Abstract
SUMMARY
Background:
Bedaquiline and delamanid are the first drugs of new classes registered for tuberculosis treatment in 40 years. Each can prolong the QTc interval, with maximum effects weeks after drug initiation. The cardiac safety and microbiologic activity of these drugs when co-administered is not well-established.
Methods:
ACTG A5343 is a phase 2, open-label trial in which adults with multidrug- or rifampicin-resistant tuberculosis (MDR/RR-TB) receiving multidrug background treatment were randomized 1:1:1 using centrally, computer-generated randomization, using permuted blocks to receive bedaquiline, delamanid, or both for 24 weeks. Individuals with QTc >450ms were excluded. HIV-positive participants received dolutegravir-based ART. Clofazimine was disallowed, and levofloxacin replaced moxifloxacin. Triplicate ECG and sputum cultures were performed fortnightly. The primary endpoint was mean QTcF change from baseline (averaged over weeks 8–24).
Findings:
84 participants (28 in each treatment group, and 31 in total with HIV) were enrolled in South Africa and Peru. Mean (95% CI) change (ms) in QTc from baseline was 12.3 (7.8, 16.7) (bedaquiline), 8.6 (4.0, 13.1) (delamanid), and 20.7 (16.1, 25.3) (bedaquiline+delamanid). There were no Grade 3 or 4 QTc prolongation events and no deaths during study treatment. Cumulative culture conversion by Week 8 was 88% (21/24, bedaquiline), 83% (20/24 delamanid), and 95% (19/20, bedaquiline+delamanid) and was 92%, 91%, and 95%, respectively at 24 weeks. Among participants with HIV on dolutegravir at 24 weeks, 22 of 26 (85%) had undetectable HIV-1 viral load.
Interpretation:
Combining bedaquiline and delamanid has a modest, no more than additive, effect on the QTc interval, and initial microbiology data are encouraging. This study provides supportive evidence for use of these agents together in patients with MDR/RR-TB with normal baseline QTc values.
Keywords: Tuberculosis, Multidrug resistance, QT interval, Delamanid, Bedaquiline
INTRODUCTION
Multidrug-resistant or rifampicin-resistant (MDR/RR-) TB, TB resistant to rifampicin with or without concurrent isoniazid resistance, a is a growing public health threat, with over 484,000 incident cases in 2018 (1). Globally, only about 55–60% of patients treated for MDR/RR-TB experience treatment success (2, 3), with many patients dying of their disease. Having effective treatments with high completion rates for MDR/RR-TB is, thus, essential for global TB control. The best drugs to use to compose a 4–5 drug regimen, as recommended by WHO and other groups, and the potential role of new drugs, is unclear, owing to critical gaps in safety and efficacy data (4, 5).
Bedaquiline, a diarylquinoline, and delamanid, a nitroimidazole, are new anti-TB drugs with novel, and distinct mechanisms of action. Both are oral drugs and are well-tolerated (6). In 2018, bedaquiline was newly classified by the World Health Organization (WHO) as a Group A drug that should be offered to all patients with MDR/RR- TB (7), following evidence from an individual patient data meta-analysis that its use in MDR/RR-TB regimens improves treatment success and reduces mortality (2, 8). As only phase 2 trials have been completed, there are limited controlled safety data on bedaquiline (9). Delamanid is currently a Group C drug. In a Phase 3 trial, it added only modest activity to a full multidrug MDR/RR-TB treatment regimen but was well-tolerated (10); however, it is of interest as a component drug of experimental shorter, all-oral, more compact regimens, such as in the endTB trial (11). Bedaquiline and delamanid both cause modest QTc prolongation, presumably related to inhibitory effect on the rapidly activating delayed rectifier potassium channels (iKR) in the cardiac myosite, and predominantly caused by their metabolites, M2 and DM-6705, respectively (12, 13). The long terminal half-lives of M2 and DM-6705 result in delayed maximal QTc effects after 5–8 weeks of delamanid and 24 weeks of bedaquiline (10, 14). Cardiac safety is an area of particular concern given that other second-line TB drugs, such as moxifloxacin and clofazimine, may cause QTc prolongation (15, 16).
The effect of co-administered bedaquiline and delamanid on the QTc interval is incompletely characterized. Given that QTc prolongation is a necessary precondition for torsades de pointes (17), a life-threatening cardiac arrhythmia, there was a critical need to characterize the combined QTc effect of bedaquiline and delamanid before these drugs could be used together. However, while there is guidance on assessing the QTc effects of single drugs (18), there is no validated preclinical method for predicting the combined QTc effect of two drugs, nor is there standardized guidance for assessing combined QTc effect in humans. Further, there are limited data on the combined microbiologic effects of bedaquiline and delamanid, when used together. The main objective of AIDS Clinical Trials Group (ACTG) A5343 was to characterize the effects of bedaquiline, delamanid, or both on the QTc interval, longitudinally over 6 months of multidrug treatment, among patients with MDR/RR-TB taking multidrug background therapy (MBT).. We also assessed culture conversion over time and followed HIV treatment outcomes among patients with HIV, to whom we provided dolutegravir based-antiretroviral therapy (ART) to avert drug interactions with bedaquiline (19).
METHODS
Study design and participants.
DELamanId BEdaquiline for ResistAnt TubErculosis (DELIBERATE, NCT02583048) is a Phase 2, randomized, open-label study in adults with MDR/RR-TB, with or without HIV. The trial was approved by local ethics committees at each site, and participants gave written informed consent.
Three sites (two in South Africa, one in Peru) participated in the study. Adult participants (age ≥ 18) with pulmonary TB with a strain genotypically or phenotypically resistant to rifampicin (RR-TB) or to both rifampicin and isoniazid (MDR-TB) were eligible to participate. Participants with HIV had to have a CD4+ count ≥ 100 cells/mm3. Individuals with TB caused by M. tuberculosis resistant to fluoroquinolones or aminoglycosides; previous treatment for MDR/RR-TB; Karnofsky performance score <50; baseline QTc interval >450ms; cardiovascular disease (second or third degree AV block, prolongation of QRS over 120 ms, or clinically important arrhythmia, heart failure, coronary heart disease); creatinine > 1.4 times the upper limit of normal (ULN); ALT > 2.5 x ULN; potassium <3.4 or >5.6, magnesium < 0.59, calcium <1.75 mmol/L; or hepatitis B or C infection were excluded. Repletion and recheck of electrolytes was permitted.
Randomisation and masking.
Eligible participants were randomly assigned 1:1:1 to bedaquiline, delamanid, or bedaquiline plus delamanid arms, using permuted blocks, computer-generated centrally. Randomization was stratified by HIV status, with balancing by institution. Participants and site investigators were not masked to allocation, but central ECG laboratory staff were masked to treatment arm assignment
Procedures.
Participants had to have been receiving MBT for MDR/RR-TB for at least 7 but not more than 60 days prior to enrolment. They continued to receive MBT through the local TB program during the study. On-study MBT had to include at least 3 drugs to which the organism was thought or known to be susceptible (with study drug(s) added to comprise a regimen of at least 4–5 active drugs). Clofazimine was not allowed; moxifloxacin was switched to levofloxacin. Bedaquiline was given at a dose of 400mg daily for 14 days, followed by 200 mg thrice-weekly, for 24 weeks. Delamanid was given at a dose of 100 mg twice daily for 24 weeks. MBT continued beyond the 24 weeks of study treatment, with duration determined by the local program. For HIV-positive participants, dolutegravir was supplied by the study (together with two locally sourced nucleoside reverse transcriptase inhibitors, NRTI), to avoid drug interactions with bedaquiline.
Participants underwent sputum collection for AFB smear, culture, and susceptibility testing, HIV testing (including HIV-1 viral load and CD4+ counts for HIV-positive individuals), chest x-ray, ECG, safety laboratory testing (chemistry, hematology, liver function tests (LFT)), and clinical assessments at screening. Chemistry, hematology, and LFT were performed frequently throughout the study. ECG were performed in triplicate (three ECGs taken at least 5 minutes apart) at baseline (done at least 3 days after stopping moxifloxacin and 7 days after stopping clofazimine); every 2 weeks until Week 24; then at Week 28. Corrected QT using the Fridericia formula, QTc, was reported by a central reader blinded to study arm. Serial sputum samples were collected for mycobacterial culture. Intensive and sparse PK sampling for determination of bedaquiline and delamanid concentrations was performed. Viral load was re-tested at Week 24 for participants with HIV. Criteria for permanent premature treatment discontinuation included: failure to attend three consecutive visits, requirement for prohibited concomitant medications, pregnancy, or protocol-specified drug-related toxicities (grade 4 QTc event, grade 3 QTc event that does not resolve with drug holiday and electrolyte repletion, grade 3 or 4 drug-related allergic reaction, grade 3 or 4 rash, and protocol-defined liver toxicity). Grade 3 QTc toxicity was defined as absolute QTc > 500 ms or absolute QTc >480 together with QTc change from baseline >60 ms. Initially, participants were required to be inpatients during the first eight weeks of study treatment because the cardiac risk was completely uncharacterized. This requirement was relaxed to two weeks following an interim analysis.
Following completion of study TB drugs, participants continued treatment through the local TB program. Participants with HIV were invited to continue dolutegravir, provided through the study, for up to two years (until such time as the drug is available locally). Study follow-up for TB and HIV outcomes will continue through Week 128; results from the first 28 weeks (24 weeks of treatment with study TB drugs, plus four weeks of follow-up for safety) are presented here.
Outcomes.
The primary outcome measures were: (1) Arm-specific mean baseline QTc and mean on-treatment QTc (with on-treatment QTc averaged over weeks 8–24); and (2) Arm-specific mean QTc changes from baselineQTc. Secondary outcome measures included occurrence of absolute QTc above 500ms during study treatment; occurrence of QTc change from baseline > 60ms or 30–60ms; visit-specific QTc changes from baseline during treatment and at Week 28; grade ≥3 adverse events; and study TB drug discontinuation. Exploratory outcomes included culture conversion results and HIV viral load at 24 weeks (See Supplementary materials for exploratory outcome methodology.) Outcomes that will be reported in future manuscripts include: participant-specific PK parameters for BDQ and DLM and their metabolites and their relationship to QTcF, drug concentrations in hair and cerebrospinal fluid, final TB treatment outcomes (week 128), and acquired drug resistance.
Statistical considerations.
The primary objectives were addressed via estimation of arm-specific QTc values at Baseline (on MBT but not on study TB drugs) and on-treatment (Weeks 8–24) and estimation of changes from baseline. A mixed-effects analysis of variance (ANOVA) model was used for this estimation. In the mixed-effects ANOVA model, the dependent variable was QTc value, with fixed effects for period and random effects for participant. Period was defined as baseline (prior to initiating BDQ and/or DLM; for each participant, three separate QTc values for the triplicate of results at the single baseline visit) vs. post-baseline (while taking BDQ and/or DLM; all available QTc values from weeks 8 through 24, with a separate record for each of the three triplicate values at each visit where the participant was taking BDQ and/or DLM). Analyses included all participants who initiated study TB treatment (modified intent to treat, mITT) population. All participants with baseline and at least one post-baseline QTcF collected at a scheduled visit while taking study TB drugs were included in QTcF descriptive analyses (evaluable for the QTcF endpoints).
A sample size of at least 20 evaluable participants per arm would give >95% power to detect a 10ms difference between QTc changes in the bedaquiline+delamanid arm compared with the bedaquiline or the delamanid arm, assuming triplicate ECGs. A sample size of 25 participants per arm would give precision (half-width of the 95% confidence interval) of 6.0ms for comparisons of bedaquiline+delamanid vs. bedaquiline-alone or delamanid-alone QTc interval changes from baseline.The accrual target was set at 84 participants, to achieve 75 evaluable participants, assuming drop-out of about 10%. The primary analysis was performed when all participants completed their Week 28 visit.
Role of the funding source.
This trial was funded by the Division of AIDS, National Institutes of Health. The corresponding author had full access to all the data in the study.
RESULTS
Demographics.
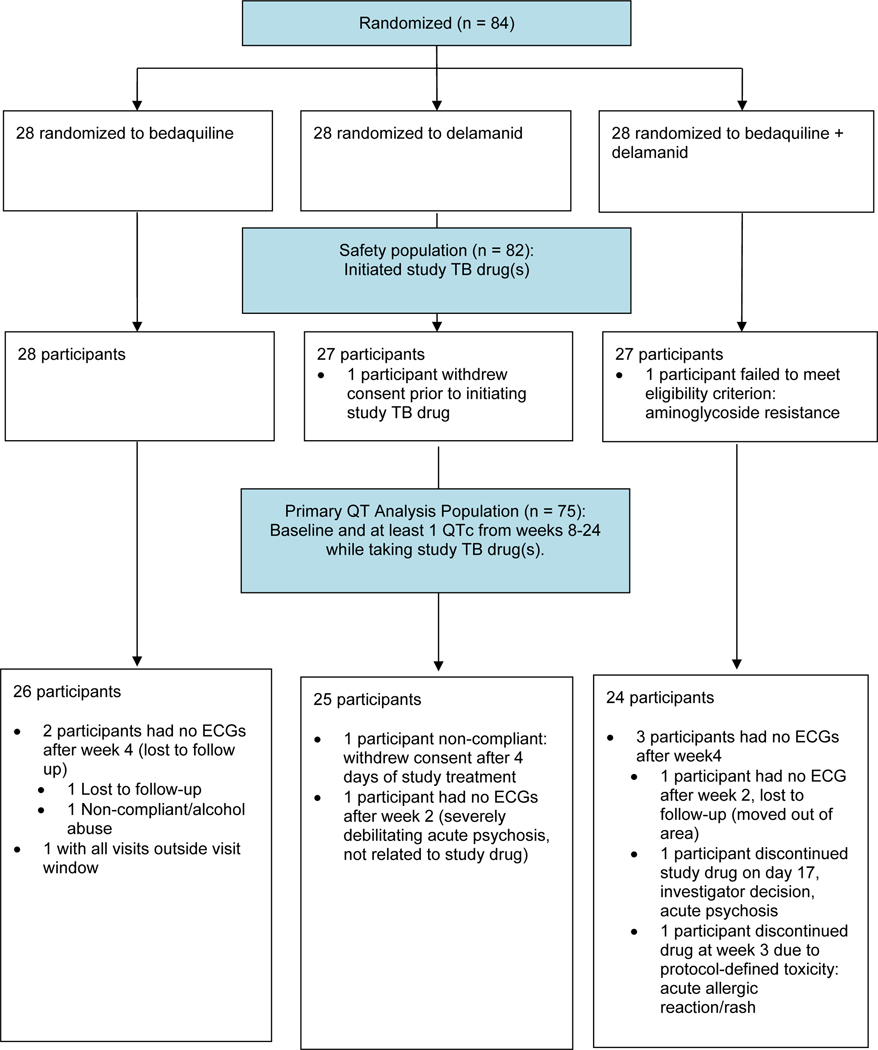
Between August 2016 and July 2018, 84 participants, 28 in each arm, enrolled at three sites—TASK in Cape Town and South African Tuberculosis Vaccine Initiative (SATVI) in Worcester, both in South Africa, and Barranco in Peru (Figure 1). The most common reason for screen failure was disqualifying drug resistance. Two participants did not initiate study treatment. One withdrew consent prior to starting study drug (delamanid arm), and one had a Mycobacterium tuberculosis (M.tb) strain resistant to aminoglycosides that was discovered after enrolment (bedaquiline+delamanid arm). The median age was 34 years, 21 (25%) were women, and 31 (37%) were HIV-positive (Table 1). Median baseline QTc was 396ms and was similar across arms. Participants had been receiving TB treatment for a median of 28 days prior to randomization. Of 84 participants, 61 (73%) were receiving standard-duration MBT (18–24 months), while 23 (27%) were taking the short-course regimen (9–12 months, with study drug(s) replacing clofazimine, and levofloxacin substituted for moxifloxacin). A summary of other drugs in the MBT regimens can be found in Supplemental materials (Table S1).
Figure 1.
CONSORT diagram, participant flow in the DELIBERATE trial.
Table 1:
Baseline characteristics
| Treatment Arm | |||||
|---|---|---|---|---|---|
| Total (N=84) | BDQ (N=28) | DLM (N=28) | BDQ+DLM (N=28) | ||
| Age (yrs) | N | 84 | 28 | 28 | 28 |
| Median (10, 90%) | 34 (20, 49) | 34.5 (21, 48) | 32 (19, 56) | 34 (19, 49) | |
| Sex | M | 63 (75%) | 22 (79%) | 21 (75%) | 20 (71%) |
| F | 21 (25%) | 6 (21%) | 7 (25%) | 8 (29%) | |
| Race | White | 1 (1%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Black African | 38 (45%) | 16 (57%) | 11 (39%) | 11 (39%) | |
| Mestizo | 6 (7%) | 1 (4%) | 2 (7%) | 3 (11%) | |
| Coloured | 38 (45%) | 11 (39%) | 13 (46%) | 14 (50%) | |
| Other | 1 (1%) | 0 (0%) | 1 (4%) | 0 (0%) | |
| HIV-1 Positive | Yes | 31 (37%) | 10 (36%) | 11 (39%) | 10 (36%) |
| No | 53 (63%) | 18 (64%) | 17 (61%) | 18 (64%) | |
| Of HIV-1 Positive, HIV treatment status | Naïve | 11 (35%) | 3 (30%) | 4 (36%) | 4 (40%) |
| Experienced | 20 (65%) | 7 (70%) | 7 (64%) | 6 (60%) | |
| Baseline QTc | Mean (s.d.) | 398 (20.3) | 398 (24.2) | 404 (19.4) | 391 (14.4) |
| Median (10, 90%) | 396 (372, 422) | 394 (370, 434) | 406 (379, 429) | 387 (374, 415) | |
| Days on TB Treatment Prior to Randomization | Median (10, 90%) | 28 (15, 45) | 26 (13,42) | 31 (16, 49) | 28 (16, 42) |
| Taking Short-Course Regimen Prior to Randomization | Yes No | 23 (27%) 61 (73%) | 9 (32%) 19 (68%) | 6 (21%) 22 (79%) | 8 (29%) 20 (71%) |
Cardiac safety results.
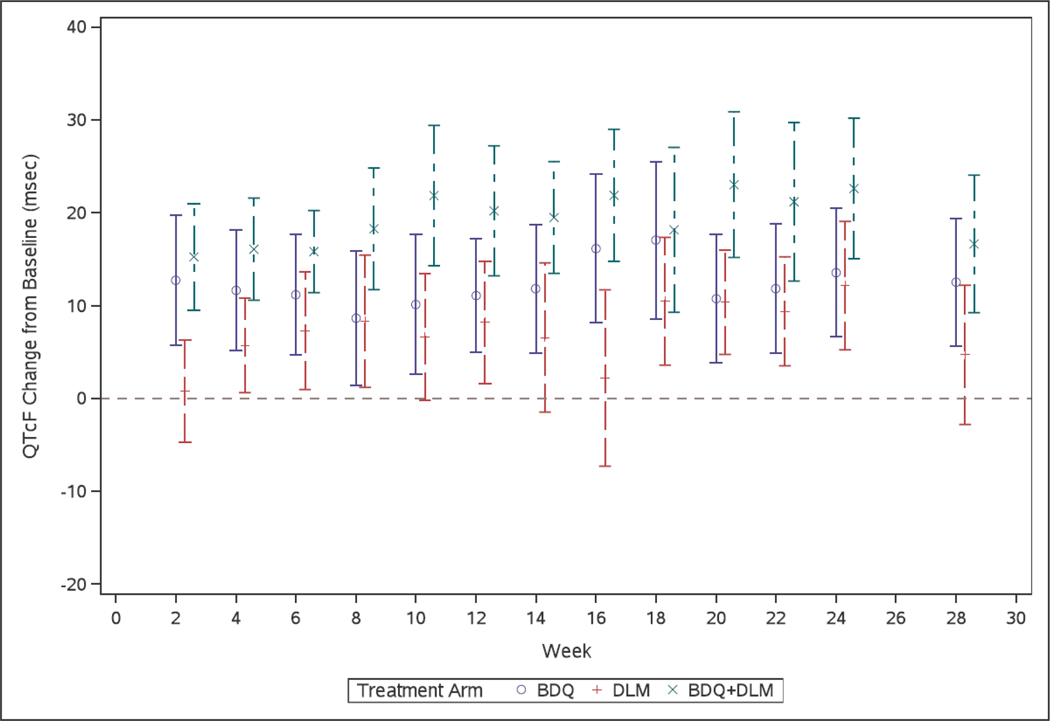
Seventy-five participants had at least one post-baseline QTc from Weeks 8–24 while taking study TB drugs and were included in the primary analyses (2099 unique ECGs). Baseline characteristics of these 75 were similar to the 84 participants who enrolled (Supplemental Table S2). Mean (95% CI) QTc change from baseline at each study visit, is shown in Figure 2. The largest median and maximum changes occurred late in treatment (Supplemental Table S3), with maximum change from baseline in any patient of 72 ms (bedaquiline arm, Week 18), 49 ms (delamanid arm, Week 14), and 75 ms (bedaquiline+delamanid, Week 22). For the primary outcome, mean (95% CI) on-treatment QTc value (in ms) was 409.7 (402.5, 416.8) in the bedaquiline arm, 413.4 (406.1, 420.8) in the delamanid arm, and 412.4 (405.0, 419.9) in the bedaquiline+delamanid arm (Table 2). Mean change in QTc (ms) from baseline, with 95% CI, by arm, were: bedaquiline 12.3 (7.8, 16.7), delamanid 8.6 (4.0, 13.1), and bedaquiline plus delamanid 20.7 (16.1, 25.3) (Table 2). There were no Grade 3 or 4 QTc prolongation events (Table 3). In exploratory analyses, for participants in the delamanid (but not the delamanid plus bedaquiline) arm, there was a trend towards increased maximum QTc change from baseline among those with albumin < 3.4 vs. ≥ 3.4 g/L, but the magnitude of the difference was small (Supplemental Table S4).
Figure 2.
Mean QTc change from baseline (with 95% confidence interval) by treatment arm, over 24 weeks of study treatment (and then four weeks following study treatment completion)
Table 2.
Estimates of QTc intervals, by arm and period, and estimates of changes from baseline and differences in changes from baseline, with 95% confidence intervals.
| Arm(s) | Period or comparison | Mean | 95% CI |
|---|---|---|---|
| Bedaquiline | Baseline | 397.4 | (389.3, 405.6) |
| On treatment | 409.7 | (402.5, 416.8) | |
| Delamanid | Baseline | 404.9 | (396.6, 413.2) |
| On treatment | 413.4 | (406.1, 420.8) | |
| Bedaquiline plus delamanid | Baseline | 391.7 | (383.2, 400.2) |
| On treatment | 412.4 | (405.0, 419.9) | |
| Bedaquiline | Change from baseline | 12.3 | (7.8, 16.7) |
| Delamanid | Change from baseline | 8.6 | (4.0, 13.1) |
| Bedaquiline plus delamanid | Change from baseline | 20.7 | (16.1, 25.3) |
| Bedaquiline plus delamanid vs. bedaquiline | Difference in change from baseline | 8.4 | (2.0, 14.8) |
| Bedaquiline plus delamanid vs. delamanid | Difference in change from baseline | 12.1 | (5.7, 18.6) |
| Bedaquiline plus delamanid vs. sum of changes on bedaquiline alone plus delamanid alone | Difference in change from baseline | -0.1 | (−8.0, 7.7) |
Table 3:
QTc adverse events, by grade.
| Arm | Total | Number (%) of Participants with QTc Prolongation... | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||||
| Bedaquiline | 28 | 9 | (32.1%) | 1 | (3.6%) | 0 | (0.0%) | 0 | (0.0%) |
| Delamanid | 27 | 11 | (40.7%) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) |
| Bedaquiline plus delamanid | 27 | 10 | (37.0%) | 2 | (7.4%) | 0 | (0.0%) | 0 | (0.0%) |
| Total | 82 | 30 | (36.6%) | 3 | (3.7%) | 0 | (0.0%) | 0 | (0.0%) |
Grade 1= 1) Absolute QTc >480 and ≤500 ms and QTc change from baseline >0 ms and ≤30 ms; or (2) absolute QTc ≤480 ms and QTc change from baseline >30 and ≤60 ms; Grade 2 = (1) Absolute QTc >480 ms and ≤500 ms and QTc change from baseline >30 ms and ≤ 60 ms; or (2) absolute QTc ≤480 and QTc change from baseline >60 ms. Grade 3 = (1) Absolute QTc >500 ms; or (2) absolute QTc >480 and QTc change from baseline >60 ms; Grade 4 = Life-threatening consequence, e.g., torsades de pointes or other associated serious ventricular dysrhythmia.
Overall safety and tolerability.
There were no deaths in the study during study treatment (one death by homicide at Week 96, delamanid arm). There were no pregnancies. Only grade 3 or higher adverse events were routinely collected. Non-cardiac grade 3 or higher adverse events occurring during study treatment in the bedaquiline arm included anaemia (n=2 participants), elevated aspartate aminotransferase (AST)(2), elevated uric acid (1), hypertension (1), peripheral neuropathy (1), hearing loss (3), weight loss (1), myalgias/arthralgias (1). In the Delamanid arm, Grade 3 or higher events were elevated creatine kinase (CK) (1), uric acid (1), glucose (1), lipase (1), peripheral neuropathy (1), psychosis (1). In the bedaquine plus delamanid arm, grade 3 or higher events were anemia (1), hyperglycemia (2), lymphopenia (1), elevated CK (4), elevated alanine aminotransferase (ALT) (1), high bilirubin (1), hearing loss (1), allergic reaction with rash and urticaria (1), seizure (2, one of which was associated with psychosis and akisthesia), hemoptysis (1), pneumothorax due to a stab wound (1). Many of the adverse events were likely attributable, at least in part, to companion drugs [e.g. acute psychoses (in the delamanid arm the participant was receiving levofloxacin and isoniazid and had adjustment issues and signs of methamphetamine use, in the bedaquiline+delamanid arm, the participant was also taking terizidone)]. Of the 82 randomized participants who initiated study TB medication(s), 68 (83%) participants completed the 24 weeks of study treatment. Most common reasons for early discontinuations were non-adherence to study procedures (n=7) or relocation/incarceration (n=2).
Sputum culture data.
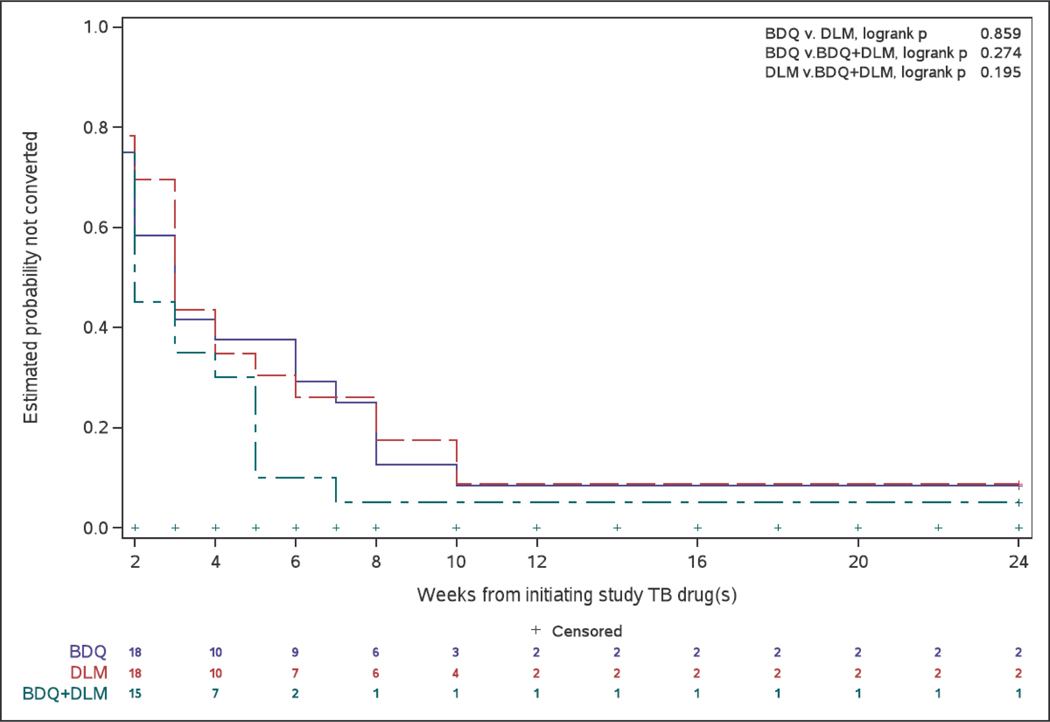
Sputum culture conversion by week 8, which was assessed in the 67 participants with a positive culture result at screening or enrolment, by study arm was: 88% (21/24) (BDQ), 83% (20/24) (DLM), and 95% (19/20) (BDQ+DLM). By Week 24, these numbers were 92%; 91%, and 95% (Figure 3). The last positive culture was seen at a median of 5, 5, and 2 weeks, in the BDQ, DLM, and BDQ+DLM arms, respectively.(Supplementary Figure S1).
Figure 3.
Cumulative culture conversion over time with treatment, by Arm.
HIV endpoints.
Median screening CD4 count was 259 cells/mm3 in the 30 participants who were HIV positive and started dolutegravir-based ART; 10 (33%) were ART-naïve and 20 (67%) were treatment-experienced at start of dolutegravir. Of these 30 patients, baseline HIV-1 viral load was available for 28. Baseline viral load was undetectable (<50 copies/mL) in 50%. Of 26 participants still on-study and still taking dolutegravir at Week 24, viral load was undetectable in 85%.
DISCUSSION
In our study, we determined that the QTc prolongation of delamanid combined with bedaquiline wass not more than additive. Moreover, no participants had a grade 3 or 4 QTc event, and even among the outliers, the maximum QTc increase seen was similar for bedaquiline+delamanid vs. bedaquiline alone. These results are reassuring and provide evidence of the safety of concurrent bedaquiline and delamanid use in patients with MDR/RR-TB. We used a novel strategy for assessing the combined cardiac risk of two drugs, which was complex owing to the very long half-lives of the metabolites that prolong QTc. To our knowledge, this is the only study that has characterized the combined QTc effects of two drugs with such precision (use of triplicate ECGs, core ECG laboratory, ECG every two weeks for six months). Finally, exploratory analyses demonstrated high rates of eight-week culture conversion in all groups, with early and sustained culture results seen in the combined treatment group.
A recent systematic review describes the data about bedaquiline’s effect in prolonging QTc as ‘scant or anecdotal,’ and data regarding bedaquiline and delamanid together is limited (20). Thus more information on the real risk to patients is needed to inform policy and practice (13). Our study featured an initial inpatient period to ensure there was not supra-additive QTc risk or arrhythmia with bedaquiline and delamanid at a time when little was known about use of these drugs together. ECGs were collected systematically and frequently (over 3000 ECGs were done in this study), thus filling a critical gap in our knowledge of the safety of these two drugs when used together. All participants were receiving stable MBT regimens excluding drugs with significant QTc-prolonging effects, so change from baseline represented the independent effects of bedaquiline or delamanid or both. Our data corroborate safety data emerging from observational studies, largely conducted in program settings, across a broader group of patients across the world (5, 21). In TBNet, a study involving 41 member European countries, for example, among 1044 patients treated with bedaquiline and 220 patients treated with delamanid (with 75–90% being dosed with another drug known to prolong the QTc interval), there was no documented case of fatal cardiac arrhythmia (22). In a retrospective study conducted by Medecins Sans Frontieres in 28 patients in Armenia, India, and South Africa receiving bedaquiline and delamanid, no patient had a QTc longer than 500 ms (23). In the largest cohort described to date of patients taking bedaquiline and delamanid together, the endTB observational study, only 2.7% of patients had a QTc > 500 ms (many of whom were getting additional QTc prolonging drugs); in data described in the WHO 2020 guidelines, there were two cardiac deaths in the endTB cohort in patients taking bedaquiline, clofazimine, capreomycin, p-aminosalicylic acid, and both had hypokalemia (neither received delamanid). (24, 25). Under compassionate use, 1 of 58 patients receiving delamanid and bedaquiline concurrently for at least 24 weeks had a QTc > 500 ms (26).
The WHO MDR/RR-TB treatment guidelines are in rapid flux. Currently, the guidelines are based on a meta-analysis of individual patient data that examined the association of treatment success with individual drugs (2, 5). While Group A drugs (bedaquiline, linezolid, levofloxacin or moxifloxacin) are strongly recommended, with moderate certainty in the evidence, Group B and C drugs are recommended conditionally with very low certainty in the estimates of effect. Currently enrolling trials (e.g. endTB, STREAM 2) will help us determine the best way to construct a regimen that has bactericidal and sterilizing activity, is robust to resistance, and is well tolerated (27). Delamanid was well tolerated in a phase 3 trial. It has some advantages over other Group B or C drugs, which commonly cause the following: skin discoloration (clofazimine), nausea and vomiting (ethionamide), neuropsychiatric side effects (cycloserine), or deafness (amikacin), plus it can be given orally (unlike meropenem). Ongoing studies suggest that regimens containing bedaquiline plus a nitroimidazole (delamanid or pretomanid) are highly active (26, 28, 29), but further data are needed to understand the role of this class of drugs in the therapy of rifampicin-resistant TB and potential synergy between the two drugs. In our study, among those randomized to receive bedaquiline and delamanid, culture conversion was 95% by Week 8, with no positive cultures after 10 weeks of therapy in contrast with the other arms. However, we did not have the power to compare microbiologic outcomes across arms. In other observational studies, high rates of culture conversion (>80%) with concomitant bedaquiline and delamanid have been seen (23, 26, 29).
In patients with HIV and MDR/RR-TB, treatment is challenging owing to drug interactions, pill burden, overlapping toxicities, and HIV-related complications. We felt it was important to include patients with HIV in our study. However, participants needed to receive ART that was compatible with the study TB drugs. We provided dolutegravir to study participants, as efavirenz and boosted protease inhibitors significantly alter bedaquiline exposure. Since DELIBERATE started, bedaquiline has become Group A for MDR/RR-TB and dolutegravir has become recommended in first-line ART by WHO for global use. The use of these drugs together was well tolerated by participants and achieved high rates of virologic suppression. Inclusion of participants with HIV provided some evidence of safety in this important population.
A key safety monitoring question for TB programs is when should ECG monitoring be done among patients taking bedaquiline, with or without delamanid? In our study, QTc in the bedaquiline-containing arms increased measurably by Week 2, and peak QTc occurred between Weeks 18 and 22. Depending on resources available, ECGs obtained at Weeks 2, 8, and 16 (with another later in treatment if the Week 16 QTc is rising) are likely to be the most informative. Four weeks after stopping bedaquiline, delamanid, or bedaquiline+delamanid, the QTc only was reduced 25%, 56%, and 26% respectively compared with the largest observed median change from baseline, highlighting the fact that the QTc effects of bedaquiline persist over weeks to months, unlike those of moxifloxacin, which do not persist past 24 hours. No evidence from our study or others suggests that QTc monitoring is required for delamanid use unless it is paired with drugs that prolong the QTc more significantly. In patients with MDR/RR-TB who develop QTc prolongation, careful questioning regarding concomitant medications and correction of low serum electrolytes is generally prudent.
Our study has limitations. Study participants were carefully selected with a normal QTc interval at baseline, had frequent ECGs, were carefully monitored for other conditions that prolong QTc (e.g. hypothyroidism, electrolyte abnormalities), and concomitant QTc prolonging drugs (including clofazimine) were not permitted. QTc was determined with precision in our study and our estimates of QTc prolongation by bedaquiline and delamanid are consistent with larger randomised controlled trials. Given the modest QT prolonging effect we found in the bedaquiline+delamanid arm, we feel that our results are generalisable to the clinic, provided baseline and monitoring ECGs are done. Thus, clinicians can consider that delamanid will add 6–8ms to an MDR/RR-TB treatment regimen, an effect that is generally not clinically important. This is in contrast to the combined QTc risk of bedaquiline and clofazimine, which has not been characterized in a systematic way but appears to be higher in magnitude than bedaquiline plus delamanid (30), and yet these drugs are recommended for concurrent use. Our microbiologic outcomes were only exploratory and the study lacked power to detect differences between study arms; participants were on MBT for a median of 28 days prior to study start, which resulted in negative sputum cultures at baseline in some, further reducing the sample size for this outcome. The early and sustained culture conversion seen in the bedaquiline plus delamanid arm will need to be confirmed in larger trials. Finally, the use of these drugs together over longer time periods and among children was not assessed and so these areas merit study.
In conclusion, the QTc effects of bedaquiline and delamanid are not more than additive and under clinical trial conduct were not associated with Grade 3 or 4 QTc prolongation. Our study adds to the emerging evidence that supports the safety of use of these two drugs together. We provide an effective framework for studying QTc interaction effects of drugs with long half-lives, metabolite effects, and increasing QTc effect with drug accumulation. Finally, although the study was not powered for efficacy outcomes and so these results remain inconclusive, high rates of early, sustained culture conversion were seen in participants receiving bedaquiline plus delamanid, which needs to be confirmed in larger clinical trials.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Bedaquiline and delamanid were the first new drugs approved for tuberculosis in >40 years. They are used for treatment of TB resistant to first line agents. Each modestly prolongs the QTc interval on the electrocardiogram (ECG), and so the World Health Organization recommended against their concurrent use because of concerns about possible increase in QTc-related cardiac risk. In small cohorts in which patients received the drugs together under compassionate use under program conditions, variable proportions of patients had QTc prolongation, but few events requiring treatment discontinuation. Many patients in those cohorts received concomitant clofazimine and/or moxifloxacin, or did not start bedaquiline and delamanid concurrently. There has been no prospective randomized assessment of the QTc effects of co-administered bedaquiline and delamanid vs. each drug alone. The added clinical benefit of delamanid, added to bedaquiline-containing regimens, is also uncertain.
Added value of this study
We conducted a randomized controlled trial of bedaquiline, delamanid, or both added to background therapy in participants with rifampicin-resistant TB to accurately determine QTc effects (the primary endpoint). Triplicate ECG read by a central reader and sputum cultures were taken at baseline and frequently over 24 weeks; participants were initially hospitalized. Adding delamanid to bedaquiline resulted in no more than additive QTc effects, no participants had grade 3 or 4 QTc events, and the study drugs were well-tolerated, including among people living with HIV. In exploratory analyses, eight-week culture conversion was high in all groups, and sustained in the combined treatment arm.
Implications of all the available evidence
Data collected prospectively in our trial and retrospective data collected under program conditions all point to acceptable cardiac safety of co-administration of bedaquiline and delamanid. Preliminary microbiology data are also encouraging. The restriction on their concurrent use should be lifted.
ACKNOWLEDGMENTS
We are grateful first and foremost to study participants and their families. We acknowledge the DELIBERATE study team members who contributed to the trial, listed in the Appendix. This study was funded by the National Institutes of Health, Division of AIDS of the National Institutes of Health under Award Numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701. This work was supported in part by the Barranco CRS grant #UM1 AI069438, SATVI CRS grant # UM1 AI069519, and TASK CRS grant #UM1AI069521. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Bedaquiline was donated by Janssen, Delamanid was donated by Otsuka, Dolutegravir was donated by ViiV Healthcare. The South African Department of Health approved purchase of linezolid at tender price, which allowed access to this drug for many trial patients who needed it.
Funding: DAIDS, NIH
Footnotes
DATA SHARING STATEMENT
Deidentified participant data and data dictionary will be available upon request, and upon approval by the sponsor, following completion of all study visits and publication of final manuscripts.
Members listed in the Appendix
DECLARATION OF INTERESTS
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Organization WH. Global Tuberculosis Report 2019 WHO/CDS/TB/2019.15. 2019. [Google Scholar]
- 2.Collaborative Group for the Meta-Analysis of Individual Patient Data in MDRTBt, Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet (London, England). 2018;392(10150):821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastos ML, Lan Z, Menzies D. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. The European respiratory journal. 2017;49(3): 10.1183/13993003.00803-2016. Print 2017 Mar. [DOI] [PubMed] [Google Scholar]
- 4.Nahid P, Mase SR, Migliori GB, Sotgiu G, Bothamley GH, Brozek JL, et al. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. American journal of respiratory and critical care medicine. 2019;200(10):e93–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH. WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment. 2020. [PubMed] [Google Scholar]
- 6.Lan Z, Ahmad N, Baghaei P, Barkane L, Benedetti A, Brode SK, et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8(4):383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health O. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment: WHO/CDS/TB/2019.3. 2019. Contract No.: Report. [Google Scholar]
- 8.Ndjeka N, Schnippel K, Master I, Meintjes G, Maartens G, Romero R, et al. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. The European respiratory journal. 2018;52(6): 10.1183/13993003.01528-2018. Print 2018 Dec. [DOI] [PubMed] [Google Scholar]
- 9.Cohen K, Maartens G. A safety evaluation of bedaquiline for the treatment of multi-drug resistant tuberculosis. Expert opinion on drug safety. 2019;18(10):875–82. [DOI] [PubMed] [Google Scholar]
- 10.von Groote-Bidlingmaier F, Patientia R, Sanchez E, Balanag V, Ticona E, Segura P, et al. Efficacy and safety of delamanid in combination with an optimised background regimen for treatment of multidrug-resistant tuberculosis: a multicentre, randomised, double-blind, placebo-controlled, parallel group phase 3 trial. The LancetRespiratory medicine. 2019;7(3):249–59. [DOI] [PubMed] [Google Scholar]
- 11.Seung KJ, Khan P, Franke MF, Ahmed S, Aiylchiev S, Alam M, et al. Culture conversion at six months in patients receiving delamanid-containing regimens for the treatment of multidrug-resistant tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Geiter LJ, Hafkin J, Wells CD. Delamanid and QT prolongation in the treatment of multidrug-resistant tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(10):1261–2. [DOI] [PubMed] [Google Scholar]
- 13.Pontali E, Sotgiu G, Tiberi S, D’Ambrosio L, Centis R, Migliori GB. Cardiac safety of bedaquiline: a systematic and critical analysis of the evidence. The European respiratory journal. 2017;50(5): 10.1183/13993003.01462-2017. Print 2017 Nov. [DOI] [PubMed] [Google Scholar]
- 14.Pym AS, Diacon AH, Tang SJ, Conradie F, Danilovits M, Chuchottaworn C, et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. The European respiratory journal. 2016;47(2):564–74. [DOI] [PubMed] [Google Scholar]
- 15.Moon SJ, Lee J, An H, Yim DS, Chung JY, Yu KS, et al. The effects of moxifloxacin on QTc interval in healthy Korean male subjects. Drugs R D. 2014;14(2):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, et al. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. American journal of respiratory and critical care medicine. 2015;191(8):943–53. [DOI] [PubMed] [Google Scholar]
- 17.Roden DM. Predicting drug-induced QT prolongation and torsades de pointes. The Journal of physiology. 2016;594(9):2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food US, Drug A. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. 2005. Contract No.: Report. [Google Scholar]
- 19.Dooley KE, Park JG, Swindells S, Allen R, Haas DW, Cramer Y, et al. Safety, tolerability, and pharmacokinetic interactions of the antituberculous agent TMC207 (bedaquiline) with efavirenz in healthy volunteers: AIDS Clinical Trials Group Study A5267. Journal of acquired immune deficiency syndromes (1999). 2012;59(5):455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pontali E, Sotgiu G, Tiberi S, Tadolini M, Visca D, D’Ambrosio L, et al. Combined treatment of drug-resistant tuberculosis with bedaquiline and delamanid: a systematic review. The European respiratory journal. 2018;52(1): 10.1183/13993003.00934-2018. Print 2018 Jul. [DOI] [PubMed] [Google Scholar]
- 21.Olayanju O, Esmail A, Limberis J, Dheda K. A regimen containing bedaquiline and delamanid compared to bedaquiline in patients with drug-resistant tuberculosis. Eur Respir J. 2020;55(1). [DOI] [PubMed] [Google Scholar]
- 22.Guglielmetti L, Tiberi S, Burman M, Kunst H, Wejse C, Togonidze T, et al. QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: a Tuberculosis Network European Trialsgroup (TBnet) study. The European respiratory journal. 2018;52(2): 10.1183/13993003.00537-2018. Print 2018 Aug. [DOI] [PubMed] [Google Scholar]
- 23.Ferlazzo G, Mohr E, Laxmeshwar C, Hewison C, Hughes J, Jonckheere S, et al. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. The LancetInfectious diseases. 2018;18(5):536–44. [DOI] [PubMed] [Google Scholar]
- 24.end TBi. Bedaquiline- and delamanid containing regimens achieve excellent interim treatment response without safety concerns. 2018. Contract No.: Report. [Google Scholar]
- 25.Khan U, Huerga H, Khan AJ, Mitnick CD, Hewison C, Varaine F, et al. The endTB observational study protocol: treatment of MDR-TB with bedaquiline or delamanid containing regimens. BMC infectious diseases. 2019;19(1):733–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafkin J, Hittel N, Martin A, Gupta R. Compassionate use of delamanid in combination with bedaquiline for the treatment of multidrug-resistant tuberculosis. The European respiratory journal. 2019;53(1): 10.1183/13993003.01154-2018. Print 2019 Jan. [DOI] [PubMed] [Google Scholar]
- 27.Pontali E, Raviglione MC, Migliori GB, and the writing group members of the Global TBNCTC. Regimens to treat multidrug-resistant tuberculosis: past, present and future perspectives. Eur Respir Rev. 2019;28(152). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keam SJ. Pretomanid: First Approval. Drugs. 2019. [DOI] [PubMed] [Google Scholar]
- 29.Sarin R, Vohra V, Singla N, Singla R, Puri MM, Munjal SK, et al. Early efficacy and safety of Bedaquiline and Delamanid given together in a “Salvage Regimen” for treatment of drug-resistant tuberculosis. The Indian journal of tuberculosis. 2019;66(1):184–8. [DOI] [PubMed] [Google Scholar]
- 30.Food US, Drug A. Sirturo (bedaquiline) package label. 2012. Contract No.: Report. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.