Abstract
Food products contaminated by foodborne pathogens (bacteria, parasites, and viruses) cause foodborne diseases. Today, great efforts are being allocated to the development of novel and effective agents against food pathogenic microorganisms. These efforts even might have a possible future effect in coronavirus disease 2019 (COVID-19) pandemic. Nanotechnology introduces a novel food packaging technology that creates and uses nanomaterials with novel physiochemical and antimicrobial properties. It could utilize preservatives and antimicrobials to extend the food shelf life within the package. Utilizing the antimicrobial nanomaterials into food packaging compounds typically involves incorporation of antimicrobial inorganic nanoparticles such as metals [Silver (Ag), Copper (Cu), Gold (Au)], and metal oxides [Titanium dioxide (TiO2), Silicon oxide (SiO2), Zinc oxide (ZnO)]. Alternatively, intelligent food packaging has been explored for recognition of spoilage and pathogenic microorganisms. This review paper focused on antimicrobial aspects of nanopackaging and presented an overview of antibacterial properties of inorganic nanoparticles. This article also provides information on food safety during COVID-19 pandemic.
Keywords: food packaging, nanoparticles, foodborne pathogens, antimicrobial, COVID-19
Introduction
Foods or beverages contaminated by foodborne pathogens (bacteria, parasites, and viruses) cause foodborne diseases that are generally classified into foodborne infection and foodborne intoxication. Foodborne infection occurs when a pathogen is ingested with food and establishes and multiplies itself in the human host. Foodborne intoxication occurs when a toxigenic pathogen establishes itself in a food and produces a toxin, which is then ingested by the human host (Bintsis, 2017). World Health Organization (WHO) reports also indicate foodborne diarrheal diseases cause 550 million cases and 230,000 deaths worldwide a year (WHO, 2015). The overall rate of foodborne diseases outbreak leads to a great concern about the infectious food products; therefore great efforts are being allocated to the development of novel and effective agents against food pathogenic microorganisms. These efforts might have a possible future effect in more critical conditions, such as pandemic diseases like influenza viruses, Severe Acute Respiratory Syndrome (SARS), and its newer form, SARS-CoV-2 (corona virus disease 2019, COVID-19).
When considering foods safety, the original source of the food, the microbiological quality of the raw food, the microbiological quality of the processed food, and subsequently the packaging, storage, and distribution are all important. Packaging alone has become a separate area for extensive research into the prevention of foodborne illnesses. Food packaging is universally used to preserve the food quality and to extend the shelf life. Proper packaging could protect food products from microbial damage or from any other type of environmental contamination while a poor quality food packaging increases food waste and foodborne illnesses. Traditional food packages are passive barriers which can only delay the adverse effects of environmental contamination (Brody et al., 2008). In fact the key safety objective for traditional packaging materials is to be inert as possible in contact with food (Biji et al., 2015).
Today, the development of non-conventional packaging is becoming a key research field. The next generation of food packaging can play an important role in reducing the risk of pathogen contamination and extending the shelf-life of foods. Application of nanotechnology presents novel opportunities for exploring the bactericidal effect of nanomaterials with a marked bioactivity (Singh et al., 2017). Nanotechnology is the study and use of matter and structures sized from 1 to 100 nm (the word “nano” means 10–9 or one billionth of something) (Sujithra and Manikkandan, 2019).
Nanotechnology introduce a novel food packaging technology for the food industry (Dasgupta et al., 2015). The term nanopackaging comes from this combination of nanotechnology and food packaging shows a direct application of nanotechnology in food science. Nano-sciences create and use nanomaterials with novel physiochemical properties that offer many new opportunities for food industries (Gupta et al., 2016; Singh et al., 2017). More potent food coloring, flavoring, nutritional additives, and antibacterial ingredients for food packaging are the new opportunities for food industries.
Utilizing the antimicrobial nanomaterials into food packaging compounds typically involves incorporation of antimicrobial inorganic nanoparticles (NPs) (Rhim and Ng, 2007). Materials in the nanoscale range with a higher surface-to-volume ratio can attach more copies of microorganism, which confers greater efficiency (Luo and Stutzenberger, 2008). The antimicrobial nanomaterials are especially interesting because of their barrier properties and desirable structural integrity leads to decrease the spoilage and pathogenic microorganism growth (Rhim and Ng, 2007). In antimicrobial films, nanomaterials can be employed as growth inhibitors, killing agents, or even antibiotic carriers. Several studies show the biological effectiveness of inorganic NPs with a strong antibacterial activity in low concentrations (Jafarzadeh et al., 2020). The typical inorganic NPs used for food packaging are silver nanoparticles (AgNPs) that are popular for its excellent toxicity to many microorganisms, with low volatility and high-temperature stability (Shahbazzadeh et al., 2009; Ahari et al., 2020; Kavakebi et al., 2021). The most common method for AgNPs preparation is chemical reduction. The reduction of silver ions in aqueous solution leads to the formation of silver atoms and aggregation into colloidal clusters. These clusters form the Ag particles with nanometer dimensions. Smaller AgNPs have larger surface area to interact with microbial cells that results in better bactericidal efficiency (Azeredo, 2009). Other antimicrobial NPs which have been applied in food packaging are titanium dioxide NPs (TiO2NPs). TiO2NPs have a photocatalytic activity that creates the polyunsaturated phospholipids peroxidation in the microbial cell membranes. This property has been applied to inactivate several foodborne pathogens (Chorianopoulos et al., 2011). Combining TiO2 with metal increases its photocatalytic bacterial inactivation. The combination of TiO2/AgNPs has been used in several studies (Barani et al., 2018; Lotfi et al., 2019). In addition to AgNPs and TiO2NPs, there are a wide range of nanomaterials introduced to the food industries. Due to the fundamental differences in structural and physicochemical properties, each nanomaterial has different applications in the food nanopackaging. Many informative studies have addressed the practical applications of various nanomaterials in the food packaging industry. However, the number of studies focusing on the application of nanopackaging technology in food microbiology is limited. Despite the antimicrobial effectiveness aspects of nanopackaging, it is important to stress the nanomaterial interactions with food ingredients and possible alterations of food quality ranged from sensory features to safety aspects (Ahari and Lahijani, 2021). This largely depends on the nanomaterial doses and concentrations used in fabrication of nanopackages. Optimizing the right dose and concentration that leads to controlled release of nanoparticle can preserve antimicrobial properties without any alteration in the food quality and safety.
The goals of this study are to focus on antimicrobial aspects of nanopackaging. Here, we present an overview of antibacterial properties of inorganic NPs and highlight their specific effectiveness. Finally, the toxicity of inorganic NPs and their possible danger to human health are discussed.
Foodborne Pathogens
Foodborne pathogenic microorganisms are a branch of food microbes that may not alter the appearance, taste, and quality of products, but they can contaminate foods and cause foodborne illnesses. Therefore, the food safety could not be assessed based on product appearance alone. According to the Centers for Disease Control and Prevention (CDC), every year, 48 million people get sick in the United States from a foodborne illness, 128,000 are hospitalized, and 3,000 die. Table 1 presents the list of foodborne pathogens involved in foodborne diseases.
TABLE 1.
Types of food pathogens.
|
Microorganism | ||
| Foodborne bacteria | Foodborne parasites | Foodborne viruses |
| Aeromonas hydrophila | Cryptosporidium parvum | Astrovirus |
| Bacillus anthracis | Cyclospora cayetanensis | Avian influenza |
| Bacillus cereus | Cystoisospora belli | Hepatitis A virus |
| Brucella sp. | Entamoeba histolytica | Hepatitis E virus |
| Campylobacter sp. | Giardia intestinalis | Norovirus |
| Clostridium botulinum | Helminths | Rotavirus |
| Clostridium perfringens | Taenia solium/saginata | |
| Cronobacter sakazakii | Toxoplasma gondii | |
| Escherichia coli | Trematodes | |
| Escherichia coli O157:H7 | Trichinella spiralis | |
| Listeria monocytogenes | ||
| Mycobacterium paratuberculosis | ||
| Salmonella sp. | ||
| Shigella sp. | ||
| Staphylococcus aureus | ||
| Vibrio sp. | ||
| Yersinia enterocolitica | ||
Based on the inherent function of the pathogens, foodborne diseases are classified into “intoxication,” “toxicoinfection,” and “infection.” Intoxication occurs when water or a food product contaminated by pathogenic toxin is ingested. The symptoms of this class appear very quickly. Staphylococcus aureus, Clostridium botulinum, and Bacillus cereus are the most important pathogens cause food intoxication. “Toxicoinfection” results when an ingested pathogen produces a toxin inside the host body. The symptoms of this type are diarrhea and occasional vomiting. Clostridium perfringens, Escherichia coli, and Vibrio cholera are the examples of this class. “Foodborne infection” occurs when an invasive pathogen is ingested. Salmonella enterica, Campylobacter jejuni, Escherichia coli (E. coli O157:H7), Shigella sp., Yersinia enterocolitica, and Listeria monocytogenes are the most important bacteria involved in the foodborne infections (Bhunia, 2018).
Campylobacter sp. mostly associated with raw or undercooked poultry while Salmonella sp. mostly found in meat, poultry, and eggs. Shigella sp., and Escherichia coli mostly found in meat and unpasteurized milk. Clostridium botulinum often found in improperly home-canned foods. Clostridium perfringens, Yersinia, Vibrio sp., Staphylococcus aureus, Bacillus sp., and Listeria are found in uncooked meats, vegetables, unpasteurized milk, and soft cheese (Safavieh et al., 2015).
Toxoplasma gondii, Cryptosporidium parvum, Cyclospora cayetanensis, Giardia intestinalis, Taenia solium, Trichinella spiralis, and Norovirus, hepatitis A virus, and Rotavirus are the most marked parasites and viruses which cause foodborne diseases (Table 1; Bhunia, 2018).
COVID-19
COVID-19 is an infectious disease caused by SARS-CoV-2 which is manifested by symptoms ranging from mild influenza to severe pneumonia and acute respiratory distress syndrome (Petrosillo et al., 2020). COVID-19 is an enveloped single-stranded RNA virus that its outbreak had become a pandemic in 2020 (Asselah et al., 2021). The beginning of the SARS-CoV-2 outbreak has been attributed to the seafood market in Wuhan, Hubei, China (Chan et al., 2020). According to the WHO, the main route for COVID-19 transmission is respiratory droplets generated by coughing, sneezing, speaking, and breathing of an infected person (Tang et al., 2020). There is no evidence suggesting food is transmission route for COVID-19. The Food and Drug Administration (FDA) (U.S. Food and Drug Administration [FDA], 2020) and CDC emphasize that the risk of infection by the COVID-19 from food products, and food packaging is thought to be very low. Up to now, no cases of COVID-19 have been reported where infection was thought to have occurred by consuming and touching food or food packaging.
Previous studies on coronaviruses family indicated that these viruses can persist in the environment for a long time and may be transmitted through the package surfaces (Geller et al., 2012). Currently, the proposed route for the possible transmission of SARS-CoV-2 through food is the consumption of infected animal foods or the consumption of cross-contaminated foods (Olaimat et al., 1854).
To date, no studies have investigated the survival of SARS-CoV-2 in various foods and food packaging. Therefore, it is not possible to comment at this time on the potential survival of SARS-CoV-2 in food products.
The COVID-19 virus can live on inanimate objects for 72 h (van Doremalen et al., 2020). If the respiratory secretions of a person with corona are secreted onto a food product, it may become a virus carrier. Contact with these food carriers can cause the virus to enter the respiratory system. Since each food product travels a long distance from farm to fork, and along this route they may be contaminated with infectious droplets, regular hand washing and the proper use of sanitizers, and disinfectants are recommended (Olaimat et al., 1854). Besides, the use of online food delivery is advised to create a physical distance between customers and sales staff (U.S. Food and Drug Administration [FDA], 2020). It is also recommended to use cooked foods because coronaviruses do not survive in high temperatures. Raw foods should be washed first and then frozen; because the virus can survive up to 2 years at freezing temperatures (Olaimat et al., 1854).
Food nanopackaging can be a promising approach to maintaining food health against SARS-CoV-2. However, the number of studies that have been done on this subject can be summarized as one where it reported that coatings or films containing copper, silver and zinc NPs have the ability to fight the virus (Sportelli and Izzi, 2020). Antiviral potency of NPs is an open field of research that needs to be fully addressed.
Antimicrobial Nanopackaging
Types of Antimicrobial Nanopackaging
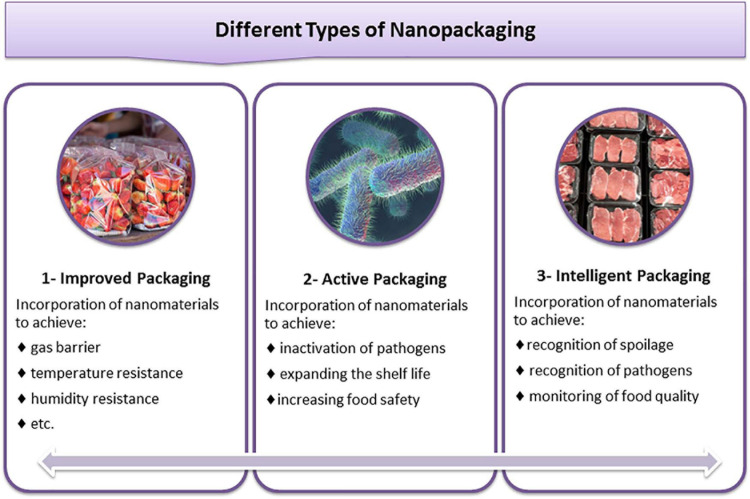
Nanopackaging can be divided into three main categories (Figure 1); (i) Improved packaging: These packages contain NPs and are resistant to temperature and humidity; (ii) Active packaging: Packages containing preservatives such as inorganic NPs that can interact directly with food and provide antimicrobial properties; (iii) Intelligent/smart packaging: Packages that are designed for sensing biochemical or microbial changes and specific pathogen developing in the food.
FIGURE 1.
Classification of food nanopackaging and their application for food safety.
The basic goal of improved packaging is to increase the mechanical and physical properties of the packages. Various nanocomposites are manufactured for various food products such as beverages and oils to reduce the oxygen and carbon dioxide permeation for up to 80–90%. One of the most popular NPs used in improved packaging is nanoclay. Nanoclay incorporated into the polymer can prevent the penetration of oxygen up to 50% and water vapor up to 90% (Kim and Cha, 2014). Antimicrobial properties are not part of the properties of improved packaging.
The development of nanomaterials leads to manufacturing of antimicrobial active packaging that is capable to preserve foods and extending their shelf life. Since the main focus of this study is on antimicrobial nanopackages, we review the active packages in more detail.
Active Packaging
Food spoilage and foodborne diseases represent a critical health problem. In addition, it will incur a lot of economic and medical costs. The development of new nanotechnologies and nanomaterials for food safety enhancement is required (Mustafa and Andreescu, 2020). Active NPs are nanoscaled materials with intrinsic preservatives antimicrobial and/or antioxidant properties that can be able to exert their activity or releasing the active functions. An active package can release antimicrobials or antioxidants into the package or absorb oxygen or water vapor from inside the package. Incorporation of active materials such as antimicrobial agents and preservatives to conventional non-degradable or biodegradable packaging polymer creates active nanocomposites that are used for food products packaging with the aim of increasing the quality and shelf life of storage food. In addition to increasing microbial safety, active nanopackages can regulate moisture, absorb ethylene, carbon dioxide, oxygen, and water vapor, and act as thermal insulators (Drago et al., 2020). Among the NPs used in the packaging industry, metals [Silver (Ag), Copper (Cu), Gold (Au)], and metal oxides [Titanium dioxide (TiO2), Silicon oxide (SiO2), Zinc oxide (ZnO)] are the most widely used NPs in active packaging applications (Bikiaris and Triantafyllidis, 2013). Table 2 lists some of the most important articles about active nanopackaging published between 2018 and February 2021.
TABLE 2.
Some of the most important nanopacking articles from 2018 to February 2021.
| Nanocomposite | Nanoparticle concentration (method)* | Tested foodborne pathogens | Types of food** | Effect | References |
| Gelatin/Cellulose nanofibers/ZnONPs/ and or Selenium NPs | Different concentrations (Casting method) | E. coli, L. monocytogenes, P. fluorescens, S. aureus | – | A stronger antibacterial effect of ZnONPs compared with SeNPs. The bacterial susceptibility to the antibacterial films was as follows: L. monocytogenes > E. coli > S. aureus > P. fluorescens | Ahmadi et al., 2021 |
| Plasticized polylactide/Polyethylene glycol/Ag-Cu NPs/Cinnamon essential oil | 4% Ag-Cu NPs with 50% Cinnamon essential oil (Compression-molding method) | C. jejuni, L. monocytogenes, S. Typhimurium | Chicken meat | Maximum antibacterial action during 21 days at the refrigerated condition | Ahmed et al., 2018 |
| Low-density polyethylene/AgNPs/TiO2NPs | 0, 1, 3, and 5% of AgNPs (Melt mixing and sol–gel methods) | A. niger, C. albicans, E. coli, S. aureus | Pikeperch filets | Effectiveness against all examined bacteria in 3% of AgNPs | Barani et al., 2018 |
| Low-density polyethylene/AgNPs | 1.50, 3.75, 7.50, 15.00, 30.00, 60.00, and 75.00 μg/ml of AgNPs | Apple-isolated Penicillium expansum, E. coli, Enterococcus faecalis, Salmonella enterica subsp. enterica serovar Typhimurium, and S. aureus | – | Antimicrobial effects against all the microorganisms studied, although more notably for fungi and gram-negative bacteria than the gram-positive bacteria | Brito et al., 2020 |
| Cellulose/AgNPs | 0.005, 0.01, 0.02, 0.04, and 0.08 g of AgNPs (Using N,N-dimethylacetamide as a reducing agent in the presence of PVP-K30) | E. coli and S. aureus | – | Remarkable antibacterial activities. The sterilization effect of produced film with 0.04 g of AgNPs against both bacteria exceeds 99.9% | Chen et al., 2020 |
| Low-density polyethylene/AgNPs + CuNPs/TiO2NPs | 0.1, 0.3, 0.5, 1, 3, and 5% of AgNPs + CuNPs (Melt mixing masterbatch method) | E. coli and L. monocytogenes | Nile Tilapia fish | a film containing 2.5% of AgNPs and 2.5% of CuNPs had the most significant antimicrobial effect on the Nile Tilapia fish | Efatian et al., 2021 |
| Carboxymethyl cellulose/Cellulose nanocrystal-AgNPs | 1, 3, 5, and 7% of AgNPs | E. coli and S. aureus | Strawberries | The best antibacterial activities against the two bacterial strains Better maintenance of strawberries quality compared with unpackaged strawberries and extending the shelf-life of strawberries to 7 days | He et al., 2021 |
| Chitosan/Nigella sativa extract-AgNPs | 0.1% w/v, 0.2% w/v, 0.3% w/v of AgNPs | B. subtilis, E. coli, P. aeruginosa, S. aureus | – | A good antibacterial activity against gram-negative bacteria compared to the gram-positive bacteria For both gram-positive and gram-negative bacteria, antibacterial activity significantly influenced by the AgNPs concentration | Kadam et al., 2019 |
| Chitosan-TiO2NPs/Red apple pomace extract | 10% TiO2NPs | E. coli and S. aureus | – | More effective antimicrobial activities against S. aureus than E. coli | Lan et al., 2021 |
| Carboxymethyl cellulose/Glycerol/Dioscorea opposita mucilage from Chinese yam/ZnONPs | “CMC to DOM weight ratio” of approximately 1:1, 2.0 g ZnONPs (Casting method) | E. coli, S. aureus | – | Antibacterial effects against both E. coli and S. aureus | Li et al., 2021 |
| Low-density polyethylene/AgNPs/TiO2NP and Low-density polyethylene/nanoclay/TiO2NPs | 0.1, 0.3, 0.5, 1, 3, and 5% of AgNPs or nanoclay (Melt mixing and sol–gel methods) | E. coli, S. aureus | Chicken meat | Greatest antimicrobial effect on gram-positive and gram-negative bacteria for films containing 5% silver and 5% titanium dioxide nanoparticles | Lotfi et al., 2019 |
| Polyvinyl alcohol/Boiled rice starch/AgNPs | 6.8 μg/mg AgNPs (Photo-assisted method) | S. aureus and S. typhimurium | – | Stronger antibacterial activity against S. typhimurium than S. aureus | Mathew et al., 2019 |
| Cellulose/CuNPs | 5, 25, 125, and 250 mM (Simple casting ethanol regeneration method) | E. coli and Bacillus sp. | – | Remarkable biocidal activity against E. coli in 250 mM concentration | Muthulakshmi et al., 2019 |
| Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/Biogenic SiO2NPs | Different concentrations (Solution casting method) | E. coli and S. aureus | – | Progressively improvement of antibacterial activity of nanocomposites upon increasing SiO2NPs concentration | Ojha and Das, 2020 |
| Poly lactic acid/Oligomeric lactic acid/Chitosan-AgNPs | 0.5 wt%, 1 wt%, 3 wt%, 5 wt% of AgNPs (Facile and green synthesis method) | E. coli, S. aureus | – | Antimicrobial activities against the both bacteria. However, the low content of chitosan-AgNPs was not effective against E. coli but was against S. aureus bacteria | Sonseca et al., 2019 |
| Carrageenan/Laponite on the oxygen plasma surface modified polypropylene film/AgNPs | 20 μg/ml AgNPs (Green synthesis method from the Digitalis purpurea plant) | E. coli and S. aureus | – | The excellent antimicrobial activity against the both bacteria | Vishnuvarthanan and Rajeswari, 2019 |
| Chitosan/ZnONPs/Gallic acid | 30, 50, and 70 mg of ZnONPs (Facile green method using solution casting technique) | B. subtilis and E. coli | – | Antimicrobial activities against both bacterial strains | Yadav et al., 2021 |
| Cellulose nanofibril/AgNPs | 50–1,000 μg/ml of AgNPs (Reduction method using NaBH4) | E. coli O157:H7, L. monocytogenes | – | Greater inhibitory effect on the growth of E. coli O157:H7 than on L. monocytogenes | Yu et al., 2019 |
| Poly(lactic acid)/3-(4′-epoxyethyl-benzyl)-5,5-dimethylhydantoin/SiO2NPs | 1, 3, 5, 7, and 9% of SiO2NPs | E. coli and S. aureus | – | Strong antibacterial activities against both bacterial strains | Zhao et al., 2020 |
*The nanocomposite fabrication method referred to it directly by the authors was mentioned in the parentheses.
**In addition to routine microbiological tests, some studies have tested the antibacterial properties of fabricated nanocomposites in the packaging of various foods. The type of food studied is listed in this column.
Silver Nanoparticles
Nanosilver is the most common NPs used for food packaging because of its high stability and its strong toxicity to a wide range of microorganisms. The mechanism of antimicrobial activity of AgNPs is not fully understood, but the following has been suggested by a number of studies. Silver NPs can release silver ions which can adhere to the cytoplasmic membrane and enhance its permeability. Up taking of free silver ions into bacterial cells inactivate respiratory enzymes and generate reactive oxygen species (ROS) but interrupt the adenosine triphosphate (ATP) synthesis. The generated ROS can cause DNA damage and bacterial death (Yin et al., 2020). Numerous studies have improved the shelf life of different food products by application of the antimicrobial activity of AgNPs. Mahdi et al. (2012) reported that trays coated with silver NPs could extend the minced meat shelf life value to 7 days at refrigerator temperature compared with common packaging. Different cellulose types could carry stable silver NPs formed in situ by physical and chemical reduction methods. Hybrid materials produced by ultraviolet light and heat were effective against pathogenic microorganisms (mesophiles as well as lactic acid bacteria) on chicken exudates (Fernández et al., 2009). The chitosan silver oxide nanocomposite film showed excellent antibacterial performance against E. coli, B. subtilis, P. aeruginosa, and S. aureus microorganisms. This nanocomposite may be used to package foods that are highly sensitive to microbial growth or directly as a surface coating on perishable fruits and vegetables (Tripathi et al., 2011). Different concentrations of AgNPs to reduce total microbial count, mold, and coliform counts in nuts were also reported (Tavakoli et al., 2017). Silver-montmorillonite (Ag-MMT) NPs have also been considered to increase the fresh-cut carrots shelf life up to more than 2 months (Costa et al., 2012). Several studies have investigated the antimicrobial effects of nanosilver in combination with low density polyethylene (LDPE). Becaro et al. (2015) have reported the antibacterial effect of films containing silver NPs embedded in silica or titanium dioxide and were mixed with LDPE. Their films showed antimicrobial properties against S. aureus and E. coli, presenting better activity against S. aureus (Becaro et al., 2015). Lower concentrations of AgNPs incorporated in LDPE had antibacterial performance against mesophilic aerobic and coliforms in fresh-cut carrots packaged. Ascorbic acid content of fresh-cut carrots was also maintained (Becaro et al., 2016). A recent study reported that LDPE/AgNP films had a greater effect on gram-negative bacteria and fungi than gram-positive bacteria (Brito et al., 2020). Additional, antimicrobial properties of nanocomposites of carrageenan/AgNPs/Laponite was demonstrated against the both gram-negative E. coli and gram-positive S. aureus (Vishnuvarthanan and Rajeswari, 2019). It is well documented that AgNPs have strong antibacterial activity toward gram-negative than gram-positive bacteria (Shankar and Rhim, 2015; Orsuwan et al., 2016). Mathew et al. (2019) observed that both polyvinyl alcohol/AgNPs and polyvinyl alcohol/starch from the boiled red rice/sAgNPs films had stronger antibacterial activity against S. typhimurium than S. aureus. The difference in the cell wall structure of gram-negative and gram-positive bacteria causes this difference. In fact, the cell wall peptidoglycan layer in gram-positive bacteria makes it difficult for silver NPs to penetrate (Shankar and Rhim, 2017).
Copper Nanoparticles
Copper NPs are considered to be an antimicrobial agent for medicine and food. The potential biocidal activity of copper is low; however, copper NPs are preferred to silver NPs because of lower cost, easier mixing with polymers, and relatively more physicochemical characteristics.
Copper shows an excellent antimicrobial activity against a wide range of microorganisms. Three different methods including disc diffusion test, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) have been used by Ruparelia et al. (2008) to study the effects of CuNPs against E. coli. This study showed that copper NPs have great promise as antimicrobial agent against E. coli, B. subtilis, and S. aureus. Càrdenas et al. (2009) tested the antimicrobial activity of colloidal CuNPs/chitosan composite film against S. aureus and Salmonella enterica serovar Typhimurium. They showed the composite film was effective in alteration of cell wall and reduction of microbial concentration in the liquid culture for both bacteria tested (Càrdenas et al., 2009). Copper NPs synthesized using different types of copper salts had antimicrobial activity against L. monocytogenes as a gram-positive, and E. coli as a gram-negative foodborne pathogens (Shankar and Rhim, 2014). The antifungal activity as well as the antimicrobial activity against Saccharomyces cerevisiae has been illustrated in cellulose films containing copper oxide NPs (Llorens et al., 2012). Regardless of the type of microorganism in CuNPs bioactivity experiments, Cu-nano-antimicrobials proved to kill or inhibit the microorganism growth. Laser-generated CuNPs embedded in a biodegradable polymer matrix (polylactic acid) possess good antibacterial activity against Pseudomonas sp. (Longano et al., 2012). Polylactic acid films activated by CuNPs showed good antibacterial activity for fresh dairy products (Conte et al., 2013).
Gold Nanoparticles
Gold NPs have a great antimicrobial activity against several ranges of microorganisms depend upon their size and shape (Lima et al., 2013). Therefore, the biofilms containing gold NPs are very promising to be used as active food packaging for the extension of the food shelf life. Lima et al. (2013) showed that 5 nm AuNPs eliminated 90–95% of E. coli and Salmonella typhi colonies at short times. Active biofilms of quinoa (Chenopodium quinoa, W.) starch exhibited strong antibacterial activity against E. coli and S. aureus foodborne pathogens with inhibition percentages of 99 and 98%, respectively (Pagno et al., 2015). Gold NPs probably affect respiratory-chain enzymes which leading to cell death (Zawrah and Abd El-Moez, 2011). AuNPs in combination with bacteriocin had increased antimicrobial activity against four food spoiling organism of Micrococcus luteus, Bacillus cereus, S. aureus, and E. coli (Thirumurugan et al., 2013).
Titanium Dioxide Nanoparticles
Titanium dioxide is a photocatalytic agent used to inactivate a wide range of microorganisms. It is probably able to kill many microorganisms due to its strong oxidizing power and the production of free hydroxyl radicals in near-UV light (Chorianopoulos et al., 2011). The photocatalytic activity of TiO2 has been used to purify contaminated water (Chaleshtori et al., 2009). Bodaghi et al. (2013) prepared a TiO2 nanocomposite thin film by the extrusion method. They observed that when the film was exposed to UVA light, Pseudomonas sp., R. mucilaginosa and mesophilic bacteria were inactivated in saline and pear solution (Bodaghi et al., 2013). The antimicrobial activity of the TiO2 nanoparticle-coated films has been found at various concentrations (0–0.11 g/100 ml organic solvent) under fluorescent and ultraviolet light (Othman et al., 2014). TiO2NPs in gelatin-based films also showed excellent antimicrobial activity against S. aureus and E. coli (Nassiri and MohammadiNafchi, 2013). According to the Gumiero et al. (2013) study, the high-density polyethylene + CaCO3 + TiO2 composite matrix was able to provide a greater maintenance of the original cheese structure due to the inhibition of lactic acid bacteria and coliforms. Compared to the TiO2 nanoparticle-incorporated film, a hydrothermally synthesized sodium alginate film containing functional Au-TiO2 nanocomposites improved the antimicrobial activity by 60 and 50% against S. aureus and E. coli, respectively (Tang et al., 2018). TiO2-ZnO-MgO mixed oxide nanomaterials is another type of TiO2 nano-alloy that has shown good antibacterial properties against E. coli, Salmonella paratyphi, S. aureus, and L. monocytogenes (Anaya-Esparza et al., 2019). Ansari et al. (2020) studied the synthesis of electrospun TiO2 nanofibers, and showed TiO2 nanofibers were more active against gram-negative P. aeruginosa cells than gram-positive S. aureus. They also demonstrated that TiO2 nanofibers inhibited biofilm formation of methicillin-resistant S. aureus and P. aeruginosa in a dose-dependent manner (Ansari et al., 2020). In a recent study, nanosized TiO2 and red apple pomace utilized as a potential extraction source to develop an intelligent chitosan-based film for packaging of the freshness of salmon filets. It was showed that this film had a synergistic enhancement of the antimicrobial activity as well as antioxidant property and pH responsive color-changing indicator (Lan et al., 2021).
Silicon Dioxide Nanoparticles
Silicon dioxide (SiO2, silica) NPs possess several advantages such as high strength, thermal stability, high abundance, and low cost used in active polymer-inorganic composite materials (Hou et al., 2019). Recently, the antimicrobial activities of the gellan gum-sodium carboxymethyl cellulose (GC)-SiO2 and GC-SiO2-octadecyldimethyl-(3-trimethoxysilylpropyl)- ammonium chloride (ODDMAC) nanocomposites were tested against S. aureus, Bacillus cereus, Cronobacter sakazakii, Salmonella enterica, Salmonella typhimurium, and E. coli. The results indicate that the GC-SiO2-ODDMAC film had a broad spectrum of antimicrobial activities for both gram-positive and gram-negative pathogens (Rukmanikrishnan et al., 2020). In another study, the nano-SiOx/chitosan complex coating film was applied for improving the tomatoes shelf life and preservation of tomatoes quality. The developed nano film slowed moisture loss, gas exchange, and respiration rates. It also limited bacterial growth, and prevented the increase of malondialdehyde and total polyphenol content. The novel nano-SiOx/chitosan complex film has been proposed for packaging the postharvested tomatoes (Zhu et al., 2019).
Zinc Oxide Nanoparticles
Zinc oxide is one of the generally recognized as safe (GRAS) material listed by the FDA (Espitia et al., 2012). Recently, a chitosan and zinc oxide NPs loaded gallic-acid films has been proposed for active food packaging for black grape, apple, mango fruits, tomato etc. (Yadav et al., 2021). For extending the shelf life of guava fruits, Kalia et al. (2021) fabricated a chitosan-based nanocomposite film containing CuO and ZnO NPs synthesized from the nettle leaf extract. The antioxidant and antimicrobial activity of biologically synthesized NPs was in order of CuONPs > ZnONPs > nettle extract (Kalia et al., 2021). Foodborne pathogens including E. coli O157:H7 can easily grow in white brined cheese. The chitosan and chitosan-ZnONPs coating reduced the initial numbers of E. coli O157:H7 in white brined cheese (Al-Nabulsi et al., 2020). Further, functionalized absorbing pads containing ZnONPs have also been successfully applied for controlling C. jejuni in raw chicken meat, where the reduced C. jejuni count to an undetectable level by immobilized ZnONPs was reported (Hakeem et al., 2020).
Intelligent Packaging
Intelligent food packaging is a packaging structure that utilize the internal molecules or external conditions of the packed food as information to warn of any changes in the environment of the packages (Drago et al., 2020). Microbiological and chemical tests are regularly carried out on food products, but there is not adequate control after delivery to the supermarket. This unfilled space can be charged with intelligent packaging (Ghaani et al., 2016). Intelligent packaging can be used as a detection method for recognition of spoilage and pathogenic microorganisms. The intelligent packaging can provide information about both the food product status and the environment surrounding it (Vanderroost et al., 2014). They can be equipped with nanosensors classified as freshness indicators, time-temperature indicators, moisture indicators, optical oxygen sensors, optochemical CO2 indicators, toxins indicators, ph contaminants indicators, and spoilage and pathogens indicators (Alfei et al., 2020). Most of these sensors or indicators are based on colorimetric methods. Natural dyes such as anthocyanins, curcumin, chlorophyll, and b-carotene found in many fruits and vegetables as well as synthetic dyes based on azo-compounds can be used as sensors in the packaging materials. Enzymatic processes that are capable to catalyze color chemical reactions are good candidate as sensors in intelligent packaging (Halonen et al., 2020). Currently, the selective interaction between antibody and antigen is one of the explored strategies used for intelligent packaging to identify microorganisms. The methods are based on antibodies conjugated with NPs, such as quantum dots (QDs). QDs are nanomaterials made from inorganic semiconductors and possess specific intrinsic characteristics. QDs are a promising class of fluorescent labels for biological detection because they can absorb wide and continuous wavelengths spectra and produce narrow fluorescence emission spectra depending on their size and composition (Valizadeh et al., 2012). QDs conjugated with antibodies are mainly employed due to their specific characteristics for detection of bacteria (Mihindukulasuriya and Lim, 2014). Mohamadi et al. (2017) presented a method to detect distribution of E. coli labeled with CdSe-QDs both on an agar nutrient and ground fish substrates. They showed that E. coli growth on food products can be easily monitored by CdSe-QDs (Mohamadi et al., 2017). Wang et al. (2011) developed a multiplex immunoassay by integrating magnetic nanobeads (MNBs) for immunoseparation with QDs as fluorescent labels for detection of Salmonella Typhimurium, E. coli O157:H7, and L. monocytogenes, in food products. Their presented multiplex immunoassay method detected simultaneously all three pathogens at levels of 20–50 CFU/ml or lower in food samples (Wang et al., 2011). Recently, a peptide-mediated immunomagnetic separation technique and an immunofluorescence quantum dot technique have been presented for detection of E. coli O157:H7, S. aureus, and V. parahaemolyticus. The method is able to detect three kinds of foodborne pathogens at the same time (Wang et al., 2020). Researchers have recently developed a bacteria-detecting device to detect L. monocytogenes in opened food packages. These devices use strips on which gold or palladium nanoparticle-labeled antibodies detect the presence of bacteria (Yousefi et al., 2019).
From the above reports, it can be concluded that the development of new nanomaterials and devices are potential options for producing intelligent labels that can detect pathogens and toxins in food matrix. More future studies need to be done to establish this technique in food antimicrobial nanopackaging application.
Toxicological Aspects
Comprehensive information regarding the interface between NPs and the human body, particularly in relation to possible NPs hazards to human health must be obtained. NPs incorporated to the nano-packed food products may enter into the body through inhalation, ingestion or cutaneous exposure (Maisanaba et al., 2015). NPs are not soluble in biological fluids, so if they enter the bloodstream, they accumulate in organelles. A very few studies have investigated possible toxicity of NPs combined with food packages. A migration of low molecular mass constituents of nanopackaging to food products is a matter of high concern, both for researchers and consumers. The migration of NPs to food matrix mainly relies on the concentration and particle size of them and composition of food. The migration of metal NPs into the food matrix depends on temperature and acidity (Huang et al., 2015). Few studies points out toward the genotoxicity and carcinogenicity of NPs. Aschberger et al. (2011) observed that toxicity of nanosilver and nano TiO2 can be present in high doses. ZnONPs even at low concentrations may possess a genotoxic potential in human epidermal cells which could be mediated through lipid peroxidation and oxidative stress (Sharma et al., 2009). NPs increase surface to volume ratio which leads to more interaction with biomolecules and create adverse biological responses. It is reported that cationic NPs may be quite toxic than neutral or anionic ones due to their high affinity toward the negatively charged biomolecules (Love et al., 2012). NPs may have adverse effects on circulation, especially affecting microcirculation. If NPs enter the circulatory system, they may affect the blood vessel lining or function and promote blood clot formation or may be associated with cardiovascular effects (Dimitrijevic et al., 2015).
In several in vitro studies, genotoxic, cytotoxic, and even carcinogenic aspects of AgNPs were evaluated. It has been observed that the exposure of normal human lung fibroblast cells (IMR-90) and human glioblastoma cells (U251) to 6–20 nm AgNPs disrupted the mitochondrial respiratory chain leading to ROS production and interruption of ATP synthesis. It also induced DNA damage and G2/M phase cell cycle arrest (AshaRani et al., 2009). Proliferating human intestinal cells (Caco-2 cell line) exposure to peptide-coated silver NPs induced ROS production and decreasing adherence capacity (Böhmert et al., 2012). It has been reported that AgNPs were internalized into the cytoplasm of Caco-2 cells and depolarized the mitochondrial membrane potential. In addition, AgNPs depleted the total intracellular glutathione level, induced the activation of Nrf2 (a stress-responsive gene), and increased the expression of heme oxygenase-1 (HO-1) (Aueviriyavit et al., 2014). It concluded that AgNPs induced acute cytotoxicity and cellular responses via the activation of Nrf2/HO-1 signaling pathway in Caco-2 cells (Aueviriyavit et al., 2014). In another study, Miranda et al. (2017) investigated the toxicological interactions of AgNPs (size = 1–2 nm; zeta potential = –23 mV) in human hepatoma HepG2 cells. The co-exposure to AgNPs and metals potentiated cell death mainly by necrosis (Miranda et al., 2017). The synthesized green silver NPs may induce cytotoxicity and cause DNA damage and apoptosis. Bin-Jumah et al. (2020) showed that higher concentrations of green AgNPs reduced cell viability. They also demonstrated following a higher concentration exposure of green AgNPs, count of apoptotic and necrotic cells was increased. These kinds of AgNPs induced more ROS in the HuH-7 cells than in the CHANG cells (Bin-Jumah et al., 2020). Recently, it was reported that AgNPs altered morphology of freshly isolated circulating human peripheral blood mononuclear cells (hPBMC). They induced apoptosis and cell death in a dose- and time-dependent manner (Vuković et al., 2020).
CuNPs have been associated with cytotoxicity mediated through oxidative stress-dependent pathways. Recently, Fahmy et al. (2020) have reported that Cu/CuO NPs suppressed proliferation and viability of normal and carcinoma lung cells. Treatment of both cell types with Cu/CuO NPs resulted in the generation of a state of oxidative stress (Fahmy et al., 2020). In another study, the toxic potentials of CuONPs were evaluated in the two types of human cell lines (HepG2 hepatocarcinoma and Caco-2 colorectal adenocarcinoma). CuONPs were found to cause cytotoxicity, genotoxicity, and oxidative and apoptotic effects in both cell lines (Abudayyak et al., 2020).
AuNPs cytotoxicity was also evaluated in human erythrocytes, murine fibroblasts (NIH3T3), human cervix carcinoma cells (HeLa), and melanoma cells (B16F10) recently. It seems that the physicochemical properties and concentration of AuNPs and also cell type were limiting factors for the cytotoxic effect of AuNPs (Vechia et al., 2020). It has also been found that greatest concentrations of Ag and Ag-Au bimetallic NPs were toxic to both H4IIE-luc (rat hepatoma) and HuTu-80 (human intestinal) cells but AuNPs were not toxic alone. This study suggests that the toxic potency of Ag-Au bimetallic NPs is greater than AuNPs (Botha et al., 2019). However, Enea et al. (2020) showed that the 13 nm AuNPs are toxic to a cell line derived from normal human kidney (HK-2).
According to the studies using TiO2 anatase NPs, TiO2NPs are cytotoxic or genotoxic. When inhalation is a major rout for entrance of nano TiO2 into the human body, the most important adverse effect of TiO2NPs in experimental models is pulmonary inflammatory responses and lung cancers (Shi et al., 2013). As reported by Koeneman et al. (2010), TiO2NPs at 10 μg/ml and above, can go through the epithelial lining by transcytosis. TiO2 could penetrate into and through the cells without disrupting junctional complexes. Low concentrations of TiO2 do not disrupt epithelial integrity and cell death (Koeneman et al., 2010). It seems that low doses of nano TiO2 are non-toxic.
According to the study conducted by Tarantini et al. (2015) silica NPs penetrate into the cytoplasm but not the nucleus of the human intestinal Caco-2 cell line and have toxic effects in the cells. They proposed that genotoxic effects of silica NPs are likely to be mediated through oxidative stress rather than a direct interaction with the DNA (Tarantini et al., 2015). It has been reported that silica NPs with 100 μg/ml concentration and 24-h exposure are safe for GES-1 and Caco-2 cells. However, at a higher concentration and longer exposure period, they arrest cell cycle and inhibit the cell growth (Yang et al., 2014). In contrast to the above reports, Schübbe et al. (2012) study showed neither cytotoxic nor genotoxic effects were detected for either 32 or 83 nm fluorescent silica NPs.
A significant inhibition of Caco-2 cell viability exposed to ZnONPs (3, 6, and 12 mM) for a 24-h exposure has been reported. Alteration in cell shape, abnormal nuclear structure, membrane blebbing, and cytoplasmic deterioration were the observed changes (Mao et al., 2016). In another study, treatment of rats with 50 and 100 mg/kg ZnONPs induced significantly intestinal injury, while treatment with 5 mg/kg ZnONPs normalized intestinal functions and structure. The authors concluded that ZnONPs have synergistic effects on liver enzyme, oxidative stress, apoptosis, inflammation, morphological changes and cell toxicity (Abbasi-Oshaghi et al., 2018). Caco-2 cells could be protected from ZnONP exposure by myricetin through modulating the apoptosis-ER stress pathway. In fact, co-exposure to myricetin and ZnONPs led to a significantly reduced ratio of cleaved caspase-3/pro-caspase-3 compared with the exposure to ZnONPs (Wu et al., 2019).
From the above reports, it can be concluded that there is no definite answer to the question of whether NPs are toxic. Studies have reported that NPs characteristics including shape, size, size distribution, structure, composition, surface functionality, porosity, surface area, surface charge, agglomeration, concentration, and solubility are all important in the toxicity of NPs (McCracken et al., 2016). For evaluation of NPs toxicity, biological and pathological effects of NPs should be determined by a number of variables, including NPs physiochemical properties, concentration, dose, exposure route, and duration. It seems that the size of NPs, the dose and the exposure time have a great effect on their toxicity. Current data are based on in vitro cell culture studies and/or in vivo animal model experiments. The potential and power of these models for predicting the NPs toxicity in humans is debated. Great care must be taken in using existing models to investigate and understand the biological and pathophysiological mechanisms of NPs toxicity. Full risk evaluations for various routes of exposure to different types of NPs are required.
Conclusion
Consumption of foods contaminated with foodborne microorganisms leads to foodborne diseases. The need to avoid foodborne pathogens contamination has led to the development of new preservative methods and innovative packaging. Antimicrobial packaging can play an important role in reducing the risk of pathogen contamination, and improving the quality and shelf life of foods. This review underlines the capability of active and intelligent packages as antimicrobial agents against foodborne pathogens. Large numbers of scientific studies have demonstrated that active and intelligent packaging has many advantages in terms of food safety. Nanomaterials can provide new antimicrobials with wide spectrum of activity and improve durability and shelf life of food products. It is predicted that nanotechnology and nanopackaging could become part of the food industry and change the packaging process and fabrication in the coming years forward. However, a gap still exists, where the toxicity of NPs and their safe applications is controversial. The safety issues and environmental impact should be concerned before releasing the NPs to the market in order to guarantee the human’s health. Given the many benefits of nanopackaging in preserving foods, increasing the shelf-life, and preventing humans from foodborne illnesses, more focused research in the area of optimization of variables caused NPs toxicity must be carried out. A successful participation and collaboration between research activities and industry will promote the antimicrobial nanopackaging technologies.
Author Contributions
MA assisted in compilation and proper editing of this review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abbasi-Oshaghi E., Mirzaei F., Mirzaei A. (2018). Effects of ZnO nanoparticles on intestinal function and structure in normal/high fat diet-fed rats and Caco-2 cells. Nanomedicine 13 2791–2816. 10.2217/nnm-2018-0202 [DOI] [PubMed] [Google Scholar]
- Abudayyak M., Guzel E., Özhan G. (2020). Cupric oxide nanoparticles induce cellular toxicity in liver and intestine cell lines. Adv. Pharm. Bull. 10 213–220. 10.34172/apb.2020.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahari H., Amanolah Nejad Z., Magharehei M. A., Paidari S. (2020). Incresing Shelf Life of of Penaeus semisulcatus in NanoSilver coatings based on titanium dioxide. J. Food Technol. Nutr. 17 91–98. [Google Scholar]
- Ahari H., Lahijani L. K. (2021). Migration of silver and copper nanoparticles from food coating. Coatings 11:380. 10.3390/coatings11040380 [DOI] [Google Scholar]
- Ahmadi A., Ahmadi P., Sani M. A., Ehsani A., Ghanbarzadeh B. (2021). Functional biocompatible nanocomposite films consisting of selenium and zinc oxide nanoparticles embedded in gelatin/cellulose nanofiber matrices. Int. J. Biol. Macromol. 175 87–97. 10.1016/j.ijbiomac.2021.01.135 [DOI] [PubMed] [Google Scholar]
- Ahmed J., Arfat Y. A., Bher A., Mulla M., Jacob H., Auras R. (2018). Active chicken meat packaging based on polylactide films and bimetallic Ag-Cu Nanoparticles and Essential Oil. J. Food Sci. 83 1299–1310. 10.1111/1750-3841.14121 [DOI] [PubMed] [Google Scholar]
- Alfei S., Marengo B., Zuccari G. (2020). Nanotechnology application in food packaging: a plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 137:109664. 10.1016/j.foodres.2020.109664 [DOI] [PubMed] [Google Scholar]
- Al-Nabulsi A., Osaili T., Sawalha A., Olaimat A. N., Albiss B. A., Mehyar G., et al. (2020). Antimicrobial activity of chitosan coating containing ZnO nanoparticles against E. coli O157:H7 on the surface of white brined cheese. Int. J. Food Microbiol. 334:108838. 10.1016/j.ijfoodmicro.2020.108838 [DOI] [PubMed] [Google Scholar]
- Anaya-Esparza L. M., Montalvo-González E., González-Silva N., Méndez-Robles M. D., Romero-Toledo R., Yahia E. M., et al. (2019). Synthesis and characterization of TiO2-ZnO-MgO mixed oxide and their antibacterial activity. Materials 12:698. 10.3390/ma12050698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. A., Albetran H. M., Alheshibri M. H., Timoumi A., Algarou N. A., Akhtar S., et al. (2020). Synthesis of Electrospun TiO2 nanofibers and characterization of their antibacterial and antibiofilm potential against gram-positive and gram-negative bacteria. Antibiotics 9:572. 10.3390/antibiotics9090572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschberger K., Micheletti C., Sokull-Klüttgen B., Christensen F. M. (2011). Analysis of currently available data for characterising the risk of engineered nanomaterials to the environment and human health–lessons learned from four case studies. Environ. Int. 37 1143–1156. 10.1016/j.envint.2011.02.005 [DOI] [PubMed] [Google Scholar]
- AshaRani P. V., Low Kah Mun G., Hande M. P., Valiyaveettil S. (2009). Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3 279–290. 10.1021/nn800596w [DOI] [PubMed] [Google Scholar]
- Asselah T., Durantel D., Pasmant E., Lau G., Schinazi R. F. (2021). COVID-19: discovery, diagnostics and drug development. J. Hepatol. 74 168–184. 10.1016/j.jhep.2020.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aueviriyavit S., Phummiratch D., Maniratanachote R. (2014). Mechanistic study on the biological effects of silver and gold nanoparticles in Caco-2 cells–induction of the Nrf2/HO-1 pathway by high concentrations of silver nanoparticles. Toxicol. Lett. 224 73–83. 10.1016/j.toxlet.2013.09.020 [DOI] [PubMed] [Google Scholar]
- Azeredo H. M. C. D. (2009). Nanocomposites for food packaging applications. Food Res. Int. 42 1240–1253. [Google Scholar]
- Barani S., Ahari H., Bazgir S. (2018). Increasing the shelf life of pikeperch (Sander lucioperca) fillets affected by low-density polyethylene/Ag/TiO 2 nanocomposites experimentally produced by sol-gel and melt-mixing methods. Int. J. Food Prop. 21 1923–1936. 10.1080/10942912.2018.1508162 [DOI] [Google Scholar]
- Becaro A. A., Puti F. C., Correa D. S., Paris E. C., Marconcini J. M., Ferreira M. D. (2015). Polyethylene films containing silver Nanoparticles for applications in food packaging: characterization of Physico-chemical and anti-microbial properties. J. Nanosci. Nanotechnol. 15 2148–2156. 10.1166/jnn.2015.9721 [DOI] [PubMed] [Google Scholar]
- Becaro A. A., Puti F. C., Panosso A. R., Gern J. C., Brandão H. M., Correa D. S., et al. (2016). Postharvest quality of fresh-cut carrots packaged in plastic films containing silver nanoparticles. Food Bioprocess Technol. 9 637–649. 10.1007/s11947-015-1656-z [DOI] [Google Scholar]
- Bhunia A. K. (2018). “Introduction to foodborne pathogens,” in Foodborne Microbial Pathogens. Food Science Text Series, (New York, NY: Springer; ). Available online at: https://link.springer.com/chapter/10.1007/978-1-4939-7349-1_1 [Google Scholar]
- Biji K. B., Ravishankar C. N., Mohan C. O., Srinivasa Gopal T. K. (2015). Smart packaging systems for food applications: a review. J. Food Sci. Technol. 52 6125–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikiaris D. N., Triantafyllidis K. S. (2013). HDPE/Cu-nanofiber nanocomposites with enhanced antibacterial and oxygen barrier properties appropriate for food packaging applications. Mater. Lett. 93 1–4. 10.1016/j.matlet.2012.10.128 [DOI] [Google Scholar]
- Bin-Jumah M., Al-Abdan M., Albasher G., Alarifi S. (2020). Effects of green silver nanoparticles on apoptosis and oxidative stress in normal and cancerous human hepatic cells in vitro. Int. J. Nanomed. 15 1537–1548. 10.2147/ijn.s239861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bintsis T. (2017). Foodborne pathogens. AIMS Microbiol. 3 529–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodaghi H., Mostofi Y., Oromiehie A., Zamani Z., Ghanbarzadeh B., Costa C., et al. (2013). Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT Food Sci. Technol. 50 702–706. 10.1016/j.lwt.2012.07.027 [DOI] [Google Scholar]
- Böhmert L., Niemann B., Thünemann A. F., Lampen A. (2012). Cytotoxicity of peptide-coated silver nanoparticles on the human intestinal cell line Caco-2. Arch. Toxicol. 86 1107–1115. 10.1007/s00204-012-0840-4 [DOI] [PubMed] [Google Scholar]
- Botha T. L., Elemike E. E., Horn S., Onwudiwe D. C., Giesy J. P., Wepener V. (2019). Cytotoxicity of Ag, Au and Ag-Au bimetallic nanoparticles prepared using golden rod (Solidago canadensis) plant extract. Sci. Rep. 9:4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito S. D. C., Bresolin J. D., Sivieri K., Ferreira M. D. (2020). Low-density polyethylene films incorporated with silver nanoparticles to promote antimicrobial efficiency in food packaging. Food Sci. Technol. Int. 26 353–366. 10.1177/1082013219894202 [DOI] [PubMed] [Google Scholar]
- Brody A., Bugusu B., Han J. H., Sand C. K., Mchugh T. (2008). Innovative food packaging solutions. J. Food Sci. 73 1750–3841. [DOI] [PubMed] [Google Scholar]
- Càrdenas G., Dìaz J. V., Melèndrez M. F., Cruzat C. C., Garcìa Cancino A. (2009). Colloidal Cu nanoparticles/chitosan composite film obtained by microwave heating for food package applications. Polym. Bull. 62 511–524. 10.1007/s00289-008-0031-x [DOI] [Google Scholar]
- Chaleshtori M. Z., Masud S. M. S., Saupe G. B. (2009). Using new porous nanocomposites for photocatalytic water decontamination. MRS Online Proc. Libr. 1145:436. [Google Scholar]
- Chan J. F.-W., Yuan S., Kok K.-H., To K. K.-W., Chu H., Yang J., et al. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395 514–523. 10.1016/s0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. Y., Xiao S. L., Shi S. Q., Cai L. P. (2020). A one-pot synthesis and characterization of antibacterial silver nanoparticle-cellulose film. Polymers 12:440. 10.3390/polym12020440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorianopoulos N. G., Tsoukleris D. S., Panagou E. Z., Falaras P., Nychas G. J. E. (2011). Use of titanium dioxide (TiO2) photocatalysts as alternative means for Listeria monocytogenes biofilm disinfection in food processing. Food Microbiol. 28 164–170. 10.1016/j.fm.2010.07.025 [DOI] [PubMed] [Google Scholar]
- Conte A., Longano D., Costa C., Ditaranto N., Ancona A., Cioffi N., et al. (2013). A novel preservation technique applied to fiordilatte cheese. Innov. Food Sci. Emerg. Technol. 19 158–165. 10.1016/j.ifset.2013.04.010 [DOI] [Google Scholar]
- Costa C., Conte A., Buonocore G. G., Lavorgna M., Del Nobile M. A. (2012). Calcium-alginate coating loaded with silver-montmorillonite nanoparticles to prolong the shelf-life of fresh-cut carrots. Food Res. Int. 48 164–169. 10.1016/j.foodres.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Dasgupta N., Ranjan S., Mundekkad D., Chidambaram R., Shanker R., Kumar A. (2015). Nanotechnology in agro-food: from field to plate. Food Res. Int. 69 381–400. 10.1016/j.foodres.2015.01.005 [DOI] [Google Scholar]
- Dimitrijevic M., Karabasil N., Boskovic M., Teodorovic V., Vasilev D., Djordjevic V., et al. (2015). Safety aspects of nanotechnology applications in food packaging. Procedia Food Sci. 5 57–60. [Google Scholar]
- Drago E., Campardelli R., Pettinato M., Perego P. (2020). Innovations in smart packaging concepts for food: an extensive review. Foods 9:1628. 10.3390/foods9111628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efatian H., Ahari H., Shahbazzadeh D., Nowruzi B., Yousefi S. (2021). Fabrication and characterization of LDPE/silver-copper/titanium dioxide nanocomposite films for application in Nile Tilapia (Oreochromis niloticus) packaging. J. Food Meas. Charact. 15 2430–2439. 10.1007/s11694-021-00836-7 [DOI] [Google Scholar]
- Enea M., Pereira E., de Almeida M. P., Araújo A. M., de Lourdes Bastos M., Carmo H. (2020). Gold nanoparticles induce oxidative stress and apoptosis in human kidney cells. Nanomaterials 10:995. 10.3390/nano10050995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espitia P. J. P., Soares N. D. F. F., Coimbra J. S. D. R., de Andrade N. J., Cruz R. S., Medeiros E. A. A. (2012). Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 5 1447–1464. 10.1007/s11947-012-0797-6 [DOI] [Google Scholar]
- Fahmy H. M., Ebrahim N. M., Gaber M. H. (2020). In-vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and genotoxicity in normal and cancer lung cell lines. J. Trace Elem. Med. Biol. 60:126481. 10.1016/j.jtemb.2020.126481 [DOI] [PubMed] [Google Scholar]
- Fernández A., Soriano E., López-Carballo G., Picouet P., Lloret E., Gavara R., et al. (2009). Preservation of aseptic conditions in absorbent pads by using silver nanotechnology. Food Res. Int. 42 1105–1112. 10.1016/j.foodres.2009.05.009 [DOI] [Google Scholar]
- Geller C., Varbanov M., Duval R. E. (2012). Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 4 3044–3068. 10.3390/v4113044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaani M., Cozzolino C. A., Castelli G., Farris S. (2016). An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 51 1–11. 10.1016/j.tifs.2016.02.008 [DOI] [Google Scholar]
- Gumiero M., Peressini D., Pizzariello A., Sensidoni A., Iacumin L., Comi G., et al. (2013). Effect of TiO2 photocatalytic activity in a HDPE-based food packaging on the structural and microbiological stability of a short-ripened cheese. Food Chem. 138 1633–1640. 10.1016/j.foodchem.2012.10.139 [DOI] [PubMed] [Google Scholar]
- Gupta A., Eral H. B., Hatton T. A., Doyle P. S. (2016). Nanoemulsions: formation, properties and applications. Soft Matter 12 2826–2841. 10.1039/c5sm02958a [DOI] [PubMed] [Google Scholar]
- Hakeem M. J., Feng J., Nilghaz A., Ma L. (2020). Active packaging of immobilized zinc oxide nanoparticles controls Campylobacter jejuni in raw chicken meat. Appl. Environ. Microbiol. 86:e01195-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen N., Pálvölgyi P. S., Bassani A., Fiorentini C., Nair R., Spigno G., et al. (2020). Bio-based smart materials for food packaging and sensors – a review. Front. Mater. 7:82. 10.3389/fmats.2020.00082 [DOI] [Google Scholar]
- He Y., Li H., Fei X., Peng L. (2021). Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 252:117156. 10.1016/j.carbpol.2020.117156 [DOI] [PubMed] [Google Scholar]
- Hou X., Xue Z., Xia Y., Qin Y., Zhang G., Liu H., et al. (2019). Effect of SiO(2) nanoparticle on the physical and chemical properties of eco-friendly agar/sodium alginate nanocomposite film. Int. J. Biol. Macromol. 125 1289–1298. 10.1016/j.ijbiomac.2018.09.109 [DOI] [PubMed] [Google Scholar]
- Huang J.-Y., Weibiao Z., Xu L. (2015). Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 45 187–199. 10.1016/j.tifs.2015.07.002 [DOI] [Google Scholar]
- Jafarzadeh S., Salehabadi A., Jafari S. M. (2020). “10 - Metal nanoparticles as antimicrobial agents in food packaging,” in Handbook of Food Nanotechnology, ed. Jafari S. M. (Cambridge, MA: Academic Press; ), 379–414. 10.1016/b978-0-12-815866-1.00010-8 [DOI] [Google Scholar]
- Kadam D., Momin B., Palamthodi S., Lele S. S. (2019). Physicochemical and functional properties of chitosan-based nano-composite films incorporated with biogenic silver nanoparticles. Carbohydr. Polym. 211 124–132. 10.1016/j.carbpol.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Kalia A., Kaur M., Shami A., Jawandha S. K., Alghuthaymi M. A., Thakur A., et al. (2021). Nettle-Leaf Extract Derived ZnO/CuO nanoparticle-biopolymer-based antioxidant and antimicrobial nanocomposite packaging films and their impact on extending the post-harvest shelf life of guava fruit. Biomolecules 11:224. 10.3390/biom11020224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavakebi E., Anvar A., Ahari H., Motalebi A. (2021). Green biosynthesized Satureja rechingeri Jamzad-Ag/poly vinyl alcohol film: quality improvement of Oncorhynchus mykiss fillet during refrigerated storage. Food Sci. Technol. 41 267–278. 10.1590/fst.62720 [DOI] [Google Scholar]
- Kim S. W., Cha S. H. (2014). Thermal, mechanical, and gas barrier properties of ethylene—Vinyl alcohol copolymer-based nanocomposites for food packaging films: effects of nanoclay loading. J. Appl. Polym. Sci. 131:40289. [Google Scholar]
- Koeneman B. A., Zhang Y., Westerhoff P., Chen Y., Crittenden J. C., Capco D. G. (2010). Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 26 225–238. 10.1007/s10565-009-9132-z [DOI] [PubMed] [Google Scholar]
- Lan W., Wang S., Zhang Z., Liang X., Liu X., Zhang J. (2021). Development of red apple pomace extract/chitosan-based films reinforced by TiO(2) nanoparticles as a multifunctional packaging material. Int. J. Biol. Macromol. 168 105–115. 10.1016/j.ijbiomac.2020.12.051 [DOI] [PubMed] [Google Scholar]
- Li X., Ren Z., Wang R., Liu L., Zhang J., Ma F., et al. (2021). Characterization and antibacterial activity of edible films based on carboxymethyl cellulose, Dioscorea opposita mucilage, glycerol and ZnO nanoparticles. Food Chem. 349:129208. 10.1016/j.foodchem.2021.129208 [DOI] [PubMed] [Google Scholar]
- Lima E., Guerra R., Lara V., Guzmán A. (2013). Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem. Cent. J. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens A., Lloret E., Picouet P., Fernandez A. (2012). Study of the antifungal potential of novel cellulose/copper composites as absorbent materials for fruit juices. Int. J. Food Microbiol. 158 113–119. 10.1016/j.ijfoodmicro.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Longano D., Ditaranto N., Cioffi N., Di Niso F., Sibillano T., Ancona A., et al. (2012). Analytical characterization of laser-generated copper nanoparticles for antibacterial composite food packaging. Anal. Bioanal. Chem.. 403 1179–1186. 10.1007/s00216-011-5689-5 [DOI] [PubMed] [Google Scholar]
- Lotfi S., Ahari H., Sahraeyan R. (2019). The effect of silver nanocomposite packaging based on melt mixing and sol–gel methods on shelf life extension of fresh chicken stored at 4 °C. J. Food Saf. 39:e12644. [Google Scholar]
- Love S. A., Maurer-Jones M. A., Thompson J. W., Lin Y. S., Haynes C. L. (2012). Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. 5 181–205. 10.1146/annurev-anchem-062011-143134 [DOI] [PubMed] [Google Scholar]
- Luo P. G., Stutzenberger F. J. (2008). Nanotechnology in the detection and control of microorganisms. Adv. Appl. Microbiol. 63 145–181. 10.1016/s0065-2164(07)00004-4 [DOI] [PubMed] [Google Scholar]
- Mahdi S. S., Vadood R., Nourdahr R. (2012). Study on the antimicrobial effect of nanosilver tray packaging of minced beef at refrigerator temperature. Glob. Vet. 9 284–289. [Google Scholar]
- Maisanaba S., Pichardo S., Puerto M., Gutiérrez-Praena D., Cameán A. M., Jos A. (2015). Toxicological evaluation of clay minerals and derived nanocomposites: a review. Environ. Res. 138 233–254. 10.1016/j.envres.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Mao X., Nguyen T. H., Lin M., Mustapha A. (2016). Engineered nanoparticles as potential food contaminants and their toxicity to Caco-2 Cells. J. Food Sci. 81 T2107–T2113. [DOI] [PubMed] [Google Scholar]
- Mathew S., Jayakumar A., Kumar V. P., Mathew J., Radhakrishnan E. K. (2019). One-step synthesis of eco-friendly boiled rice starch blended polyvinyl alcohol bionanocomposite films decorated with in situ generated silver nanoparticles for food packaging purpose. Int. J. Biol. Macromol. 139 475–485. 10.1016/j.ijbiomac.2019.07.187 [DOI] [PubMed] [Google Scholar]
- McCracken C., Dutta P. K., Waldman W. J. (2016). Critical assessment of toxicological effects of ingested nanoparticles. Environ. Sci. Nano 3 256–282. 10.1039/c5en00242g [DOI] [Google Scholar]
- Mihindukulasuriya S. D. F., Lim L. T. (2014). Nanotechnology development in food packaging: a review. Trends Food Sci. Technol. 40 149–167. 10.1016/j.tifs.2014.09.009 [DOI] [Google Scholar]
- Miranda R. R., Bezerra A. G., Jr., Oliveira Ribeiro C. A., Randi M. A. F., Voigt C. L., Skytte L., et al. (2017). Toxicological interactions of silver nanoparticles and non-essential metals in human hepatocarcinoma cell line. Toxicol. In Vitro 40 134–143. 10.1016/j.tiv.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Mohamadi E., Moghaddasi M., Farahbakhsh A., Kazemi A. (2017). A quantum-dot-based fluoroassay for detection of food-borne pathogens. J. Photochem. Photobiol. B Biol. 174 291–297. 10.1016/j.jphotobiol.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Mustafa F., Andreescu S. (2020). Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 10 19309–19336. 10.1039/d0ra01084g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthulakshmi L., Varada Rajalu A., Kaliaraj G. S., Siengchin S., Parameswaranpillai J., Saraswathi R. (2019). Preparation of cellulose/copper nanoparticles bionanocomposite films using a bioflocculant polymer as reducing agent for antibacterial and anticorrosion applications. Comp. Part B Eng. 175:107177. 10.1016/j.compositesb.2019.107177 [DOI] [Google Scholar]
- Nassiri R., MohammadiNafchi A. (2013). Antimicrobial and barrier properties of bovine gelatin films reinforced by Nano TiO2. J. Chem. Health Risks 3 12–28. [Google Scholar]
- Ojha N., Das N. (2020). Fabrication and characterization of biodegradable PHBV/SiO(2) nanocomposite for thermo-mechanical and antibacterial applications in food packaging. IET Nanobiotechnol. 14 785–795. 10.1049/iet-nbt.2020.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaimat A. N., Shahbaz H. M., Fatima N., Munir S., Holley R. A. (1854). Food safety during and after the Era of COVID-19 pandemic. Front. Microbiol. 11:1854. 10.3389/fmicb.2020.01854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsuwan A., Shankar S., Wang L.-F., Sothornvit R., Rhim J.-W. (2016). Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll. 60 476–485. 10.1016/j.foodhyd.2016.04.017 [DOI] [Google Scholar]
- Othman S. H., Abd Salam N. R., Zainal N., Kadir Basha R., Talib R. A. (2014). Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. Int. J. Photoenergy 2014:945930. [Google Scholar]
- Pagno C. H., Costa T. M. H., de Menezes E. W., Benvenutti E. V., Hertz P. F., Matte C. R., et al. (2015). Development of active biofilms of quinoa (Chenopodium quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chem. 173 755–762. 10.1016/j.foodchem.2014.10.068 [DOI] [PubMed] [Google Scholar]
- Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. (2020). COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 26 729–734. 10.1016/j.cmi.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. W., Ng P. K. (2007). Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 47 411–433. 10.1080/10408390600846366 [DOI] [PubMed] [Google Scholar]
- Rukmanikrishnan B., Jo C., Choi S., Ramalingam S., Lee J. (2020). Flexible ternary combination of gellan gum, sodium carboxymethyl cellulose, and silicon dioxide nanocomposites fabricated by quaternary ammonium silane: rheological, thermal, and antimicrobial properties. ACS Omega 5 28767–28775. 10.1021/acsomega.0c04087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparelia J. P., Chatterjee A. K., Duttagupta S. P., Mukherji S. (2008). Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 4 707–716. 10.1016/j.actbio.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Safavieh M., Nahar S., Zourob M., Ahmed M. U. (2015). “15 - Microfluidic biosensors for high throughput screening of pathogens in food,” in High Throughput Screening for Food Safety Assessment, eds Bhunia A. K., Kim M. S., Taitt C. R. (Cambridge: Woodhead Publishing; ), 327–357. 10.1016/b978-0-85709-801-6.00015-0 [DOI] [Google Scholar]
- Schübbe S., Schumann C., Cavelius C., Koch M., Müller T., Kraegeloh A. (2012). Size-dependent localization and quantitative evaluation of the intracellular migration of silica nanoparticles in Caco-2 Cells. Chem. Mater. 24 914–923. 10.1021/cm2018532 [DOI] [Google Scholar]
- Shahbazzadeh D., Ahari H., Rahimi N., Dastmalchi F., Soltani M., Rahmannya J., et al. (2009). The effects of Nanosilver (Nanocid®) on survival percentage of rainbow trout (Oncorhynchus mykiss). Pak. J. Nutr. 8 1178–1179. 10.3923/pjn.2009.1178.1179 [DOI] [Google Scholar]
- Shankar S., Rhim J.-W. (2014). Effect of copper salts and reducing agents on characteristics and antimicrobial activity of copper nanoparticles. Mater. Lett. 132 307–311. 10.1016/j.matlet.2014.06.014 [DOI] [Google Scholar]
- Shankar S., Rhim J.-W. (2015). Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 130 353–363. 10.1016/j.carbpol.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Shankar S., Rhim J.-W. (2017). Preparation and characterization of agar/lignin/silver nanoparticles composite films with ultraviolet light barrier and antibacterial properties. Food Hydrocoll. 71 76–84. 10.1016/j.foodhyd.2017.05.002 [DOI] [Google Scholar]
- Sharma V., Shukla R. K., Saxena N., Parmar D., Das M., Dhawan A. (2009). DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 185 211–218. 10.1016/j.toxlet.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Shi H., Magaye R., Castranova V., Zhao J. (2013). Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 10:15. 10.1186/1743-8977-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T., Shukla S., Kumar P., Wahla V., Bajpai V. K., Rather I. A. (2017). Application of nanotechnology in food science: perception and overview. Front. Microbiol. 8:1501. 10.3389/fmicb.2017.01501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonseca A., Madani S., Rodríguez G., Hevilla V., Echeverría C., Fernández-García M. (2019). Multifunctional PLA blends containing Chitosan mediated silver nanoparticles: thermal, mechanical, antibacterial, and degradation properties. Nanomaterials 10:22. 10.3390/nano10010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportelli M. C., Izzi M. (2020). Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? Nanomaterials 10:802. 10.3390/nano10040802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujithra S., Manikkandan T. R. (2019). Application of nanotechnology in packaging of foods : a review. Int. J. ChemTech Res. 12 07–14. 10.20902/ijctr.2019.120402 [DOI] [Google Scholar]
- Tang D., Comish P., Kang R. (2020). The hallmarks of COVID-19 disease. PLoS Pathog. 16:e1008536. 10.1371/journal.ppat.1008536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Wang Z., Li P., Li W., Li C., Wang Y., et al. (2018). Degradable and photocatalytic antibacterial Au-TiO2/sodium alginate nanocomposite films for active food packaging. Nanomaterials 8:930. 10.3390/nano8110930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini A., Lanceleur R., Mourot A., Lavault M. T., Casterou G., Jarry G., et al. (2015). Toxicity, genotoxicity and proinflammatory effects of amorphous nanosilica in the human intestinal Caco-2 cell line. Toxicol. In Vitro 29 398–407. 10.1016/j.tiv.2014.10.023 [DOI] [PubMed] [Google Scholar]
- Tavakoli H., Rastegar H., Taherian M., Samadi M., Rostami H. (2017). The effect of nano-silver packaging in increasing the shelf life of nuts: an in vitro model. Italian J. Food Saf. 6:6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumurugan A., Ramachandran S., Gowri A. (2013). Combined effect of bacteriocin with gold nanoparticles against food spoiling bacteria - an approach for food packaging material preparation. Int. Food Res. J. 20 1909–1912. [Google Scholar]
- Tripathi S., Mehrotra G. K., Dutta P. K. (2011). Chitosan–silver oxide nanocomposite film: preparation and antimicrobial activity. Bull. Mater. Sci. 34 29–35. 10.1007/s12034-011-0032-5 [DOI] [Google Scholar]
- U.S. Food and Drug Administration [FDA] (2020). Food Safety and the Coronavirus Disease 2019 (COVID-19). White Oak Campus: FDA. [Google Scholar]
- Valizadeh A., Mikaeili H., Samiei M., Farkhani S. M., Zarghami N., Kouhi M., et al. (2012). Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 7:480. 10.1186/1556-276x-7-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D. H. (2020). Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 382 1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderroost M., Ragaert P., Devlieghere F., De Meulenaer B. (2014). Intelligent food packaging: the next generation. Trends Food Sci. Technol. 39 47–62. 10.1016/j.tifs.2014.06.009 [DOI] [Google Scholar]
- Vechia I. C. D., Steiner B. T., Freitas M. L., dos Santos Pedroso Fidelis G., Galvani N. C., Ronchi J. M., et al. (2020). Comparative cytotoxic effect of citrate-capped gold nanoparticles with different sizes on noncancerous and cancerous cell lines. J. Nanopart. Res. 22:133. [Google Scholar]
- Vishnuvarthanan M., Rajeswari N. (2019). Preparation and characterization of carrageenan/silver nanoparticles/Laponite nanocomposite coating on oxygen plasma surface modified polypropylene for food packaging. J. Food Sci. Technol. 56 2545–2552. 10.1007/s13197-019-03735-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuković B., Milić M., Dobrošević B., Milić M., Ilić K., Pavičić I., et al. (2020). Surface stabilization affects toxicity of silver nanoparticles in human peripheral blood mononuclear cells. Nanomaterials 10:1390. 10.3390/nano10071390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Lian F., Yao S., Liu Y., Wang J., Song X., et al. (2020). Simultaneous detection of three foodborne pathogens based on immunomagnetic nanoparticles and fluorescent quantum dots. ACS Omega 5 23070–23080. 10.1021/acsomega.0c02833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li Y., Wang A., Slavik M. (2011). Rapid, sensitive, and simultaneous detection of three foodborne pathogens using magnetic nanobead-based immunoseparation and quantum dot-based multiplex immunoassay. J. Food Prot. 74 2039–2047. 10.4315/0362-028x.jfp-11-144 [DOI] [PubMed] [Google Scholar]
- WHO (2015). Word Health Organization. Estimates of the Global Burden of Foodborne Diseases. Available online at: https://www.who.int/activities/estimating-the-burden- of-foodborne-diseases (accessed March 13, 2020). [Google Scholar]
- Wu C., Luo Y., Liu L. (2019). Toxicity of combined exposure of ZnO nanoparticles (NPs) and myricetin to Caco-2 cells: changes of NP colloidal aspects, NP internalization and the apoptosis-endoplasmic reticulum stress pathway. Toxicol. Res. 8 613–620. 10.1039/c9tx00127a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Mehrotra G. K., Dutta P. K. (2021). Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 334:127605. 10.1016/j.foodchem.2020.127605 [DOI] [PubMed] [Google Scholar]
- Yang Y. X., Song Z. M., Cheng B., Xiang K., Chen X. X., Liu J. H., et al. (2014). Evaluation of the toxicity of food additive silica nanoparticles on gastrointestinal cells. J. Appl. Toxicol. 34 424–435. 10.1002/jat.2962 [DOI] [PubMed] [Google Scholar]
- Yin I. X., Zhang J., Zhao I. S., Mei M. L., Li Q., Chu C. H. (2020). The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 15 2555–2562. 10.2147/ijn.s246764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi H., Su H.-M., Imani S. M., Alkhaldi K. M., Filipe C. D., Didar T. F. (2019). Intelligent food packaging: a review of smart sensing technologies for monitoring food quality. ACS Sens. 4 808–821. 10.1021/acssensors.9b00440 [DOI] [PubMed] [Google Scholar]
- Yu Z., Wang W., Kong F., Lin M., Mustapha A. (2019). Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 129 887–894. 10.1016/j.ijbiomac.2019.02.084 [DOI] [PubMed] [Google Scholar]
- Zawrah M., Abd El-Moez S. (2011). Antimicrobial activities of gold nanoparticles against major foodborne pathogens. Life Sci. J. 8 37–44. [Google Scholar]
- Zhao Y., Wei B., Wu M., Zhang H., Yao J., Chen X., et al. (2020). Preparation and characterization of antibacterial poly(lactic acid) nanocomposites with N-halamine modified silica. Int. J. Biol. Macromol. 155 1468–1477. 10.1016/j.ijbiomac.2019.11.125 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Li D., Belwal T., Li L. (2019). Effect of Nano-SiOx/Chitosan complex coating on the physicochemical characteristics and preservation performance of green tomato. Molecules 24:4552. 10.3390/molecules24244552 [DOI] [PMC free article] [PubMed] [Google Scholar]