excretion (intestine, liver and kidney) and low levels in non-metabolizing organs (lung and muscle)32. Since the prostate is also not a metabolizing organ, this may explain low Q levels found in the present study. Knowledge of the human prostate tissue concentrations will be informative in designing and comparing findings in future trials.
Although there was no significant difference in prostate tissue levels, there was a trend for increased plasma EGC (p = 0.066) levels in the GT + Q group when comparing the 3-week T0 vs. T2 (2 hours after capsule intake) time points relative to the GT + PL group. Redan BW et al. previously reported an increase in GT flavan-3-ol uptake resulting from prior exposure of intestinal cells to GT and grape seed extract.33 Therefore, we hypothesize that prior intestinal exposure to Q and GTP might lead to increased intestinal absorption of GTPs. In addition, there was a trend for decreased urine EGC levels in men consuming GT + Q compared to GT + PL in urine collected on the morning of surgery (p = 0.053). These findings point to an effect of Q on the metabolism of GTPs. In general, plasma concentration and urinary excretion of GTPs and Q depends on their conjugation. Q and non-gallated GTPs (EC, EGC) are mostly detected in plasma in glucuronidated and sulfated form enhancing renal excretion.34 Glucuronidation of polyphenols mostly takes place in intestinal epithelium and liver.35 Possibly, when consumed at the same time GTPs and Q compete for UDP-glucuronosyltransferase and sulfotransferase enzyme activity, which might lead to a decrease in glucuronidation and renal excretion of EGC and EC, explaining the changes in plasma and urine EGC and 4′-MeEGC concentration. In addition it has been observed previously by radioactive labeling of EGCG, that EGCG in the rat intestine is converted to EGC and phenylvalerolactone by intestinal bacteria.36 Possibly the simultaneous presence of tea catechins and Q in the intestine might increase the bacterial activity and enhance the conversion of EGCG to EGC leading to the observed increase in plasma EGC. Overnight fasting prior to surgery may also potentially impact on GTP metabolism and urinary excretion. A lack of fluid intake might lead to more concentrated urine and increased concentrations rather than the observed decreased urine EGC concentration (Fig. 2A). However, GTPs and Q were expressed per gram of creatinine, which should compensate for difference in urine volume.
GTPs and Q have been shown to inhibit DNA and catechol methylation in tissue culture studies and mouse models.20,21 Our previous mouse study also demonstrated that administration of GT together with Q inhibited the DNMT1 and COMT activity compared to GT alone.26 In the present human study, however, we did not observe any changes in methylation activity when men consumed GT + Q compared to GT + PL. Differences in mouse and human methylation activity may contribute to these disparate findings. There are basic differences in the degree of methylation of Q and GTP between human and mice. The human methylation rate was reported to be similar to pigs.32 In mice, prostate tumor isorhamnetin, the methylated form of Q, was 5-fold higher when Q was administered with GT,26 whereas in the present human study, plasma and prostate isorhamnetin was 10-fold lower compared to Q in men consuming GT + Q.32 The 10-fold lower concentration of isorhamnetin compared to Q in blood after intake of a dietary Q source (onions), was also observed by other investigators.37 In addition, in the present study the methylated form of EGC was about 3 to 4 fold lower compared to EGC while in our mouse study methylated GTP was present in about the same concentration as non-methylated GTP. This difference in methylation of polyphenols between human and mice might have contributed to the lack of effect of the combined GT + Q supplementation in the present study compared to our previous mouse study.
Our study had a number of shortcomings. We did not record participant food intake. Dietary intake of foods containing GT or Q would have been useful to interpret inter-individual variation in plasma, urine and prostate concentrations of GTP or Q. We determined total GTPs and therefore do not know the degree of glucuronidation/sulfation, which might have affected renal excretion. In addition the delayed time between capsule intake and prostate tissue collection might have lowered prostate GTP concentrations since the last intake of GT and Q capsules was the evening prior to the day of surgery. Another potential problem with the study design might be that men in the trial all had prostate cancer. It has been shown that multidrug resistance-associated proteins (MRP1/MRP2) are overexpressed in human tumors and therefore our results might not be generalizable to men without prostate cancer.38 In addition, the lack of control groups receiving placebo only, or placebo plus quercetin would have added additional information on quercetin tissue uptake and metabolism. In hindsight, basing our power calculation on previous mouse tissue concentrations did not provide appropriate group size numbers. Larger numbers of participants or possibly higher doses of Q will be required in future investigations.
5. Concluding remarks
In a three-week pre-prostatectomy intervention, 800 mg of Q supplementation combined with 1000 mg of GTE for 4 weeks did not result in a significant increase in EGCG and ECG concentrations or a decrease in methylated GTPs in prostate tissue as compared to men receiving placebo with GTE. Q may effect GTP metabolism (glucuronidation) as evidenced by decreased urinary levels of EGC and 4′-MeEGC on the day of surgery, and a trend for increased plasma EGC levels as compared to the placebo group. Further studies are warranted evaluating Q effects on GTP metabolism and biological and chemopreventive effects of the Q + GTP combination on prostate tissue.
Supplementary Material
Acknowledgements
We thank Dr Steve Wood from Pharmanex/NuSkin and Nature’s Life for providing the supplements and advice on study design and capsule content. We also thank the men who participated in the study.
SMH, WJA and PW designed and performed the study. RPL and AT performed HPLC analyses. GH, JY, EMG performed methylation studies. AL, MH and TG performed data analysis. SMH, WJA, DH and ZL prepared the manuscript. All authors have contributed to and approved the final manuscript. This work was supported by National Institute of Health [R03CA171583; P50CA092131 and RO1CA231219].
Abbreviations
- C
Catechin
- COMT
Catechol-O-methyltransferase
- DNMT
DNA (cytosine-5) methyltransferases
- EGCG
Epigallocatechin gallate
- EC
Epicatechin
- ECG
Epicatechin gallate
- EGC
Epigallocatechin
- IsoR
Isorhamnetin
- Q
Quercetin
- GTE
Green tea extract
- PL
Placebo
- 4′-MeEGC
4′-O-Methyl epigallocatechin
- GTP
Green tea polyphenols
- RBC
Red blood cell
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0fo00565g
Conflicts of interest
There are no conflicts of interest to declare.
References
- 1.Henning SM, Wang P, Said J, Magyar C, Castor B, Doan N, Tosity C, Moro A, Gao K, Li L. and Heber D, J. Nutr. Biochem, 2012, 23, 1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang CS, Wang H, Li GX, Yang Z, Guan F. and Jin H, Pharmacol. Res, 2011, 64, 113–122. [DOI] [PubMed] [Google Scholar]
- 3.Hashibe M, Galeone C, Buys SS, Gren L, Boffetta P, Zhang ZF and La Vecchia C, Br. J. Cancer, 2015, 113, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee PMY, Ng CF, Liu ZM, Ho WM, Lee MK, Wang F, Kan HD, He YH, Ng SSM, Wong SYS and Tse LA, Prostate Cancer Prostatic Dis., 2017, 20, 318–322. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi N, Ohmori K, Shimazu T, Nakaya N, Kuriyama S, Nishino Y, Tsubono Y. and Tsuji I, Br. J. Cancer, 2006, 95, 371–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YF, Xu Q, Lu J, Wang P, Zhang HW, Zhou L, Ma XQ and Zhou YH, Eur. J. Cancer Prev, 2015, 24, 353–362. [DOI] [PubMed] [Google Scholar]
- 7.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G. and Corti A, Cancer Res., 2006, 66, 1234–1240. [DOI] [PubMed] [Google Scholar]
- 8.Kumar NB, Pow-Sang J, Egan KM, Spiess PE, Dickinson S, Salup R, Helal M, McLarty J, Williams CR, Schreiber F, Parnes HL, Sebti S, Kazi A, Kang L, Quinn G, Smith T, Yue B, Diaz K, Chornokur G, Crocker T. and Schell MJ, Cancer Prev. Res, 2015, 8, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henning SM, Wang P, Said JW, Huang M, Grogan T, Elashoff D, Carpenter CL, Heber D. and Aronson WJ, Prostate, 2015, 75, 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT and Yang CS, Drug Metab. Dispos, 2003, 31, 452–461. [DOI] [PubMed] [Google Scholar]
- 11.Lu H, Meng X. and Yang CS, Drug Metab. Dispos, 2003, 31, 572–579. [DOI] [PubMed] [Google Scholar]
- 12.Henning SM, Choo JJ and Heber D, J. Nutr, 2008, 138, 1529S–1534S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D. and Henning SM, Cancer Prev. Res, 2010, 3, 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, Chan TH and Dou QP, J. Cell. Physiol, 2007, 213, 252–260. [DOI] [PubMed] [Google Scholar]
- 15.Manach C, Scalbert A, Morand C, Remesy C. and Jimenez L, Am. J. Clin. Nutr, 2004, 79, 727–747. [DOI] [PubMed] [Google Scholar]
- 16.Shoskes DA and Nickel JC, Urol. Clin. North Am, 2011, 38, 279–284. [DOI] [PubMed] [Google Scholar]
- 17.Cialdella-Kam L, Ghosh S, Meaney MP, Knab AM, Shanely RA and Nieman DC, Nutrients, 2017, 9(7), pii: E773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Zhang M, Feng J, Fan A, Zhou Y. and Xu Y, Int. J. Environ. Res. Public Health, 2017, 14, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K, Kouretas D, Tzanakakis G, Nikitovic D, Anisimov NY, Spandidos DA, Tsatsakis AM and Rezaee R, Oncol. Rep, 2017, 38, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee WJ, Shim JY and Zhu BT, Mol. Pharmacol, 2005, 68, 1018–1030. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez MC, Maso V, Torello CO, Ferro KP and Saad STO, Clin. Epigenet, 2018, 10, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK and Yang CS, Clin. Cancer Res, 2005, 11, 7033–7041. [DOI] [PubMed] [Google Scholar]
- 23.Pandey M, Shukla S. and Gupta S, Int. J. Cancer, 2010, 126, 2520–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz M, Paul F, Moobed M, Baumann G, Zimmermann BF, Stangl K. and Stangl V, Eur. J. Pharmacol, 2014, 740, 645–651. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, Heber D. and Henning SM, Nutr. Cancer, 2012, 64, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P, Vadgama JV, Said JW, Magyar CE, Doan N, Heber D. and Henning SM, J. Nutr. Biochem, 2014, 25, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi EH, Lee DY, Kim S, Chung JO, Choi JK, Joo KM, Jeong HW, Kim JK, Kim WG and Shim SM, Food Funct, 2017, 8, 3664–3674. [DOI] [PubMed] [Google Scholar]
- 28.Lu NT, Crespi CM, Liu NM, Vu JQ, Ahmadieh Y, Wu S, Lin S, McClune A, Durazo F, Saab S, Han S,Neiman DC, Beaven S. and French SW, Phytother. Res, 2016, 30, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhagwat S, Haytowitz DB and Holden JM, USDA Database for the Flavonoid Content of Selected Foods, https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03-1.pdf. [Google Scholar]
- 30.Henning SM, Yang J, Hsu M, Lee RP, Grojean EM, Ly A, Tseng CH, Heber D. and Li Z, Eur. J. Nutr, 2018, 57, 2759–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuomainen P, Reenila I. and Mannisto PT, J. Pharm. Biomed. Anal, 1996, 14, 515–523. [DOI] [PubMed] [Google Scholar]
- 32.Bieger J, Cermak R, Blank R, de Boer VC, Hollman PC, Kamphues J. and Wolffram S, J. Nutr, 2008, 138, 1417–1420. [DOI] [PubMed] [Google Scholar]
- 33.Redan BW, Chegeni M. and Ferruzzi MG, Food Funct, 2017, 8, 111–121. [DOI] [PubMed] [Google Scholar]
- 34.Kawai Y, J. Med. Invest, 2018, 65, 162–165. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka S, Oyama M, Nishikawa M, Ikushiro S. and Hara H, Biosci., Biotechnol., Biochem, 2018, 82, 2118–2129. [DOI] [PubMed] [Google Scholar]
- 36.Kohri T, Matsumoto N, Yamakawa M, Suzuki M, Nanjo F, Hara Y. and Oku N, J. Agric. Food Chem, 2001, 49, 4102–4112. [DOI] [PubMed] [Google Scholar]
- 37.Burak C, Brull V, Langguth P, Zimmermann BF, Stoffel-Wagner B, Sausen U, Stehle P, Wolffram S. and Egert S, Eur. J. Nutr, 2017, 56, 343–353. [DOI] [PubMed] [Google Scholar]
- 38.Hong J, Lambert JD, Lee SH, Sinko PJ and Yang CS, Biochem. Biophys. Res. Commun, 2003, 310, 222–227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.