Abstract
Background:
Depression and antidepressant medications are associated with increased risk for type 2 diabetes. It is not known if diabetes can be prevented in the setting of depression. Cambodian Americans have high rates of both depression and diabetes. This paper reports intervention development, experimental design, baseline characteristics, and process outcomes of diabetes prevention interventions for Cambodian Americans with depression, “Diabetes Risk Reduction through Eat, Walk, Sleep and Medication Therapy Management” (DREAM).
Methods:
Participants were aged 35–75, Khmer speaking, at high risk for developing diabetes, and met criteria for likely depression by either a) antidepressant medication and/or b) elevated depressive symptoms at two time-points during a study eligibility period. Treatment arms were: 1) community health educator (CHE) delivered lifestyle intervention called Eat, Walk, Sleep (EWS), 2) EWS plus pharmacist/CHE-delivered medication therapy management (EWS + MTM), and, 3) social services (SS; control).
Results:
188 participants were randomized. Treatment fidelity was high (98% checklist adherence) and on a scale from 0 to 3, participants reported high EWS treatment satisfaction (M = 2.9, SD = 0.2), group cohesion (M = 2.9, SD = 0.3), and therapeutic alliance to CHEs (M = 2.9, SD = 0.2) and to pharmacists (2.9, SD = 0.3). Attendance was challenging but highly successful; in EWS, 99% attended ≥ one session and 86% completed ≥ 24 sessions, M = 27.3 (SD = 3.7) sessions. Of those randomized to EWS + MTM, 98% attended at least one MTM session and 77%) completed ≥ 4 sessions. Retention was high, 95% at 12-month and 96% at 15-month assessments.
Conclusions:
The interventions were successfully implemented. Lessons learned and suggestions for future trials are offered.
ClinicalTrials.gov identifier: NCT02502929
Keywords: Diabetes prevention, Depression, Cambodian, Implementation, Community health worker, Community based participatory research
1. Introduction
Cambodian refugees, who were resettled in the USA approximately 40 years ago fleeing the genocidal Pol Pot regime, have age- and sex-adjusted rates of type 2 diabetes more than double the US national average [1,2]. Depression is associated with increased risk for type 2 diabetes, with relative risks (RR) ranging from 1.26 to 1.60 [3,4]. Rates of past-year major depressive disorder are high in the general population 10% [5], but among Cambodian Americans, the rate is 51% [6]. For many Cambodian Americans the depression becomes chronic, with pre-migration trauma increasing odds of major depressive disorder even decades after resettlement (odds ratio = 1.56) [6].
Numerous lifestyle risk factors for diabetes are worsened by the presence of depression. Meta-analysis shows that high intake of total dietary carbohydrate is associated with an increased risk of diabetes (RR = 1.11) [7]. Compared to individuals without depression, those with depression have less healthy dietary patterns [8,9]. Risk for diabetes is also increased by sedentary lifestyle. In meta-analysis, individuals with the highest sedentary time had higher risk of diabetes (RR = 1.12) compared to those with the lowest sedentary time [10]. Depressive symptom burden is associated with a graded reduction in the effectiveness of diabetes prevention programs at increasing physical activity [11]. Numerous studies have linked sleep problems to metabolic dysregulation. A review of prospective studies [12] found that sleeping <6 h per night conferred a relative risk of 1.28 in the incidence of diabetes. The use of antidepressant medications is also associated with risk for diabetes (RR = 1.68) [13] which is slightly higher than the relative risk of untreated depression (RR = 1.56). The Diabetes Prevention Program (DPP; [14]) showed that lifestyle intervention was superior to metformin for diabetes delay and prevention. Yet, to date, no diabetes prevention intervention has been rigorously tested in people with depression.
Diabetes prevention studies in Asia have shown efficacy of lifestyle interventions in Asian populations specifically [14–18]. Yet, few have been tested in east Asian Americans (c.f., [19,20]) and none have been rigorously tested for Cambodian Americans specifically. Any such interventions must take into account Cambodians’ significant barriers to lifestyle change including high rates of historic trauma [6,21], high carbohydrate diet [22], and social isolation [23] as well as their poor profile on the social determinants of health. For example, in a 2014 needs assessment of Southeast Asians in Connecticut, modal annual income was <$10,000, 43% reported that food ran out often or sometimes in the past year, 39% did not rate their housing as secure, and 27% of Cambodians had no formal schooling at all [24].
Thus, there are several gaps in the diabetes prevention literature. First, whereas there is some suggestion that individuals with depression [25] and serious and persistent mental illness [26,27] may benefit from diabetes prevention programs, there is a need for rigorous testing of diabetes prevention interventions for individuals with depression – both elevated symptoms of depression and/or those taking antidepressant medication [28]. Second, culturally adapted diabetes prevention programs have suffered from low reach and overall engagement of minority populations [29]. Because lifestyle factors are cultural in nature, interventions that are developed by and for the target community using participatory approaches may yield better reach and engagement than linguistic adaptions of the national DPP. Third, carefully trained and supervised lay health workers (community health workers, promotoras) may be in the best position to reach and engage these hard-to-reach populations [30–33]. Fourth, there is a lack of rigorously tested diabetes prevention interventions for east Asian Americans, and none for Cambodians specifically. This paper describes a randomized, controlled trial of a culturally derived diabetes prevention intervention for Cambodian Americans with depression, i.e., those with elevated symptoms of depression and/or taking antidepressant medication. It reports key baseline characteristics of the sample and process outcomes.
2. Methods
Eat, Walk, Sleep (EWS) is a cardiometabolic lifestyle curriculum that was created by and for Khmer (ethnic Cambodian) people and was designed to be delivered by lay health workers [34,35]. “Diabetes Risk Reduction through Eat, Walk, Sleep and Medication Therapy Management” (DREAM) is a randomized, controlled trial that aims to compare the efficacy of Eat, Walk, Sleep (EWS) vs. EWS plus medication therapy management (EWS + MTM) vs. social services (SS, control condition) (clinicaltrials.gov identifier NCT02502929). Assessments were at baseline, post-treatment (12 months) and follow-up (15 months). Primary psychosocial outcome was change from baseline in depressive symptoms. Primary biological outcomes were change from baseline in HbA1c and insulin resistance (logHOMA-IR).
Participants randomized to SS were assessed for social service needs such as food and housing assistance and then provided support for any unmet needs over 12 months as needed. Participants assigned to EWS or EWS + MTM received their interventions over the course of 12 months. A post-intervention assessment was conducted at 12 months. A ‘booster’ EWS session (and MTM session for those assigned to EWS + MTM) occurred between 12 and 15 months. A follow-up assessment was conducted 15 months after baseline. See Fig. 1 for depiction of intervention sessions.
Fig. 1.
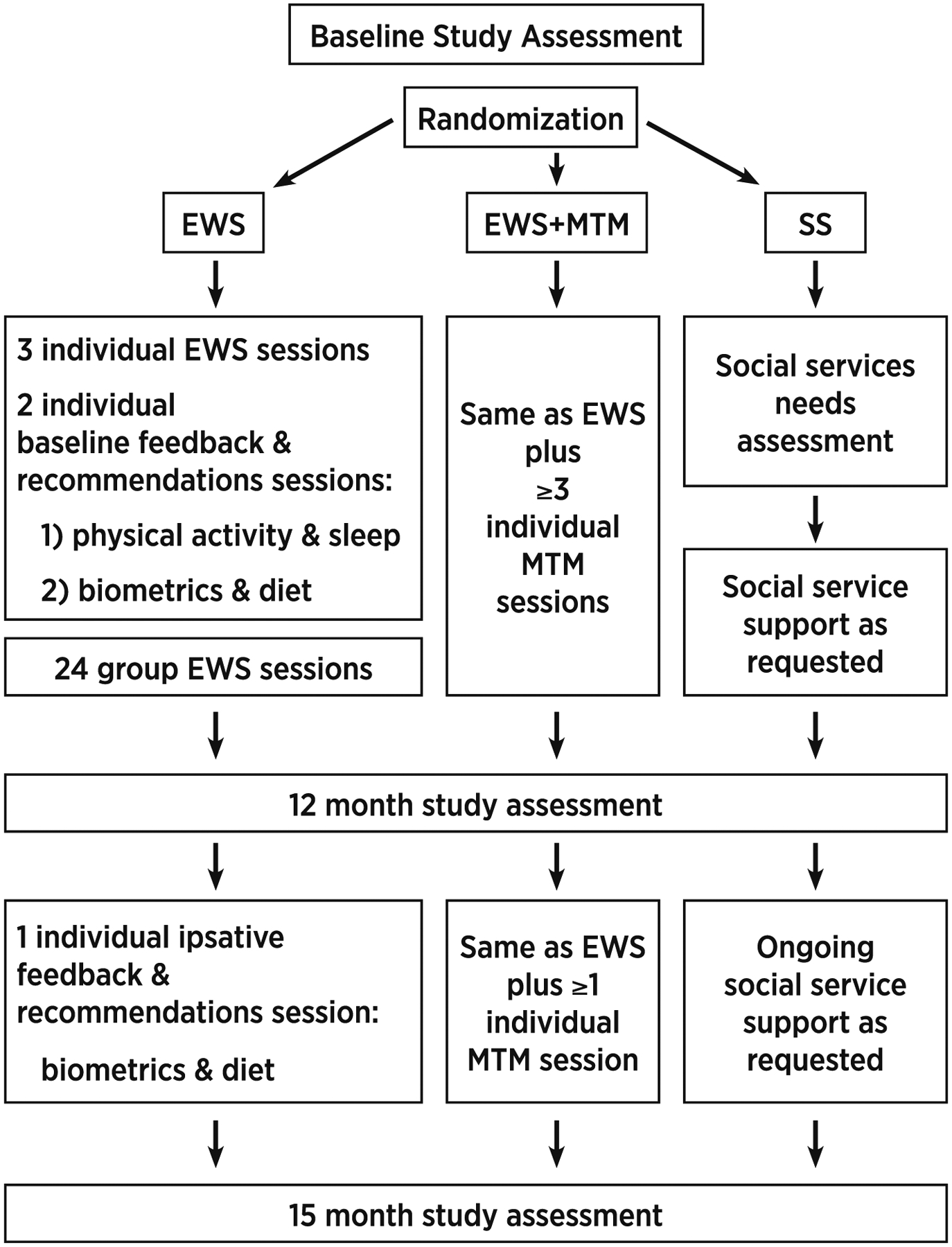
Intervention sessions.
All staff who had direct contact with participants were born in Cambodia, bilingual and bicultural. To minimize bias, lay health workers were divided into two roles. Community health workers (CHWs) conducted data collection. Community health educators (CHEs) delivered intervention sessions. Both CHWs and CHEs conducted outreach, screening, recruitment and consenting.
2.1. Participants
The study was approved by the UConn Health institutional review board and was conducted according to the World Medical Association Declaration of Helsinki. Participants signed written informed consent forms in their preferred language (Khmer or English) and provided written HIPAA authorization and a release of information for study staff contact with their healthcare provider. As in similar trials [36], DREAM employed several recruitment strategies: through referrals from clinicians and social service agencies; outreach at large cultural gatherings such as Cambodian new year celebrations; posters at local Khmer businesses; holding special events at relevant temples and churches; and recruiting through a partner-led approach, in which community partners developed and managed the recruitment efforts at their sites.
Inclusion criteria were: 1) aged 35–75; 2) Cambodian or Cambodian-American; 3) Khmer speaking; 4) currently living in Connecticut, Massachusetts, or Rhode Island (northeastern U.S.); 5) lived in Cambodia during the Pol Pot regime (1975–1979); 6) ambulatory; 7) consumed meals by mouth; 8) elevated risk for diabetes per the American Diabetes Association Risk Test [37] modified for this population [38,39]1. Note that the sample was selected for elevated diabetes risk, not for pre-diabetes per se. Participants were also required to meet criteria for depression by a) current antidepressant medication, and/or, b) elevated depressive symptoms indicative of likely major depressive disorder on the Khmer language Hopkins Symptom Checklist [40] with elevated symptoms on two occasions that were two weeks apart during a study screening and eligibility period. Exclusion criteria were: type 2 diabetes; seeing or hearing problems that would interfere with group sessions; major medical problems requiring intensive treatment; pregnancy or planning pregnancy; serious thinking or memory problems (e.g., schizophrenia or dementia); and 3 or more days in a psychiatric hospital or self-harm in the past 2 years.
2.2. Procedures
CHW data collectors conducted baseline assessments. Assessments were conducted in a private setting at a location of the participant’s choice, either in-home or at a clinic or social service agency. The CHWs administered surveys verbally and recorded responses in Remote Electronic Data Capture (REDCap) [41] using a tablet. Participants were paid $10 USD in gift cards to a local pharmacy for completing the surveys. CHWs obtained anthropometrics and blood pressures. Participants were instrumented with sleep and physical activity actigraphy devices and asked to wear them continually for 7 days and nights. Next, CHWs removed instruments from participants and compensated them $10 for actigraphy. CHWs then returned the devices to the laboratory for data retrieval. On a separate day, participants presented to a nearby Quest Diagnostics to provide a fasting blood sample and were compensated an additional $10.
2.3. Randomization
After all components of the baseline assessments were complete (i.e., surveys, anthropometrics and blood pressure, actigraphy, bloodwork), participants were individually randomized by an English-only speaking research assistant at UConn Health using an urn randomization [42] computer program that balanced the three treatment arms on gender, age, symptoms of post-traumatic stress disorder, and site (Connecticut, Rhode Island, Massachusetts). The research assistant had no contact with participants at any point during the study and did not conduct data analysis. The research assistant reported the participant’s assignment to the bilingual study coordinator who then notified the participant in Khmer. The study coordinator also telephoned to notify the site-specific CHE (interventionist) of the allocation. The CHWs, who conducted all study assessments, worked in close proximity to the CHEs so it was not possible to blind CHWs to allocation.
Power analysis based on published standard deviations of logHOMAIR yielded a target enrollment of 210 participants with an allocation ratio of 1:1:1 (EWS:EWS + MTM:SS). Recruitment began March 2016. Early in 2017 it became apparent that the enrollment goal would not be met within the timeframe of the funding period. A revised power analysis was conducted based on the actual observed standard deviation of logHOMA-IR for the 40 participants who had been randomized at that point. The power analysis showed 80% power to detect treatment effects on primary outcomes with 175 participants completing the 12-month assessment. The target sample size was reduced and the allocation ratio was changed to 2:2:1 going forward in order to gather relatively more information on the two intervention groups. All changes were approved by the funder. All other aspects of randomization remained the same.
2.4. Interventions and their development
2.4.1. Eat, walk, sleep
Khmer Health Advocates is the national Cambodian organization for survivors of war, torture and genocide from Cambodia living in the United States. Founded in 1982, Khmer Health Advocates is a founding member of the National Consortium of Torture Treatment Programs and provides clinical services, health promotion and advocacy on a national level. Staff include Khmer and American providers. With funding from the Centers for Disease Control and Prevention (CDC), Khmer Health Advocates used community based, participatory methods to develop EWS [34,35,43,44]. Five Cambodian American community organizations across the U.S. collaborated with Khmer Health Advocates to gather input from community members and develop the EWS curriculum. EWS is a trauma-informed, cardiometabolic education curriculum based on Buddhist concepts of health and disease. It was designed for delivery by Khmer CHEs to low-literacy and low-numeracy Khmer learners. EWS is a bilingual Khmer/English, manualized, educational toolkit. It includes a pictorial flipchart, hands-on tools, handouts, and recorded audio teaching material. Participants who were randomized to EWS or EWS + MTM were assigned to receive 3 individual EWS sessions, 24 group EWS sessions, two feedback/recommendation sessions at baseline and one feedback/recommendation session after their post (12 month) assessment. A booster EWS session was also scheduled between post (12 month) and follow up (15 month) assessments. The investigators prioritized maximizing participation so did not set an upper limit on the number of sessions that a participant could attend. See Fig. 1 for a schematic of the interventions.
2.4.1.1. Individual EWS teaching sessions.
The basic EWS curriculum was designed to be delivered in approximately three 1-h sessions that cover principles of nutrition, physical activity, healthy sleep, and “know your numbers” (understanding HbA1c, blood pressure, and cholesterol). DREAM participants who were randomized to either EWS or EWS + MTM received the 3 individual EWS basic sessions. Behavioral targets included eating no more than 1 small bowl of (brown) rice per meal, walking at least 30 min per day on 6 days per week, and getting 7–9 h of restful sleep per night [45]. The curriculum explicitly acknowledges Pol Pot, trauma, and loss, but the tone emphasizes cultural wisdom, resiliency, survival, and a life worth living.
2.4.1.2. Individual feedback and recommendations sessions.
Consistent with “know your numbers”, a key element of the one-on-one EWS sessions was individualized behavior change recommendations based on integrated self-reported and objective findings from assessments. Sometimes these sessions were incorporated into the individual EWS teaching sessions described above and other times there were separate depending on participant scheduling preferences and constraints. Here we report them out separately for clarity. Feedback about results and corresponding recommendations were provided in simple text and graphical format and explained to participants by the CHE in simple language. Participants were encouraged to discuss the information with their primary care provider. A separate report of the laboratory findings was sent directly to the primary care provider. Participants randomized to EWS or EWS + MTM received normative feedback after the baseline assessment. They were encouraged to track whatever parameters they could easily measure at home (e.g., weight, blood pressure). However, due to extremely low levels of literacy, participants were not asked to keep logs of behaviors or biometrics. After the post assessment they attended another session in which they were provided ipsative feedback regarding changes from baseline to post. Whereas this ipsative feedback/recommendation session could not impact 12-month changes (because they occurred after the 12-month assessment), they were important to participants and had the potential to impact the 15-month follow-up assessment. (For equity, participants in SS received recommendations during their individual EWS session which occurred after their final study assessment was complete.)
“Eat” feedback was based on laboratory findings (HbA1c, fasting glucose, lipids), blood pressure, and eating patterns. A short, tailored food frequency questionnaire focused on rice-based foods, sugar sweetened beverages, high-sodium sauces and high-fat cooking preparation methods. For example, a participant with high blood pressure who reported frequently eating fish sauce might receive the recommendation to reduce fish sauce in order to lower blood pressure. Walk feedback was based on physical activity actigraphy and corroborating self-report data [46,47]. “Sleep” feedback was primarily derived from responses on the Pittsburgh Sleep Quality Index (PSQI [48]) reviewed in conjunction with sleep actigraphy data. Responses of “very frequently” to PSQI questions assessing trouble sleeping were considered to be the greatest perceived sleep difficulty and actograms were used as an adjuvant objective measure of sleep duration, timing, and quality. For example, a participant who reports “very frequently” in response to “how often have you had trouble sleeping because you cannot get to sleep within 30 minutes?” with actigraphic sleep onsets at highly variable times would likely receive the recommendation to try going to go to sleep around the same time each night.
2.4.1.3. Group EWS sessions.
After completing individual EWS sessions, participants started EWS group sessions and were asked to attend for 12 months. Groups used rolling enrollment. Our goal was to titrate the intervention by starting with weekly sessions then moving to biweekly, triweekly, and monthly sessions, but allowed maximal flexibility in order tosuit participant schedules. To accommodate transportation needs, sessions were held in a variety of venues including a social service agency, clinic, church, and participant homes.
The diabetes prevention program established that a minimum of 16 contact hours were necessary for successful lifestyle modification in the general population. We anticipated that our low-literacy, low-numeracy sample with likely depression would require additional contacts. Therefore, we expanded the basic EWS curriculum to 24 group sessions of approximately 1 h each. Our expanded curriculum was designed to meet or exceed the published guidelines for diabetes prevention interventions set forth in the National Institute for Health and Clinical Excellence (NICE) [49] and Implementation of A European Guideline (IMAGE) [50].
EWS content was culturally derived and trauma-informed. Each topic was introduced by discussing traditional Khmer concepts of health, how those traditions were broken during Pol Pot, and are now difficult to restore or maintain in American culture. Sessions were activity based and conversational rather than didactic. Sessions for this population characterized by isolation and likely depression were also designed to promote socialization and behavioral activation. Consistent with other diabetes prevention interventions for those with mental illness [51], we emphasized instilling hope, expressing empathy, social modeling, frequent reminders, self-monitoring, and positive motivation. We also incorporated exercise and cooking components, provided information about diabetes, information about medications, and provided feedback regarding lab values [52].
CHEs used SMART goal setting to help each participant set an individualized short-term behavior change goal at each group session. If a participant had difficulty volunteering a goal, CHEs could guide the participant by referring to a standard list of possible goals such as, “try eating brown rice once this week”, “add 10 minutes of physical activity per day”, or, “limit caffeine after noon”. Goals were recorded on a form and progress toward the goal was discussed at the following session.
Participants were provided with a small bag of high quality brown rice to try at home. Particpants were given Fit4Life DVDs from the National Institute on Aging to promote home exercising. A series of Khmer language guided meditations were designed by the study team and recorded in Khmer to assist with sleep and were provided to participants in a variety of digital formats. A scale and digital sphygmomanometer were made available at all group sessions to track weight and blood pressure. See Supplemental Table 1 for group session content.
2.5. Medication therapy management
MTM followed guidelines of the American Pharmacist Association [53]. Whereas there are a handful of reports in the literature describing the use of lay health workers for pharmacist-delivered MTM [54–56], it remains a novel approach to standard MTM. Khmer Health Advocates developed and refined the practice [57]. Participants assigned to EWS + MTM received all the EWS intervention described above plus at least 3 MTM sessions between baseline and post assessment. A booster MTM session was also scheduled between post (12 month) and follow up (15 month) assessments.
The initial encounter between the CHE and participant was used to create a comprehensive medication record of prescription and non-prescription therapies for medical and mental health. The second encounter among the CHE, participant, and pharmacist was for 1) identification and potential resolution of drug-therapy problems, 2) a medication action plan, and 3) a report for the patient’s provider. The pharmacist had access to the study-related biometrics (body mass index, blood pressure and heart rate, A1c, fasting glucose, fasting insulin, lipid profile, c-reactive protein). Patients and CHEs were together face-to-face and communicated with the pharmacist via telemedicine using secure, high definition videoconferencing. Thereafter, the three met as clinically indicated, but at least two more times over the course of the 12 month intervention, and once again for a ‘booster’ session between 12 and 15 months. With a signed release of information, the pharmacist attempted to contact the prescriber and continued to work directly with the participant on education about their medications and monitoring drug therapy problems (e.g., side effects, adherence). Four pharmacists conducted MTM. All were either nationally certified in MTM or board-certified in ambulatory care, geriatric, or psychiatric pharmacy. All had extensive experience providing comprehensive medication therapy management services.
2.5.1. Social services
Participants who were assigned to SS were assessed for any social service needs. They were specifically queried about needing assistance such as food or housing assistance, referral to a healthcare provider, tax preparation, or citizenship applications. If participants endorsed a need for services, their specific requests were recorded, CHWs were tasked with following up to meet those needs, and contacts were documented over the following 12 months. If the participant denied the need for services, then they were told that they could contact the CHW in the future with any new needs. For equity, participants randomized to SS received a single, abbreviated EWS session and results of their follow-up lab work, along with results to their healthcare provider after they completed their follow-up assessment.
2.6. Lay health workers and their training
The two CHWs included a man in his 60’s with one year of college who was a pastor at an Asian church and a woman in her 30’s with a high school diploma who was a certified nursing assistant. The five CHEs included a man in his 40’s who had previously been a monk, a woman in her 50’s with a high school diploma who worked as a medical assistant, a woman in her 30’s with a certificate in human service assistance, a woman in her 50’s with a bachelor’s degree who was an insurance broker and a woman in her 60’s with high school education who was the only certified community health worker. All were active and trusted in their communities and were recruited through informal networks.
All DREAM lay health workers were naïve to research methods. Therefore they underwent extensive training to socialize them to research, principles of protocol and standardization, recruitment and screening, and train them in human subjects protection using the Harvard Catalyst materials [58].
We incorporated training principles for lay health workers addressing diabetes [59,60]. CHW training on data collection took approximately 3 full days spread across several weeks with homework and practice between training sessions. Training included a 51-page manual designed for this study and several online tutorials. CHWs completed 12 h of interactive presentations by the investigators in their particular field of expertise. The bilingual study coordinator assisted with all trainings. CHWs also observed experts conducting data collection and participated in role-play of data collection, including how to handle challenging cases. CHWs conducted a minimum of 10 supervised assessments with structured feedback and were judged to be competent by the study coordinator prior to working independently.
CHE training on EWS delivery took approximately 3 full days spread across several weeks with homework and practice between training sessions. First, CHEs studied a 30-page manual designed for this study and senior study staff met with them regularly to discuss its content. CHEs were further trained by viewing training videos of EWS group sessions in English and in Khmer, and discussing illustrative clinical examples. Next, the CHEs role-played delivering the intervention and received supervision. Ongoing training included live supervision and structured feedback from the study coordinator.
CHEs were also extensively trained to work with the pharmacists. Training materials included a 14-page manual for conducting medication reviews, a 34-page toolkit for reading prescription and non-prescription labels, a 2-page chart of commonly prescribed medications, and several online videos. Training sessions included 4 direct hours of interactive presentations by our pharmacist investigator and study coordinator, and additional hours observing experts completing the medication review form, role play, and a minimum of 10 supervised participant sessions with feedback prior to working independently.
2.7. Measures
2.7.1. Participant characteristics
Demographics included marital status, age, education, health insurance status and type. Migration history included country of birth, years spent in a refugee camp, and year of arrival in the U.S. Participants reported language spoken at home and rated their ability to read, write, and speak English and Khmer, separately, on a 4-point scale from “not at all” to “very well”.
2.7.2. Anthropometrics
Weight was measured on a hard floor surface using a calibrated electronic Seca (Chino, California) digital scale, after removing heavy clothing, pocket items, and shoes. Height was measured with the Seca portable stadiometer, with the participant’s head positioned in the Frankfurt horizontal plane, after removing shoes. Waist circumference was measured twice at the umbilicus with an inelastic tape. Following recommended procedures [61], blood pressure was measured twice with calibrated digital sphygmomanometer (Omron, Hoffman Estates, IL). For all measurements, discrepant values exceeding a predetermined allowance triggered a third measurement and the two closest values were averaged.
2.7.3. HbA1c and insulin resistance
Glycosylated hemoglobin A1c (HbA1c) was assayed at Quest laboratory using direct enzymatic assay. In this laboratory, A1c shows the following coefficient’s of variation (CVs) for normal and high values: Level 1 mean = 5.51%, CV = 3.3 based on n = 320; and Level 2 mean = 9.01%, CV = 3.2. Glucose was reported in mg/dL and insulin was reported in uiU/mL. Log transformed homeostatic model assessment of IR (logHOMA-IR) was calculated from fasting glucose and insulin values according to the standard formula [62]: log(fasting glucose × fasting insulin)/405.
2.7.4. Depressive symptoms
Symptoms of depression were assessed with the 15-item depression subscale of the Khmer language Hopkins Symptom Checklist using the published cutoff of mean = 1.75 [40] to determine likely major depressive disorder. Cronbach’s alpha in this study was 0.93. Antidepressant medication was self-reported as yes vs no.
2.7.5. Recruitment, attendance and retention
Data regarding outreach, number of screens and reasons for screen failures, number of consents, and attendance at study assessments were tracked by the study coordinator and maintained in a central database stored on REDCap. Data for attendance at individual and group sessions was tracked over time on paper by the CHEs and reasons for non-attendance were recorded. Data for recruitment, screening, consenting, assessments and session attendance were reviewed at monthly meetings of the study team.
2.7.6. Individualized recommendations
A research assistant at UConn Health organized survey data and bloodwork to formulate individual “Eat” recommendations following an algorithm set by the investigators. A research assistant at Pennsylvania State University organized survey data and actigraphy to formulate individual “Walk” and “Sleep” recommendations following separate algorithms set by the investigators. Data regarding the type and number of individual recommendations made were tracked by a research assistant at UConn Health. The count of nutrition, physical activity, and sleep recommendations was calculated.
2.7.7. Group session individualized goal setting
CHEs completed goal setting forms at each group session. Forms were delivered to the research assistant at UConn Health who entered the data regarding goal setting. The frequency of types of individual SMART goals set by participants was calculated.
2.7.8. Treatment fidelity
Two methods were used to assess fidelity which we have used in previous CHE trials [63]. The first was assessment by a supervisor who observed the delivery of the session using a 19-item checklist covering activities such as “Had supplies ready” and “Demonstrated the skill being taught”. Items were answered with yes/no/not applicable. A trained observer completed the checklist for every session for each CHE for their first several participants, after which, once the CHE’s performance reached 80%, the CHE was occasionally “spot checked”. The second method was a CHW self-assessment. The checklist mirrored the supervisor’s checklist. For sessions with paired data, agreement between CHE and observer was calculated as proportion agreement.
2.7.9. Facilitation skills
CHE facilitation skills were rated by the study coordinator on 23-item scale [63]. Sample items are, “paced the session appropriately” and “responded empathically to participants”. Response options are 1 = very little, 2 = somewhat, 3 = a lot, or not applicable. In this study, Cronbach’s alpha was 0.79 for individual sessions and 0.83 for group sessions.
2.7.10. Therapeutic cohesion and alliance
A modified version of the Outcome Alliance Scale was used to assess participant perception of cohesion in the group (e.g., “did the members of the group listen to you when you talked?”), therapeutic alliance with the CHE (e.g., “did you have confidence in your CHE?”, and for those in EWS + MTM, therapeutic alliance with the pharmacist (e.g., “did you trust the pharmacist?”) [24]. Response options were from 0 = not at all to 3 = a lot, with higher scores indicating higher cohesion and alliance. The scales were administered at the post assessment. Cronbach’s alpha for group cohesion was 0.83, CHE alliance was 0.82, and pharmacist alliance was 0.94.
2.7.11. Treatment satisfaction
Participant satisfaction was measured at the post assessment (12 months). Three items assessed importance, enjoyment, and perceived ability of the program to keep participants healthy; responses were from 0=“did not like it” to 3=“liked it a lot”. In this study, Cronbach’s alpha was 0.87.
2.8. Data analysis
This manuscript aims to describe key baseline characteristics of the sample and to report important process outcomes. Frequencies and proportions were calculated for categorical demographic and clinical characteristics, recruitment, retention, attendance, treatment fidelity, goal setting, feedback/recommendations, and social services provided. Means and standard deviations were calculated for continuous demographic and clinical characteristics, facilitations skills, cohesion, alliance, and satisfaction.
3. Results
3.1. Recruitment and retention
See Fig. 2 for CONSORTdiagram; 540 recruits were screened, 206 recruits were consented, and 188 participants were randomized. As can be seen in Table 1, the randomization procedure worked well as groups were similar at baseline along key characteristics. At the 12-month assessment 179 participants (95%) were retained and at the 15-month assessment 180 participants were retained (96%). Post assessments were disrupted due to COVID-19 for five participants. Those participants remained in the study, but their post assessments were delayed for three participants or skipped for two participants. Retention increased by one individual from 12- to 15-months because one participants who was not able to complete the 12-month assessment due to COVID lock-down was able to complete the 15-month assessment after partial re-opening.
Fig. 2.
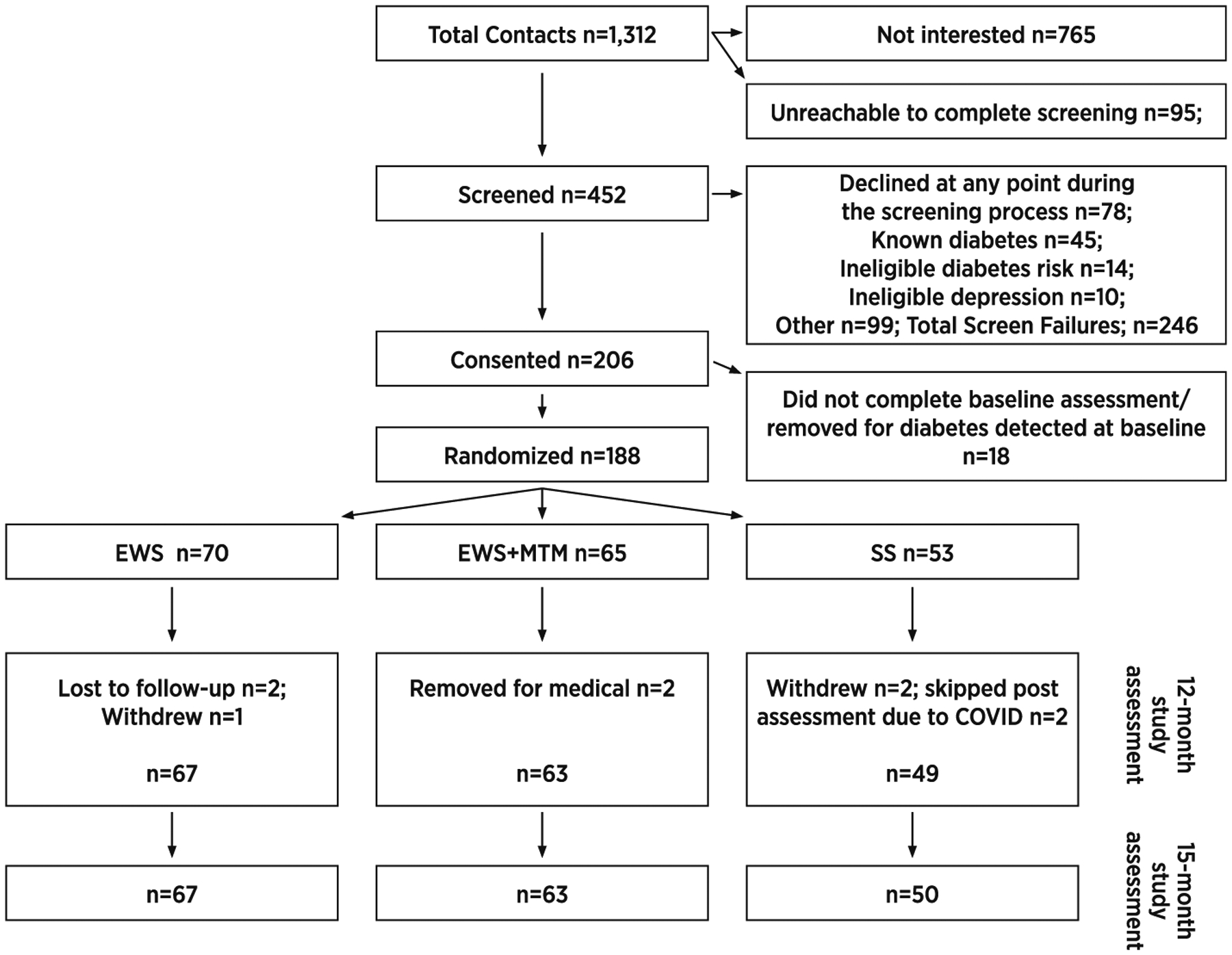
CONSORT diagram.
Table 1.
Demographic and clinical characteristics at baseline (n = 188).
| Characteristic | SS (n = 53) | EWS (n = 70) | EWS + MTM (n = 65) |
|---|---|---|---|
| Panel A: Demographic and clinical characteristics | |||
| Gender (Female) | 44 (83%) | 50 (71%) | 53 (82%) |
| Age | 54.2 ± 8.5 | 55.9 ± 9.3 | 55.3 ± 8.5 |
| Household Income | |||
| Under $20,000 | 25 (53%) | 30 (47%) | 27 (48%) |
| $20,000–30,000 | 9 (19%) | 16 (25%) | 16 (29%) |
| $31,000–40,000 | 3 (6%) | 7 (11%) | 7 (13%) |
| Over $40,000 | 10 (21%) | 11 (17%) | 16 (11%) |
| Employment Status | |||
| Full-time | 19 (37%) | 22 (32%) | 18 (28%) |
| Part-time | 3 (6%) | 2 (3%) | 4 (6%) |
| Unemployed/looking | 2 (4%) | 9 (13%) | 6 (9%) |
| Retired | 3 (6%) | 3 (4%) | 0 (0%) |
| Disabled | 18 (35%) | 21 (30%) | 19 (30%) |
| Homemaker | 3 (6%) | 6 (9%) | 7 (11%) |
| Other | 4 (8%) | 6 (9%) | 10 (16%) |
| Years Education | 7.3 ± 5.1 | 7.4 ± 4.9 | 6.2 ± 5.1 |
| Health Insurance | |||
| Medicare | 11 (22%) | 17 (25%) | 17 (27%) |
| Medicaid | 19 (37%) | 23 (34%) | 20 (32%) |
| Private | 17 (33%) | 26 (39%) | 22 (36%) |
| Other | 4 (8%) | 1 (2%) | 3 (5%) |
| Years in US | 28.4 ± 10.7 | 25.6 ± 12.4 | 30.0 ± 10.9 |
| Speak English | |||
| Not at all | 12 (23%) | 10 (14%) | 15 (23%) |
| A little bit | 24 (46%) | 37 (53%) | 29 (45%) |
| Conversational | 8 (15%) | 21 (30%) | 13 (20%) |
| Very well | 8 (15%) | 2 (3%) | 7 (11%) |
| BMI | 26.5 ± 4.7 | 27.2 ± 4.9 | 27.1 ± 3.3 |
| HbA1c% | 5.4 ± 0.4 | 5.6 ± 0.4 | 5.5 ± 0.5 |
| logHOMA-IR | 0.20 ± 0.32 | 0.13 ± 0.28 | 0.16 ± 0.26 |
| Depression | |||
| On antidepressant | 16 (30%) | 23 (33%) | 24 (37%) |
| Elevated symptoms | 26 (50%) | 39 (56%) | 36 (56%) |
| Panel B. Self-reported outcomes by treatment arm | |||
| SS | EWS | EWS + MTM | |
| Group cohesion | N/A | 2.9 ± 0.3 | 2.9 ± 0.3 |
| Treatment satisfaction | N/A | 2.9 ± 0.2 | 2.9 ± 0.3 |
| Alliance with CHE | N/A | 2.9 ± 0.2 | 2.9 ± 0.3 |
| Alliance with pharmacist | N/A | N/A | 2.9 ± 0.3 |
3.2. Attendance
3.2.1. EWS sessions
Of those randomized to EWS, 69 participants (99%) attended at least one EWS session and 60 participants (86%) completed ≥ 24 sessions. Of all participants who attended at least one session, the mean number of sessions was M = 27.3 (SD = 3.7). This includes individual EWS teaching sessions, feedback and recommendation sessions, and group EWS sessions. Reasons for non-attendance included illness, medical and social service appointments, family care obligations, unpredictable or incompatible work schedules, travel, and stressful events such as accident, illness, or death of a loved one.
3.2.2. MTM sessions
Of those randomized to EWS + MTM, 64 participants (98%) attended at least one MTM session and 50 participants (77%) completed ≥ 4 sessions.
3.3. SMART goal setting during group sessions
EWS group sessions yielded 2248 goal setting forms; 81.9% of goals were self-reported as achieved. The most common goals for Eat, Walk, and Sleep, respectively, were trying brown rice, trying a new type of physical activity, and going to sleep at the same time each night. The least common goals set were replacing white rice with brown rice, adding one day of physical activity per week, and trying a new bedtime routine. See Supplemental Table 2.
3.4. Feedback and recommendations
The most common recommendations for Eat and Sleep, respectively, were to replace white rice with brown rice, and to increase consistency of sleep timing (going to bed and waking up around the same time each day). The overall level of physical activity was so low overall that each participant received the same Walk recommendations, i.e.: Walk at least 30 min on 6 days per week; do not sit for more than 30 min straight; break up long periods of sitting with short walks; choose walking instead of the car for short trips; when you park in a parking lot, park far from the entrance and walk to the door; take the stairs rather than the elevator or escalator whenever you can. See Supplemental Table 3 for individualized recommendations.
3.5. Treatment fidelity and facilitation skills
Concordance was high between CHE self-ratings and paired observer ratings of adherence to session checklists. A total of 165 paired data points from 12 group sessions had 98% concordance. A total of 313 paired data points from 37 individual sessions had 97% concordance.
Unpaired CHE self-ratings of 323 group sessions showed mean = 98% adherence to the session checklist, with the lowest adherence (86%) for reviewing the “Know your numbers” section of the curriculum. Unpaired CHE self-ratings for 135 individual sessions showed mean = 98% adherence, with the lowest adherence (97%) for ‘greet each participant as they arrive’. Unpaired observer ratings of 22 group sessions showed 90% adherence to the checklist, with the lowest adherence for ‘admit lack of knowledge’ (77%). Unpaired observer ratings of 40 individual sessions showed 95% adherence, with the lowest adherence (97%) for ‘engaging and enthusiastic’ presentation.
Facilitation skills were high. On a scale from 1 to 3, observer ratings of CHE facilitation skills for 22 group sessions was mean = 2.25 (0.17), and for 40 individual sessions was mean = 2.32 (0.28).
3.6. Cohesion, alliance, and satisfaction
On a scale from 0 to 3, group cohesion for the EWS groups was high, mean = 2.87 (0.33). Alliance was high with the CHE, mean = 2.94 (0.21) and the pharmacist mean = 2.92 (0.28). Satisfaction with the interventions was high; EWS mean = 2.95 (0.20) EWS + MTM mean = 2.90 (0.28). See Table 1, panel B.
3.7. Social services
When prompted, 27 participants (54%) did not identify any social service needs. Participants who did request social services received mean = 12.6 (SD = 27.6) CHE contacts; the high range was largely accounted for by a handful of participants who sought frequent help with translation of letters, forms, and phone calls to social service and government agencies.
4. Discussion
The main finding reported here is successful implementation of the interventions as assessed by both objective and participant-reported process outcomes. Participants reported high therapeutic alliance and cohesion and also high satisfaction with the intervention which are likely related in part to the interventions’ cultural relevance [64]. The CHEs delivered the interventions with a high degree of treatment fidelity and skilled facilitation. Excellent attendance and a broad range of behavioral goal setting demonstrates the high level of participant engagement.
A recent review of diabetes prevention programs for minority populations in the U.S.[65] found that the lay health workers involved were generally hired from the local community, and that their qualifications and work experience varied. This was true for DREAM, also. In selecting lay health workers, we prioritized commitment to the community, bilingual reading and writing, and flexible interpersonal skills that allowed them to interact effectively with participants with various levels of acculturation. Lay health worker roles are typically limited to outreach, recruitment, translation, navigation, and occasionally intervention delivery. As in our previous studies [63] we expanded the lay health worker role to include biometric and participant self-report data collection. Ensuring quality data collection from linguistic minorities with a history of trauma requires not only careful standardization and good language skills, but also considerable patience and understanding of the community that few but lay health workers possess.
4.1. Lessons learned
4.1.1. Recruitment
Because of our unique decades long community-academic partnership, we did not experience lack of trust that can plague otherwise promising community based trials. Notwithstanding, recruitment was difficult in large part because many recruits had extant diabetes and others would not commit to such an intensive intervention spanning 15 months. Also, a large number of outreach contacts could not be reached later for screening, reflecting the instability of housing and phone service in this community. Human subjects protections may need to be modified to allow contact through methods such as Facebook. When it became apparent that the original enrollment goal of 210 participants would not be met within the timeframe of the funding period, a decision was made to reduce the number of control participants. We modified the treatment allocation ratio at that point, hence the unequal n in each arm. Future trials must allow longer recruitment periods.
4.1.2. Budget
The team had originally planned for psychiatric assessments at baseline for confirmation of major depressive disorder, and had also planned for 18-month follow-up. However, budget cuts required that we simplify our protocol so the decision was made to require 2 repeated elevated depressive symptom checklists rather than psychiatric interview for inclusion, and also to limit follow-up to 15 months. A large community agency that had agreed to participate declined when the budget was cut, challenging recruitment goals. The highest quality studies with stringent inclusion criteria, long duration of follow-up, and wide buy-in from community agencies require firm commitment from funding agencies. Like similar studies [66], DREAM was successfully implemented because the team was guided by commitment to the community, co-ownership of research endeavor, and a balance between academic rigor and community relevance.
4.1.3. Attendance
Whereas there was high satisfaction with the interventions and attendance was excellent, achieving that high attendance required considerable flexibility. Some participants had difficulty attending the sessions at the times that were convenient for the rest of the group. Individual sessions would be easier to schedule but would lack the therapeutic benefit that group sessions provide. Appointments for healthcare and social service agencies often conflicted, or exhausted participants’ limited transportation support. Extended travel to visit family in the U.S. and in Cambodia often interrupted treatment. Our CHEs remained incredibly flexible with scheduling including offering evening and weekend sessions. They invited participants to make up sessions with other groups, offered individual sessions when rescheduling a group was not possible, and maintained close contact with participants between sessions. These strategies are not easily implemented, but are necessary for high attendance. Future implementation research should carefully detail the nature of flexibility required, e.g., the number of sessions that required rescheduling, the characteristics of participants that required rescheduling, and what type of accommodations were made (e.g., evening or weekend sessions, transportation support).
4.1.4. Retention
With 96% retention at 15 months, DREAM was highly successful in retaining participants relative to other DPP-style trials with lay health workers. For example, in a sample of 178 Spanish speaking, low income participants, and an intervention of 8 sessions, 77% was retained at 12 months [67]. In a diverse sample of 402 largely foreign-born participants in east Harlem, and an intervention of 8 sessions, 75% was retained at 6 months [68]. In a sample of 174 Sikhs in New York City, and an intervention of 6 sessions, 92% was retained at 6 months [69]. In a DPP-adaptation for 13 individuals with mental illness and a 24 session intervention, 85% was retained at 6-months [51]. We attribute our successful retention to the thoughtful anticipation of barriers and the dedicated, flexible, and skilled staff who maintained communication with participants over time. Whereas medical professionals’ concerns persist about lay health worker skill level for diabetes prevention [70], there is overwhelming evidence of their effectiveness [71] and we submit that, in fact, only they can achieve such participant engagement.
4.1.5. Staffing
All staff who had direct contact with participants were bilingual in speaking, listening, reading, and writing, all of which were necessary for their tasks. However, many community members who are conversationally bilingual cannot read or write in Khmer. For this reason, the pool of potential lay health workers is relatively small. The training of each staff person was extensive. Whereas only one staff member left the project, this combination of factors made the turnover of that one CHE a particular challenge. Other small, geographically dispersed immigrant and minority groups with limited native language capacity may face similar difficulties with staffing.
4.2. Limitations and conclusions
Several limitations should be acknowledged. First, the change in sample size and treatment group allocation resulted in less precision for estimating the SS arm and comparisons involving the SS arm. Second, the self-reported process outcomes (e.g., satisfaction) were reported at post assessments, potentially positively biasing the outcomes because those participants who left the study before then may have been less satisfied. Third, although we carefully delineated roles for CHW data collection and CHE intervention delivery, CHWs were not blinded to treatment allocation and social desirability may have biased participant responses. Results also may not generalize to other ethnic/racial or sociodemographic groups. Limitations are generally outweighed by strengths including a sample with likely depression, a novel intervention, a staff of lay health workers, and an underserved population. Future studies should conduct psychiatric interviews for the determination (and resolution of) major depressive disorder, employ larger samples, allocate to all treatment conditions equally, and totally blind all data collectors.
DREAM was the first study designed to decrease diabetes risk among persons with likely depression, and did so in a non-English speaking, refugee population with high rates of trauma. Enrolling, retaining, and engaging minorities with such barriers is tremendously challenging. Yet, DREAM was successfully implemented with a high degree of treatment fidelity and was favorably rated by the target community. We attribute this success to careful training and supervision of the lay health workers, our community-based participatory approach, and the culturally derived intervention. Such approaches hold promise for decreasing diabetes risk among hard-to-reach populations and results for biological, behavioral, and psychosocial outcomes of DREAM will be forthcoming.
Supplementary Material
Acknowledgements
Outside of the current work, Orfeu M. Buxton discloses that he received subcontract grants to Penn State from Proactive Life LLC (formerly Mobile Sleep Technologies) doing business as SleepScape (NSF/STTR #1622766, NIH/NIA SBIR R43-AG056250, R44-AG056250), received honoraria/travel support for lectures from Boston University, Boston College, Tufts School of Dental Medicine, New York University and Allstate, and receives an honorarium for his role as the Editor in Chief of Sleep Health (sleephealthjournal.org). The other authors have nothing to disclose.
Funding
DK103663 to JW.
Footnotes
Human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. Informed Consent: Informed consent was obtained from all individual participants in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We modified the standard risk test by altering items and lowering the cutoff score. We modified items because the traditional items were too limiting for our specific population. For example, the traditional risk test assigns points for having first-degree relatives with known diabetes. Our participants are survivors of genocide and the vast majority have lost at least one first-degree relative (many have lost several). Also, almost participants have first-degree relatives still living in Cambodia who have extremely limited access to healthcare and so whose diabetes status is unknown. Therefore, we expanded the item to include second-degree relatives. In another example, the standard risk test assigns points for body mass index (BMI) based on the general US population. Instead, we assigned points based on Asian-specific cutoffs for waist circumference established by the International Diabetes Federation. It is well-established that waist circumference has more predictive power than BMI for diabetes in east Asians because they convert to type 2 diabetes at lower BMI than do other ethnic groups. We also added an item regarding having experienced starvation under Pol Pot since starvation has been shown to be a risk factor for development of type 2 diabetes. Finally, we lowered the cutoff score from 5 to 3 because this sample was already enriched with other risk factors for type 2 diabetes including, most importantly, elevated depressive symptoms and/or antidepressant medications. Our changes had the net effect of lowering the threshold for “at risk”.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2021.106427.
References
- [1].Marshall GN, et al. , Diabetes and cardiovascular disease risk in Cambodian refugees, J. Immigr. Minor. Health 18 (1) (2016) 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Berthold SM, et al. , Comorbid mental and physical health and health access in Cambodian refugees in the US, J. Community Health 39 (6) (2014) 1045–1052. [DOI] [PubMed] [Google Scholar]
- [3].Knol MJ, et al. , Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis, Diabetologia 49 (5) (2006) 837–845. [DOI] [PubMed] [Google Scholar]
- [4].Mezuk B, et al. , Depression and type 2 diabetes over the lifespan: a meta-analysis, Diabetes Care 31 (12) (2008) 2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hasin DS, et al. , Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States, JAMA Psychiatry 75 (4) (2018) 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marshall GN, et al. , Mental health of Cambodian refugees 2 decades after resettlement in the United States, JAMA 294 (5) (2005) 571–579. [DOI] [PubMed] [Google Scholar]
- [7].Alhazmi A, et al. , Macronutrient intakes and development of type 2 diabetes: a systematic review and meta-analysis of cohort studies, J. Am. Coll. Nutr 31 (4) (2012) 243–258. [DOI] [PubMed] [Google Scholar]
- [8].Rashidkhani B, et al. , Dietary patterns and anthropometric indices among Iranian women with major depressive disorder, Psychiatry Res. 210 (1) (2013) 115–120. [DOI] [PubMed] [Google Scholar]
- [9].Davison KM, Kaplan BJ, Vitamin and mineral intakes in adults with mood disorders: comparisons to nutrition standards and associations with sociodemographic and clinical variables, J. Am. Coll. Nutr 30 (6) (2011) 547–558. [DOI] [PubMed] [Google Scholar]
- [10].Wilmot EG, et al. , Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis, Diabetologia 55 (11) (2012) 2895–2905. [DOI] [PubMed] [Google Scholar]
- [11].Yates T, et al. , Impact of depression and anxiety on change to physical activity following a pragmatic diabetes prevention program within primary care: pooled analysis from two randomized controlled trials, Diabetes Care 42 (10) (2019) 1847–1853. [DOI] [PubMed] [Google Scholar]
- [12].Cappuccio FP, et al. , Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis, Diabetes Care 33 (2) (2010) 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rotella F, Mannucci E, Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies, J. Clin. Psychiatry 74 (1) (2013) 31–37. [DOI] [PubMed] [Google Scholar]
- [14].Knowler WC, et al. , Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin, N. Engl. J. Med 346 (6) (2002) 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pan XR, et al. , Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study, Diabetes Care 20 (4) (1997) 537–544. [DOI] [PubMed] [Google Scholar]
- [16].Li G, et al. , The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing diabetes prevention study: a 20-year follow-up study, Lancet 371 (9626) (2008) 1783–1789. [DOI] [PubMed] [Google Scholar]
- [17].Sakane N, et al. , Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance, BMC Public Health 11 (1) (2011) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramachandran A, et al. , The Indian diabetes prevention programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1), Diabetologia 49 (2) (2006) 289–297. [DOI] [PubMed] [Google Scholar]
- [19].Islam NS, et al. , A randomized-controlled, pilot intervention on diabetes prevention and healthy lifestyles in the new York City Korean community, J. Community Health 38 (6) (2013) 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yeh MC, et al. , Translation of the diabetes prevention program for diabetes risk reduction in Chinese immigrants in new York City, Diabet. Med 33 (4) (2016) 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wong EC, et al. , Characterizing the mental health care of U.S. Cambodian refugees, Psychiatr. Serv 66 (9) (2015) 980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Peterman JN, et al. , Relationship between past food deprivation and current dietary practices and weight status among Cambodian refugee women in Lowell, MA, Am. J. Public Health 100 (10) (2010) 1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Berthold SM, et al. , Social disconnection as a risk factor for health among Cambodian refugees and their offspring in the United States, J. Immigr. Minor. Health 21 (2) (2019) 290–298. [DOI] [PubMed] [Google Scholar]
- [24].Asian Pacific American Affairs Commission, Needs Assessment of Southeast Asian Population in Connecticut, 2014.
- [25].Janney CA, et al. , Does mental health influence weight loss in adults with prediabetes? Findings from the VA diabetes prevention program, Gen. Hosp. Psychiatry 53 (2018) 32–37. [DOI] [PubMed] [Google Scholar]
- [26].Quiñones MM, et al. , Case study of an adaptation and implementation of a diabetes prevention program for individuals with serious mental illness, Transl. Behav. Med 8 (2) (2018) 195–203. [DOI] [PubMed] [Google Scholar]
- [27].Schneider KL, Sullivan JC, Pagoto SL, Translation of the diabetes prevention program into a community mental health organization for individuals with severe mental illness: a case study, Transl. Behav. Med 1 (3) (2011) 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Whitegoat W, et al. , Mental health in diabetes prevention and intervention programs in American Indian/Alaska Native communities, Wash Univ. J. Am. Indian Alsk. Native Health (2015) 1(1). [PMC free article] [PubMed] [Google Scholar]
- [29].Ackermann RT, O’Brien MJ, Evidence and challenges for translation and population impact of the diabetes prevention program, Curr. Diab. Rep 20 (3) (2020) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ruggiero L, et al. , Translation of the diabetes prevention program’s lifestyle intervention: role of community health workers, Curr. Diab. Rep 12 (2) (2012) 127–137. [DOI] [PubMed] [Google Scholar]
- [31].Corkery E, et al. , Effect of a bicultural community health worker on completion of diabetes education in a Hispanic population, Diabetes Care 20 (3) (1997) 254–257. [DOI] [PubMed] [Google Scholar]
- [32].Viswanathan M, et al. , Outcomes of community health worker interventions, Evid. Rep. Technol. Assess (Full Rep.) 181 (2009), 1–144, A1–2, B1–14, passim. [PMC free article] [PubMed] [Google Scholar]
- [33].Pérez-Escamilla R, et al. , Impact of a community health workers-led structured program on blood glucose control among latinos with type 2 diabetes: the DIALBEST trial, Diabetes Care 38 (2) (2015) 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kuoch T, et al. , The National Cambodian American Town Hall Meeting: a community dialogue on “Eat, Walk, Sleep” for health, Prog. Community Health Partnersh 8 (4) (2014) 541–547. [DOI] [PubMed] [Google Scholar]
- [35].Wagner J, et al. , Patient reported outcomes of ‘Eat, Walk, Sleep’: a cardiometabolic lifestyle program for Cambodian Americans delivered by community health workers, J. Health Care Poor Underserved 26 (2) (2015) 441–452. [DOI] [PubMed] [Google Scholar]
- [36].Horowitz CR, et al. , Effective recruitment of minority populations through community-led strategies, Am. J. Prev. Med 37 (6 Suppl 1) (2009) S195–S200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].American Diabetes Association, Are You at Risk for Diabetes?. https://www.diabetes.org/risk-test.
- [38].International Diabetes Federation Epidemiology Task Force Consensus Group, The IDF Consensus Worldwide Definition of the Metabolic Syndrome. https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html, 2005.
- [39].van Abeelen AF, et al. , Famine exposure in the young and the risk of type 2 diabetes in adulthood, Diabetes 61 (9) (2012) 2255–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mollica RF, et al. , Indochinese versions of the Hopkins symptom Checklist-25: a screening instrument for the psychiatric care of refugees, Am. J. Psychiatry 144 (4) (1987) 497–500. [DOI] [PubMed] [Google Scholar]
- [41].Harris PA, et al. , Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Stout RL, et al. , Ensuring balanced distribution of prognostic factors in treatment outcome research, J. Stud. Alcohol Suppl 12 (1994) 70–75. [DOI] [PubMed] [Google Scholar]
- [43].Wagner J, Berthold SM, Buckley T, Bermudez-Millan A, Ha T, Scully M, Kuoch T, Kong S, Fraser-King L, Horn IS, Keuky L, Remote peer learning between US and Cambodian lay health workers to improve outcomes for Cambodians with type 2 diabetes: a pilot study, Int. J. Behav. Med 27 (5) (2020) 609–614, 10.1007/s12529-020-09896-3. [DOI] [PubMed] [Google Scholar]
- [44].Wagner J, et al. , Training Cambodian village health support guides in diabetes prevention: effects on Guides’ knowledge and teaching activities over 6 months, Int. J. Behav. Med 23 (2) (2016) 162–167. [DOI] [PubMed] [Google Scholar]
- [45].Hirshkowitz M, et al. , National Sleep Foundation’s sleep time duration recommendations: methodology and results summary, Sleep Health 1 (1) (2015) 40–43. [DOI] [PubMed] [Google Scholar]
- [46].Lee PH, Macfarlane DJ, Lam TH, Stewart SM, et al. , Int. J. Behav. Nutr. Phys. Act 8 (2011) 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Master L, et al. , Bidirectional, daily temporal associations between sleep and physical activity in adolescents, Sci. Rep 9 (1) (2019) 7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research, J. Psychiatr. Res 28 (2) (1989) 193–213. [DOI] [PubMed] [Google Scholar]
- [49].National Institute for Health and Clinical Excellence, Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk, National Institute for Health and Clinical Excellence, London, 2012. [Google Scholar]
- [50].Paulweber B, et al. , A European evidence-based guideline for the prevention of type 2 diabetes, Horm. Metab. Res 42 (Suppl. 1) (2010) S3–36. [DOI] [PubMed] [Google Scholar]
- [51].Aschbrenner KA, et al. , Feasibility of behavioral weight loss treatment enhanced with peer support and mobile health technology for individuals with serious mental illness, Psychiatr Q. 87 (3) (2016) 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Srebnik D, et al. , A pilot study of the diabetes prevention program on weight loss for adults at community mental health centers, Psychiatr. Serv 66 (2) (2015) 200–203. [DOI] [PubMed] [Google Scholar]
- [53].Joint Commission of Pharmacy Practitioners, Pharmacists’ Patient Care Process, in: https://jcpp.net/wp-content/uploads/2016/03/PatientCareProcess-with-supporting-organizations.pdf.
- [54].Hall-Lipsy E, et al. , Community health worker perspectives of an academic community medication therapy management collaboration, J. Am. Pharm. Assoc 60 (3) (2003) 475–480.e1, 2020. [DOI] [PubMed] [Google Scholar]
- [55].Wheat L, et al. , Utilizing a Pharmacist-Community Health Worker Collaboration to Address Medication Adherence Barriers, 2020. [DOI] [PubMed]
- [56].Segal R, et al. , Opportunities and responsibilities for pharmacists to improve their effectiveness in addressing medication adherence through culturally sensitive collaborations with community health workers, J. Am. Pharm. Assoc 60 (4) (2003) e25–e30, 2020. [DOI] [PubMed] [Google Scholar]
- [57].Buckley T, Kuoch T, Scully M, Using technology and cross-cultural teams to deliver trauma-informed medication management, Eur. J. Pub. Health (2019) 29. [Google Scholar]
- [58].Harvard Catalyst, Community-Engaged Research (CEnR), Available from: https://catalyst.harvard.edu/programs/regulatory/cenr.html, 10/26/2020.
- [59].Tang TS, et al. , Training peers to deliver a church-based diabetes prevention program, Diabetes Educ. 38 (4) (2012) 519–525. [DOI] [PubMed] [Google Scholar]
- [60].Murray NJ, et al. , A motivational peer support program for type 2 diabetes prevention delivered by people with type 2 diabetes: the UEA-IFG feasibility study, Diabetes Educ. 38 (3) (2012) 366–376. [DOI] [PubMed] [Google Scholar]
- [61].Pickering TG, et al. , Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on high blood pressure research, Circulation 111 (5) (2005) 697–716. [DOI] [PubMed] [Google Scholar]
- [62].Matthews DR, et al. , Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia 28 (7) (1985) 412–419. [DOI] [PubMed] [Google Scholar]
- [63].Wagner J, et al. , Community health workers assisting Latinos manage stress and diabetes (CALMS-D): rationale, intervention design, implementation, and process outcomes, Transl. Behav. Med 5 (4) (2015) 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Williams JH, et al. , Cultural relevancy of a diabetes prevention nutrition program for African American women, Health Promot. Pract 7 (1) (2006) 56–67. [DOI] [PubMed] [Google Scholar]
- [65].Hill J, et al. , Roles, responsibilities and characteristics of lay community health workers involved in diabetes prevention programmes: a systematic review, PLoS One 12 (12) (2017), e0189069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Horowitz CR, et al. , Effectively translating diabetes prevention: a successful model in a historically underserved community, Transl. Behav. Med 1 (3) (2011) 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Parikh P, et al. , Results of a pilot diabetes prevention intervention in East Harlem, New York City: project HEED, Am. J. Public Health 100 (Suppl. 1) (2010) S232–S239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mayer VL, et al. , Outcomes of a weight loss intervention to prevent diabetes among low-income residents of East Harlem, New York, Health Educ. Behav 46 (6) (2019) 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lim S, et al. , A culturally adapted diabetes prevention intervention in the New York City Sikh Asian Indian community leads to improvements in health behaviors and outcomes, Health Behav. Res (2019) 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gore R, et al. , Integrating community health workers into safety-net primary care for diabetes prevention: qualitative analysis of clinicians’ perspectives, J. Gen. Intern. Med 35 (4) (2020) 1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fisher EB, et al. , Peer support of complex health behaviors in prevention and disease management with special reference to diabetes: systematic reviews, Clin. Diabetes Endocrinol 3 (2017) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


