Abstract
Objective:
It is generally accepted that the incidence of birth defects in spontaneously conceived children ranges between 2.0-4.0%. However, several studies have shown that babies born after assisted reproductive technology (ART) procedures tend to present more congenital malformations than naturally conceived children, with 6.5% of the children born after intracytoplasmic sperm injection (ICSI) presenting birth defects. The use of high magnification sperm selection before ICSI was introduced in the early 2000s to allow the identification of spermatozoa with low risk of sperm DNA damage. Intracytoplasmic morphologically selected sperm injection (IMSI) is expected to change the incidence of congenital malformations, although data on the incidence of birth defects in children conceived after IMSI are still scarce.
Methods:
A systematic review based on searches performed in electronic databases (PubMed, EMBASE, Web of Science, SCOPUS, and Cochrane Central Register of Controlled Trials) including articles published by February 2021 was conducted to identify trials comparing the neonatal outcomes of ICSI and IMSI. The outcome measured was the rate of birth defects in children born after ICSI or IMSI. Three trials were included as targets for data extraction and meta-analysis.
Results:
Our meta-analysis included 3907 children conceived after IMSI (1280) or ICSI (2627). The incidence of birth defects was statistically different, with 2.5% (32/1280) in IMSI and 4.5% (119/2627) in ICSI (RR=0.59; 95% CI=0.40-0.87; p=0.007). The results demonstrated that IMSI decreased the incidence of structural defects compared to ICSI - 2.2% (18/830) vs. 3.8% (78/2049) - in a statistically significant manner (RR=0.58; 95%CI=0.35-0.96; p=0.04). No significant difference was observed in chromosomal abnormalities (Trisomy 13; 18; 21 and Triple X) between children conceived after IMSI (8/830) or ICSI (19/2049) (RR=1.07; 95%CI=0.47-2.43; p=0.87).
Conclusions:
IMSI seems to be an effective tool at reducing the incidence of structural defects compared to ICSI. However, IMSI does not change the incidence of chromosomal abnormalities.
Keywords: Intracytoplasmic Morphologically Selected Sperm Injection, IMSI, birth defects, IVI, meta-analysis
INTRODUCTION
Several authors have reported on spermatozoa morphology, including studies dating back to 1677 (Cary, 1930; Moench & Holt, 1931; Williams, 1937; Hammen, 1944; MacLeod & Gold, 1951; Franken et al., 2000; Freund, 1966; Eliasson, 1971; 1975; 1981; Katz et al., 1986; Kruger et al., 1988; Menkveld et al., 1990; 2011; van der Merwe et al., 2005; Cobb, 2006; Robertson et al., 2016; WHO, 1987; 1992; 1999). In 2010, the World Health Organization (WHO) provided guidelines for clinicians and other professionals involved in assisted reproduction based on general sperm analysis criteria. These parameters such as the Tygerberg strict criteria for morphological classification are customarily used in the selection of spermatozoa in assisted reproductive technology (ART) procedures (Cooper et al., 2010; Kruger et al., 1988; Menkveld et al., 2011; WHO, 2010).
In the early 2000s, a new technique for evaluating sperm morphology was developed by Bartoov (Bartoov et al., 2001; 2002; 2003; Berkovitz et al., 2005; 2006a; 2006b). This technique is based on a method of high sperm magnification, without fixation or staining, called MSOME (motile sperm organelle morphology examination). In addition to general evaluation of sperm cells, including the shape of the head, the intermediate piece aspect, and the insertion of the flagellum, MSOME allows the evaluation of the presence of vacuoles in the sperm head. Vacuoles are depressions on the surface of the sperm head that may vary in position, number, and size (Franco Jr. et al., 2008; Oliveira et al., 2010). This defect on the spermatozoa head is an indicator of impairment in spermatogenesis and has been associated with sperm DNA damage. Therefore, the addition of MSOME for sperm selection before ICSI (intracytoplasmic sperm injection), also known as IMSI (intracytoplasmic morphologically selected sperm injection), allows the identification of spermatozoa at low risk of sperm DNA damage (Gaspard et al., 2018). IMSI might potentially change the incidence of congenital malformations (Cassuto et al., 2014; Gaspard et al., 2018).
It is generally accepted that the incidence of birth defects in spontaneously conceived children ranges between 2.0-4.0%. However, several studies have shown that babies born after ART procedures tend to present more congenital malformations than naturally conceived children. The use of IMSI in ART cycles has been associated with better fertilization and lower miscarriage rates and described as having potential impacts on the incidence of congenital malformations (Vanderzwalmen et al., 2008; Cassuto et al., 2009; Knez et al., 2011). Nevertheless, data on the incidence of birth defects in children conceived after IMSI are still scarce (Berkovitz et al., 2007; Cassuto et al., 2014; Hershko-Klement et al., 2016; Gaspard et al., 2018).
This meta-analysis aimed to evaluate whether IMSI might decrease the incidence of birth defects, including structural defects and chromosomal abnormalities, in the offspring of patients submitted to ART cycles.
MATERIALS AND METHODS
Study inclusion criteria
Published and ongoing randomized controlled trials (RCT) and non-RCT studies comparing the incidence of congenital malformation between patients undergoing to ICSI or IMSI cycles were included.
Endpoints
The primary endpoint measured for this meta-analysis was the incidence of congenital malformations in the offspring of patients submitted to ICSI and IMSI. Secondary endpoints included the incidence of structural defects and chromosomal abnormalities in the studied population.
Identification of studies
Searches were performed in electronic databases (PubMed, EMBASE, Web of Science, SCOPUS, and Cochrane Central Register of Controlled Trials) to find RCTs and/or robust trials published by May 2021 comparing the incidence of congenital malformations in the offspring of patients submitted to ICSI and IMSI cycles. The search was restricted to articles published in English. The following medical subject headings and text words were used: IMSI, ICSI, congenital malformation, birth defects, chromosomal abnormalities, malformation, and randomized study.
Search results
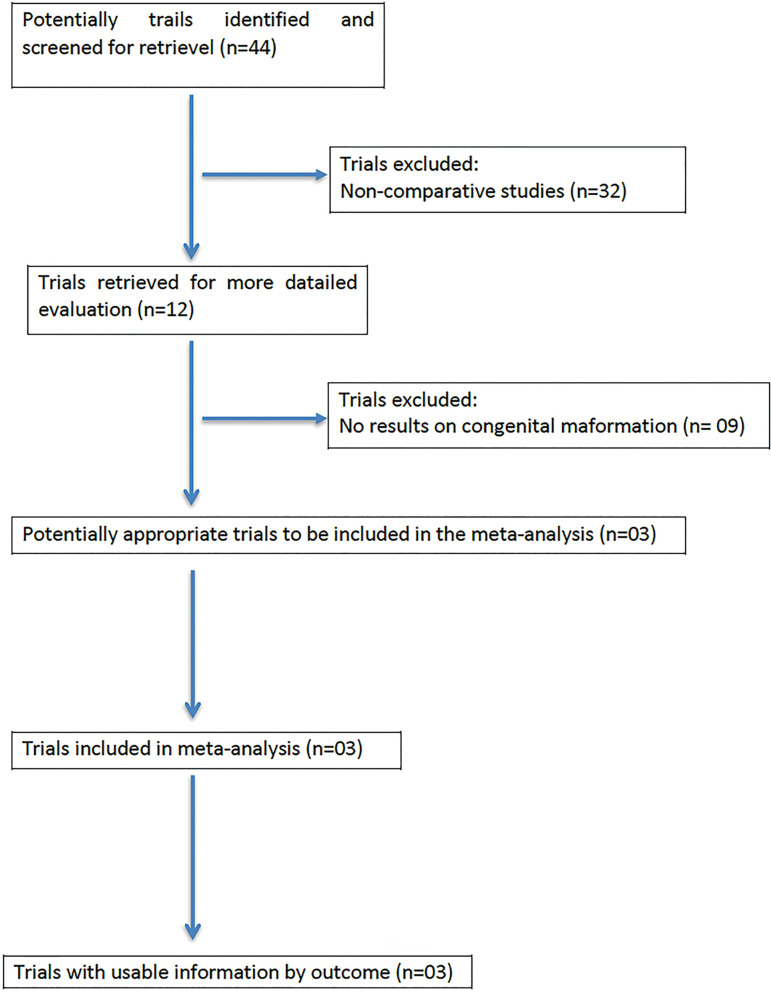
Among the 44 potentially relevant studies retrieved, three trials fulfilled the inclusion criteria. A flowchart of the selection process is shown in Figure 1.
Figure 1.
QUOROM statement flowchart illustrating the selection of trials included in this meta-analysis.
Validity assessment and data extraction
Each trial was assessed independently by three reviewers (FCD, JBAO, and JGFJr) and ranked based on methodological rigor and potential introduction of bias. Originally reported characteristics, including randomization method, presence of statistical power calculation, unit of analysis, and presence or absence of blinding were analyzed. Missing data were obtained from the authors.
Study description - Below is a brief description of each study
Cassuto et al. (2014): A prospective population-based study was conducted from 2005 to 2010 to compare the risk of major malformations in children born after ICSI and IMSI. Medical and two-year follow-up data from 1028 infants were collected. Major malformations were identified and categorized by an external independent physician. The two groups were similar concerning age of parents, treatment, number of oocytes retrieved, days of transfer, gestational age, and weight at birth. However, significantly fewer major malformations were seen in children born after IMSI (6/450, 1.33%) versus ICSI (22/578, 3.80%; adjusted odds ratio 0.35, 95% confidence interval 0.14-0.87, p=0.014); boys were preferentially affected (adjusted odds ratio 2.84, 95% confidence interval 1.24-6.53, p=0.009).
Hershko-Klement et al. (2016): This study aimed to determine the birth defect rates in pregnancies resulting from IMSI. A cohort of couples presenting infertility between January 2006 and January 2014 was retrospectively analyzed. A board-certified medical geneticist reviewed all clinical data. The main endpoints were fetal/birth defect and chromosomal abnormality rates. A total of 2,258 pregnancies were analyzed; 1669 (73.9%) resulted from ICSI and 2258 (26.1%) from IMSI. For the fresh embryo transfer cohort, the fetal/birth defect rate was 4.5%; the chromosomal aberration rate was 1.0%; and the structural malformation rate was 3.5%. Pregnancies resulting from IMSI were less likely to present fetal/birth defects (3.5% vs. 4.8% in IMSI vs. ICSI, respectively), although not significantly (OR 0.71; 95% CI 0.39-1.22). Split by multiplicity, this trend existed only for singleton pregnancies; 1.4% structural malformations rate vs. 3.8%, respectively, OR 0.35 (95% CI 0.11-0.9). The frozen embryo cohort demonstrated a significantly lower birth defect rate (OR 0.25, 95% CI 0.09-0.58).
Gaspard et al. (2018): This study aimed to compare the effects of excluding spermatozoa presenting vacuole-like structures using IMSI with ICSI in terms of neonatal outcomes. In this retrospective two-center analysis, a total of 848 successful IMSI or ICSI cycles ending in live births, induced abortion, or intrauterine fetal death (IUFD) were included. The IMSI and ICSI groups included 332 and 655 babies or fetuses, respectively. The parents were older in the IMSI group than in the ICSI group (mothers were aged 35.1 vs. 32.9 years, and fathers were aged 39.1 vs. 36.2 years). The multiple pregnancy rate was higher in the IMSI group. There was no significant difference in major congenital malformations between the two groups. However, rates were lower in the IMSI group compared to the ICSI group (1.8% vs. 3.2%), with the difference occurring mainly in singleton pregnancies (1.4% vs. 3.3%). Boys were more often affected than girls in both groups. The percentages of chromosomal abnormalities did not differ between the IMSI and ICSI groups (0.6 and 0.8%). The reported congenital malformations affected primarily the heart, urogenital, and musculoskeletal systems.
Statistical analysis
Three studies were included as targets for data extraction and meta-analysis. Data were combined in the meta-analysis using statistical package StatsDirect (Cheshire, UK). The random-effect model was used for the odds ratio (OR), and effectiveness was evaluated by the DerSimonian-Laird method. Dichotomous data were expressed as Relative Risk (RR) with a 95% confidence interval (CI), calculated using the variance formula of Robins, Breslow and Greenland. The measure of heterogeneity (non-combinability) was evaluated using Cochran’s Q, and I2 (inconsistency) tests. A non-significant result (i.e., a lack of heterogeneity) indicated that no trial had an OR significantly worse or better than the overall common OR obtained by pooling the data. p-values <0.05 were considered statistically significant.
RESULTS
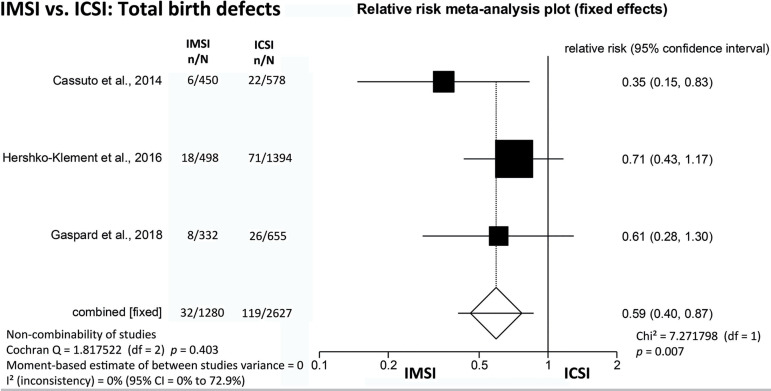
The present meta-analysis included 3907 children conceived after IMSI (1280) and ICSI (2627), and the incidence of total birth defects was 2.5% (32/1280) when IMSI was used versus 4.5% (119/2627) when ICSI was employed, showing a statistically significant difference (RR=0.59; 95% CI=0.40-0.87; p=0.007) (Figure 2).
Figure 2.
IMSI vs. ICSI: Total birth defects (structural-defects /chromosomal abnormalities).
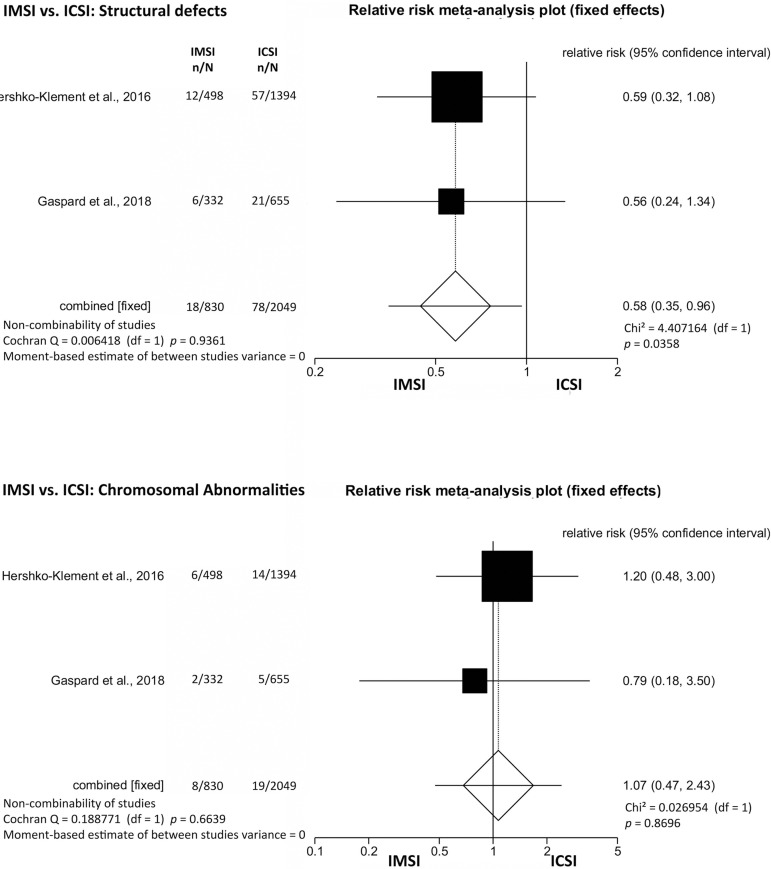
Structural defects and chromosomal abnormalities were analyzed separately. The results showed that IMSI [2.2% (18/830)] significantly decreased the incidence of structural defects when compared to ICSI [3.8% (78/2049) (RR=0.58; 95% CI=0.35-0.96; p=0.04)]. However, no significant difference was found for chromosomal abnormalities (Trisomy 13/18/21/ and Triple X) between the children conceived after IMSI (0.96%; 8/830) and ICSI (0.93%; 19/2049) (RR=1.07; 95% CI=0.47-2.43; p=0.87) (Figure 3).
Figure 3.
IMSI vs. ICSI: Structural defects and chromosomal abnormalities.
DISCUSSION
IMSI is one of the sperm selection techniques used in assisted reproduction, with application indicated for cases of male factor infertility and failed ICSI. IMSI includes strict sperm morphological investigation performed under magnifications of 6600-10000x and reportedly improves clinical and perinatal outcomes (Duran-Retamal et al., 2020). The use of MSOME for sperm selection before ICSI has been discussed by several authors regarding the improvement of clinical results including clinical pregnancy and live birth rates; however, trials rarely provide information on perinatal information (Antinori et al., 2008; Marci et al., 2013; Gatimel et al., 2016; He et al., 2018).
A biological rational has been demonstrated in the use of techniques for sperm selection, since previous studies have reported the association between low sperm quality and DNA fragmentation, and between DNA damage and various chromosomal abnormalities in the male gamete (Varghese et al., 2009; Enciso et al., 2013). These reports have demonstrated that IMSI may provide for a better selection of genetically normal spermatozoa (Hershko-Klement et al., 2016).
It has been suggested that congenital malformations are more prevalent in children born from IVF than in children born after spontaneous conception (Bonduelle et al., 2005). Studies suggest that IVF and mainly ICSI increase the risk of birth defects by 30-40% when compared with spontaneous conception (Davies et al., 2012; Wen et al., 2012; Cassuto et al., 2014). Detailed sperm selection by MSOME before oocyte injection enables the selection of spermatozoa with a lower chance of presenting DNA damage and chromatin decondensation, which might affect embryo development (Cassuto et al., 2012). Therefore, IMSI might lead to lower rates of congenital malformations.
Our meta-analysis presented data on general birth defects, structural defects and chromosomal abnormalities separately. The incidence of total birth defects and structural defects was significantly lower when MSOME was employed before ICSI. However, chromosomal abnormalities are apparently not affected by IMSI. Some authors have described a general trend of decreased incidence of birth defects in children born after IMSI, while the incidence of chromosomal aberrations was similar in children born after IMSI and ICSI (Cassuto et al., 2014; Hershko-Klement et al., 2016).
The suspicion that ART increases the risk of congenital malformations by suppressing natural male gamete selection is apparently not an absolute truth, since some trials have shown that the use of sperm selection by ICSI did not increase the risk of this undesired outcome (Fauser et al., 2014). On the other hand, studies concerning the impact of IMSI on birth defects are scarce, while the available trials have their methodological limitations, including enrollment of heterogeneous populations and lack of patient randomization.
It is important to note that randomized controlled trials on IMSI effectiveness and ART cycle safety do not include birth defects as an endpoint. Thus, studies with larger populations are needed to estimate the actual risk of birth defects associated with IMSI.
In conclusion, the present meta-analysis demonstrated that IMSI seems to decrease the incidence of structural defects in the offspring compared to ICSI. It is critical that comparative trials involving IMSI include the health status of the children after birth, a measure that might be adopted by authors of previously published studies that did not include such data in the published article, but enquired about birth defects during the course of the studies.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- Antinori M, Licata E, Dani G, Cerusico F, Versaci C, d’Angelo D, Antinori S. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reprod Biomed Online. 2008;16:835–841. doi: 10.1016/s1472-6483(10)60150-2. [DOI] [PubMed] [Google Scholar]
- Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med. 2001;345:1067–1068. doi: 10.1056/NEJM200110043451416. [DOI] [PubMed] [Google Scholar]
- Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J Androl. 2002;23:1–8. doi: 10.1002/j.1939-4640.2002.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, Artzi S, Gross M, Barak Y. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 2003;80:1413–1419. doi: 10.1016/j.fertnstert.2003.05.016. [DOI] [PubMed] [Google Scholar]
- Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, Bartoov B. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2005;20:185–190. doi: 10.1093/humrep/deh545. [DOI] [PubMed] [Google Scholar]
- Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, Bartoov B. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online. 2006a;12:634–638. doi: 10.1016/s1472-6483(10)61191-1. [DOI] [PubMed] [Google Scholar]
- Berkovitz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006b;21:1787–1790. doi: 10.1093/humrep/del049. [DOI] [PubMed] [Google Scholar]
- Berkovitz A, Eltes F, Paul M, Adrian E, Benjamin B. The chance of having a healthy normal child following intracytoplasmic morphologically-selected sperm injection (IMSI) treatment is higher compared to conventional IVF-ICSI treatment. Fertil Steril. 2007;88:S20–S20. doi: 10.1016/j.fertnstert.2007.07.083. [DOI] [Google Scholar]
- Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, Henriet S, Mau C, Victorin-Cederquist A, Van Steirteghem A, Balaska A, Emberson JR, Sutcliffe AG. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20:413–419. doi: 10.1093/humrep/deh592. [DOI] [PubMed] [Google Scholar]
- Cary WH. Sterility diagnosis: the study of sperm cell migration in female secretions and interpretation of findings. NY State J Med. 1930;30:131–136. [Google Scholar]
- Cassuto NG, Bouret D, Plouchart JM, Jellad S, Vanderzwalmen P, Balet R, Larue L, Barak Y. A new real-time morphology classification for human spermatozoa: a link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–1625. doi: 10.1016/j.fertnstert.2008.08.088. [DOI] [PubMed] [Google Scholar]
- Cassuto NG, Hazout A, Hammoud I, Balet R, Bouret D, Barak Y, Jellad S, Plouchart JM, Selva J, Yazbeck C. Correlation between DNA defect and sperm-head morphology. Reprod Biomed Online. 2012;24:211–218. doi: 10.1016/j.rbmo.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Cassuto NG, Hazout A, Bouret D, Balet R, Larue L, Benifla JL, Viot G. Low birth defects by deselecting abnormal spermatozoa before ICSI. Reprod Biomed Online. 2014;28:47–53. doi: 10.1016/j.rbmo.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Cobb M, editor. The Egg and Sperm Race: The Seventeenth-Century Scientists Who Unravelled the Secrets of Sex, Life and Growth. London: Simon & Schuster; 2006. [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- Duran-Retamal M, Morris G, Achilli C, Gaunt M, Theodorou E, Saab W, Serhal P, Seshadri S. Live birth and miscarriage rate following intracytoplasmic morphologically selected sperm injection vs intracytoplasmic sperm injection: An updated systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:24–33. doi: 10.1111/aogs.13703. [DOI] [PubMed] [Google Scholar]
- Eliasson R. Standards for investigation of human semen. Andrologia. 1971;3:49–64. doi: 10.1111/j.1439-0272.1971.tb01125.x. [DOI] [Google Scholar]
- Eliasson R. Analysis of semen. In: Behrman SJ, Kistner RW, editors. Progress in Infertility. 2nd ed. Boston, CA: Little, Brown & Co; 1975. pp. 691–713. [Google Scholar]
- Eliasson R. Analysis of semen. In: Burger H, de Kretser D, editors. The Testis. New York: Raven Press; 1981. pp. 381–399. [Google Scholar]
- Enciso M, Alfarawati S, Wells D. Increased numbers of DNA-damaged spermatozoa in samples presenting an elevated rate of numerical chromosome abnormalities. Hum Reprod. 2013;28:1707–1715. doi: 10.1093/humrep/det077. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Devroey P, Diedrich K, Balaban B, Bonduelle M, Delemarre-van de Waal HA, Estella C, Ezcurra D, Geraedts JP, Howles CM, Lerner-Geva L, Serna J, Wells D, Evian Annual Reproduction (EVAR) Workshop Group 2011 Health outcomes of children born after IVF/ICSI: a review of current expert opinion and literature. Reprod Biomed Online. 2014;28:162–182. doi: 10.1016/j.rbmo.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Franco Jr JG, Baruffi RL, Mauri AL, Petersen CG, Oliveira JB, Vagnini L. Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod Biomed Online. 2008;17:42–45. doi: 10.1016/s1472-6483(10)60291-x. [DOI] [PubMed] [Google Scholar]
- Franken DR, Barendsen R, Kruger TF. A continuous quality control program for strict sperm morphology. Fertil Steril. 2000;74:721–724. doi: 10.1016/s0015-0282(00)01498-9. [DOI] [PubMed] [Google Scholar]
- Freund M. Standards for the rating of human sperm morphology. A cooperative study. Int J Fertil. 1966;11:97–180. [PubMed] [Google Scholar]
- Gaspard O, Vanderzwalmen P, Wirleitner B, Ravet S, Wenders F, Eichel V, Mocková A, Spitzer D, Jouan C, Gridelet V, Martens H, Henry L, Zech H, d’Hauterive SP, Nisolle M. Impact of high magnification sperm selection on neonatal outcomes: a retrospective study. J Assist Reprod Genet. 2018;35:1113–1121. doi: 10.1007/s10815-018-1167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatimel N, Parinaud J, Leandri RD. Intracytoplasmic morphologically selected sperm injection (IMSI) does not improve outcome in patients with two successive IVF-ICSI failures. J Assist Reprod Genet. 2016;33:349–355. doi: 10.1007/s10815-015-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen R. Studies on impaired fertility in man with special reference to the male. Acta Obstet Gynecol Scand. 1944;24:1–206. doi: 10.3109/00016344409154563. [DOI] [Google Scholar]
- He F, Wang MJ, Li SL, Zhang CY, Hu LN. IMSI versus ICSI for male factor infertility: A meta-analysis. Zhonghua Nan Ke Xue. 2018;24:254–262. [PubMed] [Google Scholar]
- Hershko-Klement A, Sukenik-Halevy R, Biron Shental T, Miller N, Berkovitz A. Intracytoplasmic morphologically selected sperm injection and congenital birth defects: a retrospective cohort study. Andrology. 2016;4:887–893. doi: 10.1111/andr.12221. [DOI] [PubMed] [Google Scholar]
- Katz DF, Overstreet JW, Samuels SJ, Niswander PW, Bloom TD, Lewis EL. Morphometric analysis of spermatozoa in the assessment of human male fertility. J Androl. 1986;7:203–210. doi: 10.1002/j.1939-4640.1986.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Knez K, Zorn B, Tomazevic T, Vrtacnik-Bokal E, Virant-Klun I. The IMSI procedure improves poor embryo development in the same infertile couples with poor semen quality: a comparative prospective randomized study. Reprod Biol Endocrinol. 2011;9:123. doi: 10.1186/1477-7827-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- MacLeod J, Gold RZ. The male factor in fertility and infertility. IV. Sperm morphology in fertile and infertile marriage. Fertil Steril. 1951;2:394–414. doi: 10.1016/s0015-0282(16)30661-6. [DOI] [PubMed] [Google Scholar]
- Marci R, Murisier F, Lo Monte G, Soave I, Chanson A, Urner F, Germond M. Clinical outcome after IMSI procedure in an unselected infertile population: a pilot study. Reprod Health. 2013;10:16–16. doi: 10.1186/1742-4755-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–592. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Holleboom CA, Rhemrev JP. Measurement and significance of sperm morphology. Asian J Androl. 2011;13:59–68. doi: 10.1038/aja.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench GL, Holt H. Sperm morphology in relation to fertility. Am J Obstet Gynecol. 1931;22:199–210. doi: 10.1016/S0002-9378(31)90545-0. [DOI] [Google Scholar]
- Oliveira JB, Massaro FC, Baruffi RL, Mauri AL, Petersen CG, Silva LF, Vagnini LD, Franco Jr JG. Correlation between semen analysis by motile sperm organelle morphology examination and sperm DNA damage. Fertil Steril. 2010;94:1937–1940. doi: 10.1016/j.fertnstert.2010.01.042. [DOI] [PubMed] [Google Scholar]
- Robertson L, Backer J, Biemans C, van Doorn J, Krab K, Reijnders W, Smit H, Willemsen P. Antoni van Leeuwenhoek, Master of the Minuscule. Leiden: Brill; 2016. [Google Scholar]
- van der Merwe FH, Kruger TF, Oehninger SC, Lombard CJ. The use of semen parameters to identify the subfertile male in the general population. Gynecol Obstet Invest. 2005;59:86–91. doi: 10.1159/000082368. [DOI] [PubMed] [Google Scholar]
- Vanderzwalmen P, Hiemer A, Rubner P, Bach M, Neyer A, Stecher A, Uher P, Zintz M, Lejeune B, Vanderzwalmen S, Cassuto G, Zech NH. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod Biomed Online. 2008;17:617–627. doi: 10.1016/s1472-6483(10)60308-2. [DOI] [PubMed] [Google Scholar]
- Varghese AC, Bragais FM, Mukhopadhyay D, Kundu S, Pal M, Bhattacharyya AK, Agarwal A. Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia. 2009;41:207–215. doi: 10.1111/j.1439-0272.2009.00917.x. [DOI] [PubMed] [Google Scholar]
- Wen J, Jiang J, Ding C, Dai J, Liu Y, Xia Y, Liu J, Hu Z. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97:1331–7.e1-4. doi: 10.1016/j.fertnstert.2012.02.053. [DOI] [PubMed] [Google Scholar]
- WHO - World Health Organization . WHO Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction. 2nd ed. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- WHO - World Health Organization . WHO Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction. 3rd ed. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- WHO - World Health Organization . WHO Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction. 4th ed. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- WHO - World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO; 2010. [Google Scholar]
- Williams WW. Spermatic Abnormalities. N Engl J Med. 1937;217:946–951. doi: 10.1056/NEJM193712092172403. [DOI] [Google Scholar]