Abstract
Lung morphogenesis relies on a number of important processes, including proximal-distal patterning, cell proliferation, migration and differentiation, as well as epithelial-mesenchymal interactions. In mouse lung development, SOX2+ cells are localized in the proximal epithelium, whereas SOX9+ cells are present in the distal epithelium. We show that, in human lung, expression of these transcription factors differs, in that during the pseudoglandular stage distal epithelial progenitors at the tips coexpress SOX2 and SOX9. This double-positive population was no longer present by the canalicular stages of development. As in mouse, the human proximal epithelial progenitors express solely SOX2 and are surrounded by smooth muscle cells (SMCs) both in the proximal airways and at the epithelial clefts. Upon Ras-related C3 botulinum toxin substrate 1 inhibition, we noted decreased branching, as well as increased SMC differentiation, attenuated peristalsis, and a reduction in the distal double-positive SOX2/SOX9 progenitor cell population. Thus, the presence of SOX2/SOX9 double-positive progenitor cells in the distal epithelium during the pseudoglandular stage of human lung development appears to be critical to proximal-distal patterning and lung branching. Moreover, SMCs promote a SOX2 proximal phenotype and seem to suppress the SOX9+ population.
Keywords: human lung development, progenitor cells, smooth muscle cells, α-actin 2
INTRODUCTION
The developing lung is a very dynamic and complex organ composed of an epithelial and a mesenchymal compartment that closely interact. The developing lung undergoes several rounds of branching and gets remodeled during the canalicular and saccular phases to eventually reach the alveolar stage, when the alveoli form and mature. The branching process of the mouse lung has been extensively studied over the past decades, thereby identifying the major contributors to this process in rodents. Many mechanisms contribute to lung branching morphogenesis, including proximal-distal patterning, morphogen distribution, and epithelial-mesenchymal cross talk. Chief among these factors are the lung epithelial progenitor cells, which initially determine the proximal-distal patterning, and eventually the morphological features and cell types in the overall organ. In mouse, this proximal-distal patterning is defined by two transcription factors: Sox2, localized in the proximal epithelium (1), and Sox9, localized in the distal epithelium up until the saccular stage, during which time new branches exponentially increase (1). The specific distribution of these transcription factors leads to proper cell proliferation and differentiation, allowing for adequate branching to take place. Deletion of Sox2 in mouse lung results in an increase of club cells, ciliated cells, and basal cells, whereas its overexpression leads to elevated levels of committed progenitor cells, including p63+ cells and neuroendocrine cells (8). Alternatively, either a loss or gain of function of Sox9 results in branching defects characterized by decreased domain branches (10) and the presence of terminal cystic structures (12).
In conjunction with adequate proximal-distal patterning, epithelial-mesenchymal interactions play an important role in lung branching. Among the many types of mesenchymal cells present in the lung, smooth muscle cells (SMCs) are critical to the branching process. The contribution of SMCs to the developing lung has been attributed to their ability to support the epithelial structure and signal to the epithelial cells, thus controlling their proliferation and differentiation (2, 18). Another role attributed to SMCs is their ability to contract, resulting in airway peristalsis (7). A number of congenital lung branching malformations demonstrate the hallmark of atypical SMC patterning. Increased SMCs are seen in the bronchioles and pulmonary arterioles of bronchopulmonary dysplasia and asthma patients, whereas pulmonary hypoplasia is characterized by decreased SMCs (2, 5, 13).
Most of our knowledge of lung development is inferred from animal models, and little is known about human lung development. In this short report, we sought to determine the proximal-distal patterning of the human lung by assessing the spatiotemporal distribution of SOX2, SOX9, and α-actin 2 (ACTA2) and their impact on branching morphogenesis in human lung development.
METHODS
Ethics statement.
After informed consent was given, deidentified human fetal samples were collected under Institutional Review Board approval (USC-HS-13-0399 and CHLA-14-2211). The only information collected was gestational age and whether there were any known genetic or structural abnormalities.
Whole mount staining.
Whole mount staining was adapted from Metzger et al. (10). After blocking, lungs were incubated with primary antibody [cadherin 1 (CDH1), sc-1500] for at least 3–4 nights at 4°C. Lungs were then washed and incubated with secondary antibodies and Cy3-ACTA2 conjugated antibody (C6198; Sigma-Aldrich) for 3–4 days. Stained lungs were gradually dehydrated and cleared using benzyl alcohol-benzyl benzoate (1:2).
Explant cultures.
Human fetal lung explants between 10 and 12 wk gestation were cultured on air-liquid interface as previously described (4). Explants were treated with 25 μM NSC-23766 [botulinum toxin substrate 1 (RAC1) inhibitor] every 24 h for a total of 48 h.
Immunostaining.
Lungs/explants were fixed, paraffin embedded, sectioned, and processed for staining as previously described (4). Sections were incubated with the following primary antibodies overnight at 4°C: SOX2 (sc-17320; Santa Cruz), SOX9 (AB5535; Millipore), Ki-67 (RM-9106-S1; Thermo Scientific), and CDH1 [61081 (BD Biosciences) or 3195S (Cell Signaling)]. Slides were incubated with Cy3-, Cy5-, or Alexa488-conjugated antibodies (Dako or Life Technologies) in conjunction with a Cy3-ACTA2 antibody (C6198; Sigma-Aldrich) when appropriate and stained for DAPI (DE571; Life Technologies).
Live imaging.
Time lapse images were acquired using bright-field microscopy with a DMIRE2 inverted microscope (Leica Microsystems) equipped with a culture chamber. NSC-23766-treated and control explants were imaged simultaneously at each time point, at an interval of 10 min, for a total time of 48 h. The images were processed after acquisition using FIJI ImageJ software (14).
Statistical analyses.
Statistical analyses were performed using GraphPad Prism software. A paired Student’s t-test was used to compare two paired groups. Data are presented as average values ± SE. The results were considered significant if P ≤ 0.05.
RESULTS
The presence and distribution of SMCs is required for epithelial branching.
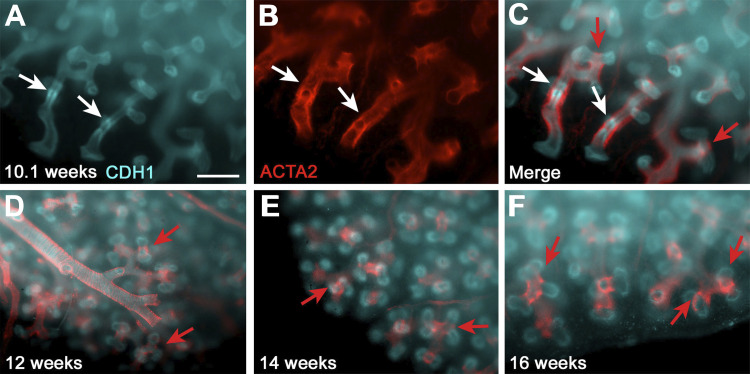
Whole mount staining using CDH1 and ACTA2 antibodies on intact lobes of human fetal lungs at different gestational ages, focusing primarily on the pseudoglandular stage of development, revealed the 3D distribution of SMCs. Human fetal lung segments showed expansion of SMCs to the very distal edge of the lung. There, they occupied the clefts of epithelial buds thus forming a “knot” around the distal epithelial branches up until at least 16 wk gestation (Fig. 1F). Interestingly, at 10.1 wk gestation, we demonstrated the presence of a ring of SMCs surrounding a condensation of epithelial cells before their producing new branches (Fig. 1, A–C). This suggests that SMCs are likely important in the formation of human fetal epithelial branches.
Fig. 1.
Smooth muscle cell (SMC) distribution in the human developing lung. A–C: whole mount staining of 10.1 wk human lung for cadherin 1(CDH1, cyan, A) and α-actin 2 (ACTA2, red, B); white arrows show epithelial condensation (A) and SMCs that gathering in a ring-like structure (B). C: merge of A and B; white arrows point to the ring of SMCs around the condensation of epithelial cells, and red arrows show the knot-forming SMCs at the cleft of very distal epithelial branches. D–F: CDH1 and ACTA2 staining on intact lung edges of 12 (D)-, 14 (E)-, and 16 (F)-wk lungs. Red arrows point to the knot-forming SMCs in the cleft. Scale: 500 μm.
Human fetal lungs in the pseudoglandular stage coexpress SOX2 and SOX9 in the distal epithelial branching tips.
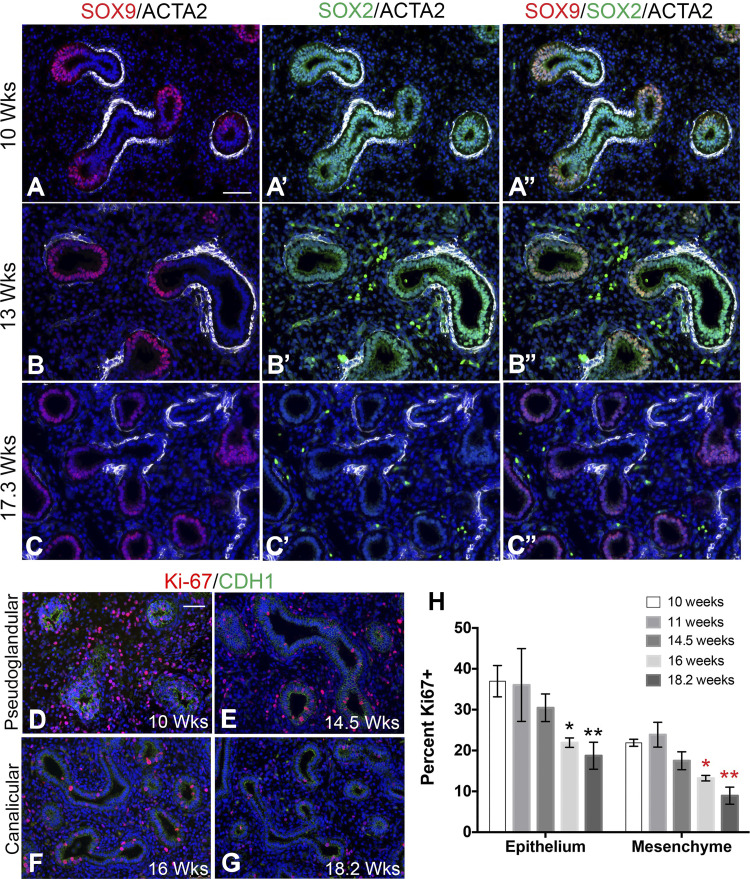
Proximal-distal patterning is another important determinant of lung branching. To determine the localization and expression pattern of SOX2 and SOX9 in the developing human lung, we examined the localization of these two transcription factors in lungs at 9–20 wk, representing the pseudoglandular (4–16 wk) to canalicular (16–20 wk) stages of development. Staining of human fetal lung tissue with SOX2, SOX9, and ACTA2 antibodies identified the presence, during the pseudoglandular stage, of a novel progenitor cell population, never before seen in mouse, coexpressing SOX2 and SOX9 and located in the epithelial tips of branching buds (Fig. 2, A–B′′). These cells appear more prominent in the earlier stages of the pseudoglandular stage (Fig. 2, A–A′′) and gradually decrease (Fig. 2, B–B′′), until reaching the canalicular stage, when they no longer appear present (Fig. 2, C–C′′). However, the proximal progenitors in the pseudoglandular human fetal lungs solely expressed SOX2, similar to mouse lungs. ACTA2+ cells surrounded these SOX2+ progenitor cells. SMCs were also present at the clefts of distal branching tips, where SOX9 was minimal. In the canalicular stage, the expression of these transcription factors was comparable to that in mouse, where SOX2 was solely expressed in the proximal airways, and SOX9 was solely expressed in the distal tips of the epithelium. ACTA2+ cells surrounded all proximal epithelium, whether SOX2+ or SOX2 negative, but never SOX9+ cells (Fig. 2, A–C′′). This transition from SOX2/SOX9 double-positive cells to SOX9-only cells occurs at the same time when cell proliferation in both epithelium and mesenchyme decreases significantly.
Fig. 2.
Epithelial cells in the branch tips of pseudoglandular stage human lungs coexpress SOX2 and SOX9 and are highly proliferative. A–C′′: immunostaining of human fetal lung tissue in the pseudoglandular (10 and 13 wk gestation) and canalicular (17.3 wk gestation) stages of development with SOX2, SOX9, and ACTA2 antibodies. A–A′′: 10 wk human fetal lung stained with SOX9/ACTA2 (red/white) (A), SOX2/ACTA2 (green/white) (A′), and the overlay of the triple stain (A′′). B–B′′: 13-wk human fetal lung stained with SOX9/ACTA2 (red/white) (B), SOX2/ACTA2 (green/white) (B′), and the overlay of the triple stain (B′′). C–C′′: 17.3-wk human fetal lung stained with SOX9/ACTA2 (red/white) (C), SOX2/ACTA2 (green/white) (C′), and the overlay of the triple stain (C′′). D–G: immunostaining of human fetal lung tissue in the pseudoglandular (10 and 14.5 wk gestation; D and E) and canalicular (16 and 18.2 wk gestation; F and G) stages of development with Ki-67 and CDH1. H: graph comparing percent of Ki-67+ epithelial cells with the total no. of epithelial cells and percent of Ki-67+ mesenchymal cells with the total no. mesenchymal cells at each gestational time point (n = 3 for each time point). Epithelium: *P = 0.0197 and **P = 0.0227; mesenchyme: *P = 0.0014 and **P = 0.0046. Scale bars: 50 μm.
Lung sections between 10 and 18.2 wk were stained with CDH1 and Ki-67 to determine the percentage of cellular proliferation in both mesenchyme and epithelium. Visually and quantitatively, it was evident there were more proliferating cells in the pseudoglandular stage, primarily within the epithelium (Fig. 2, D–E), with 36.97 ± 3.83% of cells proliferating in the 10-wk epithelium. This was followed by a gradual decline in the percentage of proliferating cells up to 16 wk, the start of the canalicular stage (Fig. 2, F–G). During this period, there was a statistically significant decrease in both epithelial and mesenchymal cell proliferation compared with 10 wk, which was further decreased at 18.2 wk in both epithelium and mesenchyme.
Decreased branching because of RAC1 inhibition is associated with SMC hypertrophy yet defective peristalsis.
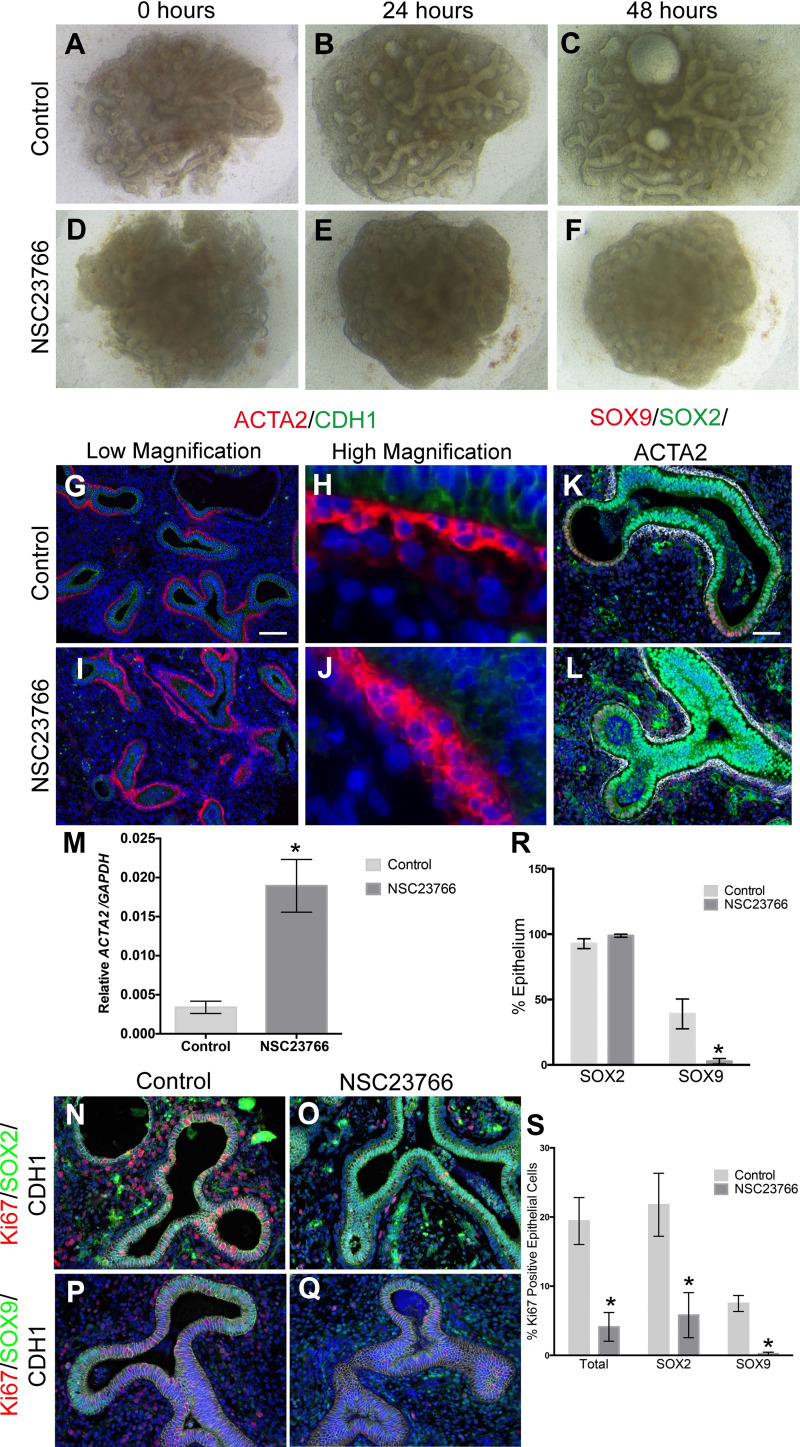
Because of the distribution of SMCs in relation to the proximal/distal progenitors, we sought to determine whether impaired SMC and/or peristalsis played an important role in human fetal lung growth. We previously showed that the RAC1 inhibitor NSC-23766 results in decreased lung branching in both mouse and human explants (4), and RAC1 inhibition is known to alter Ca2+ signaling and airway peristalsis. Human fetal lung explants (10–12 wk) were cultured in the presence (Fig. 3, D–F) and absence (Fig. 3, A–C) of NSC-23766 and ACTA2, SOX2, SOX9, and cell proliferation were assessed. Immunostaining demonstrated an increase in ACTA2 expression in the NSC-23766-treated explants, along with a thicker layer of SMC lining the proximal epithelium (Fig. 3, I and J vs. G and H). These results were validated by qRT-PCR (Fig. 3M). Furthermore, ACTA2+ cells lined many of the distal tips in the treated explants. In control explants, the distal epithelial tips preserved the double-positive SOX2/SOX9 progenitor cell population after 48 h in culture (Fig. 3K). Furthermore, SOX2 was expressed solely in the proximal epithelium underlined by a sheet of ACTA2+ cells. In the treated explants, there was a decrease in SOX9-expressing cells compared with controls (Fig. 3R) and an expansion in SOX2 domain throughout the epithelium (Fig. 3L). The expansion of SOX2 is associated with the expansion of ACTA2 around the entire epithelial structures, suggesting that SMCs promote SOX2 and repress SOX9. Although the number of SOX2+ cells between the control and treated explants remained the same, the appearance of expanded SOX2 domain could be because of changes in cell shape caused by RAC1 inhibition.
Fig. 3.
SMC function is important for human lung branching. A–F: 11.1 wk human fetal lung explants (C and F) in the presence (D–F) or absence (A–C) of NSC-23766 (25 μM). G–J: immunostaining of NSC-23766 (I and J) and control explants (G and H) with ACTA2 (red) and CDH1 (green) antibodies at low (G and I) and high (H and J) magnifications. Scale bar is 100 μm. M: qRT-PCR for ACTA2 expressed as relative expression compared with GAPDH (n = 5, P = 0.0049). K and L: immunostaining of NSC-23766 (L) and control explants (K) with SOX9 (red), SOX2 (green), and ACTA2 (white). Scale bar is 50 μm. N and O: immunostaining of NSC-23766 (O) and control (N) explants with Ki-67 (red), SOX2 (green), and CDH1 (white). P and Q: immunostaining of NSC-23766 (Q) and control (P) explants with Ki-67 (red), SOX9 (green), and CDH1 (white). R: percentage of epithelial SOX2+ cells and epithelial SOX9+ cells to total epithelial cells in control vs. NSC-23766-treated explants (n = 3, SOX9: P = 0.035). S: percentage of total epithelial Ki-67+ (n = 5, P = 0.0049), Ki-67/SOX2+ (n = 3, P = 0.046), and Ki-67/SOX9+ (n = 3, P = 0.0034) cells in control vs. NSC-23766-treated explants. *Statistically significant difference.
Total epithelial cell proliferation and SOX2 and SOX9 proliferation were investigated by staining control and treated explants with CDH1/Ki-67/SOX2 (Fig. 3, N and O) or CDH1/Ki-67/SOX9 (Fig. 3, P and Q). Quantification of the percentage of epithelial Ki-67, Ki-67/SOX2, and Ki-67/SOX9 demonstrated a significant decrease in total epithelial cell proliferation in the treated explants (Fig. 3S) compared with controls, and in proliferation of both SOX2+ (Fig. 3S) and SOX9+ (Fig. 3S) cells. These results suggest that the loss of the double-positive SOX2/SOX9 progenitor cell population is associated with decreased proliferation and impaired branching.
Human fetal lung explants in the presence and absence of the RAC1 inhibitor were live imaged for 48 h to evaluate peristalsis. In the control explants, there was continuous peristalsis throughout the explant with visible fluid flow in the large airways (V1). In contrast, in the NSC-23766-treated explant, the cells condensed, the airways constricted, likely because of the excessive SMC hypertrophy, and peristalsis was no longer visible (V2). This suggests that, in the absence of peristalsis, despite an increase in SMCs, branching fails to resume.
DISCUSSION
Proximal-distal patterning in mouse has been demarcated by the expression of two transcription factors, Sox2 and Sox9, which are exclusively expressed in the proximal and distal compartments, respectively. We have now shown that the expression of these transcription factors differs in the developing human lung (Fig. 4, A and D). During the pseudoglandular stage of development, when maximal branching occurs, a novel progenitor cell population coexpressing SOX2 and SOX9 is present in the distal tips of the branching epithelium, which is then lost in the canalicular stage of development, when differentiation starts (Fig. 4, A and B). Interestingly, the specification of SOX2/SOX9 progenitors into SOX9-only expressing cells coincides with a significant decrease in cell proliferation (Fig. 2). Interestingly, the induced loss of SOX2/SOX9 double-positive cells following RAC1 inhibition was also associated with decreased cell proliferation in both SOX2 and SOX9 cells (Fig. 4, A and C). We therefore speculate that the maintenance of this SOX2/SOX9 progenitor cell population is required for proper human lung branching.
Fig. 4.
Schematic distribution of ACTA2, SOX2, and SOX9 in the developing lung. A: SOX2/SOX9 progenitor cells are present in the distal lung epithelial tips in the pseudoglandular stage; their presence is restricted by SMCs. SMCs repress the expression of SOX9 while simultaneously allowing an expansion of SOX2. B: SOX2/SOX9 progenitor cells then disappear in the canalicular stage during which SOX2+ cells are solely localized in the proximal airways, and SOX9+ cells are localized in the distal epithelial tips, coinciding with decreased proliferation. C: in NSC3766-treated explants, an expansion of ACTA2 and SOX2 and a decrease in SOX9 and cell proliferation are observed. D: in mouse lungs, distal tip progenitors contain only SOX9+ cells.
Interestingly, airway SMCs surround SOX2+ cells in the proximal airway and in the cleft between the daughter epithelial branches, suggesting a central role for SMCs in driving branching morphogenesis in conjunction with the double-positive progenitor cell population. SMCs form a ring around a condensation of epithelial cells, before the formation of epithelial branches, suggesting that the presence of these SMCs is exerting a force on the underlying epithelial cells, likely causing them to extrude into branches.
Another role attributed to SMCs is their ability to contract and orchestrate airway peristalsis (7). In the intestine, it is proposed that epithelial buckling, leading to the formation of crypts and villi, occurs because of the fast differentiation and contraction of SMCs between embryonic days 11.5 and 14.5 (16, 17), suggesting that SMCs can exert mechanical forces that push the epithelium to fold into villi. Therefore, the presence of SMCs at the very distal tips of the lung could explain structural epithelial differences between mouse and human bronchioles through a buckling mechanism similar to that in the intestine. A recent study showed that removing SMCs from the cleft relaxes the cleft and flattens it (9), thus supporting our hypothesis that SMC force and contractility play an important role in branching. Moreover, mouse studies showed that expression of Sox2 correlated with the expression of Acta2 in the lung and that these Acta2+ cells express platelet-derived growth factor receptor-α and distinct Wnt ligands that differentiate them from other SMC in the lung (6). Our data along with data from mouse studies suggest that SOX2+ cells are tightly regulated by SMC localization. The signaling molecules contributing to that close relationship are yet to be determined.
Our studies and understanding of SMC contractility and lung branching were enhanced through the use of our optimized human lung culture system (4). Using live imaging of human fetal explants, we were able to observe in real time what happens to the lung when exposed to factors that compromise the proximal-distal axis and SMC formation and function. Inhibiting RAC1 activity in these human lung explants demonstrated an increase in SMC differentiation that in turn led to increased stiffness and decreased peristalsis, resulting in impaired branching morphogenesis. This suggests that SMC distribution and regulation are critical in proper lung development. Furthermore, the decrease in the double-positive SOX2/SOX9 progenitor cell population, in conjunction with decreased branching, suggests that there is a relationship between SMCs and SOX2/SOX9 distribution. There is an expansion of SMCs that extend to the distalmost part of the epithelial tips. Moreover, in both treated and native tissue we note that, when SMCs are localized in the immediate surrounding mesenchyme, SOX9 fails to translate, suggesting either strong negative feedback between SMCs and SOX9 expression or a positive feedback between SMCs and SOX2. Therefore, it is critical to gain a better understanding as to how SMC function and distribution interact with SOX2+ cells to suppress the distal SOX2/SOX9 progenitor cell population during the pseudoglandular stage of lung development. Such an understanding would then provide greater insight into the molecular and cellular bases of development in the early human fetal lung.
Most of our knowledge on early human lung development is inferred by extrapolation from rodents and other animal models (3, 10). Therefore, there is a lack of fundamental understanding of the mechanisms controlling early/fetal human lung development and whether regulatory pathways defined in other mammals apply to humans. Recently, significant discordances in gene expression have been identified between murine models and human diseases (11, 15). Our current study clearly demonstrates that human lung branching morphogenesis differs between mice and humans, given that a novel progenitor cell population exists in humans, never before described in mice. Identifying the similarities and differences, even subtle molecular differences, between mouse and human lung development will allow a better translational potential for mouse in vivo studies and to develop novel therapeutic targets for lung.
GRANTS
S. Danopoulos acknowledges the support of the Hastings Center of Pulmonary Research fellowship. I. Alonso acknowledges the support of the California Institute for Regenerative Medicine Bridges program. D. Al Alam acknowledges the support of the Research Career Development Award of Children’s Hospital Los Angeles.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.D., S.B., D.W., and D.A.A. conceived and designed research; S.D., I.A., M.T., B.G., and D.A.A. performed experiments; S.D., I.A., D.W., and D.A.A. analyzed data; S.D., S.B., D.W., and D.A.A. interpreted results of experiments; SD and D.A.A. prepared figures; SD and D.A.A. drafted manuscript; S.D., B.G., S.B., D.W., and D.A.A. edited and revised manuscript; S.D., I.A., M.T., B.G., S.B., D.W., and D.A.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Melissa L. Wilson (Department of Preventive Medicine, University of Southern California) and Rachel Steward (Family Planning Associates) for coordinating fetal tissue collection. We also thank G. Esteban Fernandez for guidance and assistance with live imaging.
REFERENCES
- 1.Alanis DM, Chang DR, Akiyama H, Krasnow MA, Chen J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat Commun 5: 3923, 2014. doi: 10.1038/ncomms4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badri KR, Zhou Y, Schuger L. Embryological origin of airway smooth muscle. Proc Am Thorac Soc 5: 4–10, 2008. doi: 10.1513/pats.200704-049VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc P, Coste K, Pouchin P, Azaïs JM, Blanchon L, Gallot D, Sapin V. A role for mesenchyme dynamics in mouse lung branching morphogenesis. PLoS One 7: e41643, 2012. doi: 10.1371/journal.pone.0041643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danopoulos S, Krainock M, Toubat O, Thornton M, Grubbs B, Al Alam D. Rac1 modulates mammalian lung branching morphogenesis in part through canonical Wnt signaling. Am J Physiol Lung Cell Mol Physiol 311: L1036–L1049, 2016. doi: 10.1152/ajplung.00274.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc 6: 683–692, 2009. doi: 10.1513/pats.200907-056DP. [DOI] [PubMed] [Google Scholar]
- 6.Endale M, Ahlfeld S, Bao E, Chen X, Green J, Bess Z, Weirauch MT, Xu Y, Perl AK. Temporal, spatial, and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Dev Biol 425: 161–175, 2017. doi: 10.1016/j.ydbio.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Featherstone NC, Jesudason EC, Connell MG, Fernig DG, Wray S, Losty PD, Burdyga TV. Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am J Respir Cell Mol Biol 33: 153–160, 2005. doi: 10.1165/rcmb.2005-0137OC. [DOI] [PubMed] [Google Scholar]
- 8.Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol 317: 296–309, 2008. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Kim HY, Pang MF, Varner VD, Kojima L, Miller E, Radisky DC, Nelson CM. Localized Smooth Muscle Differentiation Is Essential for Epithelial Bifurcation during Branching Morphogenesis of the Mammalian Lung. Dev Cell 34: 719–726, 2015. doi: 10.1016/j.devcel.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature 453: 745–750, 2008. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raven K. Rodent models of sepsis found shockingly lacking. Nat Med 18: 998, 2012. doi: 10.1038/nm0712-998a. [DOI] [PubMed] [Google Scholar]
- 12.Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MA, Kopp JL, Sander M, Wellik DM, Spence JR. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc Natl Acad Sci USA 110: E4456–E4464, 2013. doi: 10.1073/pnas.1311847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roos AB, Berg T, Nord M. A relationship between epithelial maturation, bronchopulmonary dysplasia, and chronic obstructive pulmonary disease. Pulm Med 2012: 196194, 2012. doi: 10.1155/2012/196194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program . Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ. Bending gradients: how the intestinal stem cell gets its home. Cell 161: 569–580, 2015. doi: 10.1016/j.cell.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shyer AE, Tallinen T, Nerurkar NL, Wei Z, Gil ES, Kaplan DL, Tabin CJ, Mahadevan L. Villification: how the gut gets its villi. Science 342: 212–218, 2013. doi: 10.1126/science.1238842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 121: 4409–4419, 2011. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]