Abstract
Objective
Dermis spheroids from different donors (40 and 50 years old) were developed from primary fibroblasts to demonstrate their capacity to synthetize and organize the main dermal structural components when cultured in 3D microenvironment, forming endogenous de novo ECM according to their potential metabolic activity.
Methods
Dermis spheroids were produced from primary human dermal fibroblasts at early passages in hanging drop culture system. Dermis models were characterized in terms of spheroid diameter, PICP release, collagen III and CD44 expression.
Results
An increase of collagen III synthesis (101%) was found in the young donor compared to the old donor (23.5%) after seven days of culture by immunofluorescence. The progressive ECM assembly over the time and dermis maturation was showed by Masson’s trichrome staining and by immunofluorescence for collagen III and CD44; both molecules significantly accumulated in the dermal compartment from day seven to day 10 of culture with a global decrease for both spheroid models after 21 days of culture.
Conclusion
Our results showed that specific culture conditions in the 3D scaffold-free microenvironment allowed the physiological and progressive ECM assembly of miniaturized dermis models reflecting phenotypic profile features of “young” and “old” native tissue from which cells were isolated with a potential application to personalized care approaches in dermatological research on aging processes and medicine.
Keywords: 3D culture, dermis, scaffold-free spheroids, intrinsic aging, extracellular matrix, tissue remodeling
Introduction
The biological mechanism of the aging process was observed for the first time in the 1960s by Hayflick who described this physiological condition as a direct consequence of cellular senescence leading to a progressive degeneration of tissues and organs over time.1 The aging phenomenon, indeed, triggers a degenerative physiological decline: the loss of postmitotic cells in the cellular self-renewal ability to sustain replication, alterations in DNA repair and shortening of telomeres are the main key events of cellular intrinsic (chronological) aging.2,3
Skin modifications induced by chronological aging are widely described in dermatological literature and mainly concern dermal cells specialized for the synthesis of main structural components of extracellular matrix (ECM) and their continuous action on dermal compartment is recognized as a fundamental mechanism. Dermal cells play a critical role in ECM assembly and enrichment in structural and functional molecules,4 improving mechanical properties and functional features.5
Despite its specific role as a natural tissue scaffold, the dermal ECM is not merely a “passive” matrix that only holds cells in place, but it also has a functional importance as a dynamic repository for morphogens, cytokines and growth factors which in vivo regulate the progressive development of a specialized ECM as a highly complex compartment.
The dermis is rich in elastic and collagen fibers embedded in an amorphous glycosaminoglycans (GAGs) matrix secreted in the extracellular space that are significantly modified during aging processes because they are directly involved in skin resistance and elasticity.5–7 Collagen fibers comprise approximately 70–80% of the dry weight of the dermis and they are responsible for the skin’s tensile strength; the elastic fiber network occupies approximately 2–4% of the total dermis volume providing resilience and suppleness.8 GAGs provide the water maintenance ensuring tissue hydration.9
The aging process has a direct effect on the ECM, impairing the texture and structure of the skin by progressive molecular alteration in structural proteins and features.8,10 In particular, during chronological skin aging, the dermis is subjected to changes in collagen rate synthesis, collagen solubility, decrease of density and thickness of the bundles, as well as fragmentation and slow degradation of elastic fibers.11 Evidences have shown that from the age of 50, the quality of the dermis gradually declines determining progressive functional and structural changes in thickness and composition with alteration of the matrix organization and architecture.12 In particular, structural and functional components, such as perlecan, hyaluronic acid and collagen fibrils’ decrease their density leading to dermis atrophy,13 due to a reduction of collagen bundle thickness and to an increase of space between bundles. As a consequence of the aging phenomenon the dermis modifications alters the texture and structure of the whole skin, by a progressive atrophy involving the quality of fibroblast anchoring in the ECM with a possible deleterious impact on traction forces and dermis cohesion.12,14
In this work, we have developed dermis spheroids from primary human dermal fibroblasts derived from donors of different ages with the aim of validating the hypothesis that it is possible to reproduce in vitro a miniaturized tissue that mimics the phenotypic features of the native tissue. In particular, we aimed at demonstrating that during long-term cultivation (up to 21 days), dermis spheroids are able to reproduce a physiological microenvironment by stimulating dermal fibroblasts to synthesize specific stromal components according to the donor’s phenotype with continuous ECM remodeling, thus mirroring the chronological aging process.
Materials and Methods
Cell Source Characterization
Primary human dermal fibroblasts from different age donors are purchased from Innoprot (Derio, Spain) and cultured in Prime PR-F medium (CELLnTEC, Bern, Switzerland), supplemented with penicillin 100 units/mL and streptomycin 100 μg/mL (Sigma Aldrich, Darmstadt, Germany). Table 1 reports primary fibroblasts characteristics. During cellular amplification, cells were cultured in monolayer for seven days at an initial density of 5,000 cells/cm2 and maintained at 37°C with 5% CO2. When cells reached a confluence of 90%, they were washed with DPBS 1X without Ca2+/Mg2+ and detached for spheroids formation with trypsin/ETDA solution 0.05%.
Table 1.
Primary Fibroblasts Characteristics and Population Doubling (PD) Values for the Two Fibroblast Donors’ Ages
Donor’s Characterization from 2D to 3D Cultures
Population doubling (PD) rate was calculated during cellular amplification in 2D culture. PD value is a specific indicator of cellular expansion speed giving an idea of senescence state and their metabolic activity. Among different cellular donors tested, the selected ones from 40 and 50 year old donors (Table 1) were used for the generation of dermis spheroids and PD was calculated. During cellular expansion, cells from both donors were counted and PD was calculated during five days of culture subsequent to thawing.
The calculation of population doubling (PD) was obtained according to the following formula:
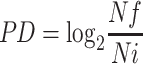 |
(1) |
Where Nf is the final cell number, and Ni is the initial cell number, counted at thawing.
Table 1 reports PD values of young and aged donors’ fibroblasts during cellular amplification in 2D monolayer before dermis spheroids production.
The PD value was considered as an early and simple discriminating parameter reflecting cellular metabolic activity and the cellular duplication rate of different age donors. As shown in the Table 2, the PD was higher for the “young” donor (2.09 for age 40) if compared to the “aged” donor (1.53 for age 50) indicating that the primary fibroblasts cultured for early passages (until P3) preserved the phenotype of native tissue, mirroring the intrinsic tissue senescence in terms of cellular duplications and cellular activity.
Table 2.
Signal Quantification of Collagen III Expression in Terms of Mean of Percent Area (Indicating the Percentage of Area Covered by Fluorescent Signal Within a Defined ROI [Region of Interest]) and Intensity Sum (Indicating the Sum of the Total Pixel Number and Intensity) in Dermis Spheroids After 10 Days of Culture
| Samples | % Area (Mean) | Intensity Sum |
|---|---|---|
| Age 40 | 3.5 | 51243718 |
| Age 50 | 2.6 | 33878044 |
Dermis Spheroid Production
After reaching 80–90% of confluence, cells were treated with 0.05% trypsin/EDTA solution and counted: 1×106 cells were seeded for each plate in the hanging drop culture systems in order to obtain 3D scaffold-free spheroids of 10,000 cells each.
3D culture system Akura® Plate by InSphero (Zurich, Switzerland) allowed the production of scaffold-free spheroids using the self-assembly approach without exogenous support: the cells, thanks to the special geometry of the plates, aggregate by themselves in compact spheroids in three to four days within the hanging drop system.
Once assembled, miniaturized dermis tissues were transferred into ultra-low attachment plates with V-shaped wells and cultured for additional days (up to 21) in order to allow the complete dermal maturation and ECM deposition.15 The production of dermis spheroids was optimized and standardized by VIAFLO Assist Plus (Integra Bioscience, Switzerland).
PICP Amount by ELISA Assay
The amount of collagen secreted by dermal fibroblasts during the dermis maturation was quantified in the culture media in terms of ng/mL of PICP by ELISA assay (Cloud Clone Corp., Katy, TX, USA). The C-terminal propeptide of type I procollagen (PICP) and the amino-terminal propeptide of type I procollagen (PINP) are enzymatically removed from the procollagen molecule by specific proteases during collagen fiber assembly. In particular, PICP is a trimeric, globular protein consisting of three polypeptide chains: two pro α1 (I) chains and one pro α2 (I) chain with a molecular mass of 100,000 Da and its cleavage is required for the initiation of collagen fibril formation. The raw data of OD are directly recorded in the microplate auto-reader (Tecan Infinite M200) and further processed by Software iControl.
Formalin-fixed Paraffin Embedded Sections: Masson’s Trichrome Staining and Immunofluorescence for CD44 and Collagen Type III
Dermis spheroids at different days were fixed in formalin buffered solution (10%) and included in paraffin blocks and 5 μm sections were obtained. Tissue slices were stained with Masson’s trichrome kit (Abcam) following producers’ instructions. Images were acquired in brightfield (40× magnification) by Leica THUNDER DMi8.
Immunofluorescence was performed for Collagen III and CD44 on tissue sections after deparaffination of samples with rabbit polyclonal primary antibody for collagen III (Abcam, 1:200) and mouse monoclonal primary antibody for CD44 (Cell Signalling, 1:200) overnight at 4°C. Alexa 555 donkey anti-rabbit and Alexa 488 goat anti-mouse (ThermoScientific, 1:500 for both) were used as secondary antibody. Nuclei were stained with DAPI Fluoroshield™ solution (Sigma Aldrich, Darmstadt, Germany). Acquisitions were performed by Leica THUNDER DMi8 and images acquired with a Leica sCMOS camera and LAS X 3.0.1 software.
Immunofluorescence on 3D Whole Mount Samples for Collagen Type III and High Content Imaging
Dermis spheroids of 10 days (D10) were washed in PBS 1X and whole fixed in formalin buffered solution 10% (Sigma Aldrich, Darmstadt, Germany) for one hour at RT. After tissue fixation, samples were prepared for clearing protocol before starting incubation with primary antibody.
A Visikol® Histo-M™ Starter kit (Visikol, Hampton, NJ, USA), was used and samples were treated according to internal procedure: pooled samples (n=12 dermis spheroids/each pool) were treated with dehydration and rehydration cycles of 15 min each by incubations with increasing concentrations of ET-OH solutions (from 50% to 100%). After permeabilization with PBS-Triton solution 0.2% and Visikol® Histo-Permeabilization Buffer, the blocking of aspecific sites was performed by incubation with Visikol® Histo Blocking Solution. Samples were incubated with rabbit polyclonal primary antibody: collagen type III (Abcam, 1:1000) and decorin (ThermoFisher, 1:500) were diluted in Visikol® Histo Antibody Buffer overnight at 4°C.
Alexa 555 donkey anti-rabbit (ThermoFisher, 1:800) was used as secondary antibody for both biomarkers while nuclei were stained with DAPI (Sigma Aldrich, 1:2500). Stained dermis spheroids were cleared with Visikol® Histo-MTM ON at 4°C. The acquisitions were performed by Leica THUNDER DMi8 and Z-stack movies were acquired with a Leica sCMOS camera and postprocessed by LAS X 3.0.1 software. Z-stacks were collected in order to observe the whole volume of 3D spheroids. Expression signal was optimized by Thunder Computational Clearing algorithm and some more informative frames were extrapolated from the Z-stack movies. The collagen type III expression signal was quantified by Leica LAS X software.
Results
Morphological Modification of Dermis Spheroids During Long-term Culture
The ECM assembly resulted in a slow process reflected by shape modification in long-term culture.
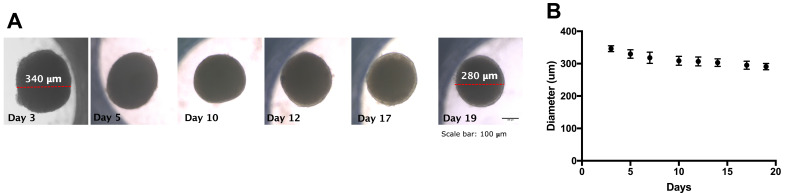
Figure 1 shows the evolution of dermis spheroids in term of shape and diameter size. During the early assembly phase (first three days) dermis spheroid boundaries appeared jagged and irregular due to the progressive cellular integration after seeding. During culture, from day five to day 19, the tissue boundaries became well defined, with a slight decrease of diameter size (340 μm to 280 μm) due to ECM assembly and its physiological contraction: the possible increasing of tensile components and elastic fibers cause a natural tissue contraction over time with a reduction of diameter size.
Figure 1.
Morphological evolution of dermis spheroids during long-term culture. (A) Diameter measurement on brightfield images (scale bar 100 μm); (B) diameter size evolution during spheroid culture.
Differences in Procollagen Type I Release on Dermis Spheroids
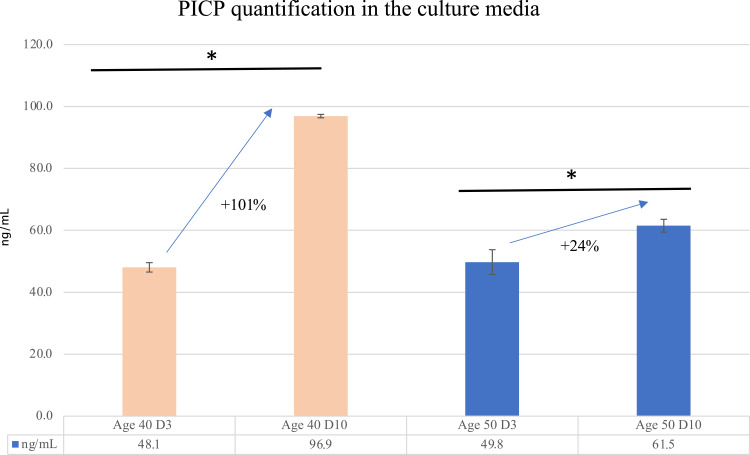
In order to figure out differences in dermis composition and organization between the two donors, an ELISA assay was performed to quantify the collagen type I synthesis by calculation of propeptide type I amount in the culture media after three and 10 days of culture. Figure 2 shows the PICP amount in relation to donor age and culture time.
Figure 2.
Quantification of PICP release in the culture medium for collagen type I synthesis evaluation. Culture media of dermis spheroids derived from age 40 and 50 cell donors were assayed for PICP quantification (ng/mL) by ELISA. *p<0.01 (by ANOVA).
The progressive ECM assembly over the time and dermis maturation started after three days of culture and continues for 10 days culture time: collagen type I synthesis reached similar values at early phases (D3) for both donors age (48.1 ng/mL and 49.8 ng/mL for 40 and 50 years old donors, respectively).
In particular, after 10 days of culture, the progressive ECM assembly was highlighted by a greater collagen synthesis in the young donor (age 40) with an increase of 101% (from 48.1 to 96.9 ng/mL) compared to the aged donor (age 50), for which a lower increase of 24% (from 49.8 to 61.5 ng/mL) has been recorded.
This evidence confirmed the dynamic behavior of dermis spheroids and the different metabolic activity of dermal fibroblasts derived from different donors: the synthesis and release of collagen molecules increased from three to 10 days of culture in young donor models, building dermal ECM progressively by the cleavage of de novo procollagen bundles. This cellular activity leaded to a greater dermis complexity and a stiffer architecture. On the contrary, during the same culture period, dermis fibroblasts of the aged donor were unable to sustain a similar de novo collagen synthesis rate, revealing a delayed enzyme activity, according to the slow physiologic metabolism associated with the chronological aging.
Our data showed that dermis spheroids are able to preserve the phenotypic features of the donor’s cells reflecting the metabolic and functional activity during culture.
Whole Mount Immunofluorescence of Collagen III on Dermis Spheroids
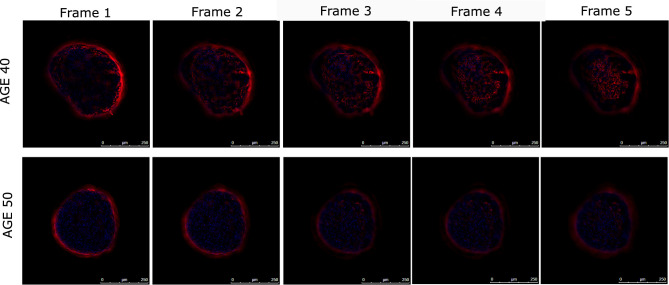
The downstream effects of ECM modulation in terms of structural composition and organization in long-term culture were examined by the evaluation of collagen type III expression by immunofluorescence on whole mount samples. Type III collagen is one of the main structural components of ECM, is synthesized from cells as a fibrillar collagen during de novo protein deposition in the ECM and represents the 15% of the total skin collagen. It may share some of the regulatory mechanisms of type I collagen playing a role in cell attachment and growth promotion.16 Dermis spheroids showed a differential collagen type III expression in relation to the age of the cell donor after 10 days of culture. Transparent dermis spheroids were visualized thanks to the clearing technique, based on serial incubation with organic reagents that aimed to modify their refractive index. Clarified samples were observed in their whole volumes by Z-stack acquisition and the more informative frames are reported in Figure 3.
Figure 3.
Collagen III expression by immunofluorescence on whole mount samples (n=3 for each condition). Dermis spheroids derived from 40 and 50 year-old donors were stained for collagen III (red signal) after 10 days of culture. The signal was obtained by Z-stack acquisitions. The most representative frames are showed. Nuclei are stained in blue. Mag. 20× (scale bar 250 μm).
The frame comparison suggests differences in collagen type III expression in relation to the age of donors: dermal models of the 40 year-old donor highlighted an increasing collagen III expression in the main focal planes if compared to the 50 year-old donor dermal models after 10 days of culture. These observations were confirmed by collagen III signal expression quantification as reported in Table 2.
The signal quantification for collagen type III confirmed results observed in the optical section images: the mean of area percentage of signal expression is higher for young dermis of age 40 (3.5%) if compared to dermis samples of the aged donor (2.6% for age 50). A similar trend was confirmed by comparing the sum of pixel intensity between two donors: a higher pixel intensity was reached in young samples.
Taken together, these results confirmed that dermis spheroids are able to preserve and maintain the phenotypic features of cellular donors’ profile by reproducing the same physiologic status of native donors in vitro, mirroring the differential ECM composition between donors of different ages.
De Novo Collagens Synthesis on Dermis Spheroids from 50 year-old Donor
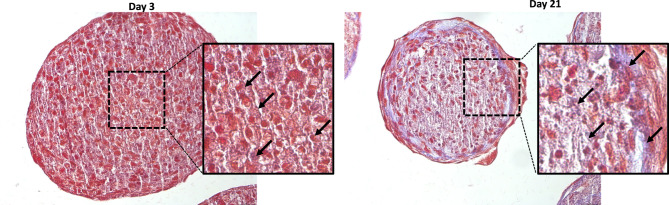
The collagen deposition during the ECM maturation was evaluated by conventional histological analysis on tissue sections. Mature collagen fibers were localized by Masson’s trichrome staining. Figure 4 shows dermal tissue compared at two time-points in terms of collagen synthesis, ECM composition and organization.
Figure 4.
Masson’s trichrome staining of dermis spheroids (50 year-old donor after 3, 21 days). At day 21 the ECM appears differently organized, less compact with more empty spaces compared to day three. Collagen fibers were stained in blue. Mag. 20×.
In the experimental window of 21 days human dermal fibroblasts have produced a more and more structured organization during long-term culture. According to the synthesis and deposition of collagen fibers, ECM composition changed: after 21 days of culture, ECM appeared differently organized, less compact with more empty spaces as a consequence of matrix remodeling and tissue rearrangement mimicking the natural evolution of tissue assembly.
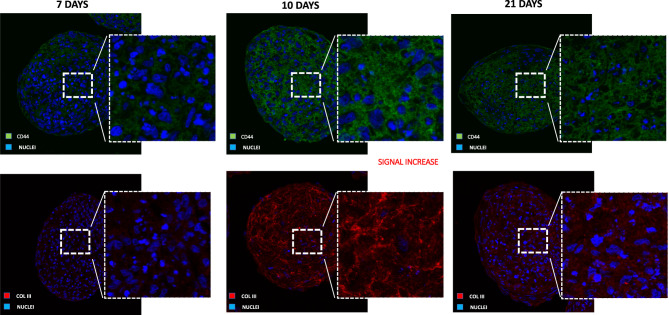
The differential ECM composition and the progressive reorganization that occurred during the dermis assembly and maturation were confirmed by immunolabeling for collagen type III and CD44 as shown in Figure 5.
Figure 5.
Immunofluorescence on dermis spheroids (50 year-old donor) for CD44 (in green, on the top) and collagen type III (in red, on the bottom) at days 7, 10 and 21 of culture. From day seven to day 10 of culture CD44 and collagen type III increased their expression while at day 21 their expression decreases. Mag. 20×. Nuclei are stained with DAPI in blue.
From day seven to day 10 of culture, CD44 and collagen type III increased their expression with a significant deposition inside the dermal compartment as a consequence of the dynamic maturation and modification of the living tissue; at day 21, remodeling processes and ECM reorganization provided substantial ECM modifications in terms of structural and functional biomarker expression, with a decrease of global expression of both molecules if compared to early time-points and a general tissue depletion of structural and functional proteins.
Discussion
Advances and new approaches in tissue engineering and regenerative medicine revealed that 2D conventional culture systems do not allow the preservation of tissue features, since the adaptation on a flat macroenvironment imposes modifications of genotypic and phenotypic cellular profile. For these reasons, 3D culture systems have been developed as alternative to 2D culture models overcoming the artifacts or aberration in protein expression, biological behavior, and metabolic activity due to the in vitro adaptation.17–19
Single cells without a three-dimensional guide are indeed not able to organize a functional 3D tissue with its complex architecture, since only cell proliferation is not sufficient. The presence of ECM as a natural scaffold allows cells to control critical process such as proliferation, differentiation and synthesis of signaling molecules and mediators playing a role in its dynamic nature: ECM is constantly enzymatically and not-enzymatically remodeled as a non-static system modifying continuously its physical and biochemical features thanks to the remodeling of dermal fibroblasts.
In conclusion, to empathize the novelty of our approach, we can underline the following achievements:
Dermis spheroids were developed according to the hanging drop culture technique starting from primary human dermal fibroblasts: primary cells at early passages are able to self-assemble, without exogenous supports, and generate a rounded shaped spheroid in three to four days by cellular proliferation and continuous synthesis of an endogenous extracellular matrix. This advanced 3D in vitro model can be applied to assess the ingredients' mechanisms of action in many therapeutic areas and in cosmetological research.
Dermis spheroids have been shown to reproduce different ECM features corresponding to the donor’s age phenotype. In particular, on 50 age donor ECM assembly over the time was observed to be modified: the immunofluorescence for CD44 and collagen type III (Figure 5) revealed in long-term culture (21 days) changes in ECM composition with a progressive increase of both biomarkers from three days of culture reaching the highest expression after 10 days. From day 10 to day 21 of culture, a progressive reduction of CD44 and collagen type III was observed. Masson’s trichrome staining (Figure 4) confirmed, at day 21 of culture, a transformation of ECM in a senescent configuration with collagen fibers which were reduced toward a progressive and physiological tissue degradation.
The relevance of our dermis spheroids was demonstrated by their capability to recapitulate the progressive dermal matrix deposition and ECM assembly preserving the main features of the native phenotype of different cell donors (age 40 and 50 years). We have demonstrated that by developing an advanced 3D culture system, the cells have a greater ability of generating a functional microenvironment responsive to external stimuli and to express ECM molecular mediators. By preserving cells phenotypical features and by enhancing the capacity to express tissue functionalities the dermis spheroid model seems an interesting candidate to perform preclinical investigations .
Furthermore, our results showed that dermis spheroids are able to recapitulate all the main steps of tissue differentiation thanks to a more physiological 3D microenvironment able to support the natural tissue evolution.
Concluding Remarks
Among all physiological phenomena affecting the dermis in terms of structural and functional modifications, the aging process is the most studied and characterized. Changes in dermal structural proteins in terms of the modification of collagen fibers, GAGs (and their receptors) and mechanical properties have been described in the intrinsic aging process (chronological aging, photoaging) and during tissue remodeling after injury.
Our data have given interesting insights on the ability of primary fibroblasts to secrete and organize the dermal ECM, with a continuous enrichment process. During the assembly of structural and functional components, the tissue increases its complexity, mechanical properties, stiffness and resistance.
Further investigations in terms of biomechanical properties by AFM and nanoindentation and the integration of perfusion systems to improve the physiological relevance of dermis spheroids could be considered in the future to move close to the Microphysiological Systems (MPS) today emerging in the biomedical sector. Static MPS models, such as dermis spheroids, can be used to gain new mechanistic insights into physiological processes where ECM is primarily involved, in the aging process as well as in disease pathogenesis and ECM fibrosis. They can provide at the same time information on cellular and ECM responses to drug therapies and personalized care approaches.
Abbreviations
ECM, extracellular matrix; GAGs, glycosaminoglycans; IF, immunofluorescence; PD, population doubling; PICP, procollagen type I carboxy-terminal propeptide; FFPE, formalin-fixed paraffin-embedded tissues.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–1435. doi: 10.1038/nm.4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lago JC, Puzzi MB, Picardo M. The effect of aging in primary human dermal fibroblasts. PLoS One. 2019;14(7):e0219165. doi: 10.1371/journal.pone.0219165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert G, Lansdorp PM. Telomeres and Aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007 [DOI] [PubMed] [Google Scholar]
- 4.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(24):4195–4200. doi: 10.1242/jcs.023820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care. 2016;5(3):119–136. doi: 10.1089/wound.2014.0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali Bahar M, Bauer B, Tredget EE, Ghahary A. Dermal fibroblasts from different layers of human skin are heterogeneous in expression of collagenase and types I and III procollagen mRNA. Wound Repair Regen. 2004;12(2):175–182. doi: 10.1111/j.1067-1927.2004.012110.x [DOI] [PubMed] [Google Scholar]
- 7.Rossetti D, Kielmanowicz MG, Vigodman S, et al. A novel anti-ageing mechanism for retinol: induction of dermal elastin synthesis and elastin fibre formation. Int J Cosmet Sci. 2011;33(1):62–69. doi: 10.1111/j.1468-2494.2010.00588.x [DOI] [PubMed] [Google Scholar]
- 8.Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol. 2006;12(3):145–154. doi: 10.1111/j.0909-752X.2006.00146.x [DOI] [PubMed] [Google Scholar]
- 9.Oh JH, Kim YK, Jung JY, et al. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci. 2011;62(3):192–201. doi: 10.1016/j.jdermsci.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Gniadecka M, Wessel S, Heidenheim M, et al. Water and protein structure in photoaged and chronically aged skin. J Invest Dermatol. 1998;111(6):1129–1133. doi: 10.1046/j.1523-1747.1998.00430.x [DOI] [PubMed] [Google Scholar]
- 11.Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61(5):427–434. doi: 10.1159/000371708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haydont V, Bernard BA, Fortunel NO. Age-related evolutions of the dermis: clinical signs, fibroblast and extracellular matrix dynamics. Mech Ageing Dev. 2019;177:150–156. doi: 10.1016/j.mad.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 13.Ahmed T, Nash A, Clark KE, et al. Combining nano-physical and computational investigations to understand the nature of “aging” in dermal collagen. Int J Nanomedicine. 2017;21(12):3303–3314. doi: 10.2147/IJN.S121400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcos-Garcés V, Aguilar PM, Serrano CB, et al. Age-related dermal collagen changes during development, maturation and ageing – a morphometric and comparative study. J Anat. 2014;225(1):98–108. doi: 10.1111/joa.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caviola E, Meloni M. Method for preparing three-dimensional scaffold-free microtissues for use in the pre-clinical screening of active substances; 2018. Patent Publication Number WO/2019/092667, Publication Date 16.05.2019. International Application No. PCT/IB2018/058870.
- 16.Mohammadzadeh E, Nikravesh MR, Jalali M, et al. Immunohistochemical study of type III collagen expression during pre and post-natal rat skin morphogenesis. Iran J Basic Med Sci. 2018;17(3):196–200. [PMC free article] [PubMed] [Google Scholar]
- 17.Duval K, Grover H, Han L-H, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017;32(4):266–277. doi: 10.1152/physiol.00036.2016 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 18.Kapałczyńska M, Kolenda T, Przbyla W, et al. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910–919. doi: 10.5114/aoms.2016.63743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravi M, Paramesh V, Kaviya SR, et al. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230(1):16–26. doi: 10.1002/jcp.24683 [DOI] [PubMed] [Google Scholar]