Abstract
Osteoarthritis (OA) and rheumatoid arthritis (RA) are two of the most common types of arthritis. Both are characterized by the infiltration of a number of proinflammatory cytokines into the joint microenvironment. miRNAs play critical roles in the disease processes of arthritic disorders. However, little is known about the effects of miRNAs on critical inflammatory cytokine production with OA and RA progression. Here, we found higher levels of proinflammatory cytokines including interleukin 1 beta (IL-1β), interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) in human OA and RA synovial fibroblasts (SFs) compared with normal SFs. Searches of open-source microRNA (miRNA) software determined that miR-let-7c-5p and miR-149-5p interfere with IL-1β, IL-6 and TNF-α transcription; levels of all three proinflammatory cytokines were lower in human OA and RA patients compared with normal controls. Anti-inflammatory agents dexamethasone, celecoxib and indomethacin reduced proinflammatory cytokine production by promoting the expression of miR-let-7c-5p and miR-149-5p. Similarly, ibuprofen and methotrexate also enhanced miR-let-7c-5p and miR-149-5p expression in human SFs. The evidence suggests that increasing miR-let-7c-5p and miR-149-5p expression is a novel strategy for OA and RA.
Keywords: miR-let-7c-5p, miR-149-5p, osteoarthritis, rheumatoid arthritis, inflammation
INTRODUCTION
Osteoarthritis (OA) and rheumatoid arthritis (RA) feature synovial inflammation and damage to articular cartilage, as well as pathological changes in subchondral bone [1]. Anti-inflammatories (NSAIDs and corticosteroids) are typically used to reduce ongoing inflammation and relieve the pain induced by arthritis [2, 3]. Patients with arthritis live with low-grade, chronic joint inflammation that perpetuates the release of inflammatory mediators, with ever-worsening damage to cartilage, bone and synovium [4, 5].
The activities of proinflammatory cytokines interleukin 1 beta (IL-1β), IL-6 and tumor necrosis factor alpha (TNF-α) contribute to the pathogenesis of arthritis by promoting proteolytic enzyme activity that damages the cartilage extracellular matrix [6, 7]. IL-1β, IL-6 and TNF-α levels in human arthritic serum and synovial fluid are higher than those of healthy controls and have been targeted by therapies including the IL-1β inhibitor canakinumab, the IL-6 inhibitor tocilizumab, and the TNF-α inhibitor infliximab [8]. Reducing proinflammatory cytokine activity has shown merit as a therapeutic strategy to reduce arthritis progression [6].
The progression of arthritis disease is regulated by several microRNAs (miRNAs), including miR-92a, miR-129-3p, miR-141-3p and miR-199a-5p [9–12], while the proinflammatory mediators IL-1β, IL-6, TNF-α and matrix metalloproteinases (MMPs) account for histological changes that occur with arthritis [13–15]. However, how miRNAs might regulate the progression of OA and RA remains unclear. We describe finding higher levels of proinflammatory cytokines in OA synovial fibroblasts (OASFs) and RASFs than in normal SFs (NSFs), while miR-let-7c-5p and miR-149-5p were associated with reductions in proinflammatory cytokine expression. Treatment of OASFs and RASFs with anti-inflammatory agents enhanced levels of miR-let-7c-5p and miR-149-5p expression and reduced proinflammatory responses. Thus, finding ways to promote the expression of these miRNAs may be of therapeutic benefit for patients with OA or RA.
RESULTS
Higher levels of proinflammatory cytokines in OA and RA patients
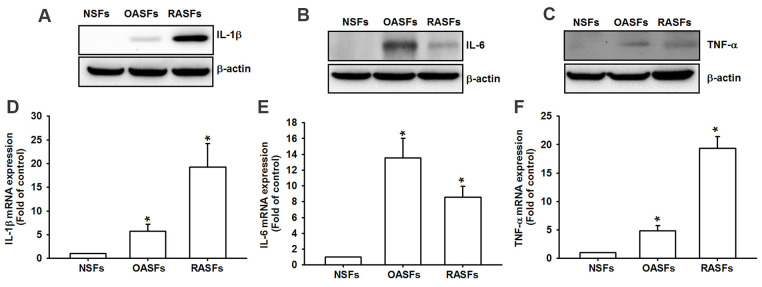
Similarly to previous research [8], we found markedly higher levels of IL-1β, IL-6 and TNF-α protein and mRNA in OASFs and RASFs than in NSFs (Figure 1), indicating that these cytokines regulate OA and RA progression.
Figure 1.
Higher levels of proinflammatory cytokines in OA and RA synovial fibroblasts. Levels of IL-1β, IL-6 and TNF-α protein (A–C) and mRNA expression (D–F) in NSFs, OASFs and RASFs were examined by Western blot and qPCR assays.
miR-let-7c-5p and miR-149-5p inhibit proinflammatory cytokine expression
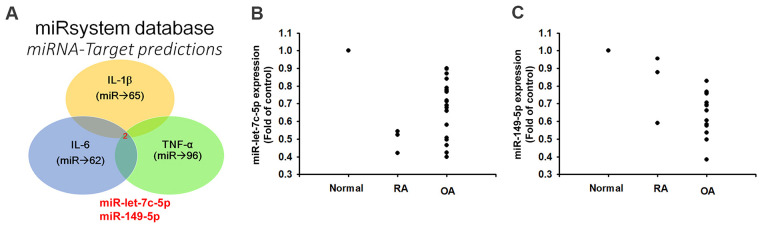
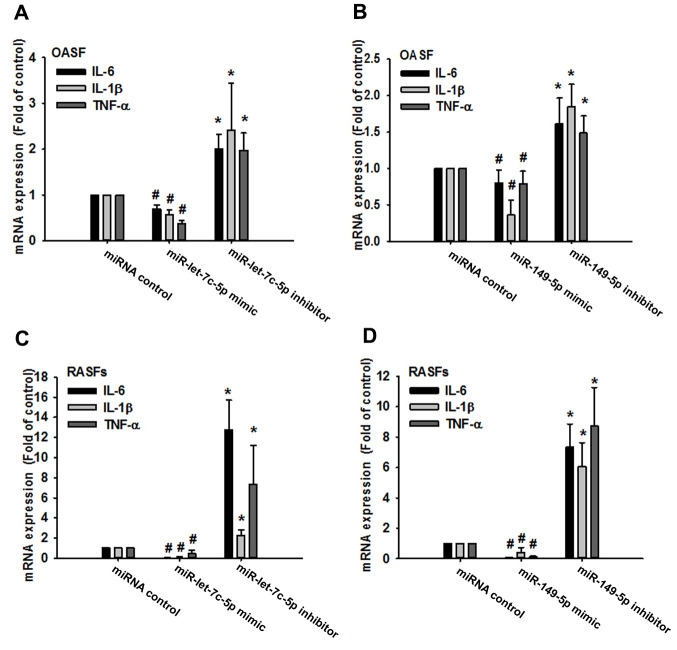
Records from the miRWalk, miRanda, and TargetScan online tools identified that 3’-UTRs of IL-1β, IL-6 and TNF-α mRNAs harbor potential binding sites for miR-let-7c-5p and miR-149-5p (Figure 2A). Levels of both miRNAs were lower in OA and RA patients than in normal controls (Figure 2B, 2C), indicating that these miRNAs are negatively correlated with the expression of IL-1β, IL-6 and TNF-α. Moreover, levels of all three cytokines were reduced in OASFs and RASFs transfected with the respective mimics of miR-let-7c-5p and miR-149-5p, whereas transfection with the respective inhibitors had the opposite effect (Figure 3), which suggests that miR-let-7c-5p and miR-149-5p negatively control the production of IL-1β, IL-6 and TNF-α.
Figure 2.
Lower levels of miR-let-7c-5p and miR-149-5p expression in OA and RA patients compared with normal controls. (A) Open-source software (miRWalk, miRanda, and TargetScan) sought to identify miRNAs that could possibly interfere with IL-1β, IL-6 and TNF-α transcription. (B, C) Levels of miR-let-7c-5p and miR-149-5p expression in normal controls (n=5), OA (n=3) and RA (n=12) patients were examined by qPCR.
Figure 3.
MiR-let-7c-5p and miR-149-5p regulate IL-1β, IL-6 and TNF-α expression in OASFs and RASFs. After transfecting OASFs (A, B) and RASFs (C, D) with the miR-let-7c-5p and miR-149-5p mimics or their respective inhibitors, IL-1β, IL-6 and TNF-α expression was examined by qPCR.
Anti-inflammatory agents reduce proinflammatory cytokine expression by increasing miR-let-7c-5p and miR-149-5p synthesis
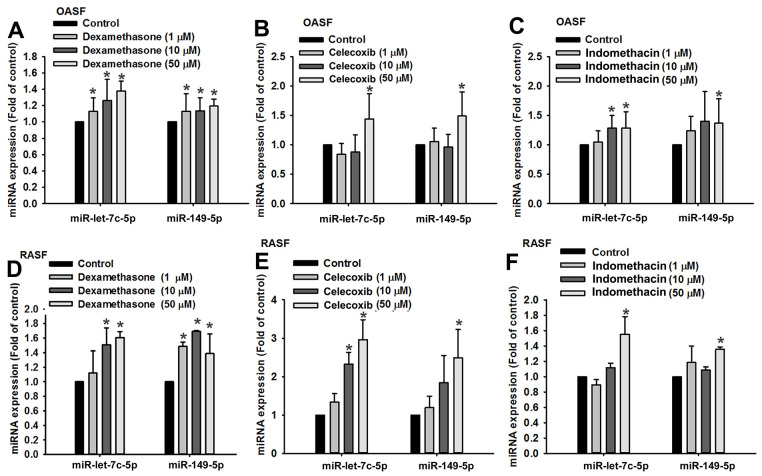
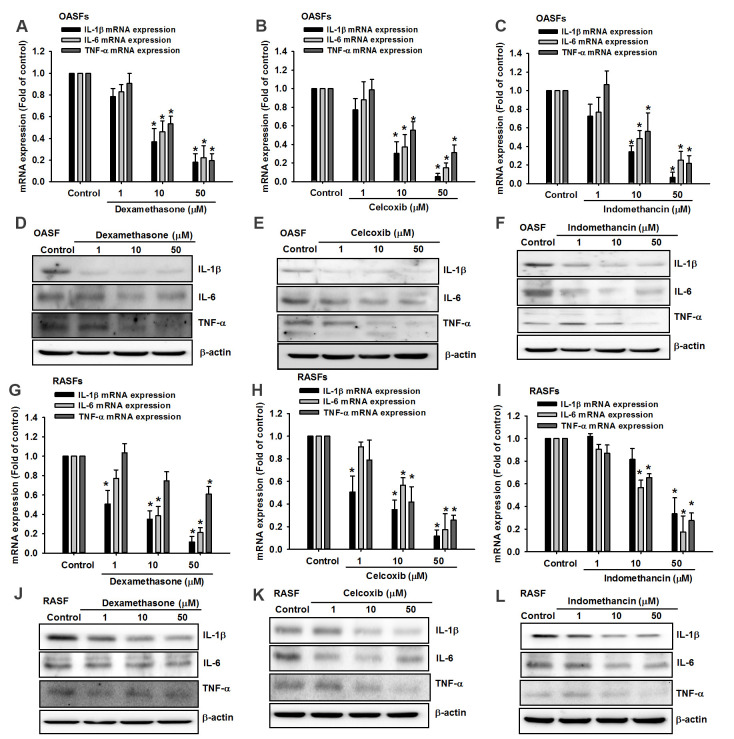
Dexamethasone (a corticosteroid), celecoxib (a selective cyclooxygenase-2 inhibitor) and indomethacin (an NSAID) are all anti-inflammatory agents that are used to treat arthritis [16]. In this study, treatment of OASFs and RASFs with dexamethasone, celecoxib, or indomethacin facilitated the synthesis of both miR-let-7c-5p and miR-149-5p (Figure 4) and concentration-dependently reduced protein and mRNA levels of IL-1β, IL-6 and TNF-α (Figure 5). Thus, these anti-inflammatory agents reduce proinflammatory cytokine production in human synovial fibroblasts by enhancing miR-let-7c-5p and miR-149-5p expression (Figure 6). Similarly, ibuprofen (another commonly used NSAID) and methotrexate (considered to be the gold standard disease-modifying antirheumatic drug [DMARD]), also enhanced the expression of these miRNAs in human synovial fibroblasts (Supplementary Figure 1).
Figure 4.
Anti-inflammatory agents upregulate miR-let-7c-5p and miR-149-5p expression. OASFs (A–C) and RASFs (D–F) were treated with dexamethasone, celecoxib, or indomethacin (1–50 μM), then subjected to qPCR quantification of miR-let-7c-5p and miR-149-5p expression.
Figure 5.
Anti-inflammatory agents reduce proinflammatory cytokine production in OASFs and RASFs. After treating OASFs (A–F) and RASFs (G–L) with dexamethasone, celecoxib, or indomethacin (1–50 μM), Western blot and qPCR assays quantified the levels of IL-1β, IL-6 and TNF-α expression.
Figure 6.
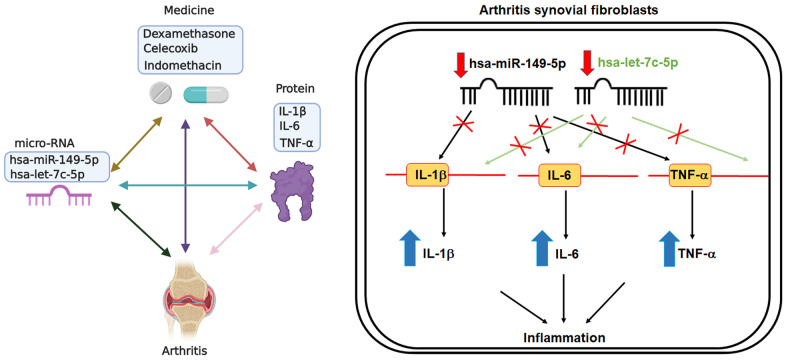
Schematic diagram summarizes the effects of miR-let-7c-5p and miR-149-5p in OA ad RA. Treatment of OASFs and RASFs with anti-inflammatory agents upregulates levels of miR-let-7c-5p and miR-149-5p expression, leading to decreases in the expression of proinflammatory cytokines (IL-1β, IL-6 and TNF-α). Thus, enhancing miR-let-7c-5p and miR-149-5p expression may be a novel therapeutic avenue for halting the progression of OA and RA disease.
DISCUSSION
OA and RA are well known for their major characteristics of synovial inflammation and joint destruction [17–19]. OASFs and RASFs in the joint microenvironment are important contributors to the disease processes, producing proinflammatory cytokines that lead to cartilage degradation and bone breakdown [20]. Several commercially available arthritis therapeutics target these proinflammatory cytokines. Amongst the many reports of different associations of human miRNAs and particular diseases, evidence attests to the efficiency of miRNA binding with their target regions and post-transcriptional regulation of target protein expression [21, 22]. However, little is known about the effect of miRNAs on critical inflammatory cytokine production and OA and RA progression. Our study results describe how OASFs and RASFs contain higher levels of proinflammatory cytokines compared with NSF levels. We also found that miR-let-7c-5p and miR-149-5p negatively regulate levels of IL-1β, IL-6 and TNF-α expression. The therapeutic effects of anti-inflammatory agents used in this study were associated with their inhibition of miR-let-7c-5p and miR-149-5p expression, indicating that modulation of these miRNAs may be a novel strategy for reducing OA and RA inflammation.
MiRNAs post-transcriptionally regulate gene expression [23]. During OA and RA progression, aberrant miRNA expression mediates inflammatory pathway signaling [24, 25]. Our examination of open-source database records identified that miR-let-7c-5p and miR-149-5p potentially interfere with the transcription of IL-1β, IL-6 and TNF-α. The levels of both miRNAs were lower in OA and RA patients than in normal controls, indicating negative correlations between miR-let-7c-5p and miR-149-5p and proinflammatory cytokine expression. There was no evidence of sequence similarity between these miRNAs. Other evidence has shown that miR-let-7c-5p inhibits the proliferation and migration of cervical carcinoma cells [26] and negatively regulates NLRC5 protein expression in ethanol-induced hepatic injury [27]. Interestingly, miR-149-5p is capable of inhibiting the growth of gastric cancer, cholangiocarcinoma and glioblastoma [28–30]. MiR-149-5p can also inhibit M1 macrophage-associated inflammation in experimental abdominal aortic aneurysm formation [31]. In this study, we enhanced miR-let-7c-5p and miR-149-5p levels in OASFs and RASFs by transfecting them with their respective miRNA mimics, which also reduced IL-1β, IL-6 and TNF-α production. In contrast, transfection of OASFs and RASFs with miR-let-7c-5p and miR-149-5p inhibitors downregulated the levels of both miRNAs. Thus, our evidence has identified novel anti-inflammatory functions in association with miR-let-7c-5p and miR-149-5p. Numerous signaling pathways, including the MAPK, PI3K/Akt and PKC pathways, control miRNA synthesis during the progression of arthritis disease [9–12]. We did not include the upstream molecules of miR-let-7c-5p and miR-149-5p in this investigation. Further research is needed to determine whether their expression is regulated by the same signaling cascades.
Anti-inflammatory agents (e.g., corticosteroids, COX-2 selective inhibitors and NSAIDs), are well recognized for their modulation of inflammatory responses in different inflammatory diseases, including arthritis [16]. Our study results confirm that dexamethasone, celecoxib and indomethacin effectively lower the synthesis of arthritic inflammatory cytokines, including IL-1β, IL-6 and TNF-α, in both OASFs and RASFs, by upregulating miR-let-7c-5p and miR-149-5p expression. Our genetic and pharmacologic investigations suggest that enhancing the levels of these miRNAs may be a novel avenue for treating OA and RA.
MATERIALS AND METHODS
Materials
Antibodies for IL-1β, IL-6 and TNF-α were obtained from GeneTex International Corporation. Lipofectamine® 2000 and Trizol® were acquired from Life Technologies. The miRNA mimic, inhibitor and negative control and Dharmafect1 were purchased from Dharmacon. β-Actin antibody and all other chemicals not already mentioned were acquired from Sigma-Aldrich.
Cell preparation
Human NSFs (primary fibroblast-like synoviocytes; 408-05A) were obtained from Cell Applications, Inc. (San Diego, CA, USA). Human RASFs were purchased from the Riken Cell Bank (Ibaraki, Japan). Human OASFs were isolated from synovial tissues of 10 OA patients by collagenase treatment, using previously detailed procedures [32]. All cells were maintained in DMEM (containing 10% FBS and antibiotics) in a 5% CO2 incubator (at 37° C).
Human synovial tissues
Study approval was granted by the Institutional Review Board of China Medical University Hospital (Taichung, Taiwan) and all patients provided written informed consent before participating in the study. Synovial tissue samples were obtained from patients undergoing total knee arthroplasty for OA or RA and also from those undergoing arthroscopic procedures for trauma/joint derangement (healthy controls).
Western blot
SDS-PAGE was used to resolve the extracted proteins, which were transferred to PVDF membranes, as described in our previous publications [33–35]. Membranes were blocked for 1 h with PBST containing 4% non-fat milk, then treated with antibodies targeting IL-1β, IL-6 and TNF-α for 1 h, before being incubated for 1 h with HRP-conjugated secondary antibodies. We visualized the blot membranes using a Fujifilm LAS-3000 imaging system.
Quantitative real-time PCR
All RNA was collected from SFs using TRIzol™ Reagent. We generated cDNA using an Invitrogen reverse transcription kit. qPCR analysis was conducted with the Taqman® One-Step RT-PCR Master Mix. Mir-X™ miRNA First-Strand Synthesis and the SYBR® RT-PCR kit were used for reverse transcription of miRNA. Analysis was carried out according to a previous protocol [36–38].
Transfection
Synthetic miRNA mimics and inhibitors (10 nM) were transfected into OASFs and RASFs following the Dharmafect1 transfection protocol. After 24 h of transfection, inflammatory cytokine expression was examined by qPCR.
Statistics
All values are presented as the mean ± standard deviation of 5 independent experiments. Differences between two experimental groups were assessed for significance using the Student’s t-test and considered to be significant if the p value was < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank Iona J. MacDonald from China Medical University, Taichung, Taiwan, for her English language revision of this manuscript.
Footnotes
AUTHOR CONTRIBUTIONS: Y.-Y. Law, J.-F.L. and C.-H.T. conceived and designed the experiments, which were performed by J.-F.L., W.-F.L. and Y.-Y.L. Reagents, materials and analysis were provided by C.J.H., C.-Y.H., C.-C.H., C.-H.T. and M.-H.W. The paper was written by J.-F.L. and C.-H.T. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This work was supported by grants from the Ministry of Science and Technology of Taiwan (108-2320-B-039-065 and MOST 107-2320-B-039-019-MY3), China Medical University Hospital (DMR-110-106 and DMR-110-022) and Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-106-0402).
REFERENCES
- 1.Wang CJ, Cheng JH, Chou WY, Hsu SL, Chen JH, Huang CY. Changes of articular cartilage and subchondral bone after extracorporeal shockwave therapy in osteoarthritis of the knee. Int J Med Sci. 2017; 14:213–23. 10.7150/ijms.17469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorais M, Martel-Pelletier J, Raynauld JP, Delorme P, Pelletier JP. Impact of oral osteoarthritis therapy usage among other risk factors on knee replacement: a nested case-control study using the Osteoarthritis Initiative cohort. Arthritis Res Ther. 2018; 20:172. 10.1186/s13075-018-1656-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012; 85:49–56. [PubMed] [Google Scholar]
- 4.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013; 21:16–21. 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 5.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013; 5:77–94. 10.1177/1759720X12467868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol. 2013; 9:400–10. 10.1038/nrrheum.2013.44 [DOI] [PubMed] [Google Scholar]
- 7.Rengel Y, Ospelt C, Gay S. Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther. 2007; 9:221. 10.1186/ar2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019; 15:612–32. 10.1038/s41584-019-0277-8 [DOI] [PubMed] [Google Scholar]
- 9.Kuo SJ, Liu SC, Huang YL, Tsai CH, Fong YC, Hsu HC, Tang CH. TGF-β1 enhances FOXO3 expression in human synovial fibroblasts by inhibiting miR-92a through AMPK and p38 pathways. Aging (Albany NY). 2019; 11:4075–89. 10.18632/aging.102038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu TJ, Fong YC, Lin CY, Huang YL, Tang CH. Glucose enhances aggrecan expression in chondrocytes via the PKCα/p38-miR141-3p signaling pathway. J Cell Physiol. 2018; 233:6878–87. 10.1002/jcp.26451 [DOI] [PubMed] [Google Scholar]
- 11.Wu MH, Tsai CH, Huang YL, Fong YC, Tang CH. Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. Int J Mol Sci. 2018; 19:190. 10.3390/ijms19010190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai CH, Liu SC, Wang YH, Su CM, Huang CC, Hsu CJ, Tang CH. Osteopontin inhibition of miR-129-3p enhances IL-17 expression and monocyte migration in rheumatoid arthritis. Biochim Biophys Acta Gen Subj. 2017; 1861:15–22. 10.1016/j.bbagen.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 13.Malemud CJ. MicroRNAs and Osteoarthritis. Cells. 2018; 7:92. 10.3390/cells7080092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan YJ, Li J, Yang X, Du S, Ding J, Gao Y, Zhang Y, Yang K, Chen Q. Evidence that miR-146a attenuates aging- and trauma-induced osteoarthritis by inhibiting Notch1, IL-6, and IL-1 mediated catabolism. Aging Cell. 2018; 17:e12752. 10.1111/acel.12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Zhao L, Fan Y, Liao L, Ma PX, Xiao G, Chen D. The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat Commun. 2019; 10:2876. 10.1038/s41467-019-10753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang CH. Research of Pathogenesis and Novel Therapeutics in Arthritis. Int J Mol Sci. 2019; 20:1646. 10.3390/ijms20071646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011; 365:2205–19. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 18.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016; 388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 19.Catrina AI, Svensson CI, Malmström V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol. 2017; 13:79–86. 10.1038/nrrheum.2016.200 [DOI] [PubMed] [Google Scholar]
- 20.Lefevre S, Meier FM, Neumann E, Muller-Ladner U. Role of synovial fibroblasts in rheumatoid arthritis. Curr Pharm Des. 2015; 21:130–41. 10.2174/1381612820666140825122036 [DOI] [PubMed] [Google Scholar]
- 21.Tüfekci KU, Oner MG, Meuwissen RL, Genç S. The role of microRNAs in human diseases. Methods Mol Biol. 2014; 1107:33–50. 10.1007/978-1-62703-748-8_3 [DOI] [PubMed] [Google Scholar]
- 22.Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013; 42:217–39. 10.1146/annurev-biophys-083012-130404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nugent M. MicroRNAs: exploring new horizons in osteoarthritis. Osteoarthritis Cartilage. 2016; 24:573–80. 10.1016/j.joca.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Wu H, Zhao M, Chang C, Lu Q. Clinical significance of miRNAs in autoimmunity. J Autoimmun. 2020; 109:102438. 10.1016/j.jaut.2020.102438 [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Yan S, Yang J, Lu H, Xu D, Wang Z. Non-coding RNAs in Rheumatoid Arthritis: From Bench to Bedside. Front Immunol. 2020; 10:3129. 10.3389/fimmu.2019.03129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao L, Wang R, Sun Y, Yue Z, Sun H, Wang X, Wang P, Sun G, Hu J, Sun H, Xie S, Li Y. Delivery of MicroRNA-let-7c-5p by Biodegradable Silica Nanoparticles Suppresses Human Cervical Carcinoma Cell Proliferation and Migration. J Biomed Nanotechnol. 2020; 16:1600–11. 10.1166/jbn.2020.2989 [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Li M, Shen Z, Bu F, Yu H, Pan X, Yang Y, Meng X, Huang C, Li J. The Long Non-coding RNA MEG3/miR-let-7c-5p Axis Regulates Ethanol-Induced Hepatic Steatosis and Apoptosis by Targeting NLRC5. Front Pharmacol. 2018; 9:302. 10.3389/fphar.2018.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hua Y, Wang H, Wang H, Wu X, Yang L, Wang C, Li X, Jin Y, Li M, Wang L, Dong C, Yin F. Circular RNA Circ_0006282 Promotes Cell Proliferation and Metastasis in Gastric Cancer by Regulating MicroRNA-144-5p/Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein β Axis. Cancer Manag Res. 2021; 13:815–27. 10.2147/CMAR.S283952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu W, Yu G, Liang J, Fan P, Dong K, Zhang B, Chen X, Zhu H, Chu L. miR-144-5p and miR-451a Inhibit the Growth of Cholangiocarcinoma Cells Through Decreasing the Expression of ST8SIA4. Front Oncol. 2021; 10:563486. 10.3389/fonc.2020.563486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu GM, Lu TC, Sun ML, Jia WY, Ji X, Luo YG. Ginsenoside Rd Inhibits Glioblastoma Cell Proliferation by Up-Regulating the Expression of miR-144-5p. Biol Pharm Bull. 2020; 43:1534–41. 10.1248/bpb.b20-00338 [DOI] [PubMed] [Google Scholar]
- 31.Shi X, Ma W, Li Y, Wang H, Pan S, Tian Y, Xu C, Li L. MiR-144-5p limits experimental abdominal aortic aneurysm formation by mitigating M1 macrophage-associated inflammation: Suppression of TLR2 and OLR1. J Mol Cell Cardiol. 2020; 143:1–14. 10.1016/j.yjmcc.2020.04.008 [DOI] [PubMed] [Google Scholar]
- 32.Huang CC, Chiou CH, Liu SC, Hu SL, Su CM, Tsai CH, Tang CH. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J Pineal Res. 2019; 66:e12560. 10.1111/jpi.12560 [DOI] [PubMed] [Google Scholar]
- 33.Lee HP, Chen PC, Wang SW, Fong YC, Tsai CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, Li TM, Tang CH. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. J Funct Foods. 2019; 52:537–44. 10.1016/j.jff.2018.11.040 [DOI] [Google Scholar]
- 34.Lee HP, Wang SW, Wu YC, Lin LW, Tsai FJ, Yang JS, Li TM, Tang CH. Soya-cerebroside inhibits VEGF-facilitated angiogenesis in endothelial progenitor cells. Food Agric Immunol. 2020; 31:193–204. 10.1080/09540105.2020.1713055 [DOI] [Google Scholar]
- 35.Lee HP, Wang SW, Wu YC, Tsai CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, Yin MC, Li TM, Tang CH. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric Immunol. 2019; 30:1033–45. 10.1080/09540105.2019.1660623 [DOI] [Google Scholar]
- 36.Lee HP, Wu YC, Chen BC, Liu SC, Li TM, Huang WC, Hsu CJ, Tang CH. Soya-cerebroside reduces interleukin production in human rheumatoid arthritis synovial fibroblasts by inhibiting the ERK, NF-κB and AP-1 signalling pathways. Food Agric Immunol. 2020; 31:740–50. 10.1080/09540105.2020.1766426 [DOI] [Google Scholar]
- 37.Liu SC, Tsai CH, Wu TY, Tsai CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, Yin MC, Wu YC, Tang CH. Soya-cerebroside reduces IL-1 beta-induced MMP-1 production in chondrocytes and inhibits cartilage degradation: implications for the treatment of osteoarthritis. Food Agric Immunol. 2019; 30:620–32. 10.1080/09540105.2019.1611745 [DOI] [Google Scholar]
- 38.Lin CC, Chen KB, Tsai CH, Tsai FJ, Huang CY, Tang CH, Yang JS, Hsu YM, Peng SF, Chung JG. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via Ras/Akt/NF-κB signaling pathways. J Food Biochem. 2019; 43:e12902. 10.1111/jfbc.12902 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.