Abstract
A wide body of evidence supports an integral role for mesolimbic dopamine (DA) in motivated behavior. In brief, drugs that increase DA in mesolimbic terminal regions, like cocaine, enhance motivation, while drugs that decrease DA concentration reduce motivation. Data from our laboratory and others shows that phasic activation of mesolimbic DA requires signaling at cannabinoid type-1 (CB1) receptors in the ventral tegmental area (VTA), and systemic delivery of CB1 receptor antagonists reduces DA cell activity and attenuates motivated behaviors. Recent findings demonstrate that cocaine mobilizes the endocannabinoid 2-arachidonoylglycerol (2-AG) in the VTA to cause phasic activation of DA neurons and terminal DA release. It remains unclear, however, if cocaine-induced midbrain 2-AG signaling contributes to the motivation-enhancing effects of cocaine. To examine this, we trained male and female rats on a progressive ratio (PR) task for a food reinforcer. Each rat underwent a series of tests in which they were pretreated with cocaine alone or in combination with systemic or intra-VTA administration of the CB1 receptor antagonist rimonabant or the 2-AG synthesis inhibitor tetrahydrolipstatin (THL). Cocaine increased motivation, measured by augmented PR breakpoints, while rimonabant dose-dependently decreased motivation. Importantly, intra-VTA administration of rimonabant or THL, at doses that did not decrease breakpoints on their own, blocked systemic cocaine administration from increasing breakpoints in male and female rats. These data suggest that cocaine-induced increases in motivation require 2-AG signaling at CB1 receptors in the VTA and may provide critical insight into cannabinoid-based pharmacotherapeutic targets for the successful treatment of substance abuse.
Keywords: endocannabinoids, cocaine, motivation, CB1 receptor, 2-arachidonoylglycerol, ventral tegmental area, dopamine
1. Introduction
Motivation is commonly conceptualized as the driving force behind goal-directed behaviors. Thus, neural mechanisms of motivation control basic survival behaviors like seeking rewards and avoiding harm, and perturbation of these systems contributes to neuropsychiatric disorders, such as substance use disorders. It is well-documented that the mesolimbic dopamine (DA) system plays a crucial role in motivated behavior (Berridge and Robinson, 1998; Salamone, 1994; Wise, 2004). In support of this, appetitive stimuli or related cues elicit burst firing of DA neurons, phasic DA release in terminal regions, and approach behavior; while DAergic antagonism attenuates goal-directed approach (Bassareo et al., 2002; Brown et al., 2011; Ikemoto and Panksepp, 1996; Mai et al., 2012; Romo and Schultz, 1990; Salamone et al., 1991).
Laboratory assessment of motivation in rodents frequently uses progressive ratio (PR) schedules of reinforcement. During PR, an exponentially escalating number of operant responses (e.g., lever presses) is required for each successive reinforcer delivery. The last schedule an animal is willing to complete for a given reinforcer is termed their “breakpoint,” with higher breakpoints reflecting greater levels of motivation. Studies using PR demonstrate that DA depletion or receptor antagonism decreases breakpoints for food reinforcers (Aberman et al., 1998; Hamill et al., 1999). Conversely, augmentation of DA signaling through knockdown or pharmacological inhibition of the membrane DA transporter (DAT) increases PR breakpoints (Cagniard et al., 2006; Sommer et al., 2014). Altogether, such studies demonstrate that DA signaling is both necessary and sufficient for motivated behavior.
A wide body of research shows that psychostimulants, such as cocaine, increase DA concentration in mesolimbic terminal regions via blockade of DAT-mediated DA reuptake activation of DA neurons (Beuming et al., 2008; Covey et al., 2016; Ferris et al., 2011; Hernandez and Hoebel, 1988; Mejias-Aponte et al., 2015; Ritz et al., 1987). Given that cocaine is a potent driver of DA, it is not surprising that it enhances motivated behavior. Indeed, acute cocaine delivery increases PR breakpoints for food (Jones et al., 1995; Sizemore et al., 2003; Thompson, 1977). Chronic cocaine administration, however, results in significant changes in mesolimbic system function and leads to an aberrant motivational state characterized by enhanced motivation for drug and drug-related stimuli and decreased motivation for alternative reinforcers (Thomas et al., 2008; Volkow et al., 2019). Because of this, it is imperative to understand the mechanisms by which cocaine activates motivational brain networks.
Recent research shows that cocaine administration results in on-demand synthesis and release of the endocannabinoid 2-Arachidonoylglycerol (2-AG) in the midbrain (Nakamura et al., 2019; Wang et al., 2015). Mobilized 2-AG binds to cannabinoid type-1 (CB1) receptors on ventral tegmental area (VTA) GABAergic terminals, resulting in reduced GABA release and consequent disinhibition of DAergic neurons (Cheer et al., 2000; Lupica and Riegel, 2005; Nakamura et al., 2019; Riegel and Lupica, 2004; Szabo et al., 1999; Szabo et al., 2002; Wang et al., 2015). These studies illustrate a previously unknown mechanism through which cocaine administration causes enhanced activation of midbrain DA cells to increase terminal DA concentration. Previous research from our lab and others suggests that 2-AG-mediated accumbal DA release is required for appetitively motivated behaviors (Melis et al., 2004; Oleson et al., 2012). However, it remains unknown if cocaine-induced augmentation of motivated behavior is mediated through a similar 2-AG-dependent mechanism.
Here we examine the novel hypothesis that cocaine-induced increases in motivation require midbrain endocannabinoid mobilization and CB1 signaling. To test this, male and female rats were trained on a PR schedule for a food reinforcer. Consistent with previous reports, we found that cocaine administration increased motivation as evidenced by increased breakpoints for food. In support of our hypothesis, systemic or intra-VTA delivery of the CB1 receptor inverse agonist rimonabant blocked cocaine-induced increases in breakpoints. Furthermore, intra-VTA infusion of the 2-AG synthesis inhibitor tetrahydrolipstatin (THL) similarly attenuated breakpoints following cocaine administration, substantiating a central role for midbrain 2-AG signaling in cocaine-mediated appetitive motivation.
2. Materials and Methods
2.1. Animals
Adult Long-Evans rats [transgene negative litter mates of the strain LE-Tg(TH-Cre)3.1Deis; 12 males, 14 females, ~6 months old] were bred on-site (University of Maryland School of Medicine, Baltimore, MD, USA). Rats were grouped housed prior to surgery or behavioral training after which they were singly-housed in plastic cages with corn-cob bedding in a temperature- and humidity-controlled facility (22°C at 40–50% humidity) on a 12-hr light/dark cycle (lights on at 07:00). Animals were food restricted to ~85% of their free feeding weight with free access to water. All methods and procedures were conducted in adherence to the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
2.2. Progressive Ratio (PR) Task
All behavioral procedures were conducted in standard Med Associates operant conditioning chambers (12.0” L × 9.5” W × 8.25”; Med Associates Inc., VT, USA) placed inside sound-attenuating cabinets. Briefly, chambers consisted of black plastic flooring, two retractable levers (one active and one inactive), white cue lights above the levers, a pellet dispenser, recessed pellet trough, a white house light, and an auditory tone generator and speaker. Behavioral programs were controlled and recorded by Med PC software.
Rats were first trained to lever press under a fixed-ratio one (FR1) schedule of reinforcement. At the beginning of each trial the cue light was illuminated above the active lever. Response on the active lever resulted in delivery of the auditory tone and one chocolate-flavored food pellet (Bio Serv, Product #F0229, NJ, USA) followed by a 15-second time-out. Responses on the inactive lever had no consequence. Once rats earned at least 40 pellets on each of three consecutive days, they were moved to a fixed-ratio five (FR5) schedule of reinforcement in which five responses on the active lever resulted in delivery of one food pellet.
Following three consecutive days of stable FR5 performance (at least 50 reinforcers earned/session), rats were moved to a PR schedule. During PR, the number of responses required on the active lever to earn one reinforcer increased exponentially over each successive trial: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, etc. If a reinforcer was not earned within 20 min after the start of a trial, the program terminated. The total number of reinforcers the animal earned was recorded as its breakpoint (Richardson and Roberts, 1996). Rats were trained on PR until breakpoints stabilized (± one response schedule or reward for three consecutive days with no increasing or decreasing trend) before undergoing drug treatment test sessions and were given at least two days to return to baseline levels of lever pressing in between drug test sessions.
2.3. Systemic Pharmacological Manipulations
Twelve male and 5 female rats received a series of systemic drug test sessions. The CB1 receptor antagonist rimonabant (Cayman Chemicals, MI, USA) was dissolved in a vehicle of ethanol, emulphor (Alkamuls EL620, Solvay Chemicals, NJ, USA), and 0.9% sterile physiological saline in a volumetric ratio of 1:1:18. Cocaine (NIDA Drug Supply Program, RTI, NC, USA) was dissolved in 0.9% sterile physiological saline. Blocks of saline or cocaine injections (e.g., 10 mg/kg cocaine, 15 mg/kg cocaine) were counter-balanced across animals, and within blocks, co-treatment with vehicle or rimonabant was pseudorandom. Rats received a dose of rimonabant (1 or 3 mg/kg, IP) or vehicle 30 min prior to cocaine (10 or 15 mg/kg, IP) or vehicle injection. All animals received a dose of cocaine or vehicle five minutes before beginning the PR task. Similar doses of rimonabant have been utilized by our laboratory and have not been found to affect locomotor behavior (Hernandez and Cheer, 2012; Hernandez et al., 2014; Morra et al., 2010; Ramiro-Fuentes and Fernandez-Espejo, 2011).
2.4. Cannula Implantation
Following completion of the systemic drug test sessions, a subset of rats (11 males, 5 females) underwent stereotaxic surgery for intra-VTA cannulation along with an additional cohort of rats (9 females) that was trained under similar behavioral conditions but did not receive systemic treatments. Bilateral guide cannula (26GA, 10 mm in length, Plastics One, VA, USA) were stereotaxically implanted in the VTA of anesthetized (Isoflurane, USP) rats at the following coordinates from bregma: −5.8 AP, +/− 0.7 ML, −7.0 DV (Paxinos, 2007). Cannulae were fixed to the skull with acrylic cement secured with stainless steel bone screws. Following implantation, a 10 mm obdurator (33GA, Plastics One) was placed inside each guide cannula and secured with a plastic dust cap (Plastics One). Before surgery, animals received carprofen for analgesia (5 mg/kg, SC) and 5 ml of 0.9% sterile physiological saline to prevent dehydration. For two days after surgery, animals received carprofen, saline, and a topical triple antibiotic ointment (bacitracin zinc, neomycin sulfate, polymyxin B sulfate) and lidocaine (2.5% lidocaine, 2.5% prilocaine) around their head cap to prevent infection and promote wound recovery. Rats were allowed 5–7 days to recover before food restriction.
2.5. Intra-VTA Micro-infusions
Rimonabant and the diacylglycerol lipase inhibitor tetrahydrolipostatin (THL, Cayman Chemicals) were each dissolved in ethanol, emulphor (Solvay Chemicals), and 0.9% sterile saline (1:1:18). For infusion, obdurators were removed and a bilateral 11 mm internal-cannula infuser (Plastics One) was inserted into the VTA guide cannula. Internal-cannula infusers were attached to Hamilton syringes (Hamilton Company, NV, USA) via PE50 tubing back-filled with rimonabant, THL, or vehicle. Microinfusion pumps (World Precision Instruments, FL, USA) controlled the infusion rate (0.25ul/min). In a within-subjects design, rats received each dose of intra-VTA rimonabant (0.5 ug/0.5 ul/side, 1.0 ug/0.5 ul/side) and THL (5 ug/0.5 ul/side) on separate test days. Blocks of saline or cocaine injections (e.g., 10 mg/kg cocaine, 15 mg/kg cocaine) were counter-balanced across animals, and within blocks, co-treatment with vehicle, rimonabant, or THL was pseudorandom. In the event of a clogged cannula, rats were removed from study. Following infusion, internal-cannulae were left in place for ~30 seconds to allow drug diffusion. After five minutes, rats received vehicle or cocaine (10 mg/kg or 15 mg/kg, IP) and were placed in the operant chamber five minutes before beginning PR sessions. Similar doses of rimonabant and THL have been shown to decrease appetitive behaviors without disrupting locomotion (Oleson et al., 2012; Wang et al., 2015; Wenzel et al., 2018), and we have previously demonstrated that the present dose of THL reduces tissue levels of 2-AG following intra-VTA administration (Wenzel et al., 2018).
2.6. Histology
Animals were deeply anesthetized with isoflurane and transcardially-perfused with 100 mL of 0.1M phosphate-buffered saline (PBS) followed by 400 mL of 4% paraformaldehyde (PFA). Brains were removed and stored in PFA for 2 hours and then stored in 30% sucrose at 4ºC for ~2 days. Brains were then frozen in powdered dry ice and coronal sections (60 μm) containing the VTA were cut using a cryostat (Leica CM1860 UV Cryostat; Leica Microsystems Inc, IL, USA) and mounted onto glass slides. Individual cannula sites were histologically verified for proper placement within the VTA.
2.7. Statistical Analyses
The primary dependent measures were total number of reinforcers earned per session and total inactive lever presses per session. Due to the within-subjects design, treatment sessions were compared to the preceding baseline sessions rather than the vehicle sessions to account for drifts in baseline over time. Data were first analyzed with 2-factor mixed ANOVA, with sex as the between-subjects factor and treatment as the repeated measure, and there were no significant interactions or main effects of sex. Thus males and females were subsequently combined and analyzed with single factor repeated-measures ANOVA. Where appropriate, planned contrasts to assess treatment effects were performed with the Sidak procedure, and results were considered significant if p < 0.05. All statistical analyses were performed using Prism (version 9.0.0 for Mac, GraphPad Software, La Jolla, CA), and data are presented as mean ± SEM.
3. Results
3.1. Systemic rimonabant attenuates cocaine-potentiated motivation
Following stable performance on the PR task, treatment with cocaine and drugs targeting the endocannabinoid system commenced. Rats were treated with systemic injections of rimonabant (1 and 3 mg/kg, IP) and vehicle in separate sessions. Despite a significant main effect of treatment (F5,75 = 4.78, p < 0.001), there was no significant main effect of sex (F1,15 = 0.5365, n.s.) or sex × treatment interaction (F5,75 = 0.5746, n.s.). Therefore, data were collapsed across sex and analyzed further with a single-factor repeated measures ANOVA. Although there remained a significant main effect of treatment (F5,80 = 5.207, p < 0.001), neither dose of rimonabant or vehicle reduced the number of reinforcers earned (Fig. 1A) compared to baseline sessions.
Figure 1.
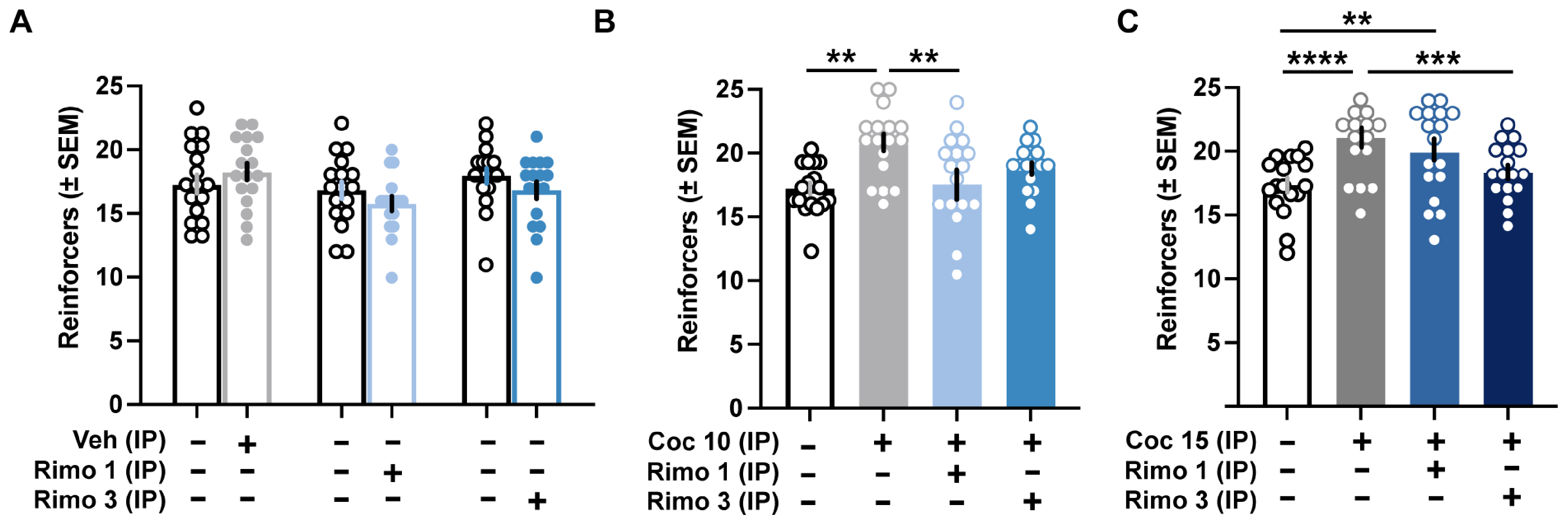
The CB1-receptor antagonist rimonabant attenuates cocaine-induced increases in motivation on a PR schedule of reinforcement. (A) Systemic administration of vehicle or rimonabant (1 and 3 mg/kg, IP) did not change the breakpoints or total number of reinforcers earned compared to baseline sessions (n = 17; 12 males, 5 females). (B) Cocaine (10 mg/kg, IP) significantly increased the total number of reinforcers, and this effect was reversed by co-administration of rimonabant (1 mg/kg, IP) (n = 17; 12 males, 5 females). (C) Rimonabant at 3 mg/kg (IP) but not 1 mg/kg (IP) reduced motivation enhanced by 15 mg/kg cocaine (IP) (n = 17; 12 males, 5 females). ** p < 0.01, *** p < 0.001, **** p < 0.0001
Systemic cocaine, however, significantly enhanced motivation. There were no significant effects of sex (10 mg/kg cocaine – sex: F1,15 = 0.9773, n.s.; treatment: F1.502,22.54 = 3.561, n.s.; sex × treatment: F3,45 = 1.750, n.s.; 15 mg/kg cocaine – sex: F1,15 = 0.007328, n.s.; treatment: F2.188,32.10 = 9.565, p < 0.001; sex × treatment: F3,45 = 1.798, n.s.), and male and female data were subsequently combined. Cocaine increased the number of reinforcers earned at both the 10 (Fig. 1B; F3,48 = 5.561, p < 0.01; p< 0.01 vs. baseline) and 15 (Fig. 1C; F3,48 = 12.83, p < 0.0001; p < 0.0001 vs. baseline) mg/kg (IP) doses. These increases in motivation were reversed by co-treatment with rimonabant at 1 mg/kg (IP) for 10 mg/kg cocaine (Fig. 1B; p < 0.01 vs. cocaine) and 3 mg/kg (IP) for 15 mg/kg cocaine (Fig. 1C; p < 0.001 vs. cocaine). Although PR performance following 3 mg/kg (IP) rimonabant and 10 mg/kg (IP) cocaine was not significantly lower than cocaine alone, it was also not statistically different than baseline levels.
Finally, there were no sex differences in inactive lever presses (IP rimonabant – sex: F1,15 = 1.056, n.s.; treatment: F5.75 = 2.179, n.s.; sex × treatment: F5,75 = 1.443, n.s.; 10 mg/kg IP cocaine + IP rimonabant – sex: F1,15 = 0.7151, n.s.; treatment: F1.779,26.69 = 3.031, n.s.; sex × treatment: F3,45 = 1.737, n.s.; 15 mg/kg IP cocaine + IP rimonabant – sex: F1,15 = 0.7151, n.s.; treatment: F1.779,26.69 = 3.031, n.s.; sex × treatment: F3,45 = 1.737, n.s.), and systemic treatment with rimonabant (F5,80 = 1.952, n.s.), cocaine, or their combination at any dose (10 mg/kg IP cocaine + IP rimonabant: F3,48 = 1.776, n.s.; 15 mg/kg IP cocaine + IP rimonabant: F3,48 = 2.435, n.s.) had no significant effect on inactive lever presses during PR sessions.
3.2. Intra-VTA rimonabant and THL prevent cocaine-potentiated motivation
Next, we examined the role of VTA CB1 receptor signaling and 2-AG mobilization in cocaine’s ability to promote food-motivated behavior. Intra-VTA microinjections of rimonabant, THL, and vehicle were administered concurrently with systemic injections of cocaine (Fig. 2D). There were no sex differences in the effects of intra-VTA rimonabant (0.5 and 1 ug/0.5 ul/side) or vehicle alone on PR task performance (sex: F1,23 = 8.454 × 10−6, n.s.; treatment: F3.099, 66.32 = 1.276, n.s.; sex × treatment: F5,107 = 1.110, n.s.). With male and female data combined, microinjections of rimonabant or vehicle into the VTA following IP saline injection had no effect on the number of reinforcers earned compared to baseline sessions (Fig. 2A; F5,112 = 1.325, n.s.).
Figure 2.
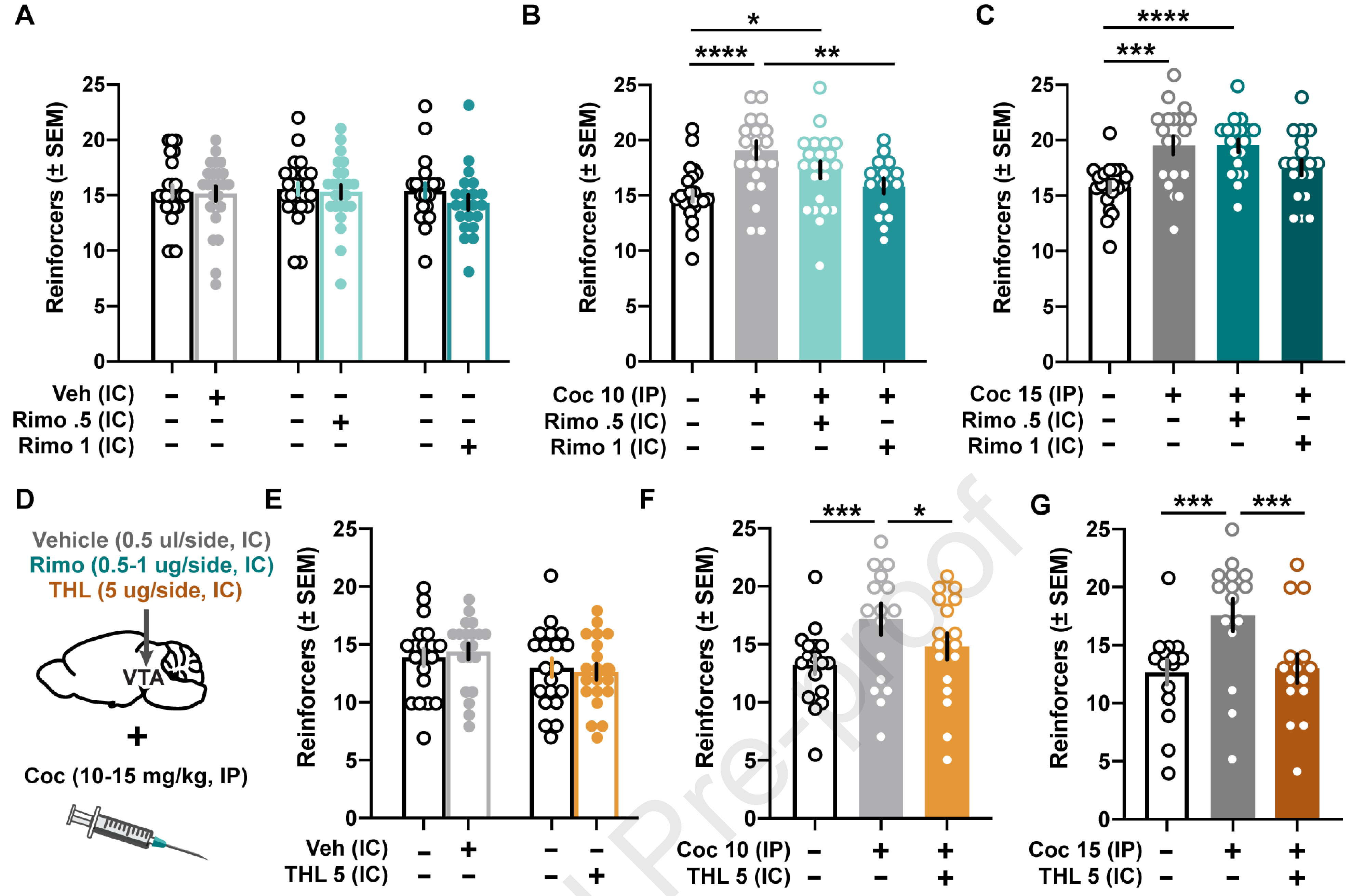
Antagonizing the CB1 receptor or preventing 2-AG mobilization in the VTA prevents cocaine-mediated increases in motivation. (A) Intracranial administration of vehicle (0.5 ul/side, IC) or rimonabant (0.5 and 1 ug/0.5 ul/side, IC) did not change the breakpoints or total number of reinforcers earned compared to baseline sessions (n = 25; 11 males, 14 females). (B) Increases in motivation following 10 mg/kg (IP) cocaine are reversed by intra-VTA administration of rimonabant (1 ug/0.5 ul/side, IC) (n = 22; 11 males, 11 females). (C) Although the intra-VTA administration of rimonabant (1 ug/0.5 ul/side, IC) did not significantly reduce motivation enhanced by 15 mg/kg (IP) cocaine, it reduced the number of reinforcers to baseline levels (n = 20; 11 males, 9 females). (D) Schematic of intracranial (IC) and systemic (IP) injections. (E) Intracranial administration of vehicle (0.5 ul/side, IC) or THL (5 ug/0.5 ul/side, IC) did not change the breakpoints or total number of reinforcers compared to baseline sessions (n = 20; 10 males, 10 females). (F) Intra-VTA THL administration reverses increases in motivation due to 10 mg/kg (IP) cocaine (n = 17; 8 males, 9 females). (G) Intra-VTA THL administration reverses increases in motivation due to 15 mg/kg (IP) cocaine (n = 15; 9 males, 6 females). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
Systemic cocaine, as before, increased motivation. Female and male data were combined following analyses yielding no significant effects of sex (10 mg/kg cocaine – sex: F1,20 = 0.1731, n.s.; treatment: F2.258,39.90 = 10.28, p < 0.0001; sex × treatment: F3,53 = 0.6815, n.s.; 15 mg/kg cocaine – sex: F1,18 = 1.169, n.s.; treatment: F2.418,42.71 = 10.52, p < 0.0001; sex × treatment: F3,53 = 1.286, n.s.). Cocaine increased motivation at both the 10 (Fig. 2B; F3,56 = 10.24, p < 0.0001; p < 0.0001 vs. baseline) and 15 (Fig. 2C; F2.339,43.67 = 10.75, p < 0.0001; p < 0.001 vs. baseline) mg/kg (IP) doses. However, intra-VTA delivery of rimonabant reduced cocaine-mediated enhancement of motivation. Rimonabant (1 ug/0.5 ul/side) co-administered with 10 mg/kg cocaine significantly decreased the number of reinforcers compared to cocaine alone (Fig. 2B; p < 0.01) and reduced the number of reinforcers following 15 mg/kg cocaine to baseline levels (Fig. 2C).
In addition to CB1 receptor antagonism, impairing mobilization of 2-AG via THL also countered the potentiating effects of systemic cocaine on motivated behavior. Intracranial microinjections of THL (5 ug/0.5 ul/side; Fig. 2D) into the VTA yielded no significant effects of sex (sex: F1,18 = 4.148, n.s.; treatment: F3,54 = 3.144, p < 0.05; sex × treatment: F3,54 = 0.4482, n.s.) and, when female and male data were combined, did not alter PR performance compared to baseline levels (Fig. 2E; F3,57 = 3.328, p < 0.05; n.s. vs. baseline).
When administered with systemic cocaine, however, THL significantly reduced PR task performance to baseline levels. Female and male data were combined following analyses yielding no significant effects of sex (10 mg/kg cocaine – sex: F1,15 = 3.844, n.s.; treatment: F1.852,27.78 = 9.328, p < 0.001; sex × treatment: F2,30 = 0.5532, n.s.; 15 mg/kg cocaine – sex: F1,13 = 1.068, n.s.; treatment: F1.404,18.25 = 11.84, p < 0.001; sex × treatment: F2,26 = 0.4821, n.s.). Co-administration of intra-VTA THL significantly reduced reinforcer counts for both the 10 (Fig. 2F; F2,32 = 9.405, p < 0.001; p < 0.05 vs. cocaine) and 15 (Fig. 2G; F2,28 = 13.70, p < 0.001; p < 0.001 vs. cocaine) mg/kg cocaine doses.
Finally, there were no sex differences in inactive lever presses (IC rimonabant – sex: F1,18 = 3.805, n.s.; treatment: F3.048,54.87 = 0.6662, n.s.; sex × treatment: F5,90 = 0.3324, n.s.; 10 mg/kg IP cocaine + IC rimonabant – sex: F1,20 = 0.9993, n.s.; treatment: F1.728,30.53 = 3.773, p < 0.05; sex × treatment: F3,53 = 1.654, n.s.; 15 mg/kg IP cocaine + IC rimonabant – sex: F1,18 = 0.03254, n.s.; treatment: F1.163,19.78 = 2.155, n.s.; sex × treatment: F3,51 = 2.296, n.s.; IC THL – sex: F1,19 = 0.6185, n.s.; treatment: F3,57 = 1.687, n.s.; sex × treatment: F3,57 = 0.2849, n.s.; 10 mg/kg IP cocaine + IC THL – sex: F1,11 = 3.401, n.s.; treatment: F1.826,20.09 = 1.464, n.s.; sex × treatment: F2,22 = 1.104, n.s.; 15 mg/kg IP cocaine + IC THL – sex: F1,15 = 0.05906, n.s.; treatment: F1.186,17.79 = 3.290, n.s.; sex × treatment: F2,30 = 1.030, n.s.), and none of the intracranial treatments alone (IC rimonabant: F5,95 = 0.6477, n.s.; IC THL: F1.541,30.81 = 1.887, n.s.), or in combination with systemic cocaine (10 mg/kg IP cocaine + IC rimonabant: F1.743, 32.53 = 3.219, n.s.; 15 mg/kg IP cocaine + IC rimonabant: F1.146,20.63 = 2.550, n.s.; 10 mg/kg IP cocaine + IC THL: F2,24 = 2.209, n.s.; 15 mg/kg IP cocaine + IC THL: F2,32 = 3.111, n.s.), had significant effects on inactive lever pressing during PR sessions.
3.3. Histological confirmation of cannula placement
Following all behavioral procedures, coronal brain sections were used to verify cannula placement in the VTA. Figure S1 shows the results of histological analyses. Rats with bilateral cannulae placed outside the indicated boundaries were removed from the study. An inlaid representative photomicrograph illustrates cannula tracks terminating above the VTA and the integrity of surrounding tissue. Any significant damage beyond that shown in the figure would warrant removal of the subject from the data analysis.
4. Discussion
In this study, we show that systemic administration of either 10 or 15 mg/kg cocaine increases operant responding for food under a PR schedule in male and female rats. This observed enhancement of PR breakpoints is reliant on CB1 receptor signaling, as pretreatment with the CB1 inverse agonist rimonabant dose-dependently attenuates cocaine-induced responding for food. Further, intra-VTA administration of doses of rimonabant that do not decrease lever pressing on their own block the ability of both 10 and 15 mg/kg cocaine to augment PR breakpoints. These data suggest that cocaine-mediated increases in PR responding require midbrain endocannabinoid mobilization. Our laboratory and others have identified 2-AG as the primary endocannabinoid responsible for facilitating neural mechanisms of goal-directed behavior (Melis et al., 2004; Oleson et al., 2012). In line with this finding, we demonstrate that inhibition of VTA 2-AG synthesis with THL similarly blocks increases in PR breakpoints following cocaine administration. Altogether, these data show that cocaine increases high-effort lever pressing for food through a VTA 2-AG-dependent mechanism, demonstrating a novel mechanism for cocaine-induced gains in motivation.
These data confirm previous reports demonstrating elevated PR breakpoints after acute cocaine administration (Sizemore et al., 2003; Thompson, 1977). Cocaine’s ability to increase food-reinforced behavior stems from enhancement of mesolimbic DA signaling. Indeed, other DA agonist drugs similarly increase PR responding for food (Cagniard et al., 2006; Randall et al., 2014; Randall et al., 2012; Rotolo et al., 2020; Sommer et al., 2014; Zhang et al., 2003), and blockade of mesolimbic DA transmission decreases such high-effort responding (Aberman et al., 1998; Hamill et al., 1999). An organism’s breakpoint reflects the outcome of a cost-benefit decision in which reinforcer value is weighed against response requirement (Hodos, 1961; Salamone et al., 2018). Thus, cocaine-induced DA agonism may increase breakpoints through increasing reinforcer efficacy, invigorating goal-directed behaviors, or both. While PR procedures cannot distinguish between these two mechanisms, other investigations have substantiated a role for DA in behavioral activation and reinforcement (Ettenberg and Camp, 1986; Horvitz and Ettenberg, 1988; Nicola, 2010; Salamone et al., 1994) (but see(Walton and Bouret, 2019). Conversely, manipulations of mesolimbic DA do not increase free feeding or hedonic responses to food (Cousins and Salamone, 1994; Randall et al., 2014; Randall et al., 2012; Treit and Berridge, 1990; Wyvell and Berridge, 2000), suggesting that while cocaine-induced DA signaling enhances food reinforcement, it does not increase food intake or food reward. On the contrary, cocaine reduces feeding behavior (Cooper and van der Hoek, 1993); although notably, rats in the present study consumed all earned food pellets. Thus, the current data replicate previous findings and support a role for cocaine in motivating positively-reinforced behaviors.
CB1 receptor antagonism with rimonabant or inhibition of 2-AG synthesis with THL prevents cocaine-induced enhancement of PR break points. These data align with previous research demonstrating a role for endocannabinoid system function in food-motivated behavior. For instance, acute administration of rimonabant dose-dependently decreases PR breakpoints, while CB1 receptor agonists (like delta-9-tetrahydrocannabinol or 2-AG) increase breakpoints (Higgs et al., 2005; Solinas and Goldberg, 2005; Wakley and Rasmussen, 2009). A wealth of evidence demonstrates that endogenous and exogenous cannabinoids increase midbrain dopamine cell activity and striatal dopamine release (Cheer et al., 2004; Cheer et al., 2007; Chen et al., 1990; French et al., 1997; Lupica and Riegel, 2005; Riegel and Lupica, 2004; Wang et al., 2015). Unlike other DA agonist drugs, however, cannabinoids also increase food consumption and hedonic responses to food (Abel, 1975; Jarrett et al., 2005; Jarrett et al., 2007; Mahler et al., 2007; Wiley et al., 2005; Williams et al., 1998). Though, cannabinoid-induced enhancement of food reward is dependent on activation of endogenous opioids rather than DA signaling, as co-administration of the opioid antagonist naloxone prevents endocannabinoid enhancement of orofacial “liking” reactions to sucrose (Mitchell et al., 2018). In this study, cocaine’s facilitation of food-reinforced behavior was blocked by doses of rimonabant or THL that did not affect operant responding for food on their own. Therefore, these drug manipulations blocked cocaine-induced increases in motivation rather than decreasing baseline food reinforcement or reward.
The field has known for decades that cocaine administration inhibits DAT function to increase forebrain dopamine levels (Ritz et al., 1987). Recent research indicates that cocaine also increases burst activity of DA cells via mobilization of endocannabinoids. Wang and colleagues (2016) demonstrate a mechanism by which cocaine causes phasic activation of midbrain DA cells. In this circuit, cocaine-induced inhibition of norepinephrine reuptake results in activation of α1-adrenergic receptors on midbrain DA cells and on-demand synthesis and release of the endocannabinoid 2-AG (Nakamura et al., 2019; Wang et al., 2015). While DA neurons express the synthetic enzyme for 2-AG (DGL-α), they do not express CB1 receptors; instead CB1 receptors are located on GABA and glutamatergic cells (Matyas et al., 2008). Binding of 2-AG to presynaptic CB1 receptors on GABA terminals inhibits GABA release onto DA cells, thereby relieving inhibitory GABAergic input to allow burst firing of DA neurons and phasic release in terminal regions (Lupica and Riegel, 2005; Nakamura et al., 2019; Riegel and Lupica, 2004; Szabo et al., 1999; Szabo et al., 2002; Wang et al., 2015). In support of this, CB1 antagonism attenuates cocaine-induced accumbal dopamine release (Cheer et al., 2007). Here we show that blockade of VTA 2-AG signaling diminishes cocaine-induced increases in PR responding. These data suggest that heightened states of motivation following cocaine delivery require endocannabinoid-mediated dopamine cell firing, and dopamine reuptake inhibition alone is not sufficient to drive increases in breakpoints following cocaine administration. However, while intra-VTA rimonabant (1 ug/0.5 ul/side, IC) diminished the motivation-enhancing effects of 10 mg/kg (IP) cocaine, it did not significantly reduce responding following administration of a higher dose of cocaine (15 mg/kg, IP). Nevertheless, this dose of intra-VTA rimonabant did reduce breakpoints to levels not significantly different than control levels. Therefore, it is possible that a higher dose of rimonabant would further reduce increases in PR breakpoints following 15 mg/kg cocaine to levels significantly lower than cocaine alone. However, this remains unknown, and future research is necessary to examine how different doses of cocaine, and possibly different schedules of cocaine administration, may affect endocannabinoid control of motivated behavior.
Altogether, our findings show that cocaine increases high-effort operant responding through mobilization of 2-AG and resultant signaling at VTA CB1 receptors in male and female rats. Previous evidence suggests that midbrain endocannabinoid signaling is integral for motivation for other natural reinforcers as well, such as exercise and sex (Canseco-Alba and Rodriguez-Manzo, 2019; Muguruza et al., 2019). Further, research indicates that endocannabinoid signaling underlies motivation for drugs of abuse and may contribute to changes in motivated behaviors following chronic drug administration (see reviews by (Maldonado et al., 2006; Parsons and Hurd, 2015; Sagheddu et al., 2015; Zlebnik and Cheer, 2016b). CB1 agonists enhance PR responding for drugs such as cocaine or heroin, while CB1 receptor blockade or deletion decreases PR breakpoints (Orio et al., 2009; Solinas and Goldberg, 2005; Solinas et al., 2003; Soria et al., 2005; Xi et al., 2008). CB1 antagonism, however, does not have significant effects on drug self-administration under FR schedules of reinforcement, but rimonabant does decrease reinstatement of cocaine-seeking behavior in the absence of cocaine reinforcement (De Vries et al., 2001; Maldonado et al., 2006; Solinas and Goldberg, 2005; Solinas et al., 2003; Solinas et al., 2005; Soria et al., 2005; Xi et al., 2008). Together, these studies suggest that endocannabinoid signaling mediates motivation for seeking drugs, as opposed to drug reinforcement. Interestingly, animals with a history of extended-access cocaine self-administration require a lower dose of rimonabant to decrease PR breakpoints for cocaine, indicating that greater drug exposure results in neuroadaptations in circuits responsible for endocannabinoid control of motivation. Our data thus add to a growing body of research demonstrating a role for endocannabinoid activation in motivated behavior and suggest that endocannabinoid manipulations may be useful for the treatment of motivational disorders (Covey et al., 2018; Oliere et al., 2013; Zlebnik and Cheer, 2016a). Potential pharmacotherapies may target endocannabinoid synthesis in order to attenuate maladaptive motivated behavior. Development of such endocannabinoid-directed treatments is of particular interest given the harmful negative side effects found to result from CB1 antagonism in randomized trials (Christensen et al., 2007). Future research is required, however, to determine if the midbrain 2-AG mechanism explored here plays an essential role in motivation for drugs of abuse and to determine how this signal may be altered by extensive drug exposure.
Supplementary Material
Highlights:
Cocaine increases food-motivated behavior through an unknown mechanism
Systemic CB1 receptor blockade decreased cocaine-induced motivation
Antagonizing CB1 receptors in the VTA reduced cocaine-induced motivation
Blocking VTA 2-AG synthesis prevented increases in cocaine-induced motivation.
Cocaine promotes motivated behavior through midbrain endocannabinoid signaling
Acknowledgements
This research was supported by the National Institute on Drug Abuse (R01 DA022340 and R01 DA042595 to JFC; F32 DA039690 to JMW; and F32DA043967 and K99 DA047419 to NEZ) and by the São Paulo Research Foundation, FAPESP (2019/23286–3 to SAE and FC). The authors would like to thank Samantha Miller, Autumn Bows, Lakota Watson, Iness Gildish, and Brian Donnellan for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL, 1975. Cannabis: Effects on hunger and thirst. Behavioral Biology 15, 255–281. [DOI] [PubMed] [Google Scholar]
- Aberman JE, Ward SJ, Salamone JD, 1998. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav 61, 341–348. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G, 2002. Differential Expression of Motivational Stimulus Properties by Dopamine in Nucleus Accumbens Shell versus Core and Prefrontal Cortex. The Journal of Neuroscience 22, 4709–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 1998. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews 28, 309–369. [DOI] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ, 2008. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11, 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF, 2011. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci 34, 1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X, 2006. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology 31, 1362–1370. [DOI] [PubMed] [Google Scholar]
- Canseco-Alba A, Rodriguez-Manzo G, 2019. Endocannabinoids Interact With the Dopaminergic System to Increase Sexual Motivation: Lessons From the Sexual Satiety Phenomenon. Front Behav Neurosci 13, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Marsden CA, Kendall DA, Mason R, 2000. Lack of response suppression follows repeated ventral tegmental cannabinoid administration: an in vitro electrophysiological study. Neuroscience 99, 661–667. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien MLAV, Phillips PEM, Wightman RM, 2004. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. Journal of Neuroscience 24, 4393–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM, 2007. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci 27, 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL, 1990. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 102, 156–162. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A, 2007. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370, 1706–1713. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, van der Hoek GA, 1993. Cocaine: a microstructural analysis of its effects on feeding and associated behaviour in the rat. Brain Research 608, 45–51. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD, 1994. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacology Biochemistry and Behavior 49, 85–91. [DOI] [PubMed] [Google Scholar]
- Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA, 2016. Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur J Neurosci 43, 1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Dantrassy HM, Yohn SE, Castro A, Conn PJ, Mateo Y, Cheer JF, 2018. Inhibition of endocannabinoid degradation rectifies motivational and dopaminergic deficits in the Q175 mouse model of Huntington’s disease. Neuropsychopharmacology 43, 2056–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN, 2001. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7, 1151–1154. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Camp CH, 1986. Haloperidol induces a partial reinforcement extinction effect in rats: Implications for a dopamine involvement in food reward. Pharmacology Biochemistry and Behavior 25, 813–821. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DC, Jones SR, 2011. Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry 69, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X, 1997. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 8, 649–652. [DOI] [PubMed] [Google Scholar]
- Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD, 1999. Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav 64, 21–27. [DOI] [PubMed] [Google Scholar]
- Hernandez G, Cheer JF, 2012. Effect of CB1 receptor blockade on food-reinforced responding and associated nucleus accumbens neuronal activity in rats. J Neurosci 32, 11467–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Oleson EB, Gentry RN, Abbas Z, Bernstein DL, Arvanitogiannis A, Cheer JF, 2014. Endocannabinoids promote cocaine-induced impulsivity and its rapid dopaminergic correlates. Biol Psychiatry 75, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG, 1988. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sciences 42, 1705–1712. [DOI] [PubMed] [Google Scholar]
- Higgs S, Barber DJ, Cooper AJ, Terry P, 2005. Differential effects of two cannabinoid receptor agonists on progressive ratio responding for food and free-feeding in rats. Behav Pharmacol 16, 389–393. [DOI] [PubMed] [Google Scholar]
- Hodos W, 1961. Progressive ratio as a measure of reward strength. Science 134, 943–944. [DOI] [PubMed] [Google Scholar]
- Horvitz JC, Ettenberg A, 1988. Haloperidol blocks the response-reinstating effects of food reward: A methodology for separating neuroleptic effects on reinforcement and motor processes. Pharmacology Biochemistry and Behavior 31, 861–865. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J, 1996. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci 110, 331–345. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Limebeer CL, Parker LA, 2005. Effect of Delta9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav 86, 475–479. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Scantlebury J, Parker LA, 2007. Effect of delta9-tetrahydrocannabinol on quinine palatability and AM251 on sucrose and quinine palatability using the taste reactivity test. Physiol Behav 90, 425–430. [DOI] [PubMed] [Google Scholar]
- Jones CA, LeSage M, Sundby S, Poling A, 1995. Effects of cocaine in pigeons responding under a progressive-ratio schedule of food delivery. Pharmacology Biochemistry and Behavior 50, 527–531. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, 2005. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48, 1105–1116. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC, 2007. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 32, 2267–2278. [DOI] [PubMed] [Google Scholar]
- Mai B, Sommer S, Hauber W, 2012. Motivational states influence effort-based decision making in rats: the role of dopamine in the nucleus accumbens. Cogn Affect Behav Neurosci 12, 74–84. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F, 2006. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29, 225–232. [DOI] [PubMed] [Google Scholar]
- Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, Freund TF, Katona I, 2008. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology 54, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Ye C, Bonci A, Kiyatkin EA, Morales M, 2015. A subpopulation of neurochemically-identified ventral tegmental area dopamine neurons is excited by intravenous cocaine. J Neurosci 35, 1965–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M, 2004. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci 24, 10707–10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Berridge KC, Mahler SV, 2018. Endocannabinoid-Enhanced "Liking" in Nucleus Accumbens Shell Hedonic Hotspot Requires Endogenous Opioid Signals. Cannabis Cannabinoid Res 3, 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JT, Glick SD, Cheer JF, 2010. Neural encoding of psychomotor activation in the nucleus accumbens core, but not the shell, requires cannabinoid receptor signaling. J Neurosci 30, 5102–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruza C, Redon B, Fois GR, Hurel I, Scocard A, Nguyen C, Stevens C, Soria-Gomez E, Varilh M, Cannich A, Daniault J, Busquets-Garcia A, Pelliccia T, Caille S, Georges F, Marsicano G, Chaouloff F, 2019. The motivation for exercise over palatable food is dictated by cannabinoid type-1 receptors. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Dryanovski DI, Kimura Y, Jackson SN, Woods AS, Yasui Y, Tsai SY, Patel S, Covey DP, Su TP, Lupica CR, 2019. Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, 2010. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci 30, 16585–16600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Beckert MV,Morra JT,Lansink CS, Cachope R, Abdullah RA, Loriaux AL, Schetters D, Pattij T, Roitman MF, Lichtman AH, Cheer JF, 2012. Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron 73, 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliere S, Joliette-Riopel A, Potvin S, Jutras-Aswad D, 2013. Modulation of the endocannabinoid system: vulnerability factor and new treatment target for stimulant addiction. Front Psychiatry 4, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Edwards S, Journal George O, Parsons LH, Koob GF, 2009. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci 29, 4846–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL, 2015. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16, 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The Rat Brain in Stereotaxic Coordinates. Academic Press. [DOI] [PubMed] [Google Scholar]
- Ramiro-Fuentes S, Fernandez-Espejo E, 2011. Sensitization to cocaine is inhibited after intra-accumbal GR103691 or rimonabant, but it is enhanced after co-infusion indicating functional interaction between accumbens D(3) and CB1 receptors. Psychopharmacology (Berl) 214, 949–959. [DOI] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Nunes EJ, Yohn SE, Nowak V, Khan B, Shah P, Pandit S, Vemuri VK, Makriyannis A, Baqi Y, Muller CE, Correa M, Salamone JD, 2014. The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: reversal with antidepressant drugs. PLoS One 9, e99320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, Lopez Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Muller CE, Correa M, Salamone JD, 2012. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One 7, e47934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66, 1–11. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR, 2004. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci 24, 11070–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ, 1987. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237, 1219–1223. [DOI] [PubMed] [Google Scholar]
- Romo R, Schultz W, 1990. Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol 63, 592–606. [DOI] [PubMed] [Google Scholar]
- Rotolo RA, Presby RE, Tracy O, Asar S, Yang JH, Correa M, Murray F, Salamone JD, 2020. The novel atypical dopamine transport inhibitor CT-005404 has pro-motivational effects in neurochemical and inflammatory models of effort-based dysfunctions related to psychopathology. Neuropharmacology 183, 108325. [DOI] [PubMed] [Google Scholar]
- Sagheddu C, Muntoni AL, Pistis M, Melis M, 2015. Endocannabinoid Signaling in Motivation, Reward, and Addiction: Influences on Mesocorticolimbic Dopamine Function. Int Rev Neurobiol 125, 257–302. [DOI] [PubMed] [Google Scholar]
- Salamone JD, 1994. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behavioural Brain Research 61, 117–133. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yang JH, Rotolo R, Presby R, 2018. Dopamine, Effort-Based Choice, and Behavioral Economics: Basic and Translational Research. Front Behav Neurosci 12, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S, 1994. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behavioural Brain Research 65, 221–229. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K, 1991. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 104, 515–521. [DOI] [PubMed] [Google Scholar]
- Sizemore GM, Cannon DG, Smith JE, Dworkin SI, 2003. The effects of acutely administered cocaine on responding maintained by a progressive-ratio schedule of food presentation. Behav Pharmacol 14, 33–40. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, 2005. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology 30, 2035–2045. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR, 2003. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther 306, 93–102. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR, 2005. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology 30, 2046–2057. [DOI] [PubMed] [Google Scholar]
- Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W, 2014. The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats. Int J Neuropsychopharmacol 17, 2045–2056. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O, 2005. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology 30, 1670–1680. [DOI] [PubMed] [Google Scholar]
- Szabo B, Muller T, Koch H, 1999. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem 73, 1084–1089. [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I, 2002. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci 15, 2057–2061. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y, 2008. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 154, 327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, 1977. Effects of cocaine and fenfluramine on progressive-ratio performance. Pharmacology Biochemistry and Behavior 7, 555–558. [DOI] [PubMed] [Google Scholar]
- Treit D, Berridge KC, 1990. A comparison of benzodiazepine, serotonin, and dopamine agents in the taste-reactivity paradigm. Pharmacology Biochemistry and Behavior 37, 451–456. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Michaelides M, Baler R, 2019. The Neuroscience of Drug Reward and Addiction. Physiol Rev 99, 2115–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Rasmussen EB, 2009. Effects of cannabinoid drugs on the reinforcing properties of food in gestationally undernourished rats. Pharmacol Biochem Behav 94, 30–36. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bouret S, 2019. What Is the Relationship between Dopamine and Effort? Trends Neurosci 42, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Treadway T, Covey DP, Cheer JF, Lupica CR, 2015. Cocaine-Induced Endocannabinoid Mobilization in the Ventral Tegmental Area. Cell Rep 12, 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Oleson EB, Gove WN, Cole AB, Gyawali U, Dantrassy HM, Bluett RJ, Dryanovski DI, Stuber GD, Deisseroth K, Mathur BN, Patel S, Lupica CR, Cheer JF, 2018. Phasic Dopamine Signals in the Nucleus Accumbens that Cause Active Avoidance Require Endocannabinoid Mobilization in the Midbrain. Curr Biol 28, 1392–1404 e1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, Martin BR, 2005. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol 145, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC, 1998. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav 65, 343–346. [DOI] [PubMed] [Google Scholar]
- Wise RA, 2004. Dopamine, learning and motivation. Nat Rev Neurosci 5, 483–494. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC, 2000. Intra-Accumbens Amphetamine Increases the Conditioned Incentive Salience of Sucrose Reward: Enhancement of Reward “Wanting” without Enhanced “Liking” or Response Reinforcement. The Journal of Neuroscience 20, 8122–8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng XQ, Gardner EL, 2008. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology 33, 1735–1745. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE, 2003. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci 117, 202–211. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Cheer JF, 2016a. Beyond the CB1 Receptor: Is Cannabidiol the Answer for Disorders of Motivation? Annu Rev Neurosci 39, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Cheer JF, 2016b. Drug-Induced Alterations of Endocannabinoid-Mediated Plasticity in Brain Reward Regions. J Neurosci 36, 10230–10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


