Abstract
Palliative care — specialized healthcare focused on improving quality of life for patients with serious illnesses — can help urologists to care for patients with unmet symptom, coping and communication needs. Society guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend incorporating palliative care into standard oncological care, based on multiple randomized trials demonstrating that it significantly improves physical well-being, patient satisfaction and goal concordant care. Misconceptions regarding the objective and ideal timing of palliative care are common; a key concept is that palliative care and treatments seeking to cure or prolong life are not mutually exclusive. Urologists are well positioned to champion the integration of palliative care into surgical urologic oncology and should be aware of palliative care guidelines, indications for palliative care use and how the field of urologic oncology can adopt best practices.
Subject terms: Surgical oncology, Urological cancer, Quality of life
Society guidelines recommend incorporating palliative care into standard oncological care. However, misconceptions regarding palliative care are common — notably, palliative care and treatments seeking to cure or prolong life are not mutually exclusive. In this article, the authors discuss the integration of palliative care into surgical urologic oncology and consider palliative care guidelines, indications for palliative care use, and how the field of urologic oncology can adopt best practices.
Introduction
Although most early-stage genitourinary cancers have favourable prognoses, patients with locally advanced or metastatic disease face burdensome lifelong symptoms and limited survival. Over the past few decades, the field of urology has reduced the physical suffering and functional limitations caused by genitourinary cancers through subspecialization, surgical innovation, regionalization, multidisciplinary care and novel or improved medications. However, no panacea is available to eliminate postoperative complications or thwart aggressive tumour biology.
Patients with cancer face a high burden of unmet needs1–4: the literature on genitourinary cancer thoroughly documents the negative long-term effects of cancer on physical, functional, psychological and socioeconomic well-being5–9. Despite using the best available treatments, in many cases cancer recurs, patients suffer disease-related symptoms or loss of function, and death ultimately follows. The use of palliative care principles or referral to a multidisciplinary palliative care team when appropriate can help the urologist to care for patients at any cancer stage with unmet symptom, coping and communication needs. The Center to Advance Palliative Care defines palliative care as specialized medical care for people living with a serious illness, focused on relieving the symptoms and stress of that illness: “the goal is to improve quality of life for both the patient and the family”10. Palliative care anticipates and relieves suffering by addressing the physical, informational, emotional, social and spiritual needs of patients and their caregivers11.
Multiple randomized controlled trials published during the past decade demonstrate the positive effects of early incorporation of palliative care into standard oncological care, particularly for patients with advanced disease (Table 1). Benefits of early concurrent palliative care include improved quality of life, physical and spiritual well-being, improved patient and caregiver satisfaction, use of goal-concordant healthcare services, and increased hospice use at the end of life12. Furthermore, no evidence is available to suggest that palliative care negatively affects patient survival. Based on these trials, both the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have published guidelines recommending early and concurrent palliative care in addition to standard oncological care for patients with advanced cancer12,13. The European Society of Medical Oncology (ESMO) states that “supportive and palliative care are areas of high importance in oncology” and maintains dozens of symptom-specific palliative care guidelines14. Unfortunately, most palliative care randomized trials include few patients with genitourinary cancers compared with patients with lung and gastrointestinal malignancies.
Table 1.
Randomized trials of early incorporation of palliative care into standard oncological care
| Study (country, year) | Design and comparison | Cohort | Intervention and outcomes | Key finding |
|---|---|---|---|---|
| Zimmermann et al.110 (Canada, 2014) | Cluster-randomization of medical oncology clinics; early palliative care team consultation and regular follow-up monitoring versus standard oncological care | Patients with advanced cancer (22% lung, 30% GI, 17% GU, 16% breast, 15% Gyn) | Baseline and 4-monthly survey measuring quality of life (FACIT-Spiritual), symptom severity (QUAL-E), and satisfaction and problems with medical interactions (CARES-MIS) | 85% completed at least one follow-up survey; at 4 months, the adjusted difference between change scores favoured the intervention group; FACIT-Sp: 6.44 (95% CI 2.1–10.8), P = 0.006, ES 0.44; QUAL-E: 3.51 (95% CI 1.3–5.7), P = 0.003, ES 0.45; CARES-MIS: −0.84 (−1.9 to 0.2), P = 0.11 |
| 228 intervention; 233 control | ||||
| Bakitas et al.111 (USA, 2015) | Randomized control trial, 1:1 block randomization by cancer type and enrolment site; early versus delayed concurrent palliative care and standard oncological care | Advanced cancer (46% lung, 24% GI, 11% breast, 10% other, 8% GU, 5% haematological) | In-person outpatient palliative care physician consultation, 6 weekly nurse-led phone coaching sessions, monthly phone follow-up monitoring; q3 monthly quality of life (FACIT-palliative care), symptom impact (QUAL-E), mood (CES-D), 1-year survival and resource use (hospital admission and ICU days, ED visits, and chemotherapy use) | 66% completed in-person consultation (early by day 24 and late by day 79 after enrolment). 88% of the intervention group completed three or more coaching sessions, compared with 69% of the control group; no significant difference in quality of life, symptom impact or mood at 2, 6 or 12 months after enrolment or 12, 6 or 3 months prior to death; 53% of the cohort died, 15% fewer intervention patients died at 1 year (P = 0.038), median survival was 18.3 and 11.8 months for intervention and control groups, respectively (NS); no difference in resource use |
| 104 early; 103 delayed | ||||
| Maltoni et al.112 (Italy, 2016) | Multicentre randomized trial, 1:1 block randomization by centre, no blinding; early versus on-demand palliative care | Newly diagnosed metastatic or locally advanced inoperable pancreatic cancer at 21 centres | Consultation within 2–4 weeks of randomization and 2–4-weekly thereafter; control group was seen by palliative care only if the patient, family or oncologist requested a consultation. Health-related quality of life (FACT-Hepatobiliary), mood (HADS), and satisfaction with care | All patients in the trial received at least one palliative care consultation but intervention patients received more consultations (mean 8.9 versus 3.9). Upon analysis, 77% of participants died and there was no difference in survival (38% in the intervention group and 32% in the control group); significantly improved quality of life in the intervention group for hepatobiliary cancer subscale (mean difference of 2.5, P = 0.013) and trial outcome index score (mean difference 3.8, P = 0.041) |
| 97 early; 89 on-demand | ||||
| Vanbutsele et al.113 (Belgium, 2018) | Randomized controlled trial, 1:1 block randomization by treating department; early, systematic palliative care versus usual multidisciplinary standard oncological care | Advanced solid malignancy (38% GI, 17% lung, 10% head and neck, 9% GU, 8% breast, 8% melanoma) | Monthly symptom assessment and incorporation of palliative care into multidisciplinary meetings. Change in overall quality of life from baseline at 12 weeks (EORTC QOL C30 and MQOL), as well as 18 and 24 weeks. Anxiety and depression measured via HADS | By 12 weeks, 89% and 27% of intervention patients had at least one palliative care nurse and one palliative care physician consultation, respectively (compared with 18% and 1% in the usual care arm) but 11% of intervention patients did not have any consultations; at 12 weeks, quality of life was significantly improved in the intervention arm via EORTC QLQ C30 (mean difference 7.6, P = 0.03) and MQOL (mean difference 1.11, P = 0.0006); at analysis, 65% of participants had died and there was no significant difference in median overall survival (312 days for intervention and 343 days for control, P = 0.97) |
| 92 early systemic; 94 usual | ||||
| Temel et al.114 (USA, 2017) | Randomized trial, 1:1 randomization stratified by cancer type; early palliative care versus usual oncological care | Incurable cancer (55% lung and 45% non-colorectal GI) | Palliative care consultation within 4 weeks of enrolment and at least monthly follow-up monitoring until death; change in overall quality of life from baseline at 12 weeks. Change in FACT-General (primary end-point), change in depression (PHQ-9) and end of life communication (secondary end points) | The mean number of palliative care visits was 6.5 and 0.9 by 24 weeks for intervention and control patients, respectively; at 12 weeks, quality of life was not significantly improved (P = 0.09); at 24 weeks, quality of life was improved overall (P = 0.002); patients with lung cancer had improved quality of life and depression at both 12 and 24 weeks (adjusted mean difference of 5.0 and −1.6/6.5 and −1.2, respectively (all P<0.05)); patients with GI cancer did not experience improved quality of life or depression; more intervention patients discussed end-of-life wishes with their oncologist (30% versus 15%, P = 0.004) |
| 175 early; 175 usual |
CARES-MIS, Cancer Rehabilitation Evaluation System Medical Interaction Subscale; CES-D, Center for Epidemiologic Studies — Depression scale; ED, emergency department; EORTC QLQ C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items; ES, effect size; ESAS, Edmonton Symptom Assessment Scale; FACIT, Functional Assessment of Chronic Illness Therapy; FACT, Functional Assessment of Cancer Therapy; GI, gastrointestinal; GU, genitourinary; Gyn, gynaecological; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; MQOL, McGill Quality of Life Questionnaire; NS, not significant; PHQ-9, Patient Health Questionaire-9; QUAL-E, Quality of Life at the End of Life.
In 2019, our group reported that only 4% of patients with muscle-invasive bladder cancer receiving the Medicare insurance benefit in the USA received subspecialty palliative care15. This low rate of palliative care use was seen even among patients with advanced bladder care — defined as tumour stage 4, lymph node positive or metastatic disease — which comprised 30% of the study cohort. Other than population-based or hospital registry studies, little work has been done on the specific structure and process of palliative care in urology. Studies are needed to provide insight into the underuse of palliative care and determine how concurrent palliative and oncological care affects outcomes for patients with genitourinary cancers.
In this Perspectives article, we describe the evolution of palliative care in oncology, review indications and guidelines for palliative care use and provide guidance on how urological oncologists can adopt best practices. We discuss the history of palliative care and the strong connection that urology shares with the development of this relatively new subspecialty. We hope to show that urologists are uniquely positioned among surgical subspecialists to champion the integration of palliative care into standard cancer care. Our ultimate aim is to further the growing dialogue surrounding early integration of palliative care into urology and bolster a call to action for researching its effect on patients with genitourinary cancers.
Palliative care origins
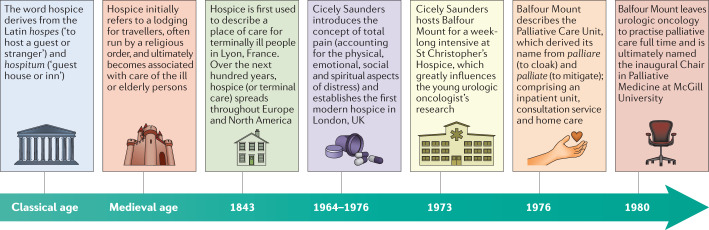
Modern palliative care emerged from the hospice movement of the 1960s and studies from the 1990s demonstrating that hospice services alone were insufficient to meet patients’ needs. The 1960s were a time of intense research interest in oncology and increasing concern over medical neglect of patients dying with cancer16. In 1964, Dame Cicely Saunders emerged as a transformative figure who revolutionized end-of-life care, when she introduced the concept of “total pain” and, 3 years later, established the world’s first modern hospice in London, UK17. St Christopher’s Hospice in South London quickly became a clinical and research centre of excellence, and Dame Saunders was critical in training the first generation of palliative care physician–scientists, while moving end-of-life care from the margins of oncology to become a central tenet of patient care18 (Fig. 1).
Fig. 1. Evolution of palliative care.
Palliative care began with a hospice tradition that dates back to the medieval period. The modern hospice care movement began in the 1960s, and modern palliative care developed during the following decade. Palliative care, with an emphasis on integration with curative or life-prolonging treatments, continues to evolve, with both death and survivorship as possible outcomes115.
On the other side of the Atlantic, a young surgeon–scientist named Balfour Mount had a growing urologic oncology practice at McGill University in Ottawa, Canada19. In the early 1970s, Mount increasingly noticed feelings of abandonment and psychological suffering in patients with cancer. He began to study the condition of terminally ill patients at the Royal Victoria Hospital and uncovered themes of “disastrous communication, isolation, abandonment, and very poor control of pain”. Mount recognized that this suffering and neglect occurred at the hands of thoughtful and well-intentioned physicians like himself16. Thus, he sought the mentorship of Cicely Saunders in order to learn more about end-of-life care19. Through his research, he uncovered discord in the doctor–patient relationship, finding that patients craved honesty and attention at the end of their life, which was often withheld by physicians in favour of protecting patients from the perceived harms of knowing the truth. Hospital staff seemed to lack self-awareness of this problem, with over half believing that co-workers avoided difficult discussions with patients but fewer than 20% personally feeling that they struggle with such situations. Mount concluded that “our personal fears influence how candidly we discuss death with our patients” and how physicians perceive patients’ feelings20. Thanks to his relationship with Cicely Saunders, Mount applied services that were typically provided by hospices to inpatient acute-care and community settings. He called this new service ‘palliative care’ in order to avoid the negative perceptions of hospice, which among the Quebecois “had a pejorative reputation [as] a very mediocre kind of care”16,21,22.
Following on from Mount’s work in the 1970s, data from the 1995 Study to Understand Prognosis and Preferences for Outcomes and Risk of Treatment (SUPPORT) demonstrated multiple care deficiencies of seriously ill hospitalized patients. SUPPORT was a multi-phase trial performed at five centres in the USA. The study aimed to determine end-of-life decision-making and outcomes for patients in the advanced stages of one of nine chronic illnesses, and subsequently design an intervention to improve these outcomes. Phase I enrolled over 4,301 hospitalized patients who had a mean 6-month survival estimate of 52%. By 6 months, 47% of the phase I cohort had died. Of these, one-third spent ≥10 days in the intensive care unit (ICU), less than half of physicians knew their patients’ preferences for a Do Not Resuscitate order, and families reported that 50% of patients had moderate to severe pain at least half the time during the last 3 days of life23. SUPPORT intensified attention paid to the unmet needs of seriously ill patients in the USA, which culminated in the 1997 Institute of Medicine report “Approaching Death: Improving Care at the End of Life”24. This report recommended urgent development of reliable, skilful and supportive care for people with potentially fatal illnesses and a commitment of health professionals to improve care of dying patients24. Responses to this report by policy-makers and health systems included the creation of hospital-based palliative care consultation services and development of a distinct hospice and palliative medicine specialty for physicians and other health professionals25.
Defining palliative care
The Center to Advance Palliative Care defines palliative care as “specialized medical care for people living with a serious illness”, focused on relieving the symptoms and stress of that illness10. Palliative care also recognizes that caregivers are included within the unit of care along with the patient and thereby seeks to identify and address caregiver needs. Palliative care skills can be differentiated from clinical skills expected from all providers by the training required to competently minister them to those in need. Primary palliative care includes the skills expected of all physicians caring for patients with uncomplicated symptom and communication needs. All physicians are expected to be competent in basic pain and symptom management, including initial treatment of anxiety and depression, as well as being capable of tactfully communicating with the patient regarding their prognosis, goals of care and code status26. Subspecialty palliative care services, on the other hand, are provided by board-certified clinicians and are usually reserved for patients with particularly complex needs. In addition to addressing advanced pain and symptom management, subspecialty palliative care facilitates communication among medical and surgical teams, ensures clear and consistent communication with patients and caregivers (especially at times of heightened emotions), coordinates transitions of care within the hospital and to post-hospitalization settings, and facilitates the consideration of hospice options when these are deemed medically appropriate and consistent with patients’ care goals (Box 1).
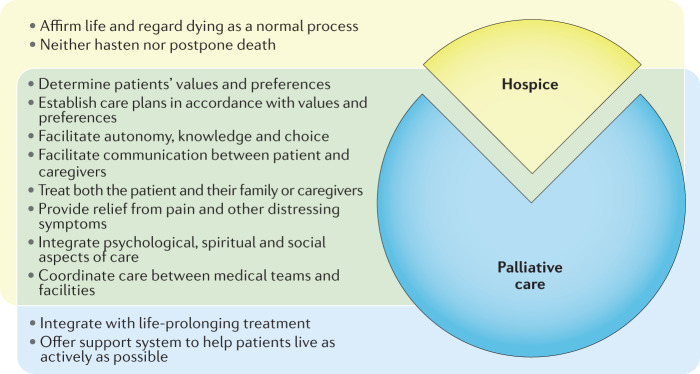
Differentiation between palliative care and hospice is important, as physicians, patients and families often confuse these two disciplines27. Differences include the distinction that patients are eligible to receive subspecialty palliative care services independent of their prognosis28, whereas hospice care is generally provided to patients at the end of life. Although no international consensus definition of hospice exists, in order to qualify for a hospice in the USA, two physicians must agree that a patient’s life expectancy is ≤6 months should their terminal diagnosis follow its expected course29. Patients can receive palliative care concurrently with disease-directed treatments, and assessment of palliative care needs begins at the time of diagnosis of a life-limiting medical condition. Over time, the balance between cancer-directed treatments and palliative services evolves in response to the patient’s condition, prognosis and preferences. By contrast, a hospice provides more focused in-home and facility-based services for patients with limited prognoses who prioritize comfort and dignity as their care goals. When contrasting hospice and palliative care for patients and their families, a useful analogy is that hospice is just one aspect of palliative care that applies to a narrow group of patients at the end of life (Fig. 2). Palliative care, on the other hand, is a more broad and comprehensive approach to care, which is applicable to patients with any stage of a potentially life-limiting illness.
Fig. 2. Tenets of palliative care.
Palliative care is often conflated with hospice care, but importantly differs by its integration or parallel administration with curative or life-prolonging treatments. Hospice is more narrow in scope, applies to a select group of patients at the end of life, and aims to affirm life while enabling a ‘good death’. A useful analogy compares palliative care to a pie and emphasizes that hospice is but a narrow slice of that pie. However, as hospice falls under the general discipline of palliative care these two disciplines share many core principles.
Box 1 Domains and skills required in primary palliative care117.
Domain: structure and processes of care
Skills: Recognize the value of palliative care
Understand basic principles and practices of palliative care
Complete palliative care assessments and address common sources of suffering
Domain: physical
Skills: Identify and treat cancer-related and treatment-related symptoms that lead to functional impairment; such as pain, nausea, constipation, urinary frequency and/or urgency, and haematuria.
Domain: psychological and psychiatric
Skills: Identify and treat uncomplicated depression and anxiety
Understand the psychological effects of cancer
Domain: social
Skills: Identify deficient housing or transportation and food insecurity
Identify caregiver burnout and isolation, and refer for social support
Domain: spiritual and religious
Skills: Conduct a spiritual screening and assessment for distress
Be aware of community resources that can help to address spiritual distress
Domain: cultural
Skills: Learn how culture influences decision-making
Explore how patients from different cultures approach symptom management
Domain: end-of-life care
Skills: Discuss dying with patients and caregivers
Understand hospice eligibility criteria
Make timely referrals for hospice evaluations
Domain: ethical and legal
Skills: Learn about advanced care planning and available resources
Facilitate advanced care planning for patients and caregivers
Know how to contact ethical or legal experts when needed
Palliative care guidelines
Many patients with cancer are referred to palliative care specialists late in their disease course, presenting with uncontrolled symptoms near the end of their life30. For example, in a 2012 report, patients with advanced cancer at MD Anderson Cancer Center were referred to palliative care an average of 1.4 months before their death, with 20 medical team encounters occurring before palliative care referral31. During the past decade, evidence has emerged to support early integration of palliative care into standard oncological care for patients with metastatic disease. A landmark study by Temel and colleagues32 randomized patients with newly diagnosed metastatic non-small-cell lung cancer to one of two arms: standard oncological care or integrated palliative and oncological care. Quality of life was the primary outcome and baseline measurement was compared with reassessment 12 weeks after enrolment. Mood, health-care utilization, documentation of resuscitation preferences and survival were also analysed. At 12 weeks, patients receiving palliative care had a significantly better quality of life and fewer depressive symptoms (P < 0.05 for all measures with moderate effect sizes). Patients in the intervention arm had fewer hospitalizations in the last month of life (37% versus 54%, no P value), improved documentation of resuscitation preferences (53% versus 28%, P = 0.05) and a greater median overall survival (11.6 versus 8.9 months, P = 0.02) than those in the control arm. Notably, measures of health-care utilization and survival are not primary objectives of palliative care, but are thought of as beneficial by-products.
Initial studies33–38 of early palliative care integration in the early 2000s led the ASCO to release a provisional clinical opinion in 2012 recommending the increased use of subspecialty palliative care39. In 2017, this document was incorporated into a clinical practice guideline that analysed data from 15 additional studies, nine of which were randomized clinical trials12. Owing to mounting evidence, the 2017 ASCO guideline recommended that multidisciplinary palliative care services be offered to patients with advanced cancer early in their disease course, concurrent with active treatment, and ideally within 8 weeks of a diagnosis of advanced cancer32. Other societies have released similar guidelines, in which the timing, indication and screening recommendations for palliative care referral vary slightly (Table 2).
Table 2.
Summary of society palliative care guidelines
| Society | Guideline publication date | Timing | Indications | Patient screening | Initial needs assessment |
|---|---|---|---|---|---|
| ASCO12 | January 2017 and May 2018 | Needs should be addressed at presentation to the oncologist, and early specialist involvement should occur within 8 weeks of an advanced cancer diagnosis | Metastatic or poor prognosis cancer, limited treatment options, estimated survival <12 months, frequent admissions owing to refractory symptoms, functional decline, failure to thrive or complex care requirements | All patients with cancer should be screened at presentation and reassessed at appropriate intervals or as clinically indicated. Screening tools include the NCCN Distress Thermometer, Edmonton Symptom Assessment Scale, Condensed Memorial Symptom Assessment Scale and Brief Pain Inventory | Identify palliative care needs with regard to quality of life and physical, functional, spiritual, psychological and social domains; evaluate basic pain and symptom management; determine patient understanding of illness and prognostic awareness; clarify treatment goals; assess medical literacy and decision-making; and coordinate care with other medical teams |
| NCCN13 | February 2020 | Can begin at cancer diagnosis and be delivered concurrently with life-prolonging therapies | Metastatic solid tumour, uncontrolled symptoms, distress related to diagnosis or therapy, serious comorbid conditions, patient or family request for palliative care, poor performance status (ECOG >2 or KPS <60) or cachexia | All patients with cancer should be screened at presentation and reassessed at appropriate intervals or as clinically indicated. If screening criteria are met, proceed to a comprehensive initial palliative care assessment. If no criteria are present, inform patient of palliative care services and rescreen at next visit | Discuss benefit and burdens of anticancer therapy; define patient/family goals, values, priorities and expectations; evaluate for psychosocial and spiritual distress; evaluate educational, cultural and informational needs; begin with initial symptom management if needed; and determine whether criteria for formal palliative care consultation are met |
| EAU72 | March 2013 | Applicable early in the course of illness in conjunction with life-prolonging therapies and be available throughout a patient’s care pathway | No specific criteria proposed, but early collaboration between the oncologist and palliative care team is emphasized | Assess pain (using the OPQRSTUV mnemonic — Onset, Provocative factors, Quality, Radiation, Severity, Timing, Understanding/impact on patient, and Values), patient’s readiness to accept palliative care and the role they expect it to have in their care | Establish excellent communication (good eye contact, open-ended questioning, responding to patient’s emotions, displaying empathy); assess pain; assess patient knowledge and establish goals of medical care; develop realistic expectations; and develop a treatment plan |
Indications less pertinent to genitourinary malignancies in general are not included in the table. ASCO, American Society of Clinical Oncology; EAU, European Association of Urology; ECOG, Eastern Cooperative Oncology Group; KPS, Karnofsky Performance Status; NCCN, National Comprehensive Cancer Network.
Although a patient’s surgeon and oncologist can provide primary palliative care services, referral to subspecialty palliative care should be considered for patients with complex and refractory symptoms, substantial emotional distress and decision-making challenges26. An analysis of 2,921 clinic visits demonstrated that palliative care clinicians who provide services to newly diagnosed patients initially focus on physical and emotional support rather than prognosis and treatment decision-making. The initial three visits addressed rapport building (86%), symptom management (71%), coping with life-threatening illness (68%) and illness understanding (47%). By contrast, treatment decisions were addressed only 5% of the time, and 0.2% of visits addressed disposition to other facilities40. Patients with a higher proportion of visits that addressed coping had significantly improved quality of life (β = 0.19, P = 0.02) and depression symptoms (β = −0.26, P = 0.002). The conclusions from this study might reassure oncologists who worry about patients receiving mixed messages from palliative care providers regarding treatment and prognosis, while showing that the patients and their oncologists continue to direct cancer care decision-making. Importantly, early palliative care referral lays the foundation for future difficult conversations when they inevitably arise. Other than referring for symptoms, emotional distress and decision-making, subspecialty palliative care consultations are helpful for navigating emotionally charged treatment decisions or when disposition (that is, hospice) becomes a greater focus than disease control.
Beyond the general recommendation of subspecialty palliative care referral for patients with advanced cancer, ASCO guidelines more specifically recommend referring patients with a life expectancy of <6 months, very poor performance status, or those enrolled in a phase I or II clinical trial41. The authors note that these referral criteria would likely overwhelm existing palliative care resources. Thus, the ASCO recommends that all physicians receive education in primary palliative care skills and the administration of needs assessments, stating that these moves would likely ameliorate the majority of basic unmet supportive care needs without the need for referral to specialist palliative care services. However, before primary palliative care can be widely implemented, barriers facing palliative care must be better understood and subsequently addressed.
Barriers to providing palliative care
Palliative and curative care are not mutually exclusive, nor do their philosophies conflict. Palliative care enables the goals of comfort and cure to coexist until cure is achieved, suffering is relieved or the disease progresses beyond control. Highlighting these goals is important because palliative care faces a persistent and considerable branding issue, a primary cause of which is prevalent misconception regarding the objectives and timing of referral42. Studies often find that palliative care referrals are made when physicians identify no other options for the patient’s treatment or that the patient is very near the end of life43. For example, a major cancer centre in the USA showed that median time between palliative care consultation and death was 1.4 months31. A systematic review of 169 studies representing 23 countries found this duration to be only 19 days, with longer durations for patients with cancer (15 days versus 6 for non-malignant conditions) and less developed countries (19 days for the most developed countries versus 34 days for all others)44. A collection of exemplar quotes from qualitative studies display the beliefs regarding providers’ negative perceptions of palliative care that can explain some of these trends (Box 2).
Surgeons and palliative care physicians often lack experience in collaborating with one another, either because they perceive divergent approaches to caring for the patient or the surgeon’s immersion in rescue culture45,46. Low acceptance of palliative care services is common among surgeons, and might be explained by the preoperative covenant (“Trust in me and I will care for you until the end”)47, their personal identity as a ‘fixer’48, a strong sense of responsibility to patients post-operatively and demanding professional expectations for excellence and operative success49,50. An additional factor contributing to low acceptance from surgeons is the worry that the palliative care team might miscommunicate diagnostic or prognostic information, or that the referral to palliative care itself will serve as a signal to the patient and their family that the surgeon has given up51.
Surgeons use palliative care less frequently than their medical counterparts52. Among 191,000 Veterans Health Administration patients from the USA who had inpatient admissions and died over a 4-year period, patients cared for on surgical services were 16% less likely to receive palliative care or hospice (odds ratio 0.84, 0.81–0.86, P < 0.001). This phenomenon might be due to a lack of formal palliative care education, lack of experience or both. A 2016 systematic review assessed surgeon underuse of palliative care and reported that, among surgical residents, palliative care training is informal and on-the-job. In fact, the vast majority of surgeons — 40–98% across 11 studies and nearly 12,000 subjects — receive no formal palliative care training at all53. The association between surgeon experience and their confidence in providing palliative care is conflicting. In one study, some veteran attending surgeons reported that they felt that palliative care was irrelevant to quality of life and symptom management, potentially owing to negative experiences with these services in the past53. However, a survey of surgeons working in the trauma ICU showed that a majority (57%) believe that palliative care is beneficial and a plurality (49%) believe that it is underused51.
In spite of the abundant data emphasizing the value of palliative care in advanced cancer, a considerable proportion of oncologists lack awareness of local palliative care resources or believe that palliative care is an alternative philosophy that conflicts with active cancer care54. In a 2012 survey of Canadian oncologists, nearly two-thirds (63.1%) of respondents (medical, radiation and surgical specialists) reported referring their patients to subspecialty palliative care only if the patient was receiving palliative chemotherapy or if all life-prolonging therapies had been stopped30. Even among academic surgical oncologists, of whom 92% had access to palliative care services at their hospital, 42% believed that palliative care was appropriate only when life expectancy was <6 months and 73% worried that their patients would feel abandoned should palliative care be consulted55. Why these physician barriers to early referral persist in the face of level 1 evidence supporting early palliative care referral remains unclear. Reticence might be partly due to an initial focus on aggressive curative therapies, a desire to avoid sending conflicting messages regarding treatment goals and worry about upsetting patients and their caregivers by giving the impression that they are giving up or that there is no hope of controlling their disease51,56. Notably, however, many medical oncologists provide primary palliative services as part of their holistic approach to the patient.
Patient awareness and misconceptions also contribute to low uptake of palliative care. Among a nationally representative sample of adults in the USA, only 30% of respondents had heard of palliative care. Of those who had heard of palliative care, nearly half conflate it with hospice. The proportion of respondents with a negative perception of palliative care did not differ between those who professed high awareness versus low awareness of palliative care57. Similar trends regarding poor awareness are seen across Europe. Over 40% of Italians had never heard of palliative care and only 24% had a “somewhat precise” idea58. Even among health professionals knowledge varies widely, with palliative care survey scores ranging from a low of 20% in Polish nursing facilities to a high of 61% in Belgian facilities59.
Access to palliative care services is also a barrier. Among patients who actually receive palliative care, stated difficulties in initially accessing this care include low personal awareness and lack of physician referral60. That racial and ethnic minority patients in particular face socioeconomic and institutional barriers to receiving many healthcare services is well known, and palliative care is no different. Patients from minority groups also have increased supportive care needs, particularly in the informational and spiritual domains61,62. For example, a study in patients with lung cancer reported that more Black and Hispanic patients than white patients believed that surgery facilitates the spread of cancer (30% of Black patients versus 14% of white and Hispanic patients, P = 0.008), that a lawyer is required in order to change a living will (a written statement detailing a patient’s desires in the event that they are no longer able to express informed consent; 52% of Black patients and 57% of Hispanic patients versus 32% of white patients, P = 0.01), that life support should never stop because “only God can decide when it is time for death” (30% of Black patients versus 14% of white and Hispanic patients, P = 0.008), and that hospice care is not needed if a patient has family at home to help care for them (30% of Black patients and 48% of Hispanic patients versus 19% of white patients, P = 0.006)61. In addition, these misunderstandings and mistrust in the health-care system are exacerbated by a history of unethical research practices and persistent inequities in access to care, poor pain control and access to opioids, worse perception of communication and lower satisfaction with end-of-life care62. Taken together, these factors might explain why non-white patients are less likely to enrol in hospice care and are more likely to die in acute-care settings63. Such inequities exist despite focus group data that show a strong desire for holistic approaches to care and additional support to decrease caregiver burden in minority populations64.
Finally, workforce limitations contribute to difficulties in addressing palliative care needs. Palliative care staffing shortages in the USA are widespread and well documented — an estimated shortage of up to 18,000 palliative care physicians means that the current number of board-certified palliative care physicians cannot meet the demands of even just the most complex patients65. Since this study estimated the provider gap in 2010, others have predicted that the number of patients eligible for palliative care will grow by 20% by 2030. This increase is troubling given that graduate medical education positions, at least in the USA, allow for only a 1% growth in the number of palliative care physicians over that same period66. This workforce shortage might exacerbate scepticism of palliative care among surgeons, as unavailability (26%) and a lack of timely access (22%) are cited as reasons for not referring to palliative care51. One interviewee felt that “their resources are sometimes a little bit limited at the times you need them the most”48. In facilities with subspecialty palliative care physician shortages, hospital chief executives report a number of barriers that perpetuate this low availability. Among respondents, 52% report poor reimbursement, 44% report budgetary shortfalls and 44% state that a lack of adequately trained providers prevents them from providing palliative care to their patients49.
Box 2 Exemplar quotations from studies of surgeon perceptions of palliative care.
- Community general surgeons from Michigan, USA (30–60-min semi-structured interviews regarding barriers to palliative approaches to care and palliative care services (n = 46))48
- “There’s lots of ways to take care of problems… I’m open to, you know, palliative care, but again, as a surgeon I’m trying to fix everybody”
- “It’s hard for me to not seem like I’m giving up or backing out on someone if things don’t go well”
- “It feels like if you start talking palliative care to family or patients, they think… ‘Oh he’s kind of written me off and he doesn’t want to take care of me anymore. He just wants to get rid of me’”
- “The expectations are kind of not realistic in that they don’t think they’ll need it and so they don’t want to talk to palliative care or hospice”
- “People don’t like being told they’re going to die. I don’t want to die, but you know, it’s all going to happen someday”
- “Palliative care I think is complicated because families often think that they’re interchangeable with hospice and they can potentially get offended if you suggest that we get a palliative care team involved”
- “I think sometimes family members and doctors and nurses view palliative care as giving up”
- “Sometimes we have to be careful because why, as soon as they get palliative care, patients sometimes take this view as, ‘Okay this is palliative. That means nothing can be done’. So we have to educate them about palliative care”
National sample of colon and rectal surgeons, USA (open-ended survey questions regarding reflections on and experiences of end-of-life care (n = 131))118
“I don’t think that we do a good job educating families about end of life. Everyone just continues to think ‘you’ve got to do something’”
“In my opinion, the biggest gap is that our country views death as a taboo subject and as a failure, instead of treating it like another part of life that has its own value and meaning”
“We need a better palliative care service. As surgeons, we just can’t fill that role adequately, though I feel we should stay involved”
-
3.
Academic and private surgeons from Wisconsin, USA (90-min focus groups on presentation of treatment options for very frail patients (n = 17))119
“Usually, I won’t tell until patients ask my opinion”
“I see my job to try and help them understand what their options are. Their job is to choose”
Delivery of palliative care
The availability and quality of hospital-based palliative care services has steadily increased over the past decade. Palliative care services can be hospital based or community based. In the USA, two-thirds of hospitals with >50 beds and 94% of hospitals with >300 beds have a palliative care programme, but these services are present in only 17% of rural hospitals67. Nearly all National Cancer Institute (NCI)-designated centres in the USA have palliative care programmes and a further 80% of non-NCI-designated cancer centres in the USA also have programmes68. In Europe, ESMO-designated centres provide a high level of palliative care infrastructure. In a 2017 electronic survey study of ESMO-designated centres — 32 from Italy, 17 from Germany, 14 from Spain and 89 from other countries around the world — 90% had inpatient palliative care consultation teams, 89% had outpatient clinics and 50% provided community-based care, such as home palliative care and hospice care69. Unfortunately, low-income and middle-income countries, and underserved regions of high-income countries uniformly lack access to palliative care. In 2015, a total of 25.5 million people who died experienced serious health-related suffering (45% of all worldwide deaths). Over 80% of these individuals were from low-income and middle-income countries, most of whom lack basic resources for pain management and palliative care70.
Hospital-based care
The inpatient consultation service is the foundation of hospital-based palliative care. At well-equipped centres, the inpatient team can consist of experts from a range of backgrounds including subspecialty-trained physicians, advance practice providers, social workers, chaplains, dietitians, pharmacists, physical and occupational therapists, and music therapists24. The overarching goals of the palliative care service are to improve coordination between services and provide recommendations to the consulting team regarding pain and symptom management, psychological care, goals of care, and reconciling of patient and family conflict71. Originally, hospital-based services were provided in a consultative model. As it is currently employed, the consultative mode “focuses on increasing the involvement and effectiveness of palliative care consultants”72. In the early 2000s, interventions using an integrative model were studied, which involves specialty training of clinicians to enhance primary palliative care skills and incorporate them into daily practice73. An integrative model reported by Lilly et al.74 involved a multidisciplinary family meeting for all ICU patients within 72 h of admission. The investigators found that the median length of stay decreased from 4 to 3 days (IQR 2–6, P = 0.01), particularly for the quartile of patients with the highest acute physiology and chronic health evaluation scores. This change occurred without an associated increase in mortality, signifying that earlier ICU discharge and transition to less-invasive goal-concordant care occurred without hastening death74. Since publication of this study, multiple trials studying integrative or consultative interventions in the ICU have been published, but no consensus exists regarding which approach is most effective. A hybrid approach involves embedding palliative care providers within hospital units in a co-management model with other specialties. In the ICU for example, embedded palliative care providers facilitate family meetings and informed decision-making, assist with prognostic assessment, and provide caregiver and bereavement support75.
Community-based care
Community-based palliative care has become the fastest growing component of this discipline76. A systematic review on the integration of palliative care and oncological care reported that outpatient interventions were the most frequently cited (55% of studies) in the literature41. Additionally, outpatient palliative care clinics at NCI-designated cancer centres in the USA increased from 59% to 95% between 2009 and 2018 (ref.77). These services can be provided in the patient’s home, nursing homes, palliative care clinics or other subspecialty practices, such as medical oncology. Benefits of widely available outpatient palliative care include improved patient satisfaction, improved symptom control and quality of life, reduced health-care utilization and improved care coordination among various specialties78. Another review showed fewer significantly improved benefits, but quality of life was still significantly improved among patients receiving integrated outpatient palliative care (standardized mean difference 0.24, 95% CI 0.13–0.35)79.
Finally, telemedicine-based palliative care has been increasingly investigated over the past decade, with citations increasing from two annually between 1999 and 2009 to more than three annually between 2010 and 2019 (ref.80). Although studies are small, telemedicine interventions have been shown to offer improved symptom control and less frequent emergency department use81. The growth of palliative telemedicine, which has been bolstered by the COVID-19 pandemic, might further improve access to these services while decreasing logistical burdens on patients and providers. For example, COVID-19 necessitated an expedient transition to telehealth palliative care at a major US cancer centre and showed an increase in goals of care discussions82.
Palliative care research in urology
The urology community has thoroughly explored the physical and psychosocial impact of genitourinary malignancies on both patients and caregivers6–8,15,83. Although minimal work has investigated how palliative care can ameliorate these effects, initial studies suggest benefits to providing subspecialty palliative care concurrent with curative intent urological care. For example, a serial cohort study of 63 patients with localized muscle-invasive bladder cancer, in which 33 patients received usual care with radical cystectomy and 30 received palliative care concurrent with radical cystectomy, showed that palliative care benefits patients with early-stage bladder cancer84. In this study, functional status, pain, fatigue, anxiety, depression, quality of life and spirituality were measured at baseline and every 2 months postoperatively for a total of 6 months. Patients receiving concurrent palliative care had significantly improved anxiety and depression (hospital anxiety and depression score, P = 0.01 for comparison of trends between groups), fatigue (cancer fatigue score, P = 0.02 for decrease among the intervention group and P = 0.002 for comparison of trends between groups), and quality of life scores postoperatively (Functional Assessment of Cancer Therapy-General, P = 0.002 for improvement among the intervention group and P = 0.01 for comparison of trends between groups). These improvements occurred despite a limited study intervention, whereby most palliative care encounters occurred over the phone and <25% of patients in the intervention arm received a new prescription from the palliative care provider. The improvement seen in the intervention arm of this study illustrates the potential benefits of a more intensive palliative care intervention in patients with localized bladder cancer.
A pilot intervention integrating urologic oncology and palliative care investigated the utility of a urology–palliative care clinic for military veterans in the USA with newly diagnosed metastatic genitourinary cancer85. The investigators offered all patients with newly diagnosed metastatic bladder, metastatic kidney and bone-metastatic prostate cancer a palliative care referral at the point of care. Quality of life and satisfaction were assessed among the 53 patients recruited to the integrated clinic over a 3.5-year period86. Outcomes were reassessed during follow-up visits occurring within varying timeframes. At the time of analysis, 68% of the cohort had died, of whom 81% died at home or as an in-patient in a hospice. Nearly all (91%) reported high satisfaction via the Patient Satisfaction Questionnaire-18, which did not significantly change over time. Brief pain inventory scores and the proportion screening positive for depression (via the Patient Health Questionaire-2), although improved over time, did not significantly change (P = 1.0 and P = 0.57, respectively). Additionally, clinicians practising in the integrated clinic completed semi-structured interviews to gauge provider perceptions of the pilot intervention. A number of important themes emerged: in particular, clinicians found that patients were receptive to palliative care referral within the urology clinic, that referral did not affect the clinic workflow and that patient care improved through better pain and symptom management. These data illustrate the feasibility of embedding subspecialty palliative care within a urology clinic setting.
As these studies suggest, future research into embedded palliative care could show benefits in patients with genitourinary cancer similar to those it has shown in patients with other malignancies. Future studies should investigate how concurrent oncological and palliative care affects outcomes for patients with genitourinary cancers, specifically focusing on quality of life, symptom burden, functional status, caregiver burden and health care utilization87,88. In addition, conducting a primary palliative care needs assessment could direct future educational initiatives for urologists at all stages of training. Existing attitudes of urologists towards palliative care, starting with expert consensus on indications and potential urology-specific primary palliative care interventions, should also be assessed in order to address barriers and promote collaborations89.
The urologist’s role in palliative care
The importance of palliating symptoms for patients with genitourinary cancer has been extensively discussed in the literature90,91. Unfortunately, these discussions have not resulted in a lasting emphasis on incorporating palliative care into standard genitourinary oncological care. This fact is evident through the underemphasis of early concurrent palliative care in urology society guidelines (Box 3). However, guidelines released by national societies increasingly reflect the importance of symptom management and quality of life. For example, in 2020, joint guidelines from the American Urological Association (AUA), American Society for Radiation Oncology and Society for Urologic Oncology for management of patients with advanced prostate cancer specifically emphasized the need to prioritize pain control and connect men with palliative care resources in their community92. In 2014, the European Association of Urology (EAU) provided guidelines for the treatment of disease-specific pain, postoperative pain management and basic palliative care for symptoms such as dyspnoea, nausea and vomiting, anorexia and cachexia, and mood disorders93,94. Although comprehensive, the EAU Guidelines on Pain Management and Palliative Care have not been updated since their initial publication.
In 2017, a plenary session on palliative and end-of-life care was held at the AUA annual meeting; this session highlighted how the urologist can improve the whole-person care of their patients with chronic or terminal illnesses95. Additionally, a quality improvement summit is to occur at the 2021 AUA annual meeting. No similarly prominent showcase to discuss the role of palliative care for patients with genitourinary cancers has been held at other national meetings, such as the EAU Congress or GU-ASCO Symposium. Continuing to discuss the evidence supporting early, concurrent palliative care is critical to improving adoption of this practice. Increased palliative care referral rates have been associated with the publication of clinical guidelines and a potential trial effect through participation in randomized trials. For example, at a large academic medical centre in Canada, referral patterns after guideline publication and trial participation significantly shifted toward earlier palliative care (OR 2.36, 95% CI 1.72–3.25, P < 0.0001). This trend held true even among surgical oncologists and for patients with genitourinary malignancies (early referral rate of 20.0% and 16.2% increased to 28.6% and 35.3%, respectively)96.
Not all practices or health care systems can afford the time required to provide primary palliative care in the outpatient setting, with these interventions averaging 17 min in duration to complete97. In these cases, individual providers should perform an efficient palliative care needs assessment for at-risk patients, with a compilation of specific signs prompting either a more in-depth needs assessment or referral to a palliative care specialist (Box 4). These triggers include high health-care resource utilization, high pain or symptom burden, advanced disease with comorbidity limiting potential treatment options, challenging communication needs and complex socioeconomic circumstances. Components of a comprehensive palliative care needs assessment are included in the ASCO, NCCN and EAU Pain Management and Palliative Care guidelines (Table 2). Above all else, these societies emphasize the need for excellent communication to establish the patient’s level of understanding regarding their disease process and prognosis, and to determine their values and preferences. Basic symptom management, particularly the treatment of pain, is also emphasized.
Surgical subspecialists such as urologists need to develop the skills necessary to assess and address basic palliative care needs98,99. Where clinics lack the infrastructure to provide robust primary palliative care, the individual urologist can focus on improving a few specific areas of their supportive care repertoire. The importance of excellent communication cannot be overstated — unmet informational needs are common among patients with cancer100. Patients who are pessimistic about their diseases or have less assertive coping styles — compared with assertive information-seeking patients or those with ‘fighting spirit’ — are more likely to report communication problems such as poor understanding, difficulty expressing feelings or asking questions, and a desire for more control over their medical care. In a study of patients with breast cancer, for example, poor patient–provider communication preoperatively was associated with increased psychological and informational problems at 3-month follow-up prior to initiating adjuvant therapy. Poor communication correlated positively with anxiety, confusion, depression and anger postoperatively (r = 0.31, 0.38. 0.21 and 0.29, respectively; all P < 0.01)101. The adequacy of communication can reasonably be gauged during routine clinic visits and can reveal unknown psychosocial stressors for the patient.
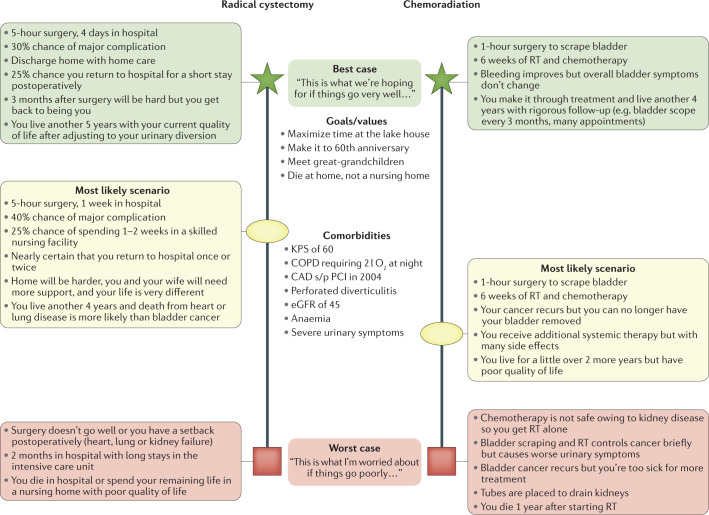
Aligning a patient’s prognosis with their treatment plan is also crucial: for patients preparing to undergo curative or palliative surgery, healthcare providers must avoid focusing discussions on the mechanics of the procedure at the expense of discussing the patient’s overall goals102. The clinician must ensure that the procedure itself aligns with the patient’s values, preferences and goals of care. To enable this approach, the best case/worst case surgical communication tool provides a patient-centred framework for discussing risks and benefits with critically ill patients or patients with comorbidities facing high-risk surgery103 (Fig. 3). This method uses scenario planning to engage the patient as the decision-maker, presenting them with multiple possible outcomes based on various assumptions104. ‘Best case/worst case’ enables the surgeon to incorporate comorbidities, patient values and preferences, and possible outcomes into a personalized depiction of how treatment decisions could affect the patient’s daily life. Tactful communication is also critical in non-operative settings, such as when a patient has cancer recurrence, progresses through systemic therapy or suffers a major complication. Outlines for guiding these challenging conversations are available on the VITALtalk Resource Page or through the VITALtalk Tips App. More in-depth education can be found in the very accessible text Mastering Communication with Seriously Ill Patients105.
Fig. 3. Best case/worst case.
This communication tool is a useful graphical aid that the surgeon can use, particularly when discussing treatment options with patients who are acutely ill or have multiple comorbidities. Use of this framework was first described by Schwarze. “To start, the surgeon names each of the patient’s treatment options and describes … the ‘best case’ outcome, the ‘worst case’ outcome, and what [they believe] is the ‘most likely’ outcome for each treatment. The verbal description of each ‘case’ incorporates rich narrative derived from clinical experience and relevant evidence, and focuses on the story of how the patient might experience an outcome, instead of quoting discrete statistical risks. The surgeon simultaneously draws the pen-and-paper diagram, and under each treatment option […] places a vertical bar. The bar length suggests a range of outcomes and the relative magnitude of difference between the ‘best case’ (star), the ‘worst case’ (box) and the physician’s estimate of the ‘most likely’ outcome (oval). After presenting the tool, the surgeon uses phrases to encourage deliberation such as, ‘what do you think about all of this?’”116. CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); KPS, Karnofsky performance status; PCI, percutaneous coronary intervention; RT, radiotherapy.
Beyond communication, numerous resources are available to improve the urologist’s comfort providing primary palliative care or initial symptom management as a patient awaits consultation with a specialist. The EAU Guideline on Pain Management and Palliative Care, which provides algorithms for treating various disease-specific symptoms, is particularly useful. The NCCN Clinical Practice Guidelines in Palliative Care and the American College of Surgeons Surgical Palliative Care: A Resident’s Guide provides high-level and pragmatic approaches to familiarizing oneself with initial symptom management106. The ESMO Clinical Practice Guidelines in Supportive and Palliative care also provide symptom-specific guidance. Finally, excellent patient-facing materials are available from numerous organizations, aiming to improve patient knowledge and beliefs regarding various aspects of palliative care. The Bladder Cancer Advocacy Network provides their ‘Get the Facts: Palliative Care’ guide on-line and the Journal of the American Medical Association offers supportive care guides on its patient page, such as palliative care, end of life care and hospice care107–109. These materials are especially valuable for patients with metastatic disease and/or poor prognosis, as misperceptions and unfamiliarity with palliative care contribute to underutilization.
For health-care providers in busy clinical practices, the single most important way to improve provision of palliative care might be to build relationships with palliative care colleagues. Seeking out these collaborations and developing referral criteria (Box 3), normalizes the role of subspecialty palliative care in providing an extra layer of support for patients, caregivers and urologists.
Future palliative care research is imperative for improving whole-person care for patients with genitourinary malignancies. We hope for an improved understanding of patient, physician and systemic barriers to palliative care use in urology. Research networks are actively working towards understanding the barriers within each of these domains. With this information, quality improvement initiatives should focus on studying how evidence-based palliative care initiatives can best be incorporated into standard urological cancer care. Such initiatives will necessitate collaboration with health economists in order to determine a time-effective and cost-effective implementation strategy, which might involve incorporating primary palliative care into the urology clinic (most likely via subspecialized urological and palliative care advanced practice providers) or fostering communication with general practitioners to address symptom management via co-primary palliative care. Evidence from ongoing randomized trials, such as CONNECT (NCT02712229), should inform the development of similar strategies in urologic oncology. CONNECT is a cluster randomized trial of an oncologist nurse-led care management intervention among patients with advanced cancer and their caregivers, with quality of life as the primary end point. The study completed in 2020 and the results are pending.
Box 3 References to palliative care in current urology guidelines.
- American Urological Association: nine cancer-specific guidelines120
- Reference to palliative care principles
-
i.Advanced prostate cancer121
- “Additional specialists may also include genitourinary pathology, genetic counselling, palliative care and holistic specialists, as appropriate, in addition to primary care. Best practices must also include clinicians comfortable describing the use of germline and somatic genetic testing, and when advanced imaging techniques could be optimally used or avoided. Radiologists and nuclear medicine specialists are valuable in helping to accurately interpret scans. Palliative care team members may also play a key role when treating men with symptomatic metastatic disease. Palliative care itself is an interdisciplinary, holistic approach to managing an advanced disease such as prostate cancer with a guarded prognosis. It can include controlling symptoms that are physical, psychological, spiritual and social. The goal of palliation is to prevent and relieve suffering and to support the best possible quality of life for the patient and family”.
-
i.
- No reference to multidisciplinary palliative care or to palliative approaches to care
-
i.Non-metastatic muscle-invasive bladder cancer
-
ii.Non-muscle-invasive bladder cancer
-
iii.Renal mass and localized renal cancer
-
iv.Follow-up for clinically localized renal cancer
-
v.Early-stage testicular cancer
-
vi.Clinically localized prostate cancer
-
vii.Adjuvant and salvage radiotherapy for prostate cancer
-
viii.Hypofractionated prostate cancer radiotherapy
-
i.
- European Association of Urology: eight cancer-specific guidelines122
- Reference to palliative care principles
-
i.Prostate cancer
- Subspecialty multidisciplinary palliative care is not mentioned but there are guidelines regarding palliative therapies and when to offer a palliative intent treatment plan, such as “Critical issues of palliation must be addressed when considering additional systemic treatment including management of pain, constipation, anorexia, nausea, fatigue and depression” and “It is important to offer standard palliative surgery, which can be effective for managing osteoblastic metastases”
-
ii.Muscle-invasive bladder cancer
- “There is limited literature describing health-related quality of life in bladder cancer patients receiving palliative care, but there are reports of bladder-related symptoms relieved by palliative surgery, radiotherapy and/or chemotherapy”
- Additional references to palliation are in the context of palliative cystectomy, radiotherapy or chemotherapy, rather than multidisciplinary palliative care
-
i.
- No reference to multidisciplinary palliative care but guideline discusses palliative therapies
-
i.Penile cancer: role of palliative radiotherapy, chemotherapy or chemoradiotherapy
-
ii.Renal cancer: palliative radiotherapy, embolization for palliation of haematuria or pain, and the palliative nature of cytoreductive nephrectomy
-
iii.Upper tract urothelial cancer: role of radical nephroureterectomy “aimed at controlling symptomatic disease” in the setting of metastatic disease
-
iv.Primary urethral cancer: “there is an urgent clinical need to better address the role of local palliative treatment strategies”
-
i.
- No mention of multidisciplinary palliative care or of palliative approaches to care
-
i.Testicular cancer
-
ii.Non-muscle-invasive bladder cancer
-
i.
- Canadian Urological Association: 11 cancer-specific guidelines123
- Reference to palliative care principles
-
i.Muscle-invasive bladder cancer
- “Palliative care consultation should be requested early on in the care of incurable/unresectable patients”
- “Patients with unresectable or metastatic disease should be offered an early palliative care referral, as a number of oncology randomized controlled trials have demonstrated improvement in health-related [quality of life] and symptom control with prompt referral”
- Additional references to palliation are made in the context of palliative transurethral resection of bladder tumour, cystectomy, radiotherapy or chemotherapy
-
i.
- No reference to multidisciplinary palliative care but guideline discusses palliative therapies
-
i.Management of castration-resistant prostate cancer: role of palliative radiotherapy and corticosteroid therapy for symptom palliation
-
ii.Guideline on androgen deprivation therapy (adverse events and management strategies: use of hormonal therapies in the palliation of hot flashes)
-
i.
- No reference to multidisciplinary palliative care or to palliative approaches to care
-
i.Guideline on metastatic castration-naive and castration-sensitive prostate cancer
-
ii.Management of non-muscle-invasive bladder cancer
-
iii.Management of small renal masses
-
iv.Management of cystic renal lesions
-
v.Recommendations on prostate cancer screening and early diagnosis
-
vi.Management of the incidentally discovered adrenal mass
-
vii.Guideline for follow-up of patients after treatment of non-metastatic renal cell carcinoma
-
viii.Guideline on genetic screening for hereditary renal cell cancers
-
i.
- Urological Society of Australia and New Zealand: one cancer-specific guideline124
- Reference to palliative care principles
-
i.Prostate cancer survivorship essentials framework (guidelines for practitioners)
- “Clear explanation is required that palliative care relates to the prevention and control of symptoms earlier in the survivorship journey as well as to end-of-life issues”
-
i.
Box 4 Triggers for palliative care consultation.
High health-care resource utilization
Frequent emergency department visits (≥2 in a month)
Any ICU-level care due to multiorgan system failure
-
2.
Persistent pain or high risk of poor pain management
Neuropathic pain
Incident or breakthrough pain
Pain with severe associated psychosocial or family distress
Rapid escalation of opioid dose
Multiple allergies or adverse reactions to pain medications
Concerns regarding substance abuse disorder
-
3.
High non-pain symptom burden or symptoms refractory to initial management
Anorexia and/or cachexia, nausea and vomiting, constipation, diarrhoea
Fatigue, weakness or asthenia, insomnia or sedation, delirium
Dyspnoea
High distress
Lymphoedema
Hormone-related
-
4.
Limited anticancer treatment options
Limited access to health-care resources
Advanced-stage disease
Severe or multiple comorbidities
Rapidly declining or poor functional status
-
5.
Need for advanced communication skills
Resistance to engage in advanced care planning
Need for clarification of goals of care
Assessment of decision-making capacity
Communication barriers (language, literacy, cognitive impairment)
Patient request for hastened death
-
6.
Complex patient and/or caregiver circumstances
High risk, or presence, of complex bereavement disorder
Inadequate social support
Substance use
Financial limitations or financial toxicity
Discordant expectations or goals of care
-
7.
Oncology care team challenges
Complex care coordination issues or involvement of multiple care teams
Intra-team conflict
Burnout and/or compassion fatigue
Moral distress or ethical concerns
Conclusions
Early integration of palliative care, especially in the outpatient setting, greatly improves the quality of life and symptom burden for patients with cancer. Despite studies in the urology literature showing the feasibility and benefit of incorporating palliative and urologic care, palliative care is seldom discussed and is underused in patients with genitourinary malignancies. We cannot hope to make strides in improving the whole-person care of our patients without reframing conversations regarding life with cancer. Palliative care can best be described as an additional layer of support that helps patients live their best lives and facilitates goal-concordant care through early and frequent discussions regarding a patient’s values and prognosis42. By continuously providing support that is grounded in achievable goals, we can relieve patient and caregiver stress while delivering quality care upon diagnosis, into survivorship and as the end of life approaches. As subspecialty palliative care is a scarce clinical resource, it is incumbent upon urologists to enhance their own primary palliative care skills in order to provide the best care to our patients.
Acknowledgements
The authors thank M. Gretchen Schwarze for assistance with creating Fig. 3.
Author contributions
L.A.H. researched data for the article. L.A.H., E.M.W.-B., G.S.W. and B.J.D. contributed substantially to discussion of the content. L.A.H. and E.M.W.-B. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Urology thanks J. Bergman, A. Mizushima and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Best case/worst case surgical communication tool: https://youtu.be/FnS3K44sbu0
Center to Advance Palliative Care: https://www.capc.org/
European Society of Medical Oncology Clinical Practice Guidelines in Supportive and Palliative Care: https://www.esmo.org/guidelines/supportive-and-palliative-care
Get the Facts: Palliative Care: https://bcan.org/wp-content/uploads/2019/05/Palliative-Care.pdf
JAMA Patient Page Hospice Care: https://jamanetwork.com/journals/jama/fullarticle/1216479
JAMA Patient Page Palliative Care: https://jamanetwork.com/journals/jamaoncology/fullarticle/2713847
VITALtalk: https://www.vitaltalk.org/resources/
References
- 1.Sanson-Fisher R, et al. The unmet supportive care needs of patients with cancer. Cancer. 2000;88:226–237. doi: 10.1002/(SICI)1097-0142(20000101)88:1<226::AID-CNCR30>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed NE, et al. Muscle invasive bladder cancer: examining survivor burden and unmet needs. J. Urol. 2014;191:48–53. doi: 10.1016/j.juro.2013.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King AJL, et al. Prostate cancer and supportive care: a systematic review and qualitative synthesis of men’s experiences and unmet needs. Eur. J. Cancer Care. 2015;24:618–634. doi: 10.1111/ecc.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenker Y, Park SY, Maciasz R, Arnold RM. Do patients with advanced cancer and unmet palliative care needs have an interest in receiving palliative care services? J. Palliat. Med. 2014;17:667–672. doi: 10.1089/jpm.2013.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman J, et al. Hospice use and high-intensity care in men dying of prostate cancer. Arch. Intern. Med. 2011;171:204–210. doi: 10.1001/archinternmed.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman J, Fink A, Kwan L, Maliski S, Litwin MS. Spirituality and end-of-life care in disadvantaged men dying of prostate cancer. World J. Urol. 2011;29:43–49. doi: 10.1007/s00345-010-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casilla-Lennon MM, et al. Financial toxicity among patients with bladder cancer: reasons for delay in care and effect on quality of life. J. Urol. 2018;199:1166–1173. doi: 10.1016/j.juro.2017.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klaassen Z, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer. 2015;121:1864–1872. doi: 10.1002/cncr.29274. [DOI] [PubMed] [Google Scholar]
- 9.Benner C, Greenberg M, Shepard N, Meng MV, Rabow MW. The natural history of symptoms and distress in patients and families following cystectomy for treatment of muscle invasive bladder cancer. J. Urol. 2014;191:937–942. doi: 10.1016/j.juro.2013.10.101. [DOI] [PubMed] [Google Scholar]
- 10.Center to Advance Palliative Care. About Palliative Care https://www.capc.org/about/palliative-care/ (2020).
- 11.National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care (3rd Edn) (2013).
- 12.Ferrell BR, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2017;35:96–112. doi: 10.1200/JCO.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. Palliative Care (Version 1.2018) NCCN Clinical Practice Guideline Oncology. 2018;vol. 1:106. [Google Scholar]
- 14.European Society of Clinical Oncology. ESMO Clinical Practice Guidelines: Supportive and Palliative Caree. https://www.esmo.org/guidelines/supportive-and-palliative-care (2020).
- 15.Hugar LA, et al. Palliative care use amongst patients with bladder cancer. BJU Int. 2019;123:968–975. doi: 10.1111/bju.14708. [DOI] [PubMed] [Google Scholar]
- 16.Martin S. Die softer: how we can give life a better ending. Globe Main. 2013;8:4–9. [Google Scholar]
- 17.Richmond C. Dame Cicely Saunders — founder of the modern hospice movement. Br. Med. J. 2005;331:238. doi: 10.1136/bmj.331.7510.238. [DOI] [Google Scholar]
- 18.Clark D. From margins to centre: a review of the history of palliative care in cancer. Lancet Oncol. 2007;8:430–438. doi: 10.1016/S1470-2045(07)70138-9. [DOI] [PubMed] [Google Scholar]
- 19.Duffy, A. A moral force: the story of Dr. Balfour Mount. Ottawa Citizen, 1–2 (2005).
- 20.Mount BM, Jones A, Patterson A. Death and dying: attitudes in a teaching hospital. Urology. 1974;4:741–748. doi: 10.1016/0090-4295(74)90264-7. [DOI] [PubMed] [Google Scholar]
- 21.Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can. Med. Assoc. J. 1976;115:119–121. [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman, M. R. Dr. Balfour Mount: The Father of Palliative Care. The Surgical Palliative Care Podcast. 10 January 2020. https://redhoffmanmd.com/podcasts/ep-01-podcast-episode-title/ (2020).
- 23.Knaus WA, Connors AF, Dawson N, Von, Lynn J. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995;274:1591–1598. doi: 10.1001/jama.1995.03530200027032. [DOI] [PubMed] [Google Scholar]
- 24.IOM (Institute of Medicine). Approaching Death (National Academies Press, 1997).
- 25.Murie J. Palliative medicine. Br. Med. J. 2006;333:s136–s137. doi: 10.1136/bmj.333.7571.s136. [DOI] [Google Scholar]
- 26.Quill TE, Abernathy AP. Generalist plus specialist palliative care-creating a more sustainable model. N. Engl. J. Med. 2013;368:1171–1173. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
- 27.Grant MS, Back AL, Dettmar NS. Public perceptions of advance care planning, palliative care, and hospice: a scoping review. J. Palliat. Med. 2020;24:46–52. doi: 10.1089/jpm.2020.0111. [DOI] [PubMed] [Google Scholar]
- 28.Morrison, R. S. & Meier, D. E. America’s Care of Serious Illness (CAPC, 2015).
- 29.Torpy J, Burke A, Golub R. Hospice care. JAMA. 2012;308:200. doi: 10.1001/jama.2012.6210. [DOI] [PubMed] [Google Scholar]
- 30.Wentlandt K, et al. Referral practices of oncologists to specialized palliative care. J. Clin. Oncol. 2012;30:4380–4386. doi: 10.1200/JCO.2012.44.0248. [DOI] [PubMed] [Google Scholar]
- 31.Hui D, et al. Access to palliative care among patients treated at a comprehensive cancer center. Oncologist. 2012;17:1574–1580. doi: 10.1634/theoncologist.2012-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temel JS, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 33.Bakitas M, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer. J. Am. Med. Assoc. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brumley R, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J. Am. Geriatr. Soc. 2007;55:993–1000. doi: 10.1111/j.1532-5415.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 35.Gade G, et al. Impact of an inpatient palliative care team: a randomized controlled trial. J. Palliat. Med. 2008;11:180–190. doi: 10.1089/jpm.2007.0055. [DOI] [PubMed] [Google Scholar]
- 36.Meyers FJ, et al. Effects of a problem-solving intervention (COPE) on quality of life for patients with advanced cancer on clinical trials and their caregivers: Simultaneous Care Educational Intervention (SCEI): linking palliation and clinical trials. J. Palliat. Med. 2011;14:465–473. doi: 10.1089/jpm.2010.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. Hospital-based palliative medicine consultation: a randomized controlled trial. Arch. Intern. Med. 2010;170:2038–2040. doi: 10.1001/archinternmed.2010.460. [DOI] [PubMed] [Google Scholar]
- 38.Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch. Intern. Med. 2004;164:83. doi: 10.1001/archinte.164.1.83. [DOI] [PubMed] [Google Scholar]
- 39.Smith TJ, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J. Clin. Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 40.Hoerger M, et al. Defining the elements of early palliative care that are associated with patient-reported outcomes and the delivery of end-of-life care. J. Clin. Oncol. 2018;36:1096–1102. doi: 10.1200/JCO.2017.75.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hui D, et al. Integration of oncology and palliative care: a systematic review. Oncologist. 2015;20:77–83. doi: 10.1634/theoncologist.2014-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry LL, Castellani R, Stuart B. The branding of palliative care. J. Oncol. Pract. 2016;12:48–50. doi: 10.1200/JOP.2015.008656. [DOI] [PubMed] [Google Scholar]
- 43.Szekendi MK, Vaughn J, Lal A, Ouchi K, Williams MV. The prevalence of inpatients at 33 U.S. hospitals appropriate for and receiving referral to palliative care. J. Palliat. Med. 2016;19:360–372. doi: 10.1089/jpm.2015.0236. [DOI] [PubMed] [Google Scholar]
- 44.Jordan, R. I. et al. Duration of palliative care before death in international routine practice: a systematic review and meta-analysis. BMC Med. 18, (2020). [DOI] [PMC free article] [PubMed]
- 45.Kamal A, Mosca P, Blazer DT, Badgwell B, Howie L. The palliative medicine-surgery interface — “How to effectively work with your surgery colleagues on complex cases” (FR409) J. Pain Symptom Manage. 2014;47:421–422. doi: 10.1016/j.jpainsymman.2013.12.081. [DOI] [Google Scholar]
- 46.Rivet EB, Del Fabbro E, Ferrada P. Palliative care assessment in the surgical and trauma intensive care unit. JAMA Surg. 2018;153:280–281. doi: 10.1001/jamasurg.2017.5077. [DOI] [PubMed] [Google Scholar]
- 47.Buchman TG, Cassell J, Ray SE, Wax ML. Who should manage the dying patient?: rescue, shame, and the surgical ICU dilemma. J. Am. Coll. Surg. 2002;194:665–673. doi: 10.1016/S1072-7515(02)01157-2. [DOI] [PubMed] [Google Scholar]
- 48.Blumenthal B, et al. Barriers to palliative care use among surgical patients: perspectives of practicing surgeons across Michigan. Ann. Palliat. Med. 2021;10:1122–1132. doi: 10.21037/apm-20-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibbs KD, Mahon MM, Truss M, Eyring K. An assessment of hospital-based palliative care in Maryland: infrastructure, barriers, and opportunities. J. Pain Symptom Manage. 2015;49:1102–1108. doi: 10.1016/j.jpainsymman.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Schwarze ML, Bradley CT, Brasel KJ. Surgical ‘buy-in’: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit. Care Med. 2010;38:843–848. doi: 10.1097/CCM.0b013e3181cc466b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karlekar M, Collier B, Parish A, Olson L, Elasy T. Utilization and determinants of palliative care in the trauma intensive care unit: results of a national survey. Palliat. Med. 2014;28:1062–1068. doi: 10.1177/0269216314534514. [DOI] [PubMed] [Google Scholar]
- 52.Olmsted CL, Johnson AM, Kaboli P, Cullen J, Vaughan-Sarrazin MS. Use of palliative care and hospice among surgical and medical specialties in the Veterans Health Administration. JAMA Surg. 2014;149:1169–1175. doi: 10.1001/jamasurg.2014.2101. [DOI] [PubMed] [Google Scholar]
- 53.Suwanabol PA, et al. Characterizing the role of U.S. surgeons in the provision of palliative care: a systematic review and mixed-methods meta-synthesis. J. Pain Symptom Manage. 2018;55:1196–1215.e5. doi: 10.1016/j.jpainsymman.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 54.Schenker Y, et al. Oncologist factors that influence referrals to subspecialty palliative care clinics. J. Oncol. Pract. 2014;10:e37–e44. doi: 10.1200/JOP.2013.001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckley de Meritens A, et al. Practice patterns, attitudes, and barriers to palliative care consultation by gynecologic oncologists. J. Oncol. Pract. 2017;13:e703–e711. doi: 10.1200/JOP.2017.021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Auret K, Bulsara C, Joske D. Australasian haematologist referral patterns to palliative care: lack of consensus on when and why. Intern. Med. J. 2003;33:566–571. doi: 10.1111/j.1445-5994.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 57.Taber JM, Ellis EM, Reblin M, Ellington L, Ferrer RA. Knowledge of and beliefs about palliative care in a nationally-representative U.S. sample. PLoS ONE. 2019;14:1–17. doi: 10.1371/journal.pone.0219074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benini F, et al. Awareness, understanding and attitudes of Italians regarding palliative care. Ann. Ist. Super. Sanita. 2011;47:253–259. doi: 10.4415/ANN_11_03_03. [DOI] [PubMed] [Google Scholar]
- 59.Smets T, et al. The palliative care knowledge of nursing home staff: the EU FP7 PACE cross-sectional survey in 322 nursing homes in six European countries. Palliat. Med. 2018;32:1487–1497. doi: 10.1177/0269216318785295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar P, et al. Utilization of supportive and palliative care services among oncology outpatients at one academic cancer center: determinants of use and barriers to access. J. Palliat. Med. 2012;15:923–930. doi: 10.1089/jpm.2011.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jonnalagadda S, et al. Racial and ethnic differences in beliefs about lung cancer care. Chest. 2012;142:1251–1258. doi: 10.1378/chest.12-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson KS. Racial and ethnic disparities in palliative care. J. Palliat. Med. 2013;16:1329–1334. doi: 10.1089/jpm.2013.9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith AK, Earle CC, Mccarthy EP. Racial and ethnic differences in end-of-life care in fee-for-service medicare beneficiaries with advanced cancer. J. Am. Geriatr. Soc. 2009;57:153–158. doi: 10.1111/j.1532-5415.2008.02081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngo-Metzger Q, et al. Older Asian Americans and Pacific Islanders dying of cancer use hospice less frequently than older white patients. Am. J. Med. 2003;115:47–53. doi: 10.1016/S0002-9343(03)00258-4. [DOI] [PubMed] [Google Scholar]
- 65.Lupu D. Estimate of current hospice and palliative medicine physician workforce shortage. J. Pain Symptom Manage. 2010;40:899–911. doi: 10.1016/j.jpainsymman.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Kamal AH, et al. Future of the palliative care workforce: preview to an impending crisis. Am. J. Med. 2017;130:113–114. doi: 10.1016/j.amjmed.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 67.Meier, D. E. & Morgan, L. Key Findings on the Perceptions of Palliative Care (Center to Advance Palliative Care, 2019).
- 68.Hui D, et al. Availability and integration of palliative care at US cancer centers. J. Am. Med. Assoc. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hui D, Cherny N, Latino N, Strasser F. The ‘critical mass’ survey of palliative care programme at ESMO designated centres of integrated oncology and palliative care. Ann. Oncol. 2017;28:2057–2066. doi: 10.1093/annonc/mdx280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knaul FM, et al. Alleviating the access abyss in palliative care and pain relief — an imperative of universal health coverage: the Lancet Commission report. Lancet. 2018;391:1391–1454. doi: 10.1016/S0140-6736(17)32513-8. [DOI] [PubMed] [Google Scholar]
- 71.Meier, D. & McCormick, E. Benefits, Services, and Modes of Subspecialty Palliative Care (UpToDate, 2019).
- 72.Nelson JE, et al. Models for structuring a clinical initiative to enhance palliative care in the intensive care unit: a report from the IPAL-ICU Project (Improving Palliative Care in the ICU) Crit. Care Med. 2010;38:1765–1772. doi: 10.1097/CCM.0b013e3181e8ad23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mercadante S, Gregoretti C, Cortegiani A. Palliative care in intensive care units: why, where, what, who, when, how. BMC Anesthesiol. 2018;18:106. doi: 10.1186/s12871-018-0574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lilly CM, et al. An intensive communication intervention for the critically ill. Am. J. Med. 2000;109:469–475. doi: 10.1016/S0002-9343(00)00524-6. [DOI] [PubMed] [Google Scholar]
- 75.Cook D, Rocker G. Dying with dignity in the intensive care unit. N. Engl. J. Med. 2014;370:2506–2514. doi: 10.1056/NEJMra1208795. [DOI] [PubMed] [Google Scholar]
- 76.Wiencek C, Coyne P. Palliative care delivery models. Semin. Oncol. Nurs. 2014;30:227–233. doi: 10.1016/j.soncn.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Hui D, et al. State of palliative care services at US cancer centers: an updated national survey. Cancer. 2020;126:2013–2023. doi: 10.1002/cncr.32738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rabow M, et al. Moving upstream: a review of the evidence of the impact of outpatient palliative care. J. Palliat. Med. 2013;16:1540–1549. doi: 10.1089/jpm.2013.0153. [DOI] [PubMed] [Google Scholar]
- 79.Fulton JJ, et al. Integrated outpatient palliative care for patients with advanced cancer: a systematic review and meta-analysis. Palliat. Med. 2019;33:123–134. doi: 10.1177/0269216318812633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hancock, S., Preston, N., Jones, H. & Gadoud, A. Telehealth in palliative care is being described but not evaluated: a systematic review. BMC Palliat. Care18, (2019). [DOI] [PMC free article] [PubMed]
- 81.Worster B, Swartz K. Telemedicine and palliative care: an increasing role in supportive oncology. Curr. Oncol. Rep. 2017;19:1–5. doi: 10.1007/s11912-017-0600-y. [DOI] [PubMed] [Google Scholar]
- 82.Lally K, Kematick BS, Gorman D, Tulsky J. Rapid conversion of a palliative care outpatient clinic to telehealth. JCO Oncol. Pract. 2021;17:e62–e67. doi: 10.1200/OP.20.00557. [DOI] [PubMed] [Google Scholar]
- 83.Jazzar U, et al. Impact of psychiatric illness on decreased survival in elderly patients with bladder cancer in the United States. Cancer. 2018;124:3127–3135. doi: 10.1002/cncr.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rabow MW, Benner C, Shepard N, Meng MV. Concurrent urologic and palliative care after cystectomy for treatment of muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2015;33:267.e23–267.e29. doi: 10.1016/j.urolonc.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 85.Bergman J, et al. Community-partnered collaboration to build an integrated palliative care clinic: the view from urology. Am. J. Hosp. Palliat. Med. 2016;33:164–170. doi: 10.1177/1049909114555156. [DOI] [PubMed] [Google Scholar]
- 86.Huen K, et al. Outcomes of an integrated urology-palliative care clinic for patients with advanced urological cancers: maintenance of quality of life and satisfaction and high rate of hospice utilization through end of life. Am. J. Hosp. Palliat. Med. 2019;36:801–806. doi: 10.1177/1049909119833663. [DOI] [PubMed] [Google Scholar]
- 87.Earle CC, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J. Clin. Oncol. 2003;21:1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 88.Earle CC, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int. J. Qual. Heal. Care. 2005;17:505–509. doi: 10.1093/intqhc/mzi061. [DOI] [PubMed] [Google Scholar]
- 89.Biondo PD, Nekolaichuk CL, Stiles C, Fainsinger R, Hagen NA. Applying the Delphi process to palliative care tool development: lessons learned. Support. Care Cancer. 2008;16:935–942. doi: 10.1007/s00520-007-0348-2. [DOI] [PubMed] [Google Scholar]
- 90.Sanford MT, Greene KL, Carroll PR. The argument for palliative care in prostate cancer. Transl. Androl. Urol. 2013;2:278–280. doi: 10.3978/j.issn.2223-4683.2013.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ok JH, Meyers FJ, Evans CP. Medical and surgical palliative care of patients with urological malignancies. J. Urol. 2005;174:1177–1182. doi: 10.1097/01.ju.0000173077.84274.01. [DOI] [PubMed] [Google Scholar]
- 92.Lowrance WT, et al. Advanced prostate cancer: AUA/ASTRO/SUO Guideline Part I. J. Urol. 2021;205:14–21. doi: 10.1097/JU.0000000000001375. [DOI] [PubMed] [Google Scholar]
- 93.Borda, A., Charnay-Sonnek, F., Fonteyne, V. & Papaioannou, E. Guidelines on Pain Management and Palliative Care. Non Traumatic Acute Flank Pain European Association of Urology. 30–42 (2014).
- 94.Bader P, et al. Prostate cancer pain management: EAU guidelines on pain management. World J. Urol. 2012;30:677–686. doi: 10.1007/s00345-012-0825-1. [DOI] [PubMed] [Google Scholar]
- 95.Griebling, T. Panel discussion: palliative and end-of-life care in urology, in American Urological Association Annual Meeting (American Urological Association, 2017).
- 96.Hausner D, et al. Timing of palliative care referral before and after evidence from trials supporting early palliative care. Oncologist. 2021;26:332–340. doi: 10.1002/onco.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Resick JM, et al. Primary palliative care for patients with advanced hematologic malignancies: a pilot trial of the SHARE intervention. J. Palliat. Med. 2020 doi: 10.1089/jpm.2020.0407. [DOI] [PubMed] [Google Scholar]
- 98.Johnson M, Fallon M. Just good care? The palliative care of those with non-malignant disease. Palliat. Med. 2013;27:803–804. doi: 10.1177/0269216313504058. [DOI] [PubMed] [Google Scholar]
- 99.Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the center to advance palliative care. J. Palliat. Med. 2011;14:17–23. doi: 10.1089/jpm.2010.0347. [DOI] [PubMed] [Google Scholar]
- 100.Osse BHP, Vernooij-Dassen MJFJ, Schade E, Grol RPTM. The problems experienced by patients with cancer and their needs for palliative care. Support. Care Cancer. 2005;13:722–732. doi: 10.1007/s00520-004-0771-6. [DOI] [PubMed] [Google Scholar]
- 101.Lerman C, et al. Communication between patients with breast cancer and health care providers. Determinants and implications. Cancer. 1993;72:2612–2620. doi: 10.1002/1097-0142(19931101)72:9<2612::AID-CNCR2820720916>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 102.Pecanac KE, et al. It’s big surgery: preoperative expressions of risk, responsibility, and commitment to treatment after high-risk operations. Ann. Surg. 2014;259:458–463. doi: 10.1097/SLA.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taylor LJ, et al. A framework to improve surgeon communication in high-stakes surgical decisions: Best Case/Worst Case. JAMA Surg. 2017;152:531–538. doi: 10.1001/jamasurg.2016.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwarze ML, Taylor LJ. Managing uncertainty — harnessing the power of scenario planning. N. Engl. J. Med. 2017;377:206–208. doi: 10.1056/NEJMp1704149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Back, A., Arnold, R. & Tulsky, J. Mastering Communication with Seriously Ill Patients (Cambridge Univ. Press, 2009).
- 106.Dunn, G. P., Martensen, R. & Weissman, D. Surgical Palliative Care: A Resident’s Guide (American College of Surgeons, 2009).
- 107.Brown TJ, Smith TJ, Gupta A. JAMA patient page. Palliative care. JAMA Oncol. 2019;5:126. doi: 10.1001/jamaoncol.2018.4962. [DOI] [PubMed] [Google Scholar]
- 108.Razmaria AA. JAMA patient page. End-of-life care. JAMA. 2016;316:115. doi: 10.1001/jama.2016.2440. [DOI] [PubMed] [Google Scholar]
- 109.Torpy JM, Burke A, Golub RM. JAMA patient page. Hospice care. JAMA. 2012;308:200. doi: 10.1001/jama.2012.6210. [DOI] [PubMed] [Google Scholar]
- 110.Zimmermann C, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 111.Bakitas MA, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J. Clin. Oncol. 2015;33:1438–1445. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maltoni M, et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur. J. Cancer. 2016;65:61–68. doi: 10.1016/j.ejca.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 113.Vanbutsele G, et al. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol. 2018;19:394–404. doi: 10.1016/S1470-2045(18)30060-3. [DOI] [PubMed] [Google Scholar]
- 114.Temel JS, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J. Clin. Oncol. 2017;35:834–841. doi: 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hawley PH. The bow tie model of 21st century palliative care. J. Pain Symptom Manage. 2014;47:2–5. doi: 10.1016/j.jpainsymman.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Kruser JM, et al. ‘Best case/worst case’: qualitative evaluation of a novel communication tool for difficult in-the-moment surgical decisions. J. Am. Geriatr. Soc. 2015;63:1805–1811. doi: 10.1111/jgs.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care (4th edn). The Kansas Nurse 79 (National Coalition for Hospice and Palliative Care, 2018).
- 118.Suwanabol PA, et al. Surgeons’ perceived barriers to palliative and end-of-life care: a mixed methods study of a surgical society. J. Palliat. Med. 2018;21:780–788. doi: 10.1089/jpm.2017.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nabozny MJ, et al. Constructing high-stakes surgical decisions: it’s better to die trying. Ann. Surg. 2016;263:64–70. doi: 10.1097/SLA.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.American Urological Association. Oncology Guidelines. https://www.auanet.org/guidelines/oncology-guidelines (2021).
- 121.Lowrance WT, et al. Advanced prostate cancer: AUA/ASTRO/SUO Guideline part II. J. Urol. 2021;205:22–29. doi: 10.1097/JU.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 122.European Association of Urology. Oncology Guidelines. https://uroweb.org/individual-guidelines/oncology-guidelines/ (2021).
- 123.Canadian Urological Association. Guidelines. https://www.cua.org/guidelines?specialty=All&topic=All&type=1&tools=All&keyword=cancer&items_per_page=10&page=1 (2021).
- 124.Urological Society of Australia and New Zealand. Position Statements and Guidelines. https://www.usanz.org.au/info-resources/position-statements-guidelines (2021).