Abstract
Genetic background has been considered one of the important contributors to the rate of cognitive decline among patients with Alzheimer’s disease (AD). We conducted a 4-year longitudinal follow-up study, recruited 255 AD and 44 mild cognitive impairment (MCI) patients, and used a data-driven trajectory analysis to examine the influence of selected AD risk genes on the age for and the rate of cognitive decline in Han Chinese population. Genotyping of selected single-nucleotide polymorphisms in the APOE, ABCA7, SORL1, BIN1, GAB2, and CD33 genes was conducted, and a Bayesian hierarchical model was fitted to analyze the trajectories of cognitive decline among different genotypes. After adjusting for sex and education years, the APOE ε4 allele was associated with an earlier mean change of −2.39 years in the age at midpoint of cognitive decline, the G allele in ABCA7 rs3764650 was associated with an earlier mean change of −1.75 years, and the T allele in SORL1 rs3737529 was associated with a later mean change of 2.6 years. Additionally, the rate of cognitive decline was associated with the APOE ε4 allele and SORL1 rs3737529. In summary, APOE and SORL1 might be the most important genetic factors related to cognitive decline in Han Chinese population.
Keywords: Alzheimer's disease, trajectory analysis, APOE ɛ4 allele, ABCA7 gene, SORL1 gene
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by progressive cognitive decline and functional impairment. The rate of cognitive decline varies substantially among different AD patients. There are many factors that contribute to the rate of cognitive decline, such as age of disease onset, gender, education, extrapyramidal signs, behavioral disorders, vascular risk factors and the most known AD susceptibility genetic locus, apolipoprotein E (APOE) genotypes [1, 2]. Among these factors, genetic background is one of the most important contributors to the rate of cognitive decline. The results from previous studies have shown that having at least one APOE ε4 allele was associated with faster cognitive decline in cognitively healthy older Taiwanese adults [3], and PDE7A and MTFR1 genes were associated with the rate of age-related cognitive decline [4]. Moreover, BDNF Val66Met was associated with faster cognitive decline and greater hippocampal atrophy in preclinical AD patients [5]. However, some evidence has revealed that these risk genes may act differently in different populations. For example, in a meta-analysis, AD was significantly associated with variants in ABCA7 in African American participants and with other genes that have been associated with AD in individuals of European ancestry [6]. In addition, the ABCA7 rs3764650 GG genotype was reported to increase the risk of AD in Caucasians and African Americans, but a protective effect was found in the Han Chinese population in our prior study [7]. In a study validating genome-wide association studies (GWAS)-identified risk loci based on Caucasians populations showed that not all of these risk loci were linked to the risk of AD in Han Chinese populations [8]. Different results regarding APOE was also found among white, black or Hispanic respondents [9]. If the association between genotypes and AD risk varies by race/ethnicity, then this may be the case for the rate of cognitive decline as well. Hence, the effect of genetic factors on the rate of cognitive decline should be addressed in different ethnic groups [10].
Recent GWAS have identified more than 20 AD susceptibility loci, including CR1, CLU, PICALM, BIN1, CD2AP, CD33, EPHA1, MS4A6A/MS4A4E, SORL1, GAB2, ABCA7, etc [11–18]. However, most of these studies have conducted in Caucasian population and data about the genetics of AD from other populations has been relatively limited [10]. In Han Chinese or Asian population, common variants in GCH1, KCNJ15 [19], and rare missense variant in the C7 genes [20] were also identified by whole-genome sequencing and whole-exome sequencing studies. In our previous studies, we identified ABCA7 rs3764650 and SORL1 rs1784933 as being associated with the risk of AD in Han Chinese individuals in Taiwan [7, 21], the association between BIN1 rs744373 and AD was reported in Asian populations [8], but the effect of these AD risk genes on the rate of cognitive decline is not clear. In this study, we used a data-driven trajectory analysis to examine the influence of selected AD risk genes on the age for and the rate of cognitive decline in the Han Chinese population.
RESULTS
A total of 299 patients—255 with AD and 44 with MCI—were included in this study. The baseline demographic and genetic data of the study participants are shown in Table 1. At baseline, the mean age of the study participants at study entry was 78.4 ± 7.0 years, 51.8% of the participants were women, and the mean MMSE score was 20.0 ± 4.9. The genotypic distributions of all SNPs were consistent with Hardy-Weinberg equilibrium (all p-values > 0.05).
Table 1. Baseline demographics and genetic characteristics of the study participants.
| Variables | Total patients (n = 299) | AD patients (n = 255) | MCI patients (n = 44) |
| Age (years) | 78.4 ± 7.0 | 79.3 ± 6.5 | 73.3 ± 7.7 |
| Sex (women) | 155 (51.8) | 134 (52.6) | 21 (47.7) |
| Education (years) | 9.5 ± 4.6 | 9.2 ± 4.6 | 11.5 ± 4.3 |
| MMSE (scores) | 20.0 ± 4.9 | 19.2 ± 4.7 | 24.7 ± 3.0 |
| APOE genotypes | |||
| ε2ε2/ε2ε3/ε3ε3 | 195 (65.2) | 166 (65.1) | 29 (65.9) |
| ε2ε4/ε3ε4 | 93 (31.1) | 81 (31.8) | 12 (27.3) |
| ε4ε4 | 11 (3.7) | 8 (3.1) | 3 (6.8) |
| ABCA7 rs3764650 | |||
| TT | 135 (45.2) | 111 (43.5) | 24 (54.6) |
| TG | 128 (42.8) | 113 (44.3) | 15 (34.1) |
| GG | 36 (12.0) | 31 (12.2) | 5 (11.4) |
| SORL1 rs3737529 | |||
| CC | 183 (61.2) | 150 (58.8) | 33 (75.0) |
| CT | 99 (33.1) | 90 (35.3) | 9 (20.5) |
| TT | 17 (5.7) | 15 (5.9) | 2 (4.6) |
| SORL1 rs1784933 | |||
| AA | 139 (46.5) | 114 (44.7) | 25 (56.8) |
| AG | 134 (44.8) | 118 (46.3) | 16 (36.4) |
| GG | 26 (8.7) | 23 (9.0) | 3 (6.8) |
| SORL1 rs2298813 | |||
| GG | 251 (84.0) | 215 (84.3) | 36 (81.8) |
| GA | 45 (15.1) | 38 (14.9) | 7 (15.9) |
| AA | 3 (1.0) | 2 (0.8) | 1 (2.3) |
| BIN1 rs744373 | |||
| AA | 104 (34.8) | 90 (35.3) | 14 (31.8) |
| AG | 142 (47.5) | 117 (45.9) | 25 (56.8) |
| GG | 53 (17.7) | 48 (18.8) | 5 (11.4) |
| GAB2 rs2373115 | |||
| CC | 139 (46.5) | 125 (49.0) | 14 (31.8) |
| CA | 120 (40.1) | 97 (38.0) | 23 (52.3) |
| AA | 40 (13.4) | 33 (12.9) | 7 (15.9) |
| CD33 rs3865444 | |||
| CC | 203 (67.9) | 173 (67.8) | 30 (68.2) |
| CA | 82 (27.4) | 71 (27.8) | 11 (25.0) |
| AA | 14 (4.7) | 11 (4.3) | 3 (6.8) |
The data are expressed as the mean ± SD or n (%).
Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; APOE, apolipoprotein E.
After adjusting for sex and education years, the effects of all AD genetic markers on the parameters M and R of the trajectories of cognitive function decline based on the MMSE are shown in Table 2. Regarding the age at the midpoint of the cognitive function decline, one copy of the ε4 allele in the APOE gene was significantly associated with an earlier mean change of −2.39 years (95% CI: −4.12, −0.64); one copy of the G allele in ABCA7 rs3764650 was significantly associated with an earlier mean change of −1.75 years (95% CI: −3.09, −0.37); one copy of the T allele in SORL1 rs3737529 was significantly associated with a later mean change of 2.60 years (95% CI: 0.38, 4.88). Moreover, the rate of cognitive decline was associated with the APOE gene (posterior mean = 0.96, 95% CI: 0.39, 1.57) and SORL1 rs3737529 (posterior mean = 0.58, 95% CI: 0.05, 1.14). No statistically significant associations were observed between either the age at midpoint or the rate of cognitive decline and the genetic markers of SORL1 rs1784933, SORL1 rs2298813, BIN1 rs744373, GAB2 rs2373115, and CD33 rs3865444.
Table 2. Effects of genetic markers on the age at the midpoint of cognitive function decline (M) and the rate of cognitive function decline (R) parameters based on the MMSE after adjusting for sex and education years (n = 299).
| Covariates | Minor allele | Regression coefficients on M | Regression coefficients on R | |||
| Posterior mean | 95% CI | Posterior mean | 95% CI | |||
| Sex (female vs. male) | — | −1.11 | (−3.05, 0.85) | 0.62 | (0.16, 1.14)* | |
| Education years | — | 0.37 | (0.15, 0.58)* | −0.05 | (−0.09, 0.001) | |
| APOE | ε4 | −2.39 | (−4.12, −0.64)* | 0.96 | (0.39, 1.57)* | |
| ABCA7 rs3764650 | G | −1.75 | (−3.09, −0.37)* | −0.20 | (−0.55, 0.14) | |
| SORL1 rs3737529 | T | 2.60 | (0.38, 4.88)* | 0.58 | (0.05, 1.14)* | |
| SORL1 rs1784933 | G | 1.04 | (−1.02, 3.03) | 0.12 | (−0.38, 0.58) | |
| SORL1 rs2298813 | A | 0.91 | (−1.58, 3.52) | −0.06 | (−0.62, 0.55) | |
| BIN1 rs744373 | G | −0.46 | (−1.74, 0.81) | −0.20 | (−0.47, 0.09) | |
| GAB2 rs2373115 | A | −0.25 | (−1.58, 1.07) | 0.10 | (−0.25, 0.47) | |
| CD33 rs3865444 | A | 1.43 | (−0.20, 3.08) | −0.07 | (−0.49, 0.39) | |
Note: The full sample of 255 AD and 44 MCI patients at baseline were analyzed.
Abbreviations: MMSE, Mini-Mental State Examination; CI, credible interval.
*Statistically significant with 95% credible interval.
To assess which variable provided the best fit to the longitudinal data from the MMSE, we then implemented a Bayesian variable selection using the Gibbs variable selection method. The results of model selection among the eight genetic markers are summarized in Table 3. Sex and education variables were included in each model. The results revealed that, compared with other models, the APOE gene and SORL1 rs3737529 in model 1 had the best fit to the data based on posterior model probabilities. We again evaluated these two genetic markers in the Bayesian hierarchical model. Significant associations and estimated directions were consistent with the full model that included all genetic markers (Table 4). Moreover, to check the fitness of a Bayesian model, we then calculated a posterior predictive p-value. The value was 0.937, which indicated that the fitted model was adequate to describe longitudinal data from the MMSE.
Table 3. Model selection among the eight genetic markers after adjusting for sex and education years (n = 299).
| Models | Variables in the model | Posterior model probabilities |
| Model 1 | APOE, SORL1 rs3737529 | 0.8956 |
| Model 2 | APOE, SORL1 rs3737529, SORL1 rs1784933 | 0.0548 |
| Model 3 | APOE, SORL1 rs3737529, CD33 rs3865444 | 0.0243 |
| Model 4 | APOE, SORL1 rs3737529, BIN1 rs744373 | 0.0113 |
| Model 5 | APOE, SORL1 rs3737529, GAB2 rs2373115 | 0.0062 |
| Model 6 | APOE, SORL1 rs3737529, SORL1 rs2298813 | 0.0056 |
| Model 7 | APOE, SORL1 rs3737529, SORL1 rs1784933, CD33 rs3865444 | 0.0023 |
| Model 8 | APOE, SORL1 rs3737529, SORL1 rs1784933, SORL1 rs2298813 | 0.0001 |
Note: The full sample of 255 AD and 44 MCI patients at baseline were analyzed.
Table 4. Effects of APOE and SORL1 rs3737529 on the age at the midpoint of cognitive function decline (M) and the rate of cognitive function decline (R) parameters based on the MMSE after Bayesian variable selection (n = 299).
| Covariates | Minor allele | Regression coefficients on M | Regression coefficients on R | |||
| Posterior mean | 95% CI | Posterior mean | 95% CI | |||
| Sex (female vs. male) | — | −1.05 | (−3.00, 0.94) | 0.61 | (0.12, 1.12)* | |
| Education years | — | 0.38 | (0.18, 0.59)* | −0.04 | (−0.10, 0.01) | |
| APOE | ε4 | −2.79 | (−4.46, −1.09)* | 0.93 | (0.44, 1.49)* | |
| SORL1 rs3737529 | T | 1.53 | (0.02, 3.04)* | 0.41 | (0.02, 0.87)* | |
Note: The full sample of 255 AD and 44 MCI patients at baseline were analyzed.
Abbreviations: MMSE, Mini-Mental State Examination; CI, credible interval.
*Statistically significant with 95% credible interval.
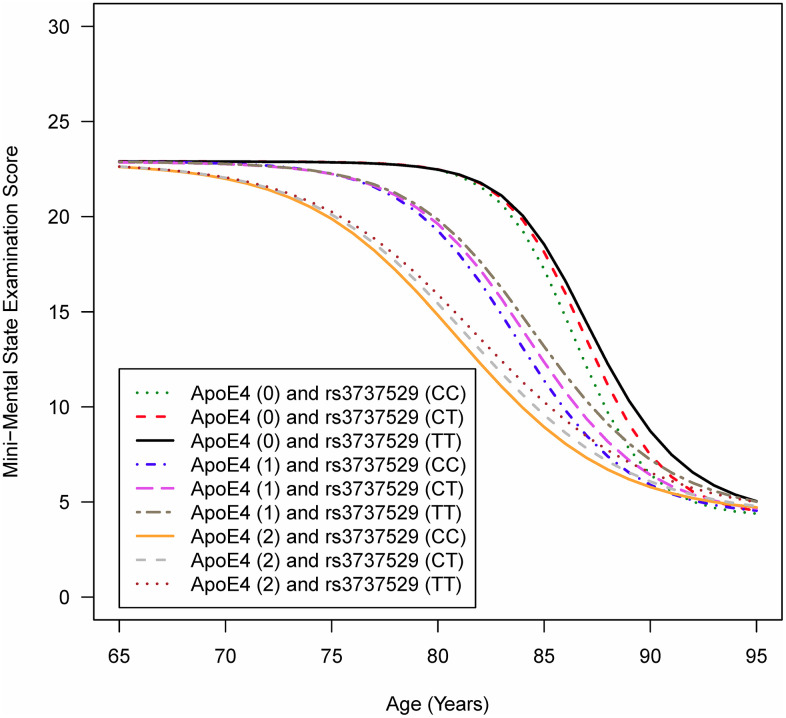
Figure 1 depicts the mean trajectories of cognitive function based on the MMSE for individuals of the same sex and education level carrying specific genotypes in the APOE gene and SORL1 rs3737529. Figure 1 shows that a more rapid decline in cognitive function was observed for individuals possessing two copies of the ε4 allele in the APOE gene and the CC genotype in SORL1 rs3737529. In other words, people with the ε4 allele and C allele tended to have earlier cognitive function decline than those with the non-ε4 allele and the T allele.
Figure 1.
Mean trajectories of cognitive function for individuals carrying specific genotypes in the APOE gene and SORL1 rs3737529 SNPa. AIn the figure legend, ApoE4 (0/1/2) means that individuals are carrying zero, one, or two copies of the ε4 allele in the APOE gene; rs3737529 (CC/CT/TT) means that individuals are carrying the CC, CT, or TT genotypes in SORL1 rs3737529. Note: The full sample of 255 AD and 44 MCI patients at baseline were analyzed.
In order to test robustness of our results, we excluded 16 stable MCI patients, who had not progressed to AD during the observation period, from the full sample, and used the subset of 283 samples to validate the results obtained from the full samples. After adjusting for sex and education years, similar results showed that the APOE ε4 allele was significantly associated with an earlier mean change of −2.10 years in the age at midpoint of cognitive decline, the G allele in ABCA7 rs3764650 was significantly associated with an earlier mean change of −1.74 years, and the T allele in SORL1 rs3737529 was significantly associated with a later mean change of 2.65 years. Moreover, the rate of cognitive decline was associated with the APOE ε4 allele and SORL1 rs3737529 (Supplementary Table 1). Model selection and estimation of effects using 283 patients are summarized in Supplementary Tables 2, 3. No matter whether the stable MCI patients were included in the analyzed sample or not, the results shown that APOE gene and SORL1 rs3737529 were associated with the age at midpoint of cognitive function decline and the rate of cognitive function decline.
To validate our results with an independent ADNI cohort, we also fitted a Bayesian hierarchical model to depict the trajectories of cognitive function decline using the ADNI data. The effects of all genetic markers on the parameters M and R of the trajectories of cognitive function decline based on the MMSE after adjusting for sex and education years are shown in Supplementary Table 4. The results revealed that only the APOE gene was associated with the age at midpoint of cognitive function decline.
DISCUSSION
In this study, we demonstrated that the APOE, ABCA7, and SORL1 genes were associated with cognitive function decline in the Han Chinese population. The results revealed that one copy of the ε4 allele in the APOE gene and one copy of the G allele in ABCA7 rs3764650 were significantly associated with earlier midpoints of cognitive decline; one copy of the T allele in SORL1 rs3737529 was significantly associated with a later midpoint of cognitive decline. Additionally, the APOE gene and SORL1 rs3737529 were associated with the rate of cognitive decline. The T allele in SORL1 rs3737529 seems to be protective against cognitive decline independent of APOE genotype. However, in ADNI population, only APOE gene was associated with the age at midpoint of cognitive function decline.
One prior study in North America found that CLU and CR1 were associated with more rapid cognitive decline and that PICALM was associated with an earlier age at the midpoint of cognitive decline in patients with AD. These associations remained after accounting for the effects of APOE and demographic factors [22]. The present study provides additional evidence that different populations have their own risk genes that may influence cognitive decline in AD and MCI patients and demonstrates that measuring cognitive trajectories is a way to test the genetic associations with both age of and rate of cognitive decline.
The relationship between APOE ε4 carriage and the rate of cognitive decline in AD patients has been examined in several studies. However, their results have been inconsistent [23], which might have been due to the distinct genetic effects in different populations and complex interactions between the APOE gene and other genetic and vascular risk factors [2]. Previous studies have shown that the effect of the APOE gene on the risk of cognitive decline and dementia was modified by other genetic factors, including ABCA7 [7], SORL1 [21, 24], PICALM [25], CR1 [26], BIN1 [16], and TREM2 [27], showing complex gene-gene interactions [28]. Although the rate of cognitive decline and the risk of AD are not identical, the presence of complex gene-gene interactions of the APOE gene in both situations should be considered. A previous study using data from the Taiwan Longitudinal Study of Aging showed an association between the APOE genotype and the rate of cognitive decline in a predominantly Han Chinese population [3]. In the present study, the APOE gene ε4 allele was significantly associated with an earlier midpoint of cognitive decline in AD and MCI patients in both the Han Chinese population in Taiwan and ADNI population in America and associated with a rapid rate of cognitive decline in the Han Chinese population.
ABCA7 is an ATP-binding cassette transporter protein mainly expressed in microglia and neurons [29, 30]. The functions of ABCA7 include lipid metabolism, regulation of phagocytosis, and amyloid β (Aβ) production and clearance [31]. The minor allele of rs3764650 in ABCA7 was associated with increased neuritic plaque formation [32] and decreased Aβ levels in cerebrospinal fluid [33]. Clinically, the ABCA7 rs3764650 minor allele was associated with cortical and hippocampal atrophy [34] and with a later age at onset and shorter disease duration [35]. In accordance with these findings in previous studies, our study demonstrated that the minor allele of rs3764650 in ABCA7 was associated with an earlier midpoint of cognitive function decline, which suggested that the ABCA7 gene affects the clinical course of AD from the preclinical stage to the dementia stage.
SORL1 encodes a multidomain-containing, membrane-bound receptor involved in endosomal sorting of proteins between the trans-Golgi network, endosomes and the plasma membrane [36]. In AD, the SORL1 encoded receptor interacts with the amyloid precursor protein (APP) and the Aβ peptide [37], participating in APP trafficking and processing and Aβ destruction [38]. Previous studies have also shown that two SORL1 polymorphisms (rs3824968-A allele and rs2282649-T allele) were related to decreased cerebrospinal fluid (CSF) concentrations of Aβ42 and Aβ40 [39], the rs2070045-G allele was associated with increased CSF tau and more hippocampal atrophy [40], and SORL1 rs11218343 was associated with cognitive performance [41]. In our previous study, SORL1 rs1784933 and rs2298813 were associated with AD and MCI risk in the Han Chinese population in Taiwan [21], and in the present study, SORL1 rs3737529 was associated with the midpoint and the rate of cognitive decline in AD and MCI patients in Han Chinese population. These results suggest that SORL1 plays many different roles in AD pathogenesis that are significantly related to clinical manifestations. The associations between ABCA7 as well as SORL1 and cognitive function decline were found in Han Chinese population, but not in the ADNI data. It might also suggest that these risk genes may act differently in different populations [6–9].
There are some limitations in this study. First, no other cohort can validate our findings in Taiwan currently. Other longitudinal follow-up studies need to be conducted to test these results. Second, the diagnoses of MCI and AD were made according to the clinical criteria without biomarker evidence of Aβ and tau, which may have influenced the diagnostic accuracy. Third, we used the MMSE to evaluate longitudinal cognitive changes, which might underestimate cognitive decline. Forth, we only tested a limited number of SNPs based on the evidence of significant genetic association in some previous studies. Other SNPs may also be associated with the trajectory of cognitive decline, and the genetic effects might be different in different populations. Finally, the sample size of our cohort is relatively small compared to the other longitudinal cohort (such as ADNI cohort). This may limit the generalizability of our findings to general population.
In summary, we used a Bayesian approach to examine the genetic effects on the trajectory of cognitive decline in AD and MCI patients in the Han Chinese population. The carriage of the APOE ε4 genotype and the G allele in ABCA7 rs3764650 were significantly associated with an earlier midpoint of cognitive decline. In contrast, the T allele in SORL1 rs3737529 was significantly associated with a later midpoint of cognitive decline. Additionally, the APOE gene and SORL1 rs3737529 were associated with the rate of cognitive function decline.
MATERIALS AND METHODS
Subjects
Participants were recruited from two teaching hospitals in the Biosignature Study of Alzheimer’s Disease (BSAD). The BSAD is carried out as a subproject of Taiwan Biobank [42] and has been designed as a prospective longitudinal follow-up study at 1-year intervals since 2012 to identify potential biomarkers for early diagnosis of AD in the Han Chinese population. The collected data included patients’ neuropsychological test outcomes, blood biomarkers, brain magnetic resonance imaging, and related clinical characteristics. Overall, 255 AD patients and 44 mild cognitive impairment (MCI) patients with at least four measurements were selected for modeling trajectories of cognitive function decline. For the 44 MCI patients at baseline, 28 patients (63.6%) would progress to AD during the consecutive follow-up period. An AD diagnosis was made during a multidisciplinary consensus meeting according to the clinical criteria for probable AD described by the National Institute on Aging–Alzheimer’s Association [43]. A diagnosis of MCI was made according to the revised criteria established from the consensus report [44, 45]. The cutoff value for the diagnosis of MCI was set at 1.5 standard deviations below the age-adjusted norm for the logical memory test of the Wechsler Memory Scale III [46]. Other inclusion criteria included an age at onset greater than 60 years and the availability of a caregiver who could provide collateral patient history. The exclusion criteria were significant neurological diseases other than AD that may affect cognition, including Parkinson’s disease, vascular cognitive impairment, normal pressure hydrocephalus, brain tumor, progressive supranuclear palsy, seizure disorder, subdural hematoma, and multiple sclerosis, a history of significant head trauma followed by persistent neurologic deficits, or other known significant structural brain abnormalities. All patients received a standardized evaluation that included a clinical interview, neuropsychological assessment, laboratory tests and brain magnetic resonance imaging. The institutional review boards of each participating hospital approved the protocol and informed consent form for this study. All participants or their legal representatives signed informed consent forms at study participation.
Cognitive test
Global cognition was annually assessed using the Mini-Mental State Examination (MMSE) [47], which was carried out to depict the trajectory of cognitive function for each participant. The total scores ranged between 0 and 30, and lower scores revealed poorer cognitive performance. The Clinical Dementia Rating (CDR) [48] was administered to determine the severity of dementia. Additionally, the 12-item memory test, modified 15-item Boston Naming Test, category verbal fluency test, and forward and backward digit span test were used at study entry to assess short-term memory, language, executive function, attention and working memory, respectively. Longitudinal follow-up was performed in AD and MCI patients at one-year intervals with the MMSE and the CDR assessment.
Genotypic data
For each participant, a Gentra Puregene kit (Qiagen, Hilden, Germany) was utilized to extract genomic DNA from whole blood samples based on standard protocols. Previous studies have reported that AD is associated with several genes, including APOE, ABCA7 [7, 11, 49], SORL1 [17, 21, 50, 51], BIN1 [52], GAB2 [18], and CD33 [11, 53]. Two single-nucleotide polymorphisms (SNPs), rs429358 and rs7412, were selected to genotype the ε2, ε3, and ε4 alleles of the APOE gene [54]. Seven SNPs were selected and considered to serve as genetic markers for these candidate genes on the basis of (1) our previous study showing ABCA7 rs3764650, SORL1 rs1784933, and SORL1 rs2298813 associated with the risk of AD or MCI in Han Chinese individuals in Taiwan [7, 21] (2) SORL1 rs3737529 being one of the most significant SNPs in Asian population [17] (3) BIN1 rs744373 and CD33 rs3865444 being the AlzGene Top Results (alzgene.org) and being associated with the risk of AD in Caucasian and Han Chinese population [8, 11] (4) GAB2 2373115 being associated with the AD risk with an odds ratio of 4.06 [18].
Genotyping of all SNPs was accomplished using the TaqMan genotyping assay (Applied Biosystems, Foster City, CA, USA) (Supplementary Table 5). Polymerase chain reaction (PCR) was carried out using 96-well microplates with an ABI 7500 real-time PCR system (Applied Biosystems). Allele discrimination was performed by identifying fluorescence using SDS software version 1.2.3 (Applied Biosystems).
ADNI dataset
ADNI data used in this study were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) [55, 56]. The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up-to-date information, see http://www.adni-info.org.
To compare with ADNI cohort, a total of 385 ADNI patients in the ADNI-1, ADNI-GO, or ADNI-2 phases—150 with AD and 235 with MCI and then progression to AD—with at least four MMSE measurements and genotypic data were selected to analyze the trajectories of cognitive function decline. Because the three candidate genetic markers—rs3737529 and rs1784933 in SORL1, and rs2373115 in GAB2— were not genotyped in the ADNI-1 and/or ADNI-GO/2 data, we imputed the two datasets separately to the 1000 Genomes Project (1000 Genomes Phase 3 v5 used as the reference panel, SHAPEIT as the phasing, and EUR as the population) using Minimac3 on the Michigan Imputation Server [PMID: 27571263].
Statistical analysis
For each SNP, Hardy-Weinberg equilibrium was checked using the goodness-of-fit test in AD and MCI subjects. A Bayesian hierarchical model with a four-parameter logistic curve [22] was fitted to depict the trajectories of cognitive function decline for patients with AD or MCI at baseline (n = 299). This model included four major parameters: the asymptotic value of cognitive function at lower ages (A), the asymptotic value of cognitive function at higher ages (B), the age at the midpoint of cognitive function decline (M) between A and B, and the rate of cognitive function decline (R) from A to B. In the model, random effects on parameters A and B were assumed to model the variation between subjects. In addition, to consider the effects of genetic markers, linear combinations of these covariates on parameters M and R were modeled adjusting for sex and education years, and an additive mode of inheritance was assumed to code the number of copies of the minor allele for each genetic marker. The distributions of observed measurements and the prior distributions of model parameters followed the assumption proposed by Sweet et al. [22]. Bayesian inference was based on an initial 2,000 iteration burn-in and a 20,000 iteration run using Gibbs sampling.
To identify an adequate fitting model of genetic markers on trajectories of cognitive function decline, model selection was carried out using Gibbs variable selection [57]. The statistical significance of all tests was evaluated using a 95% credible interval. The adequacy of the fitted Bayesian model to the data was checked by posterior predictive p-value [58]. All Bayesian analyses were performed using the open-source software WinBUGS version 1.4.3 [59]. Besides, not all MCI patients will develop clinically defined AD during the follow-up period. Therefore, we excluded 16 stable MCI patients without progression to AD and used the subset of 283 samples to validate the results obtained from the full samples. In addition, a total of 385 ADNI patients with AD or with MCI and then progression to AD were fitted to validate the trajectories of cognitive function decline obtained from our BSAD patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Taiwan Biobank and the Academia Sinica of Taiwan for their technical support for this work.
ADNI data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research and Development, LLC.; Johnson and Johnson Pharmaceutical Research and Development LLC.; Lumosity; Lundbeck; Merck and Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Abbreviations
- Aβ
amyloid β
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- APOE
apolipoprotein E
- APP
amyloid precursor protein
- BSAD
Biosignature Study of Alzheimer’s Disease
- CDR
Clinical Dementia Rating
- CSF
cerebrospinal fluid
- GWASs
genome-wide association studies
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- PCR
polymerase chain reaction
- SNPs
single-nucleotide polymorphisms
Footnotes
AUTHOR CONTRIBUTIONS: T.J.H contributed to the analysis and interpretation of the data and drafting of the manuscript. W.J.L contributed to the concept and design of the study, acquisition of the data, and drafting of the manuscript. Y.C.L contributed to the acquisition of the data and revision of the manuscript. C.C.H contributed to analysis and interpretation of the data. Y.H.F contributed to analysis and interpretation of the data. T.Y.C contributed to analysis and interpretation of the data. Y.S.L contributed to analysis and interpretation of the data. I.S.C contributed to the concept and design of the study, analysis and interpretation of the data, and revision of the manuscript. S.J.W contributed to the concept and design of the study and revision of the manuscript. C.A.H contributed to the concept and design of the study, analysis and interpretation of the data, and revision of the manuscript. J.L.F contributed to the concept and design of the study, acquisition of the data, and revision of the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: The study was supported by grants from the Academia Sinica of Taiwan (AS-BD-108-2), the Ministry of Science and Technology of Taiwan (107-2221-E-075-006, 108-2321-B-075-001 and 109-2314-B-075-052-MY2), Taipei Veterans General Hospital (V109C-061 and VGHUST109-V1-5-1), Taichung Veterans General Hospital (TCVGH-1043402C, TCVGH-1053403C, and TCVGH-1063403C), the Brain Research Center, National Yang-Ming University, from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan, and the National Health Research Institutes of Taiwan (PH-108-SP-01 and PH-109-SP-01).
REFERENCES
- 1.Ferrari C, Lombardi G, Polito C, Lucidi G, Bagnoli S, Piaceri I, Nacmias B, Berti V, Rizzuto D, Fratiglioni L, Sorbi S. Alzheimer’s Disease Progression: Factors Influencing Cognitive Decline. J Alzheimers Dis. 2018; 61:785–91. 10.3233/JAD-170665 [DOI] [PubMed] [Google Scholar]
- 2.Lee WJ, Liao YC, Wang YF, Lin YS, Wang SJ, Fuh JL. Summative Effects of Vascular Risk Factors on the Progression of Alzheimer Disease. J Am Geriatr Soc. 2020; 68:129–36. 10.1111/jgs.16181 [DOI] [PubMed] [Google Scholar]
- 3.Todd M, Schneper L, Vasunilashorn SM, Notterman D, Ullman MT, Goldman N. Apolipoprotein E, cognitive function, and cognitive decline among older Taiwanese adults. PLoS One. 2018; 13:e0206118. 10.1371/journal.pone.0206118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jager PL, Shulman JM, Chibnik LB, Keenan BT, Raj T, Wilson RS, Yu L, Leurgans SE, Tran D, Aubin C, Anderson CD, Biffi A, Corneveaux JJ, et al. , and Alzheimer’s Disease Neuroimaging Initiative. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol Aging. 2012; 33:1017.e1–15. 10.1016/j.neurobiolaging.2011.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, Harrington KD, Bourgeat P, Salvado O, Darby D, Snyder PJ, Bush AI, Martins RN, et al. , and Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group. BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2013; 34:2457–64. 10.1016/j.neurobiolaging.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 6.Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, Valladares O, Lin CF, Larson EB, Graff-Radford NR, Evans D, De Jager PL, Crane PK, et al. , and Alzheimer Disease Genetics Consortium. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ϵ4, and the risk of late-onset Alzheimer disease in African Americans. JAMA. 2013; 309:1483–92. 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao YC, Lee WJ, Hwang JP, Wang YF, Tsai CF, Wang PN, Wang SJ, Fuh JL. ABCA7 gene and the risk of Alzheimer’s disease in Han Chinese in Taiwan. Neurobiol Aging. 2014; 35:2423.e7–13. 10.1016/j.neurobiolaging.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 8.Wang HZ, Bi R, Hu QX, Xiang Q, Zhang C, Zhang DF, Zhang W, Ma X, Guo W, Deng W, Zhao L, Ni P, Li M, et al. Validating GWAS-Identified Risk Loci for Alzheimer’s Disease in Han Chinese Populations. Mol Neurobiol. 2016; 53:379–90. 10.1007/s12035-014-9015-z [DOI] [PubMed] [Google Scholar]
- 9.Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, Whites, and Hispanics. JAMA. 1998; 279:751–55. 10.1001/jama.279.10.751 [DOI] [PubMed] [Google Scholar]
- 10.Zhang DF, Xu M, Bi R, Yao YG. Genetic Analyses of Alzheimer’s Disease in China: Achievements and Perspectives. ACS Chem Neurosci. 2019; 10:890–901. 10.1021/acschemneuro.8b00435 [DOI] [PubMed] [Google Scholar]
- 11.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, et al. , and Alzheimer’s Disease Neuroimaging Initiative, and CHARGE consortium, and EADI1 consortium. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011; 43:429–35. 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011; 43:436–41. 10.1038/ng.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, et al. , and European Alzheimer’s Disease Initiative Investigators. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009; 41:1094–99. 10.1038/ng.439 [DOI] [PubMed] [Google Scholar]
- 14.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, Harold D, Schrijvers EM, Ramirez-Lorca R, et al. , and CHARGE Consortium, and GERAD1 Consortium, and EADI1 Consortium. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010; 303:1832–40. 10.1001/jama.2010.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009; 41:1088–93. 10.1038/ng.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharesouran J, Rezazadeh M, Khorrami A, Ghojazadeh M, Talebi M. Genetic evidence for the involvement of variants at APOE, BIN1, CR1, and PICALM loci in risk of late-onset Alzheimer’s disease and evaluation for interactions with APOE genotypes. J Mol Neurosci. 2014; 54:780–86. 10.1007/s12031-014-0377-5 [DOI] [PubMed] [Google Scholar]
- 17.Miyashita A, Koike A, Jun G, Wang LS, Takahashi S, Matsubara E, Kawarabayashi T, Shoji M, Tomita N, Arai H, Asada T, Harigaya Y, Ikeda M, et al. , and Alzheimer Disease Genetics Consortium. SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese, Koreans and Caucasians. PLoS One. 2013; 8:e58618. 10.1371/journal.pone.0058618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiman EM, Webster JA, Myers AJ, Hardy J, Dunckley T, Zismann VL, Joshipura KD, Pearson JV, Hu-Lince D, Huentelman MJ, Craig DW, Coon KD, Liang WS, et al. GAB2 alleles modify Alzheimer’s risk in APOE epsilon4 carriers. Neuron. 2007; 54:713–20. 10.1016/j.neuron.2007.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Chen Y, Mok KY, Zhao Q, Chen K, Chen Y, Hardy J, Li Y, Fu AK, Guo Q, Ip NY, and Alzheimer’s Disease Neuroimaging Initiative. Identification of genetic risk factors in the Chinese population implicates a role of immune system in Alzheimer’s disease pathogenesis. Proc Natl Acad Sci USA. 2018; 115:1697–706. 10.1073/pnas.1715554115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang DF, Fan Y, Xu M, Wang G, Wang D, Li J, Kong LL, Zhou H, Luo R, Bi R, Wu Y, Li GD, Li M, et al. , and Alzheimer’s Disease Neuroimaging Initiative (ADNI). Complement C7 is a novel risk gene for Alzheimer’s disease in Han Chinese. Natl Sci Rev. 2019; 6:257–74. 10.1093/nsr/nwy127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou CT, Liao YC, Lee WJ, Wang SJ, Fuh JL. SORL1 gene, plasma biomarkers, and the risk of Alzheimer’s disease for the Han Chinese population in Taiwan. Alzheimers Res Ther. 2016; 8:53. 10.1186/s13195-016-0222-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweet RA, Seltman H, Emanuel JE, Lopez OL, Becker JT, Bis JC, Weamer EA, DeMichele-Sweet MA, Kuller LH. Effect of Alzheimer’s disease risk genes on trajectories of cognitive function in the Cardiovascular Health Study. Am J Psychiatry. 2012; 169:954–62. 10.1176/appi.ajp.2012.11121815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005; 65:1888–93. 10.1212/01.wnl.0000188871.74093.12 [DOI] [PubMed] [Google Scholar]
- 24.Louwersheimer E, Cohn-Hokke PE, Pijnenburg YA, Weiss MM, Sistermans EA, Rozemuller AJ, Hulsman M, van Swieten JC, van Duijn CM, Barkhof F, Koene T, Scheltens P, Van der Flier WM, Holstege H. Rare Genetic Variant in SORL1 May Increase Penetrance of Alzheimer’s Disease in a Family with Several Generations of APOE-ɛ4 Homozygosity. J Alzheimers Dis. 2017; 56:63–74. 10.3233/JAD-160091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barral S, Bird T, Goate A, Farlow MR, Diaz-Arrastia R, Bennett DA, Graff-Radford N, Boeve BF, Sweet RA, Stern Y, Wilson RS, Foroud T, Ott J, Mayeux R, and National Institute on Aging Late-Onset Alzheimer’s Disease Genetics Study. Genotype patterns at PICALM, CR1, BIN1, CLU, and APOE genes are associated with episodic memory. Neurology. 2012; 78:1464–71. 10.1212/WNL.0b013e3182553c48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keenan BT, Shulman JM, Chibnik LB, Raj T, Tran D, Sabuncu MR, Allen AN, Corneveaux JJ, Hardy JA, Huentelman MJ, Lemere CA, Myers AJ, Nicholson-Weller A, et al. , and Alzheimer’s Disease Neuroimaging Initiative. A coding variant in CR1 interacts with APOE-ε4 to influence cognitive decline. Hum Mol Genet. 2012; 21:2377–88. 10.1093/hmg/dds054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jendresen C, Årskog V, Daws MR, Nilsson LN. The Alzheimer’s disease risk factors apolipoprotein E and TREM2 are linked in a receptor signaling pathway. J Neuroinflammation. 2017; 14:59. 10.1186/s12974-017-0835-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan J, Tao W, Li X, Li H, Zhang J, Wei D, Chen Y, Zhang Z. The Contribution of Genetic Factors to Cognitive Impairment and Dementia: Apolipoprotein E Gene, Gene Interactions, and Polygenic Risk. Int J Mol Sci. 2019; 20:1177. 10.3390/ijms20051177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim WS, Fitzgerald ML, Kang K, Okuhira K, Bell SA, Manning JJ, Koehn SL, Lu N, Moore KJ, Freeman MW. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem. 2005; 280:3989–95. 10.1074/jbc.M412602200 [DOI] [PubMed] [Google Scholar]
- 30.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006; 17:891–96. 10.1097/01.wnr.0000221833.41340.cd [DOI] [PubMed] [Google Scholar]
- 31.De Roeck A, Van Broeckhoven C, Sleegers K. The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 2019; 138:201–20. 10.1007/s00401-019-01994-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes TM, Lopez OL, Evans RW, Kamboh MI, Williamson JD, Klunk WE, Mathis CA, Price JC, Cohen AD, Snitz BE, Dekosky ST, Kuller LH. Markers of cholesterol transport are associated with amyloid deposition in the brain. Neurobiol Aging. 2014; 35:802–07. 10.1016/j.neurobiolaging.2013.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma FC, Zong Y, Wang HF, Li JQ, Cao XP, Tan L, and Alzheimer’s Disease Neuroimaging Initiative*. ABCA7 genotype altered Aβ levels in cerebrospinal fluid in Alzheimer’s disease without dementia. Ann Transl Med. 2018; 6:437. 10.21037/atm.2018.07.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez LM, Goukasian N, Porat S, Hwang KS, Eastman JA, Hurtz S, Wang B, Vang N, Sears R, Klein E, Coppola G, Apostolova LG. Common variants in ABCA7 and MS4A6A are associated with cortical and hippocampal atrophy. Neurobiol Aging. 2016; 39:82–89. 10.1016/j.neurobiolaging.2015.10.037 [DOI] [PubMed] [Google Scholar]
- 35.Karch CM, Jeng AT, Nowotny P, Cady J, Cruchaga C, Goate AM. Expression of novel Alzheimer’s disease risk genes in control and Alzheimer’s disease brains. PLoS One. 2012; 7:e50976. 10.1371/journal.pone.0050976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barthelson K, Newman M, Lardelli M. Sorting Out the Role of the Sortilin-Related Receptor 1 in Alzheimer’s Disease. J Alzheimers Dis Rep. 2020; 4:123–40. 10.3233/ADR-200177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campion D, Charbonnier C, Nicolas G. SORL1 genetic variants and Alzheimer disease risk: a literature review and meta-analysis of sequencing data. Acta Neuropathol. 2019; 138:173–86. 10.1007/s00401-019-01991-4 [DOI] [PubMed] [Google Scholar]
- 38.Yin RH, Yu JT, Tan L. The Role of SORL1 in Alzheimer’s Disease. Mol Neurobiol. 2015; 51:909–18. 10.1007/s12035-014-8742-5 [DOI] [PubMed] [Google Scholar]
- 39.Alexopoulos P, Guo LH, Kratzer M, Westerteicher C, Kurz A, Perneczky R. Impact of SORL1 single nucleotide polymorphisms on Alzheimer’s disease cerebrospinal fluid markers. Dement Geriatr Cogn Disord. 2011; 32:164–70. 10.1159/000332017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louwersheimer E, Ramirez A, Cruchaga C, Becker T, Kornhuber J, Peters O, Heilmann S, Wiltfang J, Jessen F, Visser PJ, Scheltens P, Pijnenburg YA, Teunissen CE, et al. , and Alzheimer’s Disease Neuroimaging Initiative and Dementia Competence Network. Influence of genetic variants in SORL1 gene on the manifestation of Alzheimer’s disease. Neurobiol Aging. 2015; 36:1605.e13–20. 10.1016/j.neurobiolaging.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 41.Andrews SJ, Das D, Anstey KJ, Easteal S. Late Onset Alzheimer’s Disease Risk Variants in Cognitive Decline: The PATH Through Life Study. J Alzheimers Dis. 2017; 57:423–36. 10.3233/JAD-160774 [DOI] [PubMed] [Google Scholar]
- 42.Fan CT, Lin JC, Lee CH. Taiwan Biobank: a project aiming to aid Taiwan’s transition into a biomedical island. Pharmacogenomics. 2008; 9:235–46. 10.2217/14622416.9.2.235 [DOI] [PubMed] [Google Scholar]
- 43.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7:263–69. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004; 256:183–94. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 45.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004; 256:240–46. 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 46.Wechsler D. Wechsler Memory Scale-III. San Antonio, Texas: The Psychological Corporation. 1998. [Google Scholar]
- 47.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 48.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43:2412–14. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 49.Allen M, Zou F, Chai HS, Younkin CS, Crook J, Pankratz VS, Carrasquillo MM, Rowley CN, Nair AA, Middha S, Maharjan S, Nguyen T, Ma L, et al. , and Alzheimer’s Disease Genetics Consortium (ADGC). Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology. 2012; 79:221–28. 10.1212/WNL.0b013e3182605801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng X, Hou D, Deng Y, Li W, Tian M, Yu Z. SORL1 gene polymorphism association with late-onset Alzheimer’s disease. Neurosci Lett. 2015; 584:382–89. 10.1016/j.neulet.2014.10.055 [DOI] [PubMed] [Google Scholar]
- 51.Vardarajan BN, Zhang Y, Lee JH, Cheng R, Bohm C, Ghani M, Reitz C, Reyes-Dumeyer D, Shen Y, Rogaeva E, St George-Hyslop P, Mayeux R. Coding mutations in SORL1 and Alzheimer disease. Ann Neurol. 2015; 77:215–27. 10.1002/ana.24305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Jager PL, Srivastava G, Lunnon K, Burgess J, Schalkwyk LC, Yu L, Eaton ML, Keenan BT, Ernst J, McCabe C, Tang A, Raj T, Replogle J, et al. Alzheimer’s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci. 2014; 17:1156–63. 10.1038/nn.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, Rosenkrantz LL, Imboywa S, Lee M, Von Korff A, Morris MC, Evans DA, Johnson K, et al. , and Alzheimer Disease Neuroimaging Initiative. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013; 16:848–50. 10.1038/nn.3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grupe A, Abraham R, Li Y, Rowland C, Hollingworth P, Morgan A, Jehu L, Segurado R, Stone D, Schadt E, Karnoub M, Nowotny P, Tacey K, et al. Evidence for novel susceptibility genes for late-onset Alzheimer’s disease from a genome-wide association study of putative functional variants. Hum Mol Genet. 2007; 16:865–73. 10.1093/hmg/ddm031 [DOI] [PubMed] [Google Scholar]
- 55.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement. 2005; 1:55–66. 10.1016/j.jalz.2005.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, Donohue MC, Green RC, Harvey D, Jack CR Jr, Jagust W, Morris JC, Petersen RC, et al. , and Alzheimer’s Disease Neuroimaging Initiative. Impact of the Alzheimer’s Disease Neuroimaging Initiative, 2004 to 2014. Alzheimers Dement. 2015; 11:865–84. 10.1016/j.jalz.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ntzoufras I. Gibbs variable selection using BUGS. J Stat Softw. 2002; 7:1–19. 10.18637/jss.v007.i07 [DOI] [Google Scholar]
- 58.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis. 3rd ed. Boca Raton, FL: Chapman and Hall/CRC. 2014. 10.1201/b16018 [DOI] [Google Scholar]
- 59.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS - A Bayesian modelling framework: Concepts, structure, and extensibility. Stat Comput. 2000; 10:325–37. 10.1023/A:1008929526011 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.