Abstract
Objective:
Evaluate the influence that subject-specific factors have on intracranial interictal epileptiform discharge (IED) rates in persons with refractory epilepsy.
Methods:
150 subjects with intracranial electrodes performed multiple sessions of a free recall memory task; this standardized task controlled for subject attention levels. We utilized a dominance analysis to rank the importance of subject-specific factors based on their relative influence on IED rates. Linear mixed-effects models were employed to comprehensively examine factors with high ranked importance.
Results:
Anti-seizure medication (ASM) status, time of testing, and seizure onset zone (SOZ) location were the highest-ranking factors in terms of their impact on IED rates. The average IED rate of electrodes in SOZs was 34% higher than the average IED rate of electrodes outside of SOZs (Non-SOZ) (p < 0.001). However, Non-SOZ electrodes had similar IED rates regardless of the subject’s SOZ location (p = 0.99). Subjects on older generation (p < 0.001) and combined generation (p < 0.001) ASM regimens had significantly lower IED rates relative to the group taking no ASMs; newer generation ASM regimens demonstrated a nonsignificant association with IED rates (p = 0.13). Of the ASMs included in this study, the following ASMs significantly reduced IED rates: levetiracetam (p < 0.001), carbamazepine (p < 0.001), lacosamide (p = 0.03), zonisamide (p = 0.01), lamotrigine (p = 0.03), phenytoin (p = 0.03), and topiramate (p = 0.01). We observed a nonsignificant association between time of testing and IED rates (morning-afternoon p = 0.15, morning-evening p = 0.85, afternoon-evening p = 0.26).
Significance:
The current study ranks the relative influence that subject-specific factors have on IED rates and highlights the importance of considering certain factors, such as SOZ location and ASM status, when analyzing IEDs for clinical or research purposes.
Keywords: Interictal epileptiform discharges, Epilepsy, Demographics, Clinical factors, Intracranial monitoring
Introduction
In addition to seizures, persons with epilepsy are burdened with interictal epileptiform discharges (IEDs); IEDs are transient electrographic periods where populations of pathologically connected neurons partake in hypersynchronous firing.1,2 Although the specific mechanism connecting IEDs and seizure activity remains a matter of debate, IEDs were shown to be associated with unfavorable clinical symptoms, such as increased seizure frequency and severity3–6 and reduced quality of life.7–9 Several recent studies also reported a correlation between increased IEDs and impaired cognition and memory.10–12 However, many of these studies were limited by (1) the use of scalp-EEG, which is inferior to intracranial EEG for detecting and localizing IEDs, and (2) the low number of subjects performing standardized tasks.13 The latter limitation is especially noteworthy, provided that IED rates may be dependent on cognitive activity. Thus, a particular strength of this current study was that all patients performed a standardized task, controlling for their attention levels.
These previous studies showed that IEDs were related to sleep patterns,14 mood disorders,15 hormone levels (e.g., menstrual cycle hormones16 and cortisol17), age,18 and time-dependent fluctuations,19,20 but have mostly examined factors independently. Goncharova et al. (2016) also demonstrated a relationship between anti-seizure medications (ASMs) and IEDs, where IED rates decreased with ASM tapering epochs, then increased after this tapering period in 79 subjects with scalp-EEG.3 Jointly, these findings convey that specific factors influence IED rates, and expose the need to examine these factors in aggregate, in order to better understand how they relate to IEDs.
Our study’s primary purpose was to determine if specific characteristics were correlated with differences in intracranial IED rates in refractory epilepsy. We expanded on previous work by utilizing 150 subjects with intracranial stereoelectroencephalography (Stereo-EEG) implants to rank the relative importance of subject-specific factors, in terms of their association with IED rates. We then performed a detailed analysis of the highest-ranking factors. Based on previous reports, we predicted that ASM status, age, SOZ location, and time of testing would be the highest-ranking factors associated with IED rates. However, the relative rank of these factors remains unknown. We also hypothesized that intracranial IED rates would be higher in electrodes near the seizure onset zone (SOZ) relative to areas outlying the SOZ (Non-SOZ) and that IED rates would be higher in mesial structures relative to neocortical structures. Our final hypothesis was that specific ASMs, such as levetiracetam and lamotrigine, would have higher predicted IED rate reductions than other ASMs, as these ASMs were previously shown to reduce IEDs.21,22 The results of this study are essential for understanding the influence that specific factors have on IED rates, which may otherwise confound clinical and research interpretations of intracranial IEDs.
Methods
Participants
Data was collected as part of an ongoing research collaboration registered as: “An Investigator-initiated, Prospective, Multi-center, Controlled Feasibility Study of Direct Brain Recording and Stimulation for Memory Enhancement” (ClinicalTrials.gov Identifier: NCT04286776). Informed consent was obtained from each subject, and the Institutional Review Board approved the research protocol at each of the eight testing centers (Protocol #: MEMES-001).
This data was analyzed from 150 persons with refractory epilepsy who underwent a surgical procedure to implant Stereo-EEG contacts within the brain parenchyma. Electrodes were placed to best localize the seizure onset zone. These subjects each performed a uniform free recall memory task multiple times during their long-term Stereo-EEG monitoring, resulting in a total of 399 unique experiment sessions with intracranial Stereo-EEG recordings. The median number of sessions per subject was two (IQR = 1–3). We confirmed that IED rates were stable over time (i.e., in subsequent sessions for the same subject) by including session number as a predictor after controlling for ASM status and SOZ location in a hierarchical linear model (p = 0.65).
We would like to note that the number of sessions with ASMs was limited because most subjects were taken off ASMs for intracranial monitoring, as seizures were desired to localize SOZs. Additionally, several subjects had missing ASM data and were excluded from the ASM analyses in this study. This resulted in 81 subjects on at least one ASM during the time of testing, contributing 165 unique testing sessions, and 28 subjects with a record of no ASM during the time of testing, contributing 67 unique testing sessions. Our study only included non-stimulation testing sessions collected before any brain stimulation was performed. All testing sessions were conducted at least four hours after the most recent seizure activity, and sessions were excluded if a seizure occurred during that testing session. Data collection was performed after the patient was stable and transferred to the EMU, providing a minimum recovery period of at least 24 hours post-implantation. Other subject demographic and clinical characteristics are reported in Table 1.
Table 1.
Subject characteristics
| Overall | |
|---|---|
| (N = 150) | |
| Age (years) | |
| Mean (SD) | 37.1 (11.5) |
| Sex | |
| Male | 78 (52.0%) |
| Female | 72 (48.0%) |
| Years of Education | |
| Mean (SD) | 13.7 (2.23) |
| Handedness | |
| Ambidextrous | 6 (4.0%) |
| Left | 17 (11.3%) |
| Right | 127 (84.7%) |
| Age of Seizure Onset | |
| Mean (SD) | 17.0 (13.2) |
| History of Prior Resection | |
| Yes | 30 (20.0%) |
| No | 120 (80.0%) |
| History of TBI | |
| Yes | 9 (6.0%) |
| No | 141 (94.0%) |
| Number of Implanted Channels | |
| Mean (SD) | 123 (40.4) |
| Seizure Onset Zone (SOZ) | |
| Multifocal SOZa | 77 (51.3%) |
| Focal SOZ | 73 (48.7%) |
| Normalized IED Rate (IEDs/hour)b | |
| SOZ (Mean (SD)) | 6.53 (17.47) |
| NON-SOZ (Mean (SD)) | 0.34 (0.50) |
| Combined (Mean (SD)) | 0.60 (0.72) |
Multifocal seizure onset zones (SOZs) were most commonly in neighboring brain regions (e.g. lateral temporal and mesial temporal regions).
Normalized IED Rates were calculated per session per contact by dividing initial IED rates by the total number of contacts in each region; thus, these normalized rates represent the average number of IEDs detected by each individual contact per hour. SOZ refers to average IED rates of contacts in the SOZ. NON-SOZ refers to average IED rates of contacts outside of the SOZ. Combined refers to global IED rates averaged across all contacts.
Free Recall Memory Task
Subjects performed a delayed free-recall memory task with a bedside laptop. The task consisted of an encoding phase, delay phase, and retrieval phase (Fig.1A). During the encoding phase, subjects were asked to memorize 12 random nouns from a pool of English nouns (http://memory.psych.upenn.edu/WordPools). Words were sampled without replacement from the word pool and were presented for 1600 milliseconds with blank interstimulus intervals of 800 to 1200 milliseconds. We used data from tasks where the words were categorized (categorical free recall) and completely independent (free recall). The encoding phase was followed by a 20-second distractor phase, which consisted of arithmetic problems presented as X+Y+Z=? where X, Y, and Z were set to random single-digit integers. The subjects were then given 30 seconds to recall as many words as possible from the preceding list, in any order. Each subject performed up to 25 recall lists per session and completed multiple testing sessions over the course of several days.23 Generally, the goal at each testing center enrolled in this multicenter trial was to collect as many unstimulated free-recall sessions as possible.
Figure 1. Interictal epileptiform discharge rates are higher in seizure onset zones.
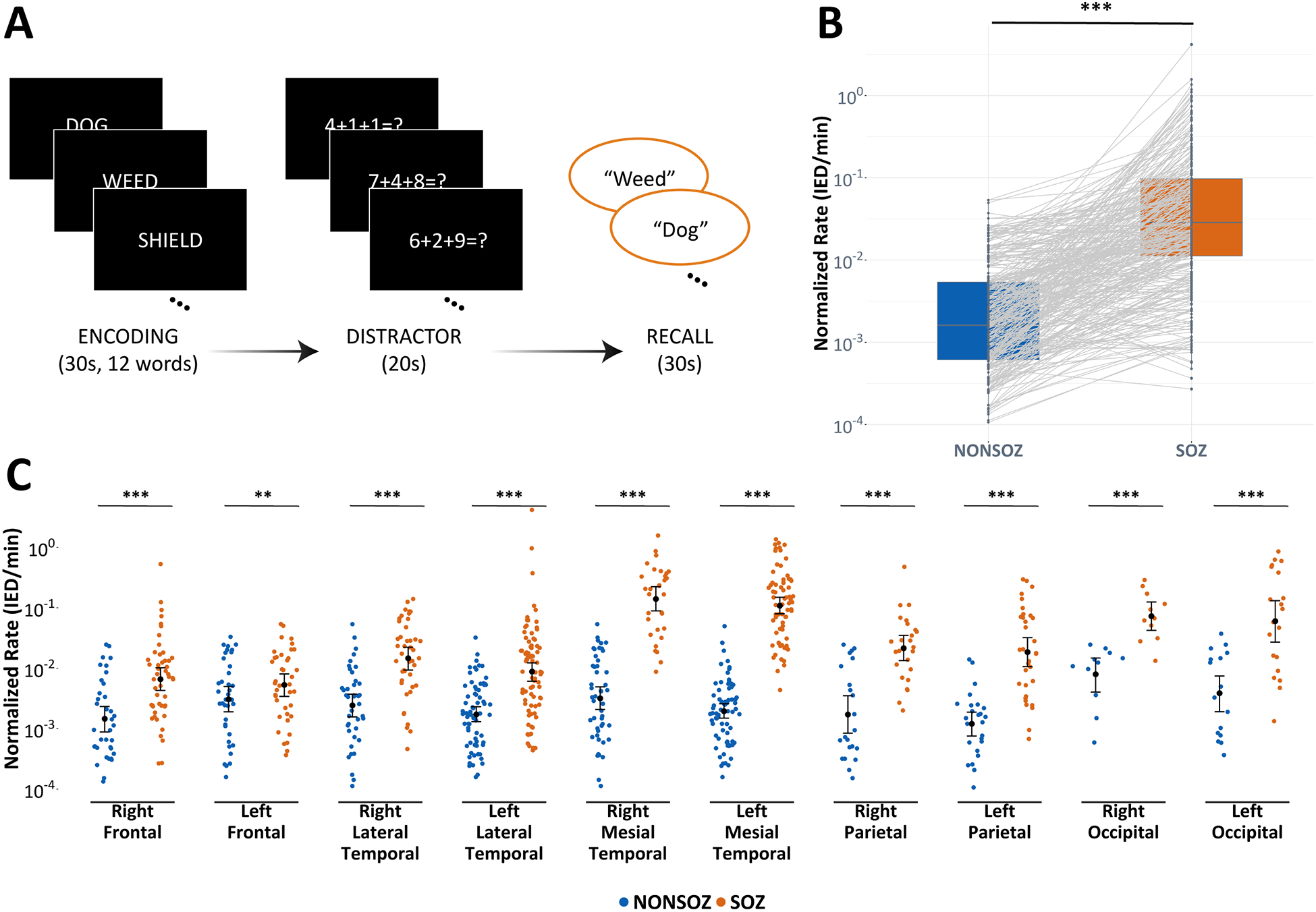
(A) For each trial of the free recall task, subjects were asked to encode 12 nouns, then performed an arithmetic distraction that was followed by the verbal recall phase. (B) Linear mixed-effects models revealed that IED rates were higher for contacts in the SOZ relative to contacts outside of the SOZ (Non-SOZ) (p < 0.001). (C) IED rates were also shown to be higher in the SOZ relative to the Non-SOZ, irrespective of the SOZ location (p < 0.01); however, there was no difference in IED rates between the stratified Non-SOZ regions (p = 0.99). Bars represent 95% confidence intervals. * p < 0.05, ** p < 0.01, *** p < 0.001
Intracranial Stereo-EEG recordings
Intracranial Stereo-EEG data was recorded using depth electrodes (contacts spaced 2.2–10 mm apart) with recording systems at each testing site. For this study, we only included subjects with depth electrodes due to the low number of subjects with surface electrodes (i.e., grids or strips) in our dataset. These recording systems included: Quantum LTM (Natus), Grass Telefactor, Nihon-Kohden, and custom Medtronic EEG systems. Electrode locations were determined by the clinical care team and were selected solely based on clinical monitoring needs. All signals were sampled at either 500, 1000, or 1600 Hz and were either bipolar referenced or referenced to a common contact placed intracranially, on the scalp, or on the mastoid process. Thus, we re-referenced all recordings to an averaged referential montage. Stereo-EEG recordings were also linearly de-trended and notch-filtered at 60Hz and its odd harmonics. The signal was then low-pass filtered with a Butterworth filter at 250Hz, and high-pass filtered with a Butterworth filter at 1Hz. Recordings were downsampled to 500Hz; then, recording channels were excluded if the signal was greater than three standard deviations from the mean value across other recording channels.
Anatomical Localization
Intracranial electrodes were localized using CT and MRI co-registration provided with the RAM dataset. Hippocampal subfields and MTL cortices were automatically labeled in a pre-implant, T2-weighted MRI using the automatic segmentation of hippocampal subfields (ASHS) multi-atlas segmentation method.24 Advanced Normalization tools were then used to co-register post-implant CT images with presurgical T1/T2 weighted scans.25 The Desikan-Killany-Tourville atlas was utilized to obtain parcellated cortical areas in different lobes.26 Two neuroradiologists then reviewed the final electrode position labels for all subjects.27 Fig.S1 depicts the spatial distribution of these localized electrodes on an average brain.
Automated Interictal Epileptiform Discharge Detection
We utilized an automated IED detector that was validated and performed comparably to multiple clinicians and other published IED detectors.28 This template-matching IED detector cross-correlated a 60-millisecond triangular template with preprocessed Stereo-EEG, then normalized the cross-correlation by the median standard deviation from one-second sliding windows.28 The absolute value of the normalized cross-correlation was then filtered with a specified detection threshold, marking local peaks above that threshold as IEDs. Our detector collapsed temporally overlapping detections into a single marked event and conservatively excluded IEDs occurring within three-seconds of another IED to handle bursts of spikes or high frequency activity.29 We tuned our IED template to minimize the number of artifactual IED detections while optimizing the number of true positive IED detections (Fig.S2). Lastly, we rejected IEDs that occurred for less than 10-milliseconds or more than 100-milliseconds to eliminate artifactual detections missed by our template-matching detector. While our detector may have over-counted IEDs, false positive IEDs were assumed to be evenly distributed, minimally biasing the true positive distribution.5 Our final IED set consisted of 43,308 IEDs.
Interictal Epileptiform Discharge Rate Calculation
IED rates were calculated by dividing the total number of IEDs that occurred during a subject testing session by the total duration of each session; this provided us with IEDs per minute. MNI coordinates for each contact were used to obtain brain locations for all implanted electrodes in the RAM dataset. The RAM dataset also contained SOZ labels for each subject, as determined by the clinical team at each of the eight testing centers. For our study, contact locations were refactored as frontal, lateral temporal, mesial temporal, parietal, or occipital. We then divided initial IED rates by the total number of contacts in each region to normalize subject IED rates. These normalized IED rates were also used to calculate average IED rates for regions inside (SOZ) and outside (Non-SOZ) the subjects’ seizure onset zones. The averaging and normalization procedures were necessary to allow for the comparison of IED rates between different brain regions and subjects.30,31 Especially, to account for differences in the Stereo-EEG coverage between neocortical and mesial regions.
Statistical Methods
We first examined if there was a difference in IED rates inside versus outside of the SOZ. We utilized linear mixed-effects models to determine statistical significance, as these models accounted for repeated subject observations and heterogeneity in the data between subjects (i.e., unequal electrode sampling between each anatomical region). The dependent variable for our model was log transformed normalized IED rates, and the fixed effect was SOZ status (SOZ, Non-SOZ). Random slopes and intercepts were added for each subject. We next examined if the location of the SOZ influenced whether there was a significant difference between IED rates inside versus outside of the SOZ. Similar linear mixed-effects models were employed after stratifying contacts based on hemisphere specific SOZ locations (e.g., left frontal, right frontal).
After comparing the IED rates of electrodes located in SOZs versus outside of SOZs (Non-SOZ), we examined if there was a difference in IED rates between the different SOZ locations. That is, we averaged the normalized IED rates for electrodes located within stratified SOZs, then used a linear mixed-effects model to test if there was an overall variation in IED rates between the different SOZs. Of note, we added an interaction term to account for whether a subject had focal or multifocal SOZs when appropriate in our models. This interaction term controlled for whether the observed effect was being driven by the presence of focal versus multifocal SOZs, while our mixed models accounted for the correlated, repeated measures produced by including subjects with multifocal SOZs in multiple groups. We performed Tukey’s honestly significant difference (HSD) test as a post-hoc, pairwise comparison of all possible SOZ combinations.
Provided the in-depth coverage of Stereo-EEG, we also evaluated if IED rates were higher in mesial structures relative to neocortical structures and if there was a difference in IED rates between common mesial structures. Common mesial structures classified in the RAM dataset include the hippocampus, amygdala, perirhinal cortex, entorhinal cortex, and parahippocampal gyrus. Similar to previous analyses, an ANOVA was utilized to assess this overall difference.
The next part of our analysis was aimed at ranking clinical and demographic characteristics in terms of which factor had the strongest influence on global IED rates. We utilized a dominance analysis to establish the relative importance of a predictor by computing the contribution a factor makes, in terms of variance, for the outcome.32–34 In other words, a factor is considered more important than another factor if it contributes a higher proportion to the outcome in models consisting of all possible combinations of predictors.35–37 In evaluating all combinations of predictors, the dominance analysis also dealt with the issue of multicollinearity. We computed the general dominance by calculating the average variance explained by a predictor and compared this to the average variance explained by other predictors obtained in other submodels. General dominance weights were averaged over 1000 bootstrapped samples for each predictor to demonstrate the relative proportion of r2 attributable to a predictor. Here, larger r2 values correspond to higher relative importance for the predictor.33 We refactored categorical variables for this analysis to prevent an overemphasis of factors with a large number of values, as this would falsely inflate a category’s relative importance; this also minimized the bias attributable to consuming varying degrees of freedom.
Following our dominance analysis, we evaluated the relationship between anti-seizure medication (ASM) status and IED rate, as this was the factor with the highest relative importance. We built linear mixed-effects models to examine the relationship between IED rates and ASM generation, in addition to specific, commonly prescribed ASMs in the RAM dataset. These models contained log transformed normalized, global IED rates as the dependent variable, ASM generation or ASM name as categorical predictors, and random effects for each unique subject. Subjects on multiple ASMs were included in multiple ASM groups; however, our mixed-effects models accounted for these repeated measures. An interaction term was also added for whether the ASM was part of a monotherapy or polytherapy treatment regimen. Predicted IED rate changes were calculated by subtracting one from the exponentiated parameter estimates and converting it to a percentage. Reference groups for these ASM models were subjects on no current ASM (n = 67). We excluded the following ASMs with less than five subjects: Gabapentin, Divalproex sodium, Perampanel, Felbamate, Rufinamide, Primidone, and ASMs categorized as Other.
Lastly, we used linear mixed-effects models to examine the relationship between IED rates and time of testing. Testing times were discretized into the following time windows: morning (6am-12pm), afternoon (12pm-5pm), and evening (5pm-9pm). This model contained log transformed normalized, global IED rates as the dependent variable, time of testing as a categorical predictor, and random effects for each unique subject while controlling for ASM status and SOZ location.
The false discovery rate was controlled at 0.05 with the Benjamini-Hochberg (BH) procedure for all models.38 Code for analyses in this study was written in R version 3.6.1 and Python version 3.6.7.
Data availability
Data is available through the Defense Advanced Research Projects Agency (DARPA) Restoring Active Memory (RAM) Public Data Release (http://memory.psych.upenn.edu/RAM). The code utilized in our study is available upon reasonable request.
Results
Determining the association between seizure onset zones and intracranial IED rates.
There were significantly higher IED rates for electrodes in SOZ regions relative to Non-SOZ regions (rate ratio = 1.34, p < 0.001) (Fig.1b). The model parameter estimates reflect the change in IED rates for each group relative to the reference group, reported here as rate ratios (RR). After categorizing SOZs based on localized brain regions, we found that all hemisphere specific SOZs, irrespective of the location, were associated with significantly higher IED rates compared to regions outside of the SOZ (p < 0.01) (Fig.1c). We also found a significant overall difference in the average IED rates between different SOZ locations (p < 0.001) (Fig.2a). When stratified by the subject’s SOZ location, there were no differences in the IED rates of electrodes in Non-SOZ regions (p = 0.99). In other words, IED rates in the SOZ were the only rates influenced by the location of the SOZ; IED rates in other brain areas were relatively constant, independent of the subject’s SOZ location. Our pairwise comparison of these SOZ locations demonstrated that (1) mesial temporal regions had significantly higher IED rates than all other ipsilateral and contralateral neocortical areas, except the occipital lobes (p < 0.01), (2) occipital lobes had higher IED rates than bilateral frontal lobes and ipsilateral lateral temporal lobes (p < 0.01), and (3) parietal lobes had markedly higher IED rates than frontal lobes (p < 0.05) (Fig.2b). All pairwise comparisons from Tukey’s HSD test are depicted in Fig.S3.
Figure 2. Seizure onset zone location influences interictal epileptiform discharge rates.
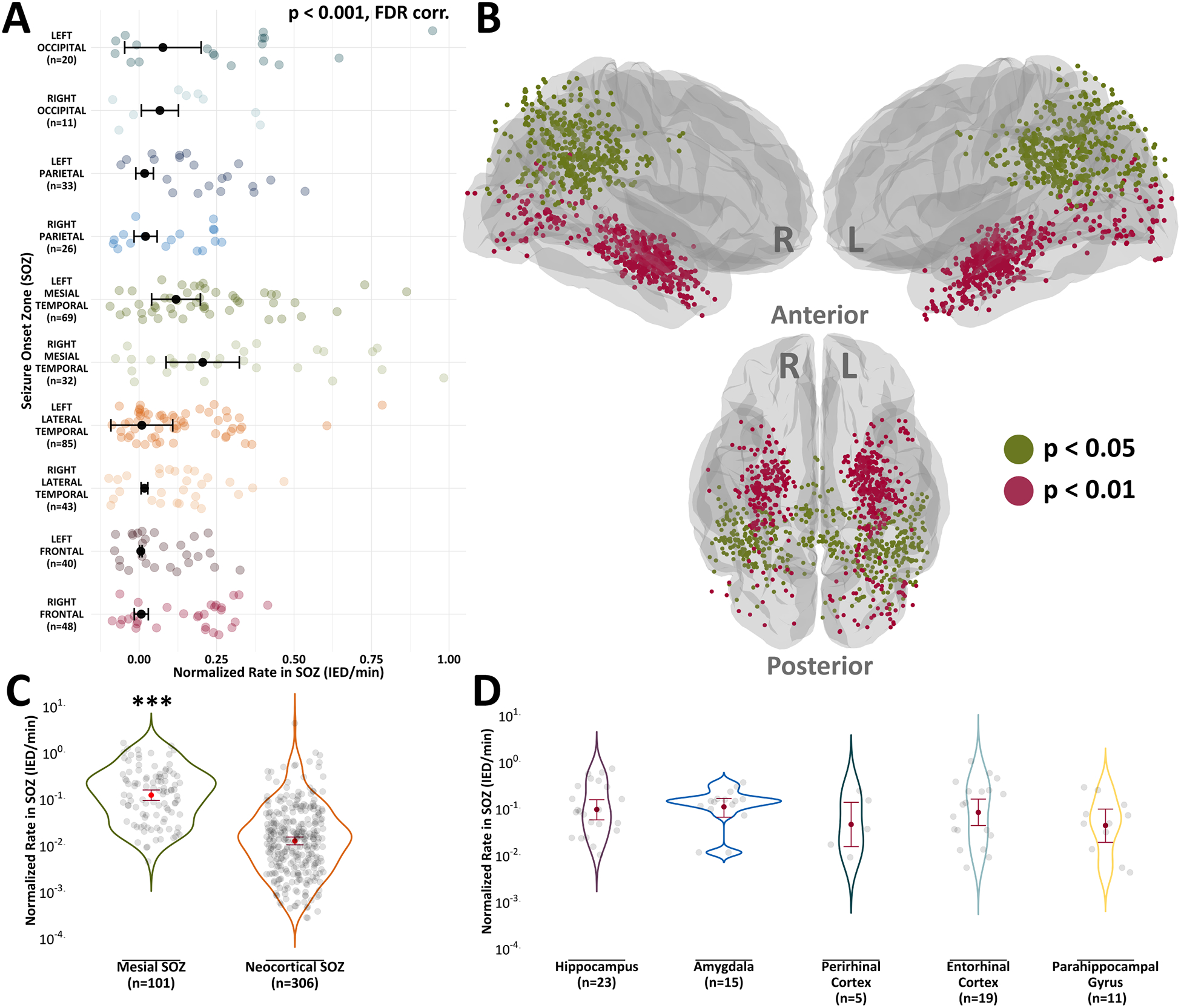
(A) Linear mixed-effects models were used to determine if there were differences in IED rates between the different SOZs, stratified by brain region. Here, we used normalized IED rates computed solely from electrode contacts in SOZs. ANOVA revealed that there were IED rate differences between the SOZs (p < 0.001). (B) Electrode contacts rendered on an average brain surface depict results from Tukey’s HSD test, a post-hoc test of the ANOVA in (A). Colors represent significance values for contacts in SOZ brain regions that had significantly higher normalized IED rates than other SOZ brain regions. (C) IED rates were higher in mesial structures compared to neocortical structures (p < 0.001). (D) There was a nonsignificant difference in IED rates between common mesial structures (p = 0.84). Bars represent 95% confidence intervals. * p < 0.05, ** p < 0.01, *** p < 0.001
We also revealed that neocortical SOZs had an 89.81% reduced likelihood of IEDs per minute compared to mesial SOZs (rate ratio = 0.1019, p < 0.001) (Fig.2c). An in-depth analysis of common mesial structures in the RAM dataset (hippocampus, amygdala, perirhinal cortex, entorhinal cortex, and parahippocampal gyrus) showed a nonsignificant difference in IED rates between mesial regions (p = 0.84) (Fig.2d). Thus, while SOZs in mesial structures had higher overall IED rates than neocortical SOZs, the specific mesial location of SOZs did not contribute to differences in IED rates.
Ranking the relative influence that subject-specific factors have on IED rates.
Our bootstrapped general dominance values revealed that ASM status had the highest value (r2 = 0.49) and generally dominated all other predictors (Fig.3). This demonstrated that ASM status had the highest relative importance for explaining differences in IED rates. Here, ASM status represented a binary classification of whether the subject was on any ASM or no ASM. The next highest value was time of testing (r2 = 0.09), followed by SOZ location (r2 = 0.01) (Fig.3). All other predictors showed minimal relative influences on IED rates (Fig.3). Of note, SOZ location and SOZ hemisphere were dichotomized for the dominance analysis to control for the degrees of freedom consumed by each predictor.
Figure 3. General dominance analysis of subject-specific features.
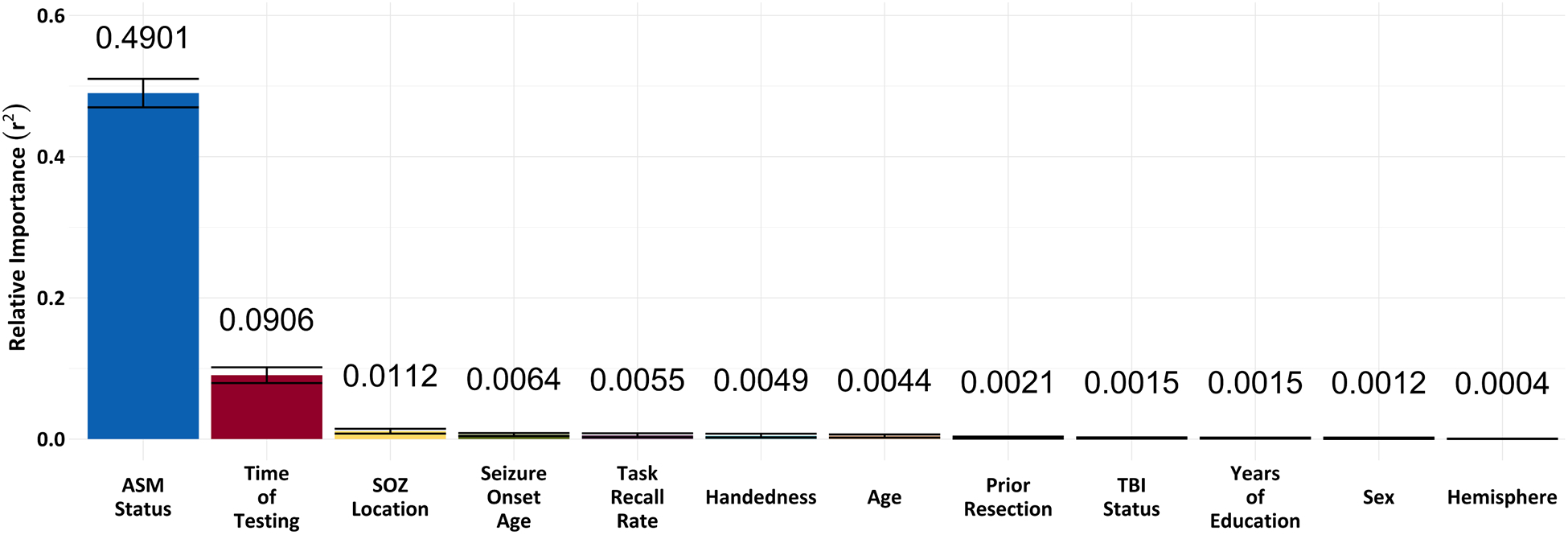
The bootstrapped general dominance analysis provided weights for each predictor to demonstrate the relative proportion of r2 attributable to that predictor. Larger r2 values represent higher relative importance of predictors relative to all possible combinations of predictors. General dominance values revealed that ASM status had the highest relative importance (r2 = 0.49) and generally dominated all other predictors, followed by time of testing (r2 = 0.09), then SOZ location (r2 = 0.01). All other predictors showed minimal relative importance for predicting IED rate changes. Bars represent standard errors.
Effect of anti-seizure medications on IED rates.
We observed a significant reduction in IED rates for subjects on older generation (rate ratio = 0.09, p < 0.001) and combined generation ASM regimens (rate ratio = 0.23, p < 0.001) (Fig.4a). However, there was a nonsignificant association between newer generation ASM regimens and IED rates (p = 0.13). As in other published works, we defined older generation ASMs as medications that were approved for the treatment of epilepsy prior to 1993.39 In evaluating ASMs independently, our models revealed that being on any of the commonly prescribed ASMs, present in the RAM dataset, was associated with reduced IED rates. However, only seven of the 13 ASMs had statistically significant IED rate reductions. These ASMs included: topiramate (rate ratio = 0.61, p = 0.01), zonisamide (rate ratio = 0.53, p = 0.01), lamotrigine (rate ratio = 0.70, p = 0.03), phenytoin (rate ratio = 0.54, p = 0.03), carbamazepine (rate ratio = 0.42, p < 0.001), levetiracetam (rate ratio = 0.54, p < 0.001), and lacosamide (rate ratio = 0.68, p = 0.03) (Fig.4b).
Figure 4. Anti-seizure medications demonstrate differences in IED rate reductions.
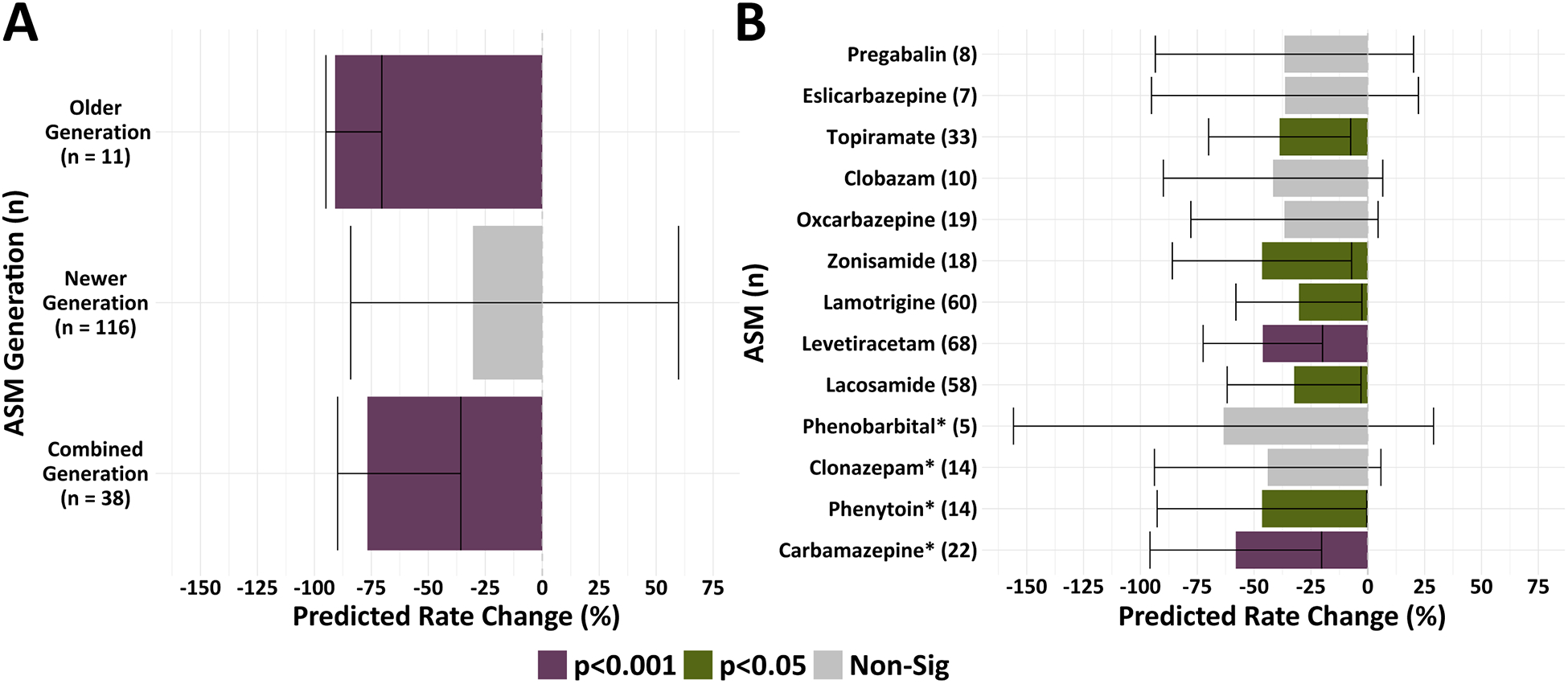
(A) There was a significant reduction in the predicted IED rate for subjects on older generation (p < 0.001) and combined generation ASM regimens (p < 0.001) relative to the group taking no ASMs. (B) Our linear mixed-effects models revealed that seven of the ASMs were associated with significant overall IED rate reductions when referenced to the group taking no ASMs. Colors reflect significance values with a false discovery rate controlled at a level of 0.05. Bars represent 95% confidence intervals. * denotes older generation ASMs. “n” corresponds to the number of unique subject sessions contributing to each stratum. ASMs excluded due to a small number in our dataset were: Gabapentin, Divalproex sodium, Perampanel, Felbamate, Rufinamide, Primidone, and ASMs categorized as Other.
Effect of time of testing on IED rates.
There was a nonsignificant difference in the IED rates between the time windows (morning-afternoon p = 0.15, morning-evening p = 0.85, afternoon-evening p = 0.26) (Fig.S4). The average normalized rate in the morning was 0.01 IEDs per minute (95% CI [0.008, 0.012]), afternoon was 0.008 IEDs per minute (95% CI [0.006, 0.01]), and evening was 0.01 IEDs per minute (95% CI [0.007, 0.013]). Fig.S4 illustrates that several of the subdivided 30-minute time bins (e.g., 10am) had higher, albeit nonsignificant average normalized IED rates.
Discussion
Our findings evince that certain factors have a stronger association with intracranial IED rates in subjects with refractory epilepsy. While we cannot establish the true direction of this association, we emphasize the importance of considering subject-specific differences in IED rates when evaluating IEDs for clinical or research purposes. We revealed that (1) the frequency of IEDs were not distributed uniformly across the brain, (2) IED rates were higher for electrodes in the SOZ, relative to electrodes outside of the SOZ, and (3) certain SOZs, especially those in mesial temporal regions, had higher IED rates. In conjunction, these results suggest that IED rates were generally higher in SOZs and that hemisphere specific SOZ locations significantly influenced IED rates. These intracranial findings align with previous observations by showing a higher percentage of IEDs per electrode in regions near the SOZ.4,8,30,40 However, we augment past studies by leveraging the power of a large Stereo-EEG dataset to comprehensively examine different focal SOZs in both mesial and neocortical regions.
In evaluating the IED rates of electrodes outside of the SOZ (Non-SOZ), but still stratified by the subject’s SOZ location, we discovered that Non-SOZ IED rates were similar, irrespective of the SOZ location. Nonetheless, previous studies demonstrated that IEDs outside of the SOZ impact memory encoding and retrieval,8,11 indicating that low IED rates in Non-SOZ brain regions are still detrimental. Our findings support the evaluation of IED rates for localizing SOZs41 and reveal that global IED rates are likely attenuated when examining all electrodes in aggregate. The paradoxically low r2 observed for SOZ location (e.g., mesial temporal, lateral temporal) in our dominance analysis and the highly significant effects shown in the detailed, factor-level analysis further revealed the importance of considering the SOZ hemisphere (e.g., right or left) when investigating IED rates.
Our validation that intracranial IED rates were higher near the SOZ enabled us to more confidently compare the relative importance of SOZs and other subject-specific factors, as they relate to IED rates. We utilized the output from a bootstrapped general dominance analysis to rank the relative importance of factors for predicting differences in IED rates. Earlier reports examined the relationship between IEDs and subject-specific factors, such as age, anti-epileptic medications, hormone levels, and multiday rhythms, yet this was mostly done in isolation.3,16,18,19 Our study is the first to address this discrepancy by examining multiple subject-specific features in aggregate. We revealed that the top three factors influencing IED rates were ASM status (i.e., whether the subject was on at least one ASM or not on any ASMs), time of testing, and SOZ location, respectively.
It was unsurprising that ASM status ranked highly, as many previous studies showed an association between ASMs and IEDs.3,21,22,42 Interestingly, we found that older generation and combined generation ASM regimens were associated with significant IED reductions. We acknowledge the possibility that subjects successfully treated with and remaining on older generation and combined generation ASM regimens may represent a different population than subjects on newer generation ASMs. Nevertheless, our findings suggest that certain ASMs may have stronger influences on IEDs, yet it remains unclear whether using ASMs with a greater impact on IEDs will result in clinically significant improvements in cognition.8,10,11
Accordingly, we examined if specific ASMs were associated with IED rate reductions relative to no ASM. Of the ASMs included in our analyses, carbamazepine, levetiracetam, lacosamide, zonisamide, lamotrigine, phenytoin, and topiramate demonstrated significant reductions in IEDs. As expected, levetiracetam and lamotrigine, two ASMs that have been previously shown to reduce IEDs, were included in this list.3,21,22 However, we observed that carbamazepine and levetiracetam had the most robust reduction in IEDs and should be considered if consistently lowering IED rates is of clinical interest – with the caveat that these considerations should not apply to specific pediatric epilepsies. Another clinical implication stems from Foster et al.’s (2020) finding that seizure frequency, rather than specific ASMs, was correlated with ASM-related cognitive dysfunction.43 In union with our results, we divulge that IED rates, which are known biomarkers for cortical excitability and seizure frequency,5 may be a potential metric for predicting cognitive side effects that coincide with ASM therapy in adults with epilepsy.
Although we observed a nonsignificant association between testing time and IED rates in our detailed, factor-level analysis, we attribute these null findings to the low temporal coverage of our data, especially since it excluded sleep states. Moreover, previous reports – explicitly designed to evaluate this question with either long-term scalp-EEG or implanted brain devices – already demonstrated that time was significantly correlated with IEDs.19,44 Specifically, that the peak IED occurrence was in the evening, likely correlated with vigilance and sleep states, and multidien IED rhythms were relatively stable within subjects.19,45 Together with evidence that IED rates affect memory during sleep, we underscore the limitations imposed by low temporal coverage in the current dataset.46
Several provisos limit the implications of this study. We acknowledge that our mode of automated IED detection could introduce bias, as automated IED detection is not 100% concordant with human review.28,47 However, this provides a method for objective signal processing of large potential deflections while avoiding potential inconsistencies of human review.48 We also did not select subjects to specifically examine the relationship between subject-level factors and IED rates, so we were missing potentially relevant subject information, such as surgical outcome data. This data was not collected systematically between the study sites included in this multicenter study, creating the possibility that Stereo-EEG missed or poorly covered SOZs. Our lack of pathology findings may bias results, as different pathologic etiologies of epilepsy could influence the rate and distribution of IEDs. We also did not have ASM blood levels at the time of testing and could not control for the impact that ASM tapering and neurosurgical procedures (e.g., Stereo-EEG implant) had on IED rates.3 ASM findings could additionally be influenced by type II error, as the effect sizes were similar between all ASMs regardless of statistical significance. Thus, nonsignificant ASM observations could be due to fewer subjects in those ASM groups, with an additional source of bias introduced due to missing ASM data.
The implications of our findings were further limited because we could not examine how ASMs impact seizure frequency and whether ASMs affect SOZ and Non-SOZ IEDs equally. We concede that subjects were not randomized to come off or stay on ASMs. Consequently, the major contribution of ASM status in the general dominance analysis could be confounded by factors other than the actual usage of ASMs. For instance, our lack of seizure information limited our ability to fully control for the relationship between ASM status and seizure frequency, a factor that may serve as a potential confound to the ASM findings. We also acknowledge potential bias from the heterogeneous number of sessions completed per subject. This arose due to several uncontrollable factors, mainly the varying lengths of implantation and the intracranial subjects’ willingness to perform repeated experiment sessions. Despite these limitations, our study explores diverse focal epilepsies and provides the foundation for future research on this topic.
In summary, the current findings demonstrated that ASM status had the strongest influence on IED rates, followed by testing time and SOZ location. We revealed that IED rates were generally higher in SOZs and that electrodes distant from SOZs had similar IED rates, irrespective of the SOZ localization. We also showed that specific ASMs more strongly influenced IED rates; however, our work encourages future studies specifically designed to examine ASMs as they relate to interictal and ictal activity. Our findings collectively provide new evidence for considering specific factors when analyzing IEDs for clinical or research purposes.
Supplementary Material
Figure S2. Sample IED detections from our automated template-matching detector. The top panel (green) depicts true IED detections, and the bottom panel (orange) depicts artifactual IED detections. We tuned the IED template used by our automated detector to minimize the number of artifactual IEDs (orange) while optimizing the number of true positive IEDs (green).
Figure S1. Spatial distribution of interictal electrodes. Electrodes aggregated across subjects in a common MNI coordinate system were plotted on an average brain. Colors indicate classified brain regions: frontal (red), parietal (blue), lateral temporal (orange), mesial temporal (yellow), occipital (green). To prevent overcrowding, this only shows electrodes that captured one or more IEDs during the memory task and were included in the mixed-effects models.
Figure S3. Post-hoc pairwise comparison of IED rates between all seizure onset zone locations. Tukey’s HSD test showed pairwise differences for all combinations of SOZ locations, with colors indicating significance values. All pairs represent a comparison of the first listed location relative to the second listed location. For instance, contacts in right parietal (RP) SOZs had significantly higher IED rates relative to contacts in right frontal (RF) SOZs. The left and right mesial temporal regions had significantly higher IED rates than most other brain regions. Bars represent 95% confidence intervals.
L Left, R Right, F Frontal Cortex, T Lateral Temporal Cortex, MTL Mesial Temporal Lobe, P Parietal Cortex, O Occipital Cortex
Figure S4. Association between time of testing and IED rates. There was a nonsignificant difference in the IED rates between the time windows (morning-afternoon p = 0.15, morning-evening p = 0.85, afternoon-evening p = 0.26). Significance was determined with linear mixed-effects models that controlled for ASM status and SOZ location, while modeling subjects as random effects. Each 30-minute time bin represents mean normalized IED rates for all experiment start times that coincided with that period, in addition to standard errors. All times within each time window were then averaged to produce mean IED rates illustrated as dashed lines and 95% confidence intervals. Time windows: morning (6am-12pm), afternoon (12pm-5pm), evening (5pm-9pm).
Key Points.
Anti-seizure medication (ASM) status and seizure onset zone (SOZ) location are important influencers of IED rates
Irrespective of the SOZ location, intracranial electrodes in the SOZ had higher overall IED rates than electrodes outside of the SOZ
Older generation and combined generation ASM regimens were associated with significant reductions in IED rates
Carbamazepine, levetiracetam, lacosamide, zonisamide, lamotrigine, phenytoin, and topiramate significantly reduced IED rates
Specific factors should be considered when analyzing IEDs for either clinical or research purposes
Acknowledgements
We would like to thank the patients, clinical staff, and research teams, without whom this study would not have been possible. We are also grateful for everyone involved in this research collaboration, which was coordinated by Dr. Michael Kahana’s Computational Memory lab at the University of Pennsylvania. This work was supported by the NIH (05-T32LM012204-03; U01NS113198-01), the NSF (Award #1632738), and a Diamond Foundation Research Development Award.
Footnotes
Disclosures
None of the authors has any conflict of interest to disclose.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;52:1–17. [DOI] [PubMed] [Google Scholar]
- 2.Ammanuel SG, Kleen JK, Leonard MK, Chang EF. Interictal Epileptiform Discharges and the Quality of Human Intracranial Neurophysiology Data. Front Hum Neurosci. 2020;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goncharova II, Alkawadri R, Gaspard N, Duckrow RB, Spencer DD, Hirsch LJ, et al. The relationship between seizures, interictal spikes and antiepileptic drugs. Clin Neurophysiol. 2016;127(9):3180–6. [DOI] [PubMed] [Google Scholar]
- 4.Gotman J Relationships Between Interictal Spiking and Seizures: Human and Experimental Evidence. Can J Neurol Sci. 1991;18(S4):573–6. [DOI] [PubMed] [Google Scholar]
- 5.Karoly PJ, Freestone DR, Boston R, Grayden DB, Himes D, Leyde K, et al. Interictal spikes and epileptic seizures: their relationship and underlying rhythmicity. Brain. 2016;139(4):1066–78. [DOI] [PubMed] [Google Scholar]
- 6.Koc G, Morkavuk G, Akkaya E, Karadas O, Leventoglu A, Unay B, et al. Latencies to first interictal epileptiform discharges in different seizure types during video-EEG monitoring. Seizure. 2019;69:235–40. [DOI] [PubMed] [Google Scholar]
- 7.Holmes GL, Lenck-Santini P-P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8(3):504–15. [DOI] [PubMed] [Google Scholar]
- 8.Ung H, Cazares C, Nanivadekar A, Kini L, Wagenaar J, Becker D, et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain. 2017;140(8):2157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes GL. What is more harmful, seizures or epileptic EEG abnormalities? Is there any clinical data? Epileptic Disord. 2014;16(S1):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horak PC, Meisenhelter S, Song Y, Testorf ME, Kahana MJ, Viles WD, et al. Interictal epileptiform discharges impair word recall in multiple brain areas. Epilepsia. 2017;58(3):373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleen JK, Scott RC, Holmes GL, Roberts DW, Rundle MM, Testorf M, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013;81(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleen JK, Kirsch HE. The nociferous influence of interictal discharges on memory. Brain. 2017;140(8):2072–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao J, Baldwin M, Hawes-Ebersole S, Ebersole J. Cortical substrates of scalp eeg epileptiform discharges. J Clin Neurophysiol. 2007;24(3):96–100. [DOI] [PubMed] [Google Scholar]
- 14.Frauscher B, Gotman J. Sleep, oscillations, interictal discharges, and seizures in human focal epilepsy. Neurobiol Dis. 2019;127:545–53. [DOI] [PubMed] [Google Scholar]
- 15.Bragatti JA, Torres CM, Cherubini PA, Leistner-Segal S, Bianchin MM. Is interictal EEG activity a biomarker for mood disorders in temporal lobe epilepsy? Clin Neurophysiol. 2014;125(10):1952–8. [DOI] [PubMed] [Google Scholar]
- 16.Herzog AG, Coleman AE, Jacobs AR, Klein P, Friedman MN, Drislane FW, et al. Interictal EEG discharges, reproductive hormones, and menstrual disorders in epilepsy. Ann Neurol. 2003;54(5):625–37. [DOI] [PubMed] [Google Scholar]
- 17.van Campen JS, Hompe EL, Jansen FE, Velis DN, Otte WM, van de Berg F, et al. Cortisol fluctuations relate to interictal epileptiform discharges in stress sensitive epilepsy. Brain. 2016;139(6):1673–9. [DOI] [PubMed] [Google Scholar]
- 18.Aanestad E, Gilhus NE, Brogger J. Interictal epileptiform discharges vary across age groups. Clin Neurophysiol. 2020;131(1):25–33. [DOI] [PubMed] [Google Scholar]
- 19.Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karoly PJ, Goldenholz DM, Freestone DR, Moss RE, Grayden DB, Theodore WH, et al. Circadian and circaseptan rhythms in human epilepsy: a retrospective cohort study. Lancet Neurol. 2018;17(11):977–85. [DOI] [PubMed] [Google Scholar]
- 21.Stodieck S, Steinhoff BJ, Kolmsee S, van Rijckevorsel K. Effect of levetiracetam in patients with epilepsy and interictal epileptiform discharges. Seizure. 2001;10(8):583–7. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson A-S et al. The Effect of Lamotrigine on Epileptiform Discharges in Young Patients with Drug-Resistant Epilepsy. Epilepsia. 2001;42(2):230–36. [DOI] [PubMed] [Google Scholar]
- 23.Solomon EA, Kragel JE, Gross R, Lega B, Sperling MR, Worrell G, et al. Medial temporal lobe functional connectivity predicts stimulation-induced theta power. Nat Commun. 2018;9(1):4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yushkevich PA, Pluta JB, Wang H, Xie L, Ding S-L, Gertje EC, et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment: Automatic Morphometry of MTL Subfields in MCI. Hum Brain Mapp. 2015;36(1):258–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–80. [DOI] [PubMed] [Google Scholar]
- 27.Solomon EA, Lega BC, Sperling MR, Kahana MJ. Hippocampal theta codes for distances in semantic and temporal spaces. PNAS. 2019;116(48):24343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horak PC, Meisenhelter S, Testorf ME, Connolly AC, Davis KA, Jobst BC. Implementation and evaluation of an interictal spike detector. Proceedings of SPIE. 2015;96000N1–11. [Google Scholar]
- 29.Noachtar S, Bilgin Ö, Rémi J, Chang N, Midi I, Vollmar C, et al. Interictal regional polyspikes in noninvasive EEG suggest cortical dysplasia as etiology of focal epilepsies. Epilepsia. 2008;49(6):1011–7. [DOI] [PubMed] [Google Scholar]
- 30.Goncharova II, Spencer SS, Duckrow RB, Hirsch LJ, Spencer DD, Zaveri HP. Intracranially recorded interictal spikes: Relation to seizure onset area and effect of medication and time of day. Clin Neurophysiol. 2013;124(11):2119–28. [DOI] [PubMed] [Google Scholar]
- 31.Gaspard N, Alkawadri R, Farooque P, Goncharova II, Zaveri HP. Automatic detection of prominent interictal spikes in intracranial EEG: Validation of an algorithm and relationsip to the seizure onset zone. Clin Neurophysiol. 2014;125(6):1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budescu DV. Dominance analysis: A new approach to the problem of relative importance of predictors in multiple regression. Psychol Bull. 1993;114(3):542–51. [Google Scholar]
- 33.Azen R, Budescu DV. The dominance analysis approach for comparing predictors in multiple regression. Psychol Methods. 2003;8(2):129–48. [DOI] [PubMed] [Google Scholar]
- 34.Luo W, Azen R. Determining Predictor Importance in Hierarchical Linear Models Using Dominance Analysis. J Educ Behav Stat. 2013;38(1):3–31. [Google Scholar]
- 35.Vize CE, Collison KL, Crowe ML, Campbell WK, Miller JD, Lynam DR. Using Dominance Analysis to Decompose Narcissism and Its Relation to Aggression and Externalizing Outcomes. Assessment. 2019;26(2):260–70. [DOI] [PubMed] [Google Scholar]
- 36.Azen R, Budescu DV. Comparing Predictors in Multivariate Regression Models: An Extension of Dominance Analysis. J Educ Behav Stat. 2006;31(2):157–80. [Google Scholar]
- 37.Johnson JW, Lebreton JM. History and Use of Relative Importance Indices in Organizational Research. Organ Res Methods. 2004;7(3):238–57. [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 39.French JA, Gazzola DM. New generation antiepileptic drugs: what do they offer in terms of improved tolerability and safety? Ther Adv Drug Saf. 2011;2(4):141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsh ED, Peltzer B, Brown III MW, Wusthoff C, Storm PB Jr, Litt B, et al. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia. 2010;51(4):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diamond JM, Chapeton JI, Theodore WH, Inati SK, Zaghloul KA. The seizure onset zone drives state-dependent epileptiform activity in susceptible brain regions. Clin Neurophysiol. 2019;130(9):1628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libenson MH, Caravale B. Do antiepileptic drugs differ in suppressing interictal epileptiform activity in children? Pediatr Neurol. 2001;24(3):214–8. [DOI] [PubMed] [Google Scholar]
- 43.Foster E, Malpas CB, Ye K, Johnstone B, Carney PW, Velakoulis D, et al. Antiepileptic drugs are not independently associated with cognitive dysfunction. Neurology. 2020;94(10):1051–61. [DOI] [PubMed] [Google Scholar]
- 44.Martins Da Silva A, Aarts JHP, Binnie CD, Laxminarayan R, Lopes Da Silva FH, Meijer JWA, et al. The circadian distribution of interictal epileptiform EEG activity. Electroencephalogr Clin Neurophysiol. 1984;58(1):1–13. [DOI] [PubMed] [Google Scholar]
- 45.Spencer DC, Sun FT, Brown SN, Jobst BC, Fountain NB, Wong VSS, et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57(9):1495–502. [DOI] [PubMed] [Google Scholar]
- 46.Lambert I, Tramoni-Negre E, Lagarde S, Roehri N, Giusiano B, Trebuchon-Da Fonseca A, et al. Hippocampal Interictal Spikes during Sleep Impact Long-Term Memory Consolidation. Ann Neurol. 2020;87(6):976–87. [DOI] [PubMed] [Google Scholar]
- 47.Janca R, Jezdik P, Cmejla R, Tomasek M, Worrell GA, Stead M, et al. Detection of Interictal Epileptiform Discharges Using Signal Envelope Distribution Modelling: Application to Epileptic and Non-Epileptic Intracranial Recordings. Brain Topogr. 2015;28(1):172–83. [DOI] [PubMed] [Google Scholar]
- 48.Kural MA, Duez L, Sejer Hansen V, Larsson PG, Rampp S, Schulz R, et al. Criteria for defining interictal epileptiform discharges in EEG: A clinical validation study. Neurology. 2020;94(20):2139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2. Sample IED detections from our automated template-matching detector. The top panel (green) depicts true IED detections, and the bottom panel (orange) depicts artifactual IED detections. We tuned the IED template used by our automated detector to minimize the number of artifactual IEDs (orange) while optimizing the number of true positive IEDs (green).
Figure S1. Spatial distribution of interictal electrodes. Electrodes aggregated across subjects in a common MNI coordinate system were plotted on an average brain. Colors indicate classified brain regions: frontal (red), parietal (blue), lateral temporal (orange), mesial temporal (yellow), occipital (green). To prevent overcrowding, this only shows electrodes that captured one or more IEDs during the memory task and were included in the mixed-effects models.
Figure S3. Post-hoc pairwise comparison of IED rates between all seizure onset zone locations. Tukey’s HSD test showed pairwise differences for all combinations of SOZ locations, with colors indicating significance values. All pairs represent a comparison of the first listed location relative to the second listed location. For instance, contacts in right parietal (RP) SOZs had significantly higher IED rates relative to contacts in right frontal (RF) SOZs. The left and right mesial temporal regions had significantly higher IED rates than most other brain regions. Bars represent 95% confidence intervals.
L Left, R Right, F Frontal Cortex, T Lateral Temporal Cortex, MTL Mesial Temporal Lobe, P Parietal Cortex, O Occipital Cortex
Figure S4. Association between time of testing and IED rates. There was a nonsignificant difference in the IED rates between the time windows (morning-afternoon p = 0.15, morning-evening p = 0.85, afternoon-evening p = 0.26). Significance was determined with linear mixed-effects models that controlled for ASM status and SOZ location, while modeling subjects as random effects. Each 30-minute time bin represents mean normalized IED rates for all experiment start times that coincided with that period, in addition to standard errors. All times within each time window were then averaged to produce mean IED rates illustrated as dashed lines and 95% confidence intervals. Time windows: morning (6am-12pm), afternoon (12pm-5pm), evening (5pm-9pm).
Data Availability Statement
Data is available through the Defense Advanced Research Projects Agency (DARPA) Restoring Active Memory (RAM) Public Data Release (http://memory.psych.upenn.edu/RAM). The code utilized in our study is available upon reasonable request.


