Abstract
Purpose
The purpose of this study is to evaluate the frequency of viral and bacterial respiratory pathogens detected by molecular methods in sputum samples of patients hospitalized for COVID-19 and to evaluate its impact on mortality and unfavorable outcomes (in-hospital death or mechanical ventilation).
Patients and Methods
The prospective cohort included patients with diagnosis of COVID-19 hospitalized at Hospital Nacional Hipólito Unanue. Sociodemographic and clinical data were collected from clinical records. Sputum samples were analyzed with the Biofire Filmarray Pneumonia plus® respiratory panel. Crude and adjusted associations with unfavorable outcomes were evaluated using logistic regression models.
Results
Ninety-three patients who were able to collect sputum samples were recruited between September 8 and December 28, 2020. The median age was 61.7 years (IQR 52.3–69-8) and 66 (71%) were male. The most frequent symptoms were dyspnea, cough, fever, and general malaise found in 80 (86%), 76 (82%), 45 (48%), and 34 (37%) patients, respectively. Fifty-three percent of patients had comorbidities. Seventy-six (82%) patients received antibiotics prior to admission and 29 (31%) developed unfavorable outcome. Coinfection was evidenced in 38 (40.86%) cases. The most frequently found bacteria were Staphylococcus aureus, Streptococcus agalactiae, Haemophilus influenzae and Klebsiella pneumoniae in 11 (11.83%), 10 (10.75%), 10 (10.75%), and 8 (8.6%) cases, respectively. Streptococcus pneumoniae was found in one case (1.08%). We neither identify atypical bacteria nor influenza virus. No association was found between the presence of viral or bacterial microorganisms and development of unfavorable outcomes (OR 1.63; 95% CI 0.45–5.82).
Conclusion
A high frequency of respiratory pathogens was detected by molecular methods in patients with COVID-19 pneumonia but were not associated with unfavorable outcomes. No atypical agents or influenza virus were found. The high use antibiotics before admission is a concern. Our data suggest that the use of drug therapy against atypical bacteria and viruses would not be justified in patients hospitalized for COVID-19.
Keywords: SARS-CoV-2, coinfection, molecular biology, mortality, COVID-19
Introduction
The COVID-19 pandemic has greatly impacted our society, pushing health systems to the limit. It has been reported that up to 17% of patients with COVID-19 attending emergency wards present serious illness,1 requiring the use of the Intensive Care Unit (ICU) and, eventually, mechanical ventilation. It is estimated that mortality from symptomatic COVID-19 can be up to 5%,2 depending on populations reported and diagnostic methods used. However, in hospitalized patients, mortality can exceed 20%,3,4 reaching around 50% in the Peruvian series.5
Known risk factors for severe disease are obesity, hypertension, diabetes mellitus, coronary heart disease, among others.6 Noteworthy, the role of co-infections by other respiratory pathogens is still unclear. Although in other viral pneumonias such as influenza, coinfection by bacteria such as S. pneumoniae and S. aureus play an important role in mortality and complications,7,8 studies on COVID-19 are still insufficient and there is no clear evidence to guide empirical antibiotic therapy currently used in many settings.
The prevalence of bacterial and viral coinfection has been reported to be up to 45% in COVID-19 cases.9 However, each geographic region has a particular microbiological profile,10 which could influence clinical characteristics of these coinfections. Since this aspect has not been explored in Latin American countries, it is necessary to study pathogens possibly associated with coinfection in COVID-19 cases. As bacterial cultures are scarce and difficult to perform in a pandemic context, the use of molecular platforms that allow rapid and accurate detection of a wide range of pathogens is an interesting option. Although the cost of such platforms precludes its routinary use in constrained-resource settings, they can be used for epidemiological surveillance purposes. The objective of our study was to evaluate the frequency of viral and bacterial respiratory pathogens detected by molecular methods in sputum samples of patients hospitalized for COVID-19 and to evaluate their impact on mortality and unfavorable outcomes including death and mechanical ventilation.
Materials and Methods
Design and Type of Study
This observational cohort study was designed to evaluate the frequency of respiratory pathogens and its association with unfavorable clinical outcomes in patients hospitalized due to COVID-19 pneumonia between September and December 2020 at Hospital Nacional Hipólito Unanue, a reference Hospital in Lima, Peru. Cohort included patients hospitalized in clinical wards specifically devoted for the care of patients with COVID-19 included within the first 2 days of hospital admission. In all of them, sociodemographic and clinical characteristics, including previous use of antibiotics and relevant laboratory results, were obtained from patient´s clinical records. Participating patients were invited to collect a sputum sample for molecular detection of viral and bacterial pathogens. Patients were subsequently followed up until discharge or death. For the main comparison, exposure was defined based on the presence or absence of respiratory pathogens at the time of the initial evaluation of the patient, while the primary outcome included mortality or the need of mechanical ventilation.
Population and Sample Size
The population included hospitalized patients diagnosed with COVID-19 pneumonia at Hospital Nacional Hipólito Unanue between September and December 2020. A non-probabilistic sampling was carried out, successively including eligible participants until the sample size was completed. We calculated sample size for a single proportion with a predefined precision level using the free software openepi (https://www.openepi.com/Menu/OE_Menu.htm). Given the lack of data at the moment of protocol development, it was assumed a coinfection prevalence of 20 ± 10% and a confidence level of 95%, giving a minimum sample size of 62 participants.
Recruitment Process
Participants were informed about the study and requested an informed consent. Spontaneous sputum samples obtained from hospitalized patients were analyzed for the presence of bacterial and viral respiratory pathogens. We included patients with a diagnosis of COVID-19 confirmed by a positive molecular test or a compatible clinical picture in the presence of a positive serology or tomographic changes suggestive of pneumonia associated with COVID-19. Patients with poor-quality (salivary) sputum samples, who decide to withdraw from the study after being included, or whose medical records did not include information about the outcomes of interest were excluded. One sputum sample per patient was included. No conventional cultures were performed.
Variables
Mortality or need for mechanical ventilation was considered as the primary combined outcome. The independent variables were the viral or bacterial coinfections detected by FilmArray® (BioMérieux, Marcy-l´Étoile, France). Respiratory pathogens detected by the platform are shown in Table 1. We also obtained information about sex, age, previous use of antibiotics, and laboratory data including leukocyte count, lymphocyte count, platelet count, aspartate (AST) and alanine (ALT) aminotransferases, albumin, alkaline phosphatase, gamma glutamyl transpeptidase, D-dimer and lactate dehydrogenase (LDH).
Table 1.
Respiratory Pathogens Analyzed Using the Molecular Platform FilmArray®
| Bacteria | Acinetobacter calcoaceticus-baumannii complex, Klebsiella oxytoca, Serratia marcescens, Enterobacter cloacae complex, Klebsiella pneumoniae group, Staphylococcus aureus, Escherichia coli, Moraxella catarrhalis, Streptococcus agalactiae, Haemophilus influenzae, Proteus spp., Streptococcus pneumoniae, Klebsiella aerogenes, Pseudomonas aeruginosa and Streptococcus pyogenes. |
| Atypical Bacteria | Chlamydia pneumoniae, Legionella pneumophila and Mycoplasma pneumoniae |
| Virus | Adenovirus, human rhinovirus/enterovirus, parainfluenza virus (1 to 4), Coronavirus (229E,HKU1,OC43,NL63), Influenza A (H1, H-2009,H3) respiratory syncytial virus, human metapneumovirus and Influenza B. |
| Antibiotic Resistance Genes | CTX-M, NDM, mecA/C and MREJ IMP, OXA-48, KPC and VIM |
Description of the Procedures and Methods
Sputum samples were placed in sterile 5 mL polypropylene tubes and transported to the National Institute of Health, maintaining the cold chain (2–8°C), for the identification of viral and bacterial etiological agents using the FilmArray ® molecular platform (BioMérieux, Marcy-l’Étoile, France). 33 respiratory pathogens were analyzed including 18 bacteria, 9 viruses, and 7 genes associated with antimicrobial resistance (Table 1) using Biofire Filmarray Pneumonia plus (https://www.biomerieux-diagnostics.com/biofire-filmarray-pneumonia-panel). The FilmArray molecular methodology is based on a multiplex PCR system certified by the FDA, CE-IVD, and the TGA, integrating 1) purification of genetic material based on magnetic beads; 2) reverse transcription and multiplex PCR, where the target nucleic acids present in the sample are enriched; 3) a nested PCR using primers internalized to the specific products (in triplicate) of the multiplex PCR step; and 4) DNA fusion analysis where a melting curve is generated and the system detects the specific melting temperatures for each etiological agent. This method includes a control of the transcription process directed to a transcript from the yeast Schizosaccharomyces pombe. Yeast is lyophilized in the system and rehydrates when the sample is loaded. Additionally, the FilmArray methodology includes a nested PCR control directed at a target DNA that is lyophilized on the system matrix. The whole process requires only 2 minutes of manipulation, with a total execution time of 1 hour. Its sensitivity and specificity for sputum samples have been calculated at 96.3% and 97.2%, respectively.11
The study included positive patients for COVID-19 confirmed by the molecular RT-PCR method at the National Institute of Health or with a compatible clinical picture and a positive rapid test for IgM antibodies or a bilateral ground glass imaging tomography compatible with pneumonia associated to COVID-19. The information about the outcomes was obtained from the review of the hospital’s medical records.
RT-qPCR SARS-CoV-2
RT-qPCR assays were performed at the National Institute of Health - Peru (NIH-Peru) following the Charité-Berlin protocol,12 according to PAHO´s recommendations.13 Briefly, RT-qPCR targeting the SARS-CoV-2-specific RNA-dependent RNA polymerase (RdRp) gene and glyceraldehyde-3-phosphate dehydrogenase gene (glyceraldehyde-3-phosphate dehydrogenase—GAPDH) (internal control), was performed in a 20 μL reaction mixture containing 5 μL of template RNA and the primers/probes (Table 2) in Capital qPCR Probe Mix 4X (Biotechrabbit GmbH, Hennigsdorf, Germany) using the Rotor Gene Q thermal cycler (Qiagen GmbH, Hilden, Germany).
Table 2.
Primers Sequences and Probes for RT-qPCR Target Genes for Detection of SARS-CoV-2
| Gene | Primer/Probe | Sequence 5’→ 3’ |
|---|---|---|
| RdRp | RdRp_SARSr-F | GTGARATGGTCATGTGTGGCGG |
| RdRp_SARSr-R | CARATGTTAAASACACTATTAGCATA | |
| RdRp_SARSr-P2 | FAM-CAGGTGGAACCTCATCAGGAGATGC- BHQ1 | |
| GAPDH | GAPDH_F | GTGAAGGTCGGAGTCAACGG |
| GAPDH_R | TCAATGAAGGGGTCATTGATG | |
| GAPDH_P | ROX – CGCCTGGTCAACAGGGTCGC- BHQ2 |
Note: Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Data from Corman et al.12
Data Analysis and Processing
The data were entered into a database in Microsoft Excel and analyzed using the statistical package STATA v15 for Windows (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Numerical variables were presented as median and interquartile range (IQR). Categorical variables were presented as frequencies and percentages. The percentage of respiratory pathogens was determined by dividing the number of patient cases in which one or more respiratory pathogens were identified by the total number of patients included. The association between the presence of respiratory bacterial or viral pathogens and the main outcome was analyzed using the Chi-square statistic or the Fisher’s exact test. The association with numerical variables was analyzed with Student’s t or Mann Whitney test according to the distribution of the data. In addition, an exploratory multivariate analysis was performed using multiple logistic regression to explore the association between the presence of respiratory pathogens and unfavorable outcomes, adjusted for possible confounding variables. Finally, the association between the presence of coinfection with any bacteria and the time until the development of unfavorable outcomes was evaluated, comparing the survival curves using the log rank test. A value of p <0.05 was considered statistically significant.
Ethical Aspects
The protocol was approved by ethics committees of the Peruvian National Institute of Health (090–2020-CIEI_INS) and Universidad Ricardo Palma (PI-018-2020). Written informed consent was obtained from all participants before recruitment. The study was conducted in accordance with the Declaration of Helsinki.
Results
Ninety-three patients were recruited between September 8 and December 28, 2020. The median age was 61.7 years (IQR 52.3 to 69.8) and 71.0% were males. The most frequent symptoms (Tables 3 and 4) were dyspnea (86.0%), cough (81.7%), fever (48.4%) and general malaise (36.6%). 52.7% of patients presented some comorbidity, the most frequent being arterial hypertension (21.5%) and diabetes mellitus (18.3%). Most of the patients received prior medication. The most widely used drug was ivermectin (72.0%). 81.7% had received at least one antibiotic and 68.8% received 2 or more antibiotics. The most widely used antibiotic was azithromycin (62.4%). Most of patients presented bilateral interstitial infiltrates on the chest radiography (Tables 5 and 6). Sixty-nine (74.2%) patients had a diagnosis confirmed by molecular testing. Twenty-nine patients (31.2%) developed unfavorable outcomes (death or mechanical ventilation) of which 20 (21.5%) died, and 13 (14%) required mechanical ventilation.
Table 3.
Sociodemographic Characteristics, Symptoms, Comorbidities, and Treatments Before Admission in Patients Hospitalized with COVID-19 Pneumonia (n=93)
| Variable | Total (n=93) | Unfavorable Outcome (n=29) | Non Unfavorable Outcome (n=64) | p value |
|---|---|---|---|---|
| Age in years | 61.7 (52.32–69.77) | 66.16 (52.99–72.86) | 61.4 (51.53–67.78) | 0.113 |
| Male gender | 66 (70.97) | 22(75.86) | 44 (68.75) | 0.486 |
| Time of symptoms | 7 (6–10) | 7 (7–10) | 7 (6–10) | 0.908 |
| Symptoms | ||||
| Dyspnea | 80 (86.02) | 28 (96.55) | 52 (81.25) | 0.057 |
| Cephalalgia | 23 (24.73) | 8 (27.59) | 15 (24.1) | 0.668 |
| Cough | 76 (81.72) | 23 (79.31) | 53 (82.81) | 0.686 |
| Fever | 45 (48.39) | 17 (58.62) | 28 (43.75) | 0.184 |
| Throat pain | 19(20.43) | 10(34.48) | 9(14.06) | 0.024 |
| Abdominal pain | 2 (2.15) | 1 (3.45) | 1 (1.56) | 0.569 |
| Diarrhea | 7 (7.53) | 1 (3.45) | 6 (9.38) | 0.428 |
| Nasal discharge | 2 (2.15) | 0 (0) | 2 (3.13) | 0.336 |
| Dysgeusia or anosmia | 4 (4.30) | 3 (10.34) | 1 (1.56) | 0.088 |
| General malaise | 34 (36.56) | 8 (27.59) | 26 (40.63) | 0.226 |
| Other symptoms | 14 (15.05) | 4 (13.79) | 10 (15.63) | 0.819 |
| Comorbidities | ||||
| Diabetes mellitus | 17 (18.28) | 5 (17.24) | 12 (18.75) | 0.862 |
| Hypertension arterial | 20 (21.51) | 7 (24.14) | 13(20.31) | 0.677 |
| Obesity | 8 (8.60) | 3 (10.34) | 5 (7.81) | 0.687 |
| Other comorbidities | 15 (16.13) | 5 (17.24) | 10 (15.63) | 0.844 |
| Any comorbidity | 49 (52.69) | 15 (51.72) | 34 (53.13) | 0.900 |
| Previous treatments | ||||
| Azithromycin | 58 (62.37) | 17 (58.62) | 41 (64.06) | 0.616 |
| Ivermectin | 67 (74.04) | 23 (79.31) | 44 (68.75) | 0.293 |
| Corticosteroids | 24 (26.37) | 7 (24.14) | 17 (27.42) | 0.741 |
| Ceftriaxone | 20 (21.51) | 7 (24.14) | 13 (20.31) | 0.677 |
| Other antibiotic | 7 (7.53) | 3 (10.34) | 4 (6.25) | 0.488 |
| Any antibiotic | 76 (81.72) | 24 (82.76) | 52 (81.25) | 0.862 |
| 2 or more antibiotics | 64 (68.82) | 19 (65.52) | 45 (70.31) | 0.644 |
| Enoxaparin | 7 (7.69) | 4 (13.79) | 3 (4.84) | 0.135 |
| NSAIDS/ASA | 2 (2.20) | 1 (3.45) | 1 (1.61) | 0.578 |
| Hydroxychloroquine | 1 (1.10) | 1 (3.45) | 0 (0.00) | 0.319 |
Abbreviation: NSAIDS/ASA, Non-steroidal anti-inflammatories/Acetylsalicylic acid.
Table 4.
Sociodemographic Characteristics, Symptoms, Comorbidities, and Treatments Before Admission in Patients Hospitalized with COVID-19 Pneumonia Confirmed by Molecular Testing (n=69)
| Variable | Total (n=69) | Unfavorable Outcome (n=24) | Non Unfavorable Outcome (n=45) | p value |
|---|---|---|---|---|
| Age in years | 61.2 (51.63–70.01) | 67.34 (52.01–73.33) | 60.05 (46.63–67.56) | 0.057 |
| Male gender | 52 (75.36) | 19 (79.17) | 33 (73.33) | 0.592 |
| Time of symptoms | 7 (6–10) | 7 (7–10) | 7 (5–10) | 0.629 |
| Symptoms | ||||
| Dyspnea | 62 (89.86) | 23 (95.83) | 39 (86.67) | 0.408 |
| Cephalalgia | 19 (27.54) | 7 (29.17) | 12 (26.67) | 0.825 |
| Cough | 58 (84.06) | 18 (75.00) | 40 (88.89) | 0.133 |
| Fever | 34 (49.28) | 14 (58.33) | 20 (44.44) | 0.272 |
| Throat pain | 17 (24.64) | 10 (41.67) | 7 (15.56) | 0.017 |
| Abdominal pain | 1 (1.45) | 1 (4.17) | 0 (0.00) | 0.348 |
| Diarrhea | 4 (5.80) | 1 (4.17) | 3 (6.67) | 1.000 |
| Nasal discharge | 1 (1.45) | 0 (0) | 1 (2.22) | 1.000 |
| Dysgeusia or anosmia | 4 (5.80) | 3 (12.50) | 1 (2.22) | 0.118 |
| General malaise | 30 (43.48) | 8 (33.33) | 22 (48.89) | 0.214 |
| Other symptoms | 11 (15.94) | 4 (16.67) | 7 (15.56) | 0.904 |
| Comorbidities | ||||
| Diabetes mellitus | 10 (14.49) | 5 (20.83) | 5 (11.11) | 0.275 |
| Hypertension arterial | 17 (24.64) | 6 (25.00) | 11 (24.44) | 0.959 |
| Obesity | 7 (10.14) | 3 (12.50) | 4 (8.89) | 0.687 |
| Other comorbidities | 11 (15.94) | 5 (20.83) | 6 (13.33) | 0.418 |
| Any comorbidity | 36 (52.17) | 14 (58.33) | 22 (48.89) | 0.454 |
| Previous treatments | ||||
| Azithromycin | 50 (72.46) | 16 (66.67) | 34 (75.56) | 0.431 |
| Ivermectin | 55 (79.71) | 19 (79.17) | 36 (80.00) | 0.935 |
| Corticosteroids | 20 (29.41) | 7 (29.17) | 13 (29.55) | 0.974 |
| Ceftriaxone | 15 (21.74) | 6 (25.00) | 9 (20.00) | 0.632 |
| Other antibiotic | 6 (8.70) | 3 (12.0) | 3 (6.67) | 0.412 |
| Any antibiotic | 62 (89.86) | 20 (83.33) | 42 (93.33) | 0.227 |
| 2 or more antibiotics | 53 (76.81) | 17 (70.83) | 36 (80.00) | 0.390 |
| Enoxaparin | 6 (8.82) | 3 (12.50) | 3 (6.82) | 0.658 |
| NSAIDS/ASA | 1 (1.47) | 0 (0.00) | 1 (2.27) | 1.000 |
| Hydroxychloroquine | 1 (1.47) | 1 (4.17) | 0 (0.00) | 0.353 |
Table 5.
Physical Examination and Radiological Findings in Patients Hospitalized with COVID-19 Pneumonia (n=93)
| Variable | Total (n=93) | Unfavorable Outcome (n=29) | Non Unfavorable Outcome (n=64) | p value |
|---|---|---|---|---|
| Physical examination | ||||
| Systolic blood pressure (mm Hg) | 112.5 (109–130) | 120 (110–130) | 112 (108–130) | 0.589 |
| Diastolic blood pressure (mm Hg) | 70 (70–80) | 70 (70–80) | 70 (70–78) | 0.237 |
| Heart rate | 98 (87.5–109) | 97 (89–110) | 99 (87–108) | 0.668 |
| Respiratory rate | 25 (24–28) | 26 (24–28) | 25 (24–28) | 0.252 |
| Temperature | 37 (37–37) | 37 (37–37) | 37 (37–37) | 0.883 |
| Oxygen saturation | 90 (85–92) | 87 (82–92) | 90 (87–92) | 0.062 |
| Laboratory Findings | ||||
| Leucocytes (x103/µL) | 12.30 (8.90–15.20) | 14.70 (11.20–15.70) | 11.15 (8.65–14.70) | 0.043 |
| Lymphocytes (x103/µL) | 0.70 (0.50–1.10) | 0.50 (0.40–0.70) | 0.80 (0.60–1.20) | 0.003 |
| D-Dimer | 0.92 (0.6–1.83) | 1.20 (0.69–4.53) | 0.85 (0.5–1.37) | 0.022 |
| Lactate Dehydrogenase | 325 (253–419) | 397 (326–554) | 290 (228–361) | <0.001 |
| C-reactive protein | 11.54 (5.07–18.41) | 11.30 (5.36–18.55) | 11.75 (5.03–17.84) | 0.938 |
| Aspartate aminotransferase | 39 (27–54.9) | 41 (31–53) | 38 (27–54.9) | 0.580 |
| Alanine aminotransferase | 44.8 (25–69.7) | 59 (32–70) | 40.5 (25–61) | 0.236 |
| Urea | 32 (23.9–41) | 37 (31.5–44.5) | 30 (22–38) | 0.007 |
| Radiographic findings | ||||
| Bilateral compromise | 76 (81.70) | 25 (86.21) | 51 (76.69) | 0.569 |
| Unilateral interstitial pattern | 19 (20.65) | 6 (20.69) | 13 (20.63) | 0.801 |
| Bilateral interstitial pattern | 65 (70.65) | 21 (72.41) | 44 (69.84) | 0.801 |
| Alveolar pattern | 15 (16.30) | 7 (24.14) | 8 (12.70) | 0.168 |
| Diagnosis of COVID-19 | 0.254 | |||
| Positive molecular test (%) | 69 (74.19) | 24 (82.76) | 45 (70.31) | |
| Positive serology + compatible clinical picture (%) | 18 (19.35) | 5 (17.24) | 13 (20.31) | |
| Typical CT scan + compatible clinical picture (%) | 6 (6.45) | 0 (0.00) | 6 (9.38) | |
Notes: Numerical variables are shown as median and interquartile range; Categorical variables are shown as frequencies and percentages.
Table 6.
Physical Examination and Radiological Findings in Patients Hospitalized with COVID-19 Pneumonia Confirmed by Molecular Testing (n=69)
| Variable | Total (n=69) | Unfavorable Outcome (n=24) | Non Unfavorable Outcome (n=45) | p value |
|---|---|---|---|---|
| Physical examination | ||||
| Systolic blood pressure (mm Hg) | 120 (111–130) | 120 (110–130) | 119 (112–130) | 0.869 |
| Diastolic blood pressure (mm Hg) | 70 (70–79) | 70 (70–80) | 70 (70–78) | 0.437 |
| Heart rate | 99 (88.5–105) | 95.5 (87.5–102.5) | 100 (89–106.5) | 0.510 |
| Respiratory rate | 25 (24–28) | 26 (24–28) | 25 (24–28) | 0.328 |
| Temperature | 37 (37–37) | 37 (37–37) | 37 (37–37.15) | 0.410 |
| Oxygen saturation | 90 (85–91.5) | 88 (82–90) | 90 (87–92) | 0.117 |
| Laboratory findings | ||||
| Leucocytes (x103/µL) | 12.00 (8.10–15.00) | 14.50 (8.55–15.50) | 10.60 (8.10–14.70) | 0.188 |
| Lymphocytes (x103/µL) | 0.70 (0.50–1.00) | 0.60 (0.50–0.80) | 0.80 (0.50–1.00) | 0.075 |
| D-Dimer | 0.87 (0.59–1.55) | 0.94 (0.62–4) | 0.72 (0.49–1.3) | 0.094 |
| Lactate Dehydrogenase | 322.5 (241–395) | 368 (321–545) | 276 (192–355) | 0.002 |
| C-reactive protein | 11.67 (5.34–17.73) | 10.79 (5.36–18.41) | 12.04 (5.32–17.68) | 0.656 |
| Aspartate aminotransferase | 41 (28–54.9) | 42 (26.5–56.5) | 40 (28–54.9) | 0.779 |
| Alanine aminotransferase | 45 (25–74) | 57.50 (27.5–72) | 42 (25–85) | 0.574 |
| Urea | 31.67 (24–39) | 37 (31–42) | 29.50 (22.50–36) | 0.022 |
| Radiographic findings | ||||
| Bilateral compromise | 60 (86.96) | 21 (87.50) | 39 (86.67) | 0.922 |
| Unilateral interstitial pattern | 7 (10.29) | 3 (12.50) | 4 (9.09) | 0.691 |
| Bilateral interstitial pattern | 54 (79.41) | 19 (79.17) | 35 (79.55) | 0.971 |
| Alveolar pattern | 9 (13.24) | 4 (16.7) | 5 (11.36) | 0.537 |
Notes: Numerical variables are shown as median and interquartile range; Categorical variables are shown as frequencies and percentages.
Respiratory pathogens were identified in 38 (40.9%) cases (Table 7), bacterial pathogens were found in 37 and viral pathogens in three cases. The most frequently found bacteria was Staphylococcus aureus (11.8%), followed by Streptococcus agalactiae (10.8%), Haemophilus influenzae (10.8%), Klebsiella pneumoniae (8.6%), Escherichia coli (6.5) and Enterobacter cloacae (5.4). Streptococcus pneumoniae was found in only 1 case (1.1%). We did not find cases of atypical bacteria such as Mycoplasma pneumoniae, Chlamydia pneumoniae, or Bordetella pertussis nor influenza, parainfluenza or respiratory syncytial virus infection.
Table 7.
Respiratory Pathogens Found in Patients Hospitalized with COVID-19 Pneumonia
| Pathogen | All Patients | Molecular Proven Diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n=93) | Unfavorable Outcome (n=29) | Non Unfavorable Outcome (n=64) | p value | Total (n=69) | Unfavorable Outcome (n=24) | Non Unfavorable Outcome (n=45) | p value | |
| S. aureus | 11 (11.83) | 2 (6.90) | 9 (14.06) | 0.493 | 8 (11.59) | 2 (8.33) | 6 (13.33) | 0.537 |
| S. agalactiae | 10 (10.75) | 3 (10.34) | 7 (10.94) | 0.932 | 7 (10.14) | 2 (8.33) | 5 (11.11) | 1.000 |
| H. influenzae | 10 (10.75) | 4 (13.79) | 6 (9.38) | 0.497 | 9 (13.04) | 4 (16.67) | 5 (11.11) | 0.514 |
| K. pneumoniae | 8 (8.6) | 0 (0) | 8 (12.5) | 0.054 | 7 (10.14) | 0 (0) | 7 (15.56) | 0.087 |
| E. coli | 6(6.45) | 3 (10.34) | 3 (4.69) | 0.371 | 6(8.70) | 3 (12.50) | 3 (6.67) | 0.412 |
| E. cloacae | 5 (5.38) | 3 (10.34) | 2 (3.13) | 0.173 | 4 (5.80) | 2 (8.33) | 2 (4.44) | 0.606 |
| S. marcescens | 2 (2.15) | 0 (0.00) | 2 (3.13) | 1.00 | 2 (2.90) | 0 (0.00) | 2 (4.44) | 0.540 |
| P. aeruginosa | 2 (2.15) | 1 (3.45) | 1 (1.56) | 0.529 | 2 (2.90) | 1 (4.7) | 1 (2.22) | 1.000 |
| S. pneumoniae | 1 (1.08) | 0 (0.00) | 1 (1.08) | 1.00 | 1 (1.45) | 0 (0) | 1 (2.22) | 1.000 |
| A. calcoaceticus-baumannii | 1 (1.08) | 0 (0.00) | 1 (1.56) | 1.00 | 0 (0.00) | 0 (0.00) | 0 (0.00) | - |
| Any bacteria | 37 (39.78) | 12 (41.38) | 25 (39.06) | 0.833 | 30 (43.48) | 10 (41.67) | 20 (44.44) | 0.825 |
| Virus* | 3 (3.23) | 1 (3.45) | 2 (3.13) | 1.00 | 0 (0.00) | 0 (0.00) | 0 (0.00) | - |
| Any pathogen | 38 (40.86) | 9 (40.9) | 23 (37.7) | 0.80 | 30 (43.48) | 10 (41.67) | 20 (44.44) | 0.825 |
Note: *Two cases of rhinovirus and one case of adenovirus.
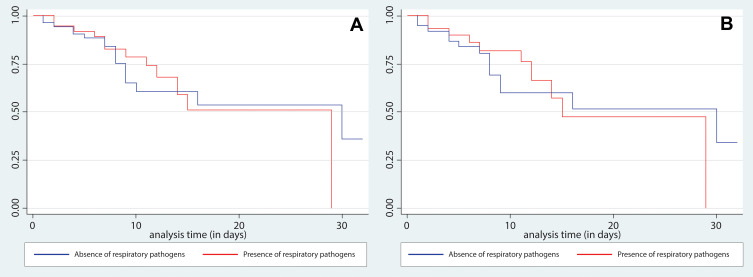
We did not found association between the presence of coinfection by any specific viral and/or bacterial microorganism and attaining the primary outcome. We neither found association between having coinfection as a whole nor having the primary outcome. In the crude analysis, the OR for the association between coinfection by pathogens was 1.03 (95% CI: 0.42–2.52; p = 0.9). In the adjusted analysis (Tables 8 and 9), the result remained non-significant (OR 1.63; 95% CI 0.45–5.82; p = 0.5). The same was found in the sub-analysis restricted to mortality, where there was no significant association (OR 1.24; 95% CI 0.46–3.37; p = 0.7). Finally, we did not find association between the presence of any bacteria and the time until development of the primary outcome (p = 0.98) in survival curves (Figure 1).
Table 8.
Association Between the Presence of Respiratory Pathogens in Sputum Samples and Unfavorable Outcomes in Patients Hospitalized with COVID-19 Pneumonia (n=93): Results of Crude and Adjusted Logistical Regression Models (Whole Population)
| Variable | ORc | 95% CI | p value | ORa | 95% CI | P value |
|---|---|---|---|---|---|---|
| Any respiratory pathogen | 1.032 | 0.423–2.516 | 0.945 | 1.626 | 0.454–5.817 | 0.455 |
| Sore throat | 3.216 | 1.136–9.106 | 0.028 | 2.865 | 0.715–11.483 | 0.137 |
| WBC x 103 | 1.079 | 0.983–1.183 | 0.110 | 1.050 | 0.928–1.189 | 0.436 |
| Lymphocytes x 103 | 0.194 | 0.054–0.697 | 0.012 | 0.394 | 0.107–1.442 | 0.159 |
| D-Dimer | 1.253 | 1.017–1.543 | 0.034 | 1.006 | 0.850–1.326 | 0.598 |
| Lactate dehydrogenase | 1.006 | 1.002–1.010 | 0.001 | 1.005 | 1.001–1.010 | 0.001 |
| Urea | 1.019 | 0.992–1.046 | 0.165 | 1.022 | 0.990–1.057 | 0.180 |
Abbreviations: ORc, Crude Odds Ratio; ORa, adjusted Odds Ratio; 95% CI, 95% confidence interval; WBC, White blood cell count.
Table 9.
Association Between the Presence of Respiratory Pathogens in Sputum Samples and Unfavorable Outcomes in Patients Hospitalized with COVID-19 Pneumonia Confirmed by Molecular Testing (n=69): Results of Crude and Adjusted Logistical Regression Models
| Variable | ORc | 95% CI | p value | ORa | 95% CI | P value |
|---|---|---|---|---|---|---|
| Any respiratory pathogen | 0.893 | 0.328–2.432 | 0.825 | 1.988 | 0.464–8.509 | 0.354 |
| Sore throat | 3.878 | 1.235–12.171 | 0.020 | 4.796 | 0.894–25.70 | 0.067 |
| WBC x 103 | 1.000 | 0.999–1.000 | 0.481 | 1.000 | 0.999–1.000 | 0.580 |
| Lymphocytes x 103 | 0.999 | 0.997–1.000 | 0.083 | 0.999 | 0.998–1.001 | 0.658 |
| D Dimer | 1.217 | 0.914–1.618 | 0.178 | 0.869 | 0.578–1.306 | 0.499 |
| Lactate dehydrogenase | 1.005 | 1.002–1.009 | 0.005 | 1.007 | 1.001–1.011 | 0.013 |
| Urea | 1.041 | 0.999–1.084 | 0.058 | 1.046 | 0991.-1.103 | 0.101 |
Abbreviations: ORc, Crude Odds Ratio; ORa, adjusted Odds Ratio; 95% CI, 95% confidence interval; WBC, White blood cell count.
Figure 1.
Comparison of Kaplan Meier Curves for survival with and without evidence of respiratory pathogens in sputum samples. Panel (A) (left) shows overall results and panel (B) (right) shows the analysis restricted to those patients with molecular confirmed diagnosis.
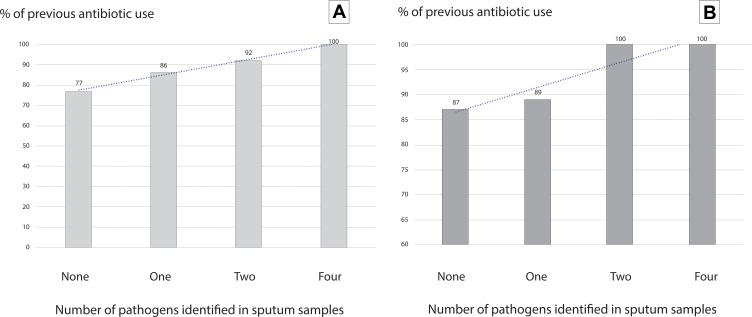
The bivariate analysis found that the presence of sore throat, D-dimer, leukocytes, and LDH on admission were associated with a higher frequency of unfavorable outcomes. Higher Lymphocyte levels, on the other hand, were associated with a lower frequency of unfavorable outcomes. However, in the adjusted analysis, only LDH levels were independently associated with unfavorable outcomes (OR 1.005; 95% CI 1.001–1.010; p = 0.001). Results were similar when the analysis was restricted to the 69 cases having diagnosis confirmed through molecular methods (Tables 3–9). The patients with the highest number of pathogens presented a non-significant trend (p = 0.12) to a higher frequency of previous antibiotics (Figure 2). Among cases with a pathogenic bacteria identified, antibiotic-resistance genes were found in six (14.2%) cases, two in those with unfavorable outcomes and in four among those not having unfavorable outcomes (p=0.97).
Figure 2.
Percentage of previous use of antibiotics according to the number of pathogens identified in sputum samples in patients with diagnosis of COVID-19 pneumonia. Panel (A) (left) shows overall results and panel (B) (right) shows the analysis restricted to those patients with molecular confirmed diagnosis.
Discussion
Our study found a high prevalence (41%) of bacterial pathogens in sputum samples from patients admitted for pneumonia associated with COVID-19 in a Peruvian referral hospital. However, we cannot ascertain that their presence implies an active role in lung damage. Moreover, the main germs involved in most cases of community-acquired pneumonia such as S. pneumoniae, M. pneumoniae or influenza virus14 had very low frequency or were not found.
This presence of respiratory pathogens in 41% of cases in our study lies in the range reported in patients with COVID-19, which has been very variable in different studies, from 0.6% to almost 50% depending on definitions and diagnostic methods employed.9 Zhou et al reported that 50% of fatal cases had secondary infections in a study of 191 patients with COVID-19 in China,3 which is comparable to the prevalence obtained in our study. Notwithstanding, this prevalence is notoriously high when compared with other studies based on conventional cultures. Two factors that may have influenced this are the enormous frequency of antibiotic medication prior to the admission of patients and the high sensitivity for detection of pathogens of the molecular technique used. In particular, the high use of azithromycin is a factor that can affect the alteration of the usual bacterial flora, facilitating colonization and subsequent infection by bacterial pathogens. On the other hand, the molecular tool used is very sensitive, presenting a greater detection capacity than conventional methods.15 Although this molecular platform has been used in few studies, a recent publication about cases of severe COVID-19 found positivity in 28 of 99 hospitalized cases (28.2%).16 It should be noted that the detection of pathogens by molecular methods in sputum samples does not necessarily imply that the identified germs are responsible for a bacterial infection, and they could only be colonizers. The presence of coinfection should be demonstrated by clinical criteria in addition to microbiological findings. Future prospective studies could complement these findings by adding metagenomic sequencing to better characterize the microbiome of these patients and give clues of which organisms are colonizing and which are indeed producing additional pathogenesis in COVID-19 patients. Interestingly, studies on metagenomic sequencing have found that S.agalactiae, one of the most common pathogens found in our study were enriched in COVID-19 patients and could stimulate the expression of ACE-2 of vero cells in vitro which may have a role in SARS CoV-2 infection.17
The finding of respiratory pathogens was not associated with the presence of unfavorable outcomes, defined in our study as the need for mechanical ventilation or death. This is consistent with other published studies.18 However, our findings could indirectly suggest that the presence of respiratory pathogens may be associated with a higher frequency of hospitalization, as studies caried out in outpatients showed lower rates of coinfection.19 This hypothesis should be corroborated with prospective outpatient coinfection studies designed to know if the presence of those pathogens increases the risk of hospitalization.
The almost absence of S. pneumoniae, considered the most frequent germ in cases of bacterial pneumonia, is striking. It was only isolated in one of the cases. This is possibly due to the high frequency of previous antimicrobial treatment. However, our findings probably imply that empirical pneumococcal therapy may not necessarily be justified. On the other hand, we did not find any case of infection by atypical germs including those of the genus Mycoplasma, Chlamydia or Legionella. Although this may be related to the high frequency of use of azithromycin, a drug with action against atypical germs, we consider that our findings suggest that empirical therapy against atypical germs should not be necessary either. No cases of influenza virus infection were found. This result is supported by previous studies in China where they reported a low prevalence (0.4%) of coinfection of Influenza viruses with SARS-CoV-2.19 This finding is also particularly important, because many clinicians, and even therapeutic guidelines used in some hospitals20,21 recommend antiviral treatment against influenza as part of the empirical therapy in cases of COVID-19. Our findings give clear evidence against this recommendation. This is consistent with other studies that do not show a high frequency of viral coinfection.22,23
One of the most important limitations for the interpretation of our results was the massive use of drugs before hospitalization, in particular antibiotics such as azithromycin, which can affect colonization of the upper respiratory tract and, therefore, affect the generalizability of our results. However, this is the (unfortunately) most common scenario faced by the clinician Latin American countries. This is particularly relevant, as resistance induction and bacterial colonization are a growing concern. Additionally, the indiscriminate use of ivermectin -a drug without evidence of efficacy against COVID-19- is striking but recommended by many physicians and even considered in the Peruvian ministry of health recommendations,24 although not endorsed by the recommendations of national25 or international26–28 medical societies. Another limitation was the small sample size, which can increase the type II error, implying a small power for detecting hypothesized associations. Finally, among variables not included in the analysis, the use of antibiotics during hospitalization may have been associated with outcomes. However, we only evaluated predictors at admission.
The absence of influenza viruses and atypical bacteria means that treatment with antivirals and agents with action against atypical bacteria should not be offered routinely to patients hospitalized for COVID-19 associated pneumonia, and this should be immediately adjusted in the national recommendations. In case of suspected bacterial co-infection or superinfection and given the relatively high frequency of H. influenzae, K. pneumoniae and methicillin-sensitive S. aureus, the use of third-generation cephalosporins such as ceftriaxone or beta-lactams associated with beta-lactamase inhibitors, such as associated amoxicillin to clavulanic acid or ampicillin/sulbactam, could be alternatives to consider in the hospitalized patient. Although we found a relatively low frequency of drug resistance genes, we cannot make definitive recommendations about this aspect, as we did not formally measure drug susceptibility patterns and our results should be viewed in the context of a high use of antibiotics before admission.
Regarding the presence of other factors related to poor outcomes, LDH levels, lymphopenia, leukocytosis, and high levels of D-dimer were associated with a higher frequency of mortality and unfavorable outcomes. Although evaluation of these factors was not the primary objective of our study, the results were consistent with other studies.5
Conclusion
We found a high frequency of bacterial respiratory pathogens detected by molecular methods in sputum samples from patients hospitalized with COVID-19 pneumonia. In contrast, the frequency of respiratory viruses was low. The high frequency of previous antibiotic use is a concern. The indiscriminate use of antibiotics in outpatients should be avoided, as it seems to directly affect the high rate of bacterial pathogens found. Finally, our data suggest that the use of therapy against atypical germs and against viruses is not justified in hospitalized patients with COVID-19 pneumonia.
Funding Statement
The research was funded by FONDECYT through the project “Coinfección por patógenos respiratorios virales y bacterianos detectados por métodos moleculares en pacientes hospitalizados por COVID-19 y su impacto en la mortalidad y desenlaces desfavorables” (Convenio N° 044-2020-FONDECYT) and Research Vice-chancellor of Universidad Ricardo Palma.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tian S, Hu N, Lou J, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Huang T, Wang Y, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalili M, Payandemehr P, Saghaei A, Sari HN, Safikhani H, Kolivand P. Characteristics and mortality of hospitalized patients with COVID-19 in Iran: a national retrospective cohort study. Ann Intern Med. 2021;174(1):125–127. doi: 10.7326/M20-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mejía F, Medina C, Cornejo E, et al. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One. 2020;15(12):e0244171. doi: 10.1371/journal.pone.0244171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198 [DOI] [PubMed] [Google Scholar]
- 7.Martin-Loeches I, Schultz MJ, Vincent JL, et al. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med. 2017;43(1):48–58. doi: 10.1007/s00134-016-4578-y [DOI] [PubMed] [Google Scholar]
- 8.Rudd JM, Ashar HK, Chow VT, Teluguakula N. Lethal synergism between influenza and Streptococcus pneumoniae. J Infect Pulm Dis. 2016;2(2). doi: 10.16966/2470-3176.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost I, Van Boeckel TP, Pires J, Craig J, Laxminarayan R. Global geographic trends in antimicrobial resistance: the role of international travel. J Travel Med. 2019;26(8):taz036. doi: 10.1093/jtm/taz036 [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Ruan SY, Pan SC, Lee TF, Chien JY, Hsueh PR. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J Microbiol Immunol Infect. 2019;52(6):920–928. doi: 10.1016/j.jmii.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan American Health Organization. Laboratory guidelines for detection and diagnosis of the novel coronavirus (2019-nCoV) infection. February 1, 2020. Available from: https://iris.paho.org/bitstream/handle/10665.2/51895/ncov-lab-recommendations-en.pdf?sequence=1&isAllowed=y. Accessed June23, 2021.
- 14.Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, Torres A. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci. 2016;17(12):2120. doi: 10.3390/ijms17122120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaku N, Hashiguchi K, Iwanaga Y, et al. Evaluation of FilmArray respiratory panel multiplex polymerase chain reaction assay for detection of pathogens in adult outpatients with acute respiratory tract infection. J Infect Chemother. 2018;24(9):734–738. doi: 10.1016/j.jiac.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolenda C, Ranc AG, Boisset S, et al. Assessment of respiratory bacterial coinfections among severe acute respiratory syndrome coronavirus 2-positive patients hospitalized in intensive care units using conventional culture and biofire, filmarray pneumonia panel plus assay. Open Forum Infect Dis. 2020;7(11):ofaa484. doi: 10.1093/ofid/ofaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong D, Muema C, Zhang X, et al. Enriched Opportunistic Pathogens Revealed by Metagenomic Sequencing Hint Potential Linkages between Pharyngeal Microbiota and COVID-19. Virol Sin. 2021:1–10. doi: 10.1007/s12250-021-00391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrasa H, Rello J, Tejada S, et al. SARS-Cov-2 in Spanish intensive care: early experience with 15-day survival in vitoria. Anaesth Crit Care Pain Med. 2020;39(5):553–561. doi: 10.1016/j.accpm.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicastri E, Petrosillo N, Bartoli TA, et al. National institute for the infectious diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep. 2020;12(1):3–9. doi: 10.4081/idr.2020.8543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T. Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0). Emerg Microbes Infect. 2020;9(1):582–585. doi: 10.1080/22221751.2020.1735265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resolución Ministerial N° 839-2020-MINSA [Internet]. Ministerio de Salud; 2020. [cited February 16, 2021]. Available from: https://www.gob.pe/institucion/minsa/normas-legales/1264399-839-2020-minsa. Accessed June23, 2021. [Google Scholar]
- 25.Vásquez-Kunze S, Soto A, Indacochea-Cáceda S, Bisso-Andrade A. Guía rápida para la evaluación, diagnóstico y el manejo del paciente con COVID-19. 2020. [cited February 16, 2021]. Available from: https://medicinainterna.net.pe/sites/default/files/Guia%20rapida%20COVID%2019%20V%203.0%20%289%20sept%29%20%20final.pdf. Accessed June23, 2021.
- 26.Ministerio de Salud y Protección Social. Lineamientos para el manejo clínico de pacientes con infección por nuevo Coronavirus COVID-19. 2020. [cited February 16, 2021]. Available from: https://www.minsalud.gov.co/Ministerio/Institucional/Procesos%20y%20procedimientos/PSSS03.pdf.
- 27.Bhimraj A, Morgan RL, Shumaker AH, et al. COVID-19 guideline, part 1: treatment and management. 2020. [cited February 16, 2021]. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed June23, 2021.
- 28.The National Institute for Health and Care Excellence. COVID-19 rapid guideline: antibiotics for pneumonia in adults in hospital [Internet]. NICE; 2020. [cited February 16, 2021]. Available from: https://www.nice.org.uk/guidance/ng173. Accessed June23, 2021. [PubMed] [Google Scholar]