Abstract
INTRODUCTION:
Gallbladder cancer exhibits striking variability in the global rates, reaching epidemic levels for some regions and ethnicities. The basis of its variability resides in differences in environmental exposure and intrinsic genetic predisposition to carcinogenesis. There is little information present regarding genetic and molecular alterations in gall bladder cancer (GBC). We, therefore, have evaluated the molecular marker expression in GBC and studied their correlation with clinicopathological staging.
MATERIALS AND METHODS:
This prospective observational study was conducted on newly diagnosed GBC patients from July 2017 to July 2020. After complete staging workup, the GBC biopsy samples paraffin block was tested for molecular markers estrogen receptor (ER), progesterone receptor (PR), p53, p16, Human epidermal growth factor receptor 2 (HER 2-neu), Survivin, Enhancer of zeste homolog-2 (EZH2), and Cyclooxygenase-2 (COX-2) expression by immunohistochemistry.
RESULTS:
Fifty newly diagnosed patients of carcinoma gall bladder were included in the present study. Age was ranged from 29 – 69 years (mean 53.42). p53 was the most common positive marker in 74% of patients, survivin in 58%, COX-2 in 44%, and p16 in 42% whereas Her 2 neu and EZH-2 were positive in 16% of patients each. None of the patients of GBC were ER or PR positive. There was a significant difference between the various groups in terms of the distribution of histological grade and Her 2 neu (χ2 = 9.886, P = 0.014) but not with other markers. Furthermore, there was a significant difference in terms of distribution of p16 and p53 with stage (χ2 = 7.017, P = 0.037 and χ2 = 5.861, P = 0.033) respectively.
CONCLUSIONS:
The present study shows the expression of molecular markers Her2 neu, p53, p16, survivin, COX-2, and EZH-2 in GBC. Now the time has come, and it is also the need of the day to establish early biomarkers of this highly lethal malignancy. It can be used in future for the detection of disease in the early phase and targeted therapy.
Keywords: Gall bladder cancer, molecular markers, expression, immunohistochemistry
Introduction
The most common malignancy of the biliary system is gall bladder cancer (GBC), and it ranks sixth among gastrointestinal cancers. It is rare though notoriously lethal malignancy with marked variation in the ethnic groups and geographical distributions.[1] In the biliary system, gall bladder cancer is the most aggressive cancer with the shortest median survival from the time of diagnosis.[2]
GBC exhibits striking variability in the global rates, reaching epidemic levels for some regions and ethnicities. The basis of its variability resides in differences in environmental exposure and intrinsic genetic predisposition to carcinogenesis.[3]
Among women of North and North-east India, GBC is one of the three leading cancers. GBC varies widely in India; North India, especially the Gangetic belt and Eastern regions of the country, had high incidence rates than other parts of the country as per population-based cancer registries.[4]
This cancer tends to increase with advancing age, with a median age of 67 years.[5] Gallstones, female gender, ethnicity, genetic susceptibility, and various lifestyle factors are associated with risk factors in GBC development. These risk factors act either as initiators, such as unknown mutagens or as promoters, including chronic inflammation and infections.
Clinical presentation of GBC is often non-specific, resulting in a delay in identification and diagnosis[6]. It is either detected incidentally at the time of cholecystectomy or due to its aggressive biological nature when it presents with complications with local spread of the malignancy in the form of jaundice, hepatomegaly, ascites, or duodenal obstruction.[6] Various ethnic, genetic, and environmental factors are responsible for this orphan cancers' poorly defined pathogenesis. The understanding of the relationship between epidemiology, molecular genetics, and pathogenesis of gallbladder cancer is needed to add new insights into its mysterious pathophysiology.[7] Historically GBC had an overall 5-year survival of <5%. At the beginning of the 21stcentury, overall survival increased from 3.6 months to 10 months.[2]
There is little information present regarding genetic and molecular alterations in GBC. Like other cancers, GBC is a multifactorial disorder involving multiple genetic alterations.[8]
Various genetic alterations in gall bladder carcinogenesis include p53, p16, Ki-67, c-erb b2/human epidermal growth factor receptor (HER), epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF)-A, cyclooxygenase-2 (COX-2), K-ras, LOH, cyclin d1/CDK4, E cadherin, loss of PTEN, and EpCAM. p53 mutations and accumulation occur in 27%-70% of GBC.[7] Deregulation caused by loss of TP53 function allows survival of genetically impaired abnormal cells, leading to a neoplastic conversion.[9]
Long-standing inflammation like cholelithiasis, the pancreaticobiliary duct' s anomalous arrangement, suggests that it may modulate tumorigenesis or carcinoma progression in long-term inflammation. Dysplastic lesions near are found frequently in the epithelia adjacent to gallbladder carcinoma associated with gallstones support this notation.[10]
The arachidonate and PGE23 levels increased in human carcinoma tissues, so now the interest is on the expression of the arachidonate cascade's inflammatory enzymes, like Cox's in carcinoma of the gallbladder. COX-2 expression varies from 59.2%-71.9% in gall bladder malignancies. Neovascularization induced by COX-2 is associated with a poor prognosis.[11]
The epigenetic modification for the inactivation of tumor suppressor genes is now being studied in various cancers and also has an essential role in gallbladder carcinogenesis.[12]
Several studies have reported that as gall bladder malignancy advances, VEGF expression increases since adequate vascularization is requisite for tumor growth. There is a highly variable expression of EGFR in gallbladder cancer. The expression ranged from 11.3% to 100%. The more expression of (EGFR) is an independent predictor of survival.[13] Chaube et al. found that the expression levels for HER2 varied depending on the tumor grade, with decreasing expression correlating with advancing a grade. The overexpression of enhancer of zeste homolog-2 (EZH2) and loss of PTEN expression might be closely related to the carcinogenesis, progression, clinical, biological behavior, and prognosis of GBC.[14] Most of the malignancy detected late, so they need adjuvant therapy. The limited chemotherapeutic therapy for gallbladder cancers opens the field for the urgent need for a new novel and more effective medical treatment options. Nowadays, various molecular markers for cancers are useful for cancer detection and prognosis and serve as crucial therapeutic targets.
We, therefore, have evaluated the molecular marker expression in GBC and studied their correlation with clinicopathological staging.
Materials and Methods
This prospective observational study was conducted from July 2017 to July 2020. The patients presenting to the out patient department (OPD) of the Department of Surgery and the hepato- pancreato-biliary clinic were enrolled as per the inclusion and exclusion criteria defined in the protocol. Institutional ethical committee clearance was taken and informed consent was taken from all the patients.
Inclusion criteria
Aged ≥18 years
Recently diagnosed and biopsy-proven patients of Gall bladder carcinoma
Exclusion criteria
Presence of synchronous/metachronous second malignancy.
A complete history and physical examination of patients of diagnosed GBC coming to the Department of Surgery and Oncology OPD was documented. Contrast-enhanced computer tomography of chest and abdomen was done. Serum tumor markers CA-19.9, CA 125, and carcinoembryonic antigen (CEA) were done for all the patients. The staging was done based on the 8th AJCC edition. Surgically resectable patients underwent radical cholecystectomy after a complete workup and staging. Surgically unresectable and metastatic disease patients were considered for ultrasound-guided biopsy from gall bladder mass lesions.
Immunohistochemistry
Immunohistochemistry was performed using commercially available antibodies for the markers.
Immunostaining methods
Serial 4μ thick sections cut from the selected representative paraffin-embedded tissue blocks and overlaid on poly-l-lysine coated slides which were used for IHC. These were deparaffinized (two changes of xylene for 5 min each and 1 change of acetone for 1 min) followed by rehydration in decreasing concentration of alcohol (95% ethanol for 3 min, 70% ethanol for 3 min, distilled water for 1 min).
Antigen retrieval
Antigen retrieval was done by heating the sections immersed in citrate buffer inside a 600-watt microwave oven at full power for 30 min. Sections were then overlaid with an adequate amount of appropriately diluted primary antibody followed by overnight incubation at 4°C in a humid chamber.
The slides were then washed in three changes (5 min each) of 0.05M Tris-HCL buffer, pH 7.4 followed by incubation for 30 min at room temperature after application of biotinylated secondary antibody of an anti-mouse immunoglobulin in phosphate-buffered saline.
After three washings (5 min each) in Tris- HCl buffer, peroxidase-conjugated streptavidin was applied to cover the sections and incubated at room temperature for 30 min.
Slides were rinsed with three changes of Tris-HCL buffer for 5 min. each. Sections were then covered with substrate chromogen solution freshly prepared by dissolving 50μl of Di-amino Benzidine (DAB) chromogen to 1 ml of DAB substrate buffer. The slides were incubated at room temperature for few minutes under microscopic control till the optimum development of the brown-colored peroxidase reactant product. During the staining of each batch, appropriate positive and negative controls were used. The patient's biopsy sample's paraffin block was tested for molecular markers estrogen receptor (ER), progesterone receptor (PR), p53, p16, c-erb b2 (HER 2/neu), Survivin, EZH 2, and COX-2 expression by IHC.
For p53, p16, survivin, EZH 2, and COX-2 >10% cell positive was considered as positive. For Her2/neu complete membranous positivity of at least 10% cells was considered as 3+, incomplete membranous positivity 2+, faint to cytoplasmic positivity 1+ or negative. ER/PR staining interpretation was done as in breast cancer as there is no other specific interpretation method for GBC. HER 2/neu testing was to be confirmed by fluorescence in situ hybridization testing in case of equivocal results by IHC (2+). The p53 was considered positive when >10% nuclei and membrane-bound/cytoplasm is stained. The positive markers analyzed were correlated with the clinicopathological staging. Statistical analysis was performed using IBM SPSS version 25.0 statistical software (SPSS Inc., Chicago, IL, United States).
Parametric data were calculated by mean and standard deviation and non-parametric data by median and interquartile range. Fischer's test was done for categorical variables, non -parametric tests (Wilcoxon-Mann-Whitney U-Test) were used to make group comparisons, and P < 0.05 was considered significant.
Results
Fifty newly diagnosed patients of carcinoma gall bladder were included in the present study. Age ranged from 29 – 69 years (mean 53.42). There were 31 females and 19 males, a ratio of 1.6:1. The clinicodemographic profile is mentioned in Table 1. Blood group A+ was the most common in 62% of patients. The body mass index (BMI) ranged from 17.3 to 34.93, mean (22.37 ± 3.97). The total bilirubin (mg/dL) ranged from 0.2-31.4 (4.65 ± 8.13). Red cell distribution width ranged from 11.23-19.3 (14.88 ± 1.98). Nearly 6% of the participants had stage I, 4% of the participants stage IIA, and 2% stage IIB. 24.4% stage IIIA, 9.6% stage IIIB, 24.4% stage IVA and 29.6% stage IVB.
Table 1.
Clinicodemographic profile of gallbladder cancer patients
| Variables | Options | Frequency (%) |
|---|---|---|
| Age (years) | 29-69 (53.42) | |
| Gender | Male | 19 |
| Female | 31 | |
| BMI | 15.8-34.93 (22.13±3.67) | |
| Obstructive jaundice | No | 36 (72) |
| Yes | 14 (28) | |
| Pain abdomen | No | 8 (16) |
| Yes | 42 (84) | |
| Lump | No | 35 (70) |
| Yes | 15 (30) | |
| Radiological presentation | Normal | 3 (6) |
| Mass | 29 (58) | |
| Wall thickness | 17 (34) | |
| Contracted | 1 (2) | |
| Gallbladder stone | No | 18 (36) |
| Yes | 32 (64) | |
| Blood group | A+ | 31 (62) |
| B+ | 7 (14) | |
| AB+ | 4 (8) | |
| O+ | 4 (8) | |
| O− | 4 (8) | |
| Stage | I | 3 (6) |
| II | 3 (6) | |
| III | 17 (34) | |
| IV | 27 (54) | |
| Histopathology | Adenocarcinoma | 48 (96) |
| Malignant round cell tumor | 2 (4) | |
| Grade | Well differentiated | 11 (22) |
| Moderately differentiated | 7 (14) | |
| Poorly/undifferentiated differentiated | 32 (64) |
BMI: Body mass index
p53 was the most common positive marker in 74% of patients, survivin in 58%, COX-2 in 44%, p16 in 42% whereas Her 2 neu and EZH-2 were positive in 16% of patients each. None of the patients of GBC were ER or PR positive. Markers and their frequency in GBC patients are detailed in Table 2. Figure 1 shows the IHC staining of markers.
Table 2.
Markers and their frequency in gallbladder cancer patients
| Marker | Frequency (%) |
|---|---|
| Her2/neu | |
| Negative | 42 (84.0) |
| 3+ | 8 (16.0) |
| p53 | |
| Positive | 37 (74.0) |
| Negative | 13 (26.0) |
| P16 | |
| Positive | 21 (42.0) |
| Negative | 29 (58.0) |
| Survivin | |
| Positive | 29 (58.0) |
| Negative | 21 (42.0) |
| COX-2 | |
| Positive | 22 (44.0) |
| Negative | 28 (56.0) |
| EZH-2 | |
| Positive | 8 (16.0) |
| Negative | 42 (84.0) |
HER2: Human epidermal growth factor receptor 2, COX-2: Cyclooxygenase-2, EZH-2: Enhancer of zeste homolog-2
Figure 1.
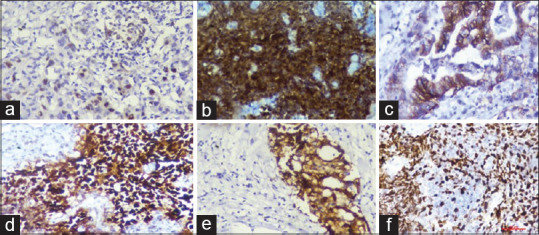
(a) Immunohistochemistry survivin nuclear staining, (b) p16 nuclear and cytoplasmic positivity, (c) human epidermal growth factor receptor 2 neu positivity, (d) p53 staining (e) cyclooxygenase-2 cytoplasmic positivity, (f) Enhancer of zeste homolog-2 nuclear positivity
Correlation of tumor markers with markers
CA-125 ranged from 1.17 to 3512 (median 21.05), CEA ranged from 0.24 to 1500 (5.05) and CA-19.9 (IU/L) ranged from 2 – 12000 (40.16). There was a significant difference between the 2 groups in terms of CEA (IU/L) (W = 202.000, P = 0.021), with the median CEA (IU/L) being highest in the p16 positive group mentioned in Figure 2, but there was no significant difference with any other marker. There was no significant correlation between CA 125 and CA 19-9 with any of the markers.
Figure 2.
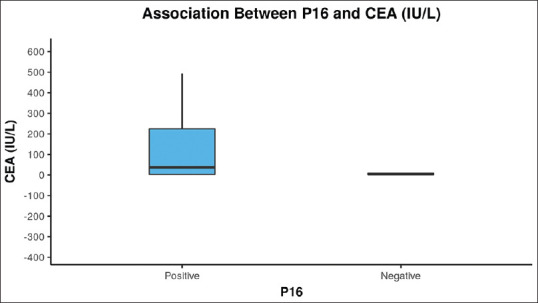
The Box-and-Whisker plot below depicts the distribution of carcinoembryonic antigen (IU/L) with p16. The middle horizontal line represents the median carcinoembryonic antigen (IU/L), the upper and lower bounds of the box represent the 75th and the 25th centile of carcinoembryonic antigen (IU/L), respectively
Association of markers and histological grade
Fisher's exact test was used to explore the association between markers and histological grade. There was a significant difference between the various groups in terms of distribution of histological grade and Her 2 neu(χ2 = 9.886, P = 0.014). However, there was no significant difference between histological grade with COX-2, survivin, EZH-2, p16 and p53 and values were χ2 = 1.806, P = 0.405; χ2 = 0.222, P = 0.895; χ2 = 1.600, P = 0.449; χ2 = 2.963, P = 0.227 and χ2 = 2.756, P = 0.252 respectively.
Association of markers and stage
Fisher's exact test was used to explore the association between markers and stages of GBC. There was no significant difference between the various groups in terms of distribution of stage and Her 2 neu, COX-2, survivin, EZH-2 and p16 values were χ2 = 1.524, P = 0.242; χ2 = 3.436, P = 0.842; χ2 = 7.608, P = 0.368; χ2 = 4.719, P = 0.694 and χ2 = 0.378, P = 0.538 respectively. There was a significant difference in terms of distribution of p16 and p53 with stage (χ2 = 7.017, P = 0.037 and χ2 = 5.861, P = 0.033) respectively.
Discussion
GBC is the most common biliary tract malignancy. It is the sixth most common malignancy in the digestive system. It has an unremarkably higher frequency in certain ethnic groups and geographic regions.[1] Notwithstanding the tremendous enhancement in diagnosis and surgical skills, gallbladder cancer prognosis is still not overwhelming. The 5-year survival for all stages of this malignancy is approximately 5%. The patients' mean age in this study was 53.42 ± 9.75, which is elder than the Western and European countries. The male to female ratio was 1:1.6. Females are at 2 to 6 times higher risk of developing cancer.[15] Among women, higher gravidity and high parity lead to a longer duration of exposure to reproductive hormones, thereby supporting the increased risk of GBC.[3]
51.5% of the participants had a BMI: 18.5-22.9 kg/m2. A higher BMI has been implicated as a risk factor in GBC. This finding contradicts the present study as more patients with a late-stage presentation presented with low BMI.[15] A recent case-control study on a large population showed an inverse relationship between GBC with BMI.[16]
Her2neu gene is located on chromosome 17q12-q21 and overexpression of Her2neu is observed in malignancies such as breast and ovarian cancers (20%)[17] and gastric cancer (12%)[18]. These are the result of either the amplification of the product of gene or transcriptional dysregulation.
An expected 10%–30% of gallbladder tumors show Her2neu protein overexpression, contingent on the standards used to interpret Her2neu. Notwithstanding, information concerning the adequacy of Her2 treatment for malignant gallbladder growth is scant.[19]
The previously done study by Kumari et al.[20] and Doval et al.[21] found marked variation in result. It could be assumed due to the use of different scoring systems adopted by different authors. Moreover, some authors have considered 2+ as well as 3+ score (cytoplasmic and membranous) as positive. In contrast, others considered only 3+ (intense membranous staining) to be Her2-neu positive (as in the present study). The present study highlights the need to evolve a uniform consensus on scoring for Her2-neu.
Her2neu overexpression has been seen to be more incessant in better-differentiated carcinomas in recent studies. Roa et al. showed a lower rate of Her2 overexpression in early carcinomas (mucosal and muscle involvement), contrasted and the severe stage carcinomas (subserosal and serosal) (7.1% versus 13.8%); in any case, this distinction was not measurably significant.[22] Unlike our findings, Chaube et al., observe a decrease in Her2/neu expression with increasing grade of the tumor.[14]
In the group of tumor suppressor genes (TSG), p53 is located on chromosome 17p13. The present study has p53 overexpression in 37 out of 50 (76%) gallbladder cancer cases. Recent studies by Chaube et al.,[14]. have shown a 20% expression of p53 and a similar percentage of expression shown by Suzuki et al., Hidalgo Grau et al. [23,24] The present study has shown a larger proportion of expression in GBC as compared to others. The higher p53 overexpression with the increasing GBC grade proposes its part in tumor progression instead of initiation. The present study has shown that overexpression of p53 protein was correlated with an advanced stage of the disease, thereby proposing p53 as an applicant marker of poor prognosis in gallbladder carcinoma.
Another member of the tumor suppressor gene group, the p16, is located on chromosome 9p21. The p16 gene forms a protein product that hinders Cyclin D binding with CDK4/6 in G1 that regulates the G1-S phase of the cell cycle.[25] Our study has shown that 21 (42%) cases out of 50 have p16 positive expression.When the association between p16 and tumor marker was studied, it was found that there was a significantly higher level of CEA (IU/L) in the p16 positive group (P = 0.001). The mean of CEA was 154.4 in the p16 positive group as compared to the negative group 11.27. However, there is a need for further study to prove this relationship. Kim et al., showed that there was no correlation between p16 expression and age, gender, tumor size, histological type, tumor location, vascular invasion, and lymph node metastasis in the present study.[25] Tadokoro et al., revealed no critical relationship between p16 protein immunoreactivity and clinicopathologic parameters.[26] Nevertheless, in the present study, out of 21 positive cases, 71.4% of the participants had stage IV, which is significant (P = 0.037). Nearly 89.7% of the participants in the group of p16- negative had stage III/IV, but this was not significant (P = 0.686). Hence, there is a mixed result. Some authors said that loss of p16 expression is associated with poor prognosis or clinicopathological results, and some said that there is no such relation.
COX-2 has played an essential role in the positive regulation of growth and genesis of tumors. They are cytoplasmic enzymes that convert arachidonic acid with phospholipase A-2 into the lipid signal transduction molecules such as prostaglandins and thromboxanes. PGE2 is a primary product of COX-2 catalyzed reaction. The use of NSAIDs in humans with adenomatous polyposis had lowered the incidence of colorectal cancers. This emphasizes the importance of COX enzymes in the process of carcinogenesis. In the present study, 44% of COX-2 overexpression was observed, results were consistent with the study by Legan et al., who identified COX-2 overexpression in 59.2% of GBC samples.[27]
The human survivin protein is encoded by the BRIC5 (baculoviral inhibitor of apoptosis repeat-containing 5) gene. Survivin protein plays a crucial role in cell death, apoptosis, and cell multiplication and proliferation. It is rarely expressed in normal cells. The expression decreases with advancing age, and it is high during fetal development and less in a healthy adult. However, the increased expression of survivin is found in malignant tumors. Subsequently, survivin has been viewed as a potential tumor marker and an essential remedial target in cancers. In the present study, survivin was expressed in 58.0% of GBC patients. Consistent with our outcomes, Gupta et al., had also found no statistically significant association with stage, grade, histopathological type, lymph node involvement, and liver metastasis with survivin expression.[28] Further investigations with a bigger sample size are required to build up its function in gallbladder cancer.
The Human EZH2 gene is located on chromosome 7q35. It is usually present in the nucleus, but it is also found in the cytoplasm. EZH2 is mainly expressed in actively dividing cells. It regulates genomic imprinting, maintains stem cell pluripotency, and determines cell destination in gene repression.
Late discoveries recommend that EZH2 enhances the development and advancement of cancers. As different types of malignant growth such as prostate, breast, bladder, stomach, liver, and pancreatic cancers have EZH2 expression, and thus EZH2 serves as a good indicator of different pathological features and results.
The present study has shown a 16% expression of EZH2 in GBC. Yamaguchi et al., found increased expression of EZH2 in GBC cell lines and identified EZH2-specific methyltransferase inhibitor as a therapeutic target.[29]
In the present study, ER/PR expression was negative in all the GBC samples and the results were similar to Shukla et al.[30] The reason being the most common etiology being an association of gall stones which leads more p53 mutations and metaplasia so they tend to have poorer differentiation of tumor which lack ER/PR expression.
Conclusions
Molecular markers and targeted therapy are the new eras of treatment of malignancy. Despite the advancement in molecular science and knowledge of the pathogenesis of different malignancies, GBC is still a gray area zone. The present study shows the expression of molecular markers such as Her2 neu, p53, p16, survivin, COX-2, and EZH-2 in GBC. These molecular markers have a considerable impact on gall bladder malignancy. Now the time has come, and it is also the need of the day to establish early biomarkers of this highly lethal malignancy. It can be used in future for the detection of disease in the early phase and targeted therapy for managing GBC.
Financial support and sponsorship
Uttarakhand Council of Science and Technology (UCOST), Dehradun India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to the Uttarakhand Council of Science and Technology (UCOST), Dehradun India for providing funding to conduct this study in the form of an extramural research grant.
References
- 1.Dutta U, Bush N, Kalsi D, Popli P, Kapoor VK. Epidemiology of gallbladder cancer in India. Chin Clin Oncol. 2019;8:33. doi: 10.21037/cco.2019.08.03. [DOI] [PubMed] [Google Scholar]
- 2.Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder Cancer in the 21st Century. J Oncol. 2015;2015:967472. doi: 10.1155/2015/967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hundal R. Gallbladder cancer: Epidemiology and outcome. Clinical Epidemiology. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phadke PR, Mhatre S S, Budukh A. M, Dikshit RP.Trends in gallbladder cancer incidence in the high- and low-risk regions of India. Indian Journal of Medical and Paediatric Oncology. 2019;40(1):90. [Google Scholar]
- 5.Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) J Surg Onco. 2008;98:485–9. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 6.Batra Y, Pal S, Dutta U, Desai P, Garg PK, Makharia G, et al. Gallbladder cancer epidemiology, pathogenesis, and molecular genetics: Recent update. World J Gastroenterol. 2017;23:397–998. doi: 10.3748/wjg.v23.i22.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis, and molecular genetics: Recent update. World J Gastroenterol. 2017;23:397–998. doi: 10.3748/wjg.v23.i22.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashid A. Cellular and molecular biology of biliary tract cancers. Surg Oncol Clin N Am. 2002;11:995–1009. doi: 10.1016/s1055-3207(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 9.Quan ZW, Wu K, Wang J, Shi W, Zhang Z, Merrell RC. Association of p53, p16, and vascular endothelial growth factor protein expressions with the prognosis and metastasis of gallbladder cancer. J Am Coll Surg. 2001;193:380–3. doi: 10.1016/s1072-7515(01)01012-2. [DOI] [PubMed] [Google Scholar]
- 10.Albores Saavedra J. Tumors of the gallbladder, extrahepatic bile ducts, and ampulla of Vater. Atlas of tumor pathology. 2000;27:259–316. [Google Scholar]
- 11.Asano T, Shoda J, Ueda T, Kawamoto T, Todoroki T, Shimonishi M, et al. Expressions of cyclooxygenase-2 and prostaglandin E-receptors in carcinoma of the gallbladder: Crucial role of arachidonate metabolism in tumor growth and progression. Clin Cancer Res. 2002;8:1157–67. [PubMed] [Google Scholar]
- 12.Letelier P, Brebi P, Tapia O, Roa JC. DNA promoter methylation as a diagnostic and therapeutic biomarker in gallbladder cancer. Clin Epigenetics. 2012;13(4):11. doi: 10.1186/1868-7083-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhi YH, Liu RS, Song MM, Tian Y, Long J, Tu W, et al. Cyclooxygenase-2 promotes angiogenesis by increasing vascular endothelial growth factor and predicts prognosis in gallbladder carcinoma. World J Gastroenterol. 2005;11:3724–8. doi: 10.3748/wjg.v11.i24.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaube A, Tewari M, Garbyal RS, Singh U, Shukla HS. Preliminary study of p53 and c-erbB-2 expression in gallbladder cancer in Indian patients. BMC Cancer. 2006;6:126. doi: 10.1186/1471-2407-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–65. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyagi B, Raina V. Risk Factors for Gallbladder Cancer: A Population Based Case-Control Study in Delhi. Indian journal of medical and paediatric oncology. 2008;29:16–26. [Google Scholar]
- 17.Mitri Z, Constantine T, O'Regan R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother Res Pract 2012. 2012:743193. doi: 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong H, Yau T. Targeted therapy in the management of advanced gastric cancer: Are we making progress in the era of personalized medicine. Oncologist? 2012;17:346–58. doi: 10.1634/theoncologist.2011-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halder S, Kundu S, Chakraborty J, Chakrabarti S. Significance of HER2 and Ki-67 in Preneoplastic Lesions and Carcinoma of Gallbladder. J Gastrointest Canc. 2019;50:848–54. doi: 10.1007/s12029-018-0162-8. [DOI] [PubMed] [Google Scholar]
- 20.Kumari N, Kapoor VK, Krishnani N, Kumar K, Baitha DK. Role of C-erbB2 expression in gallbladder cancer. Indian journal of pathology & microbiology. 2012;55:75–9. doi: 10.4103/0377-4929.94862. [DOI] [PubMed] [Google Scholar]
- 21.Doval DC, Azam S, Sinha R, Batra U, Mehta A. Expression of epidermal growth factor receptor, p53, Bcl2, vascular endothelial growth factor, cyclooxygenase-2, cyclin D1, human epidermal receptor-2 and Ki-67: Association with clinicopathological profiles and outcomes in gallbladder carcinoma. J Carcinog. 2014;13:10. doi: 10.4103/1477-3163.139450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu gene: A new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest Cancer Res. 2014;7:42–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Hidalgo Grau LA, Badia JM, Salvador CA, Monsó TS, Canaleta JF, Nogués JM, et al. Gallbladder carcinoma: The role of p53 protein overexpression and Ki-67 antigen expression as prognostic markers. HPB (Oxford) 2004;6:174–80. doi: 10.1080/13651820410025110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki T, Takano Y, Kakita A, Okudaira M. An Immunohistochemical and Molecular Biological Study of c-erbB-2 Amplification and Prognostic Relevance in Gallbladder Cancer. Pathology-Research and Practice. 1993;189:283–92. doi: 10.1016/S0344-0338(11)80511-X. [DOI] [PubMed] [Google Scholar]
- 25.Kim K, Kim DH, Chae SW, Shin JH, Kim HJ, Do SI, et al. Expression of cell cycle-related proteins, p16, p53 and p63 as important prognostic markers in gallbladder adenocarcinoma. Pathology oncology research: POR. 2014;20:409–15. doi: 10.1007/s12253-013-9710-5. [DOI] [PubMed] [Google Scholar]
- 26.Tadokoro H, Shigihara T, Ikeda T, Takase M, Suyama M. Two distinct pathways of p16 gene inactivation in gallbladder cancer. World J Gastroenterol. 2007;13:6396–403. doi: 10.3748/wjg.v13.i47.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legan M, Luzar B, Marolt VF, Cor A. Expression of cyclooxygenase-2 is associated with p53 accumulation in premalignant and malignant gallbladder lesions. World J Gastroenterol. 2006;12:3425–9. doi: 10.3748/wjg.v12.i21.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta V, Goel MM, Chandra A, Gupta P, Kumar S, Nigam J. Expression and clinicopathological significance of antiapoptotis protein survivin in gallbladder cancer. Indian J Pathol Microbiol. 2016;59:143–7. doi: 10.4103/0377-4929.182035. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi J, Sasaki M, Sato Y, Itatsu K, Harada K, Zen Y, et al. Histone deacetylase inhibitor (SAHA) and repression of EZH2 synergistically inhibit proliferation of gallbladder carcinoma. Japanese Cancer Asso. 2009;10:355–62. doi: 10.1111/j.1349-7006.2009.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla PJ, Barreto SG, Gupta P, Neve R, Ramadwar M, Deodhar K, et al. Is there a role for estrogen and progesterone receptors in gall bladder cancer? HPB (Oxford) 2007;9(4):285–8. doi: 10.1080/13651820701481489. [DOI] [PMC free article] [PubMed] [Google Scholar]


