Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a rare manifestation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children that can result in increased morbidity and mortality. The inflammatory underpinnings of MIS-C have not been examined in detail.
Methods
We examined the plasma levels of acute phase proteins and microbial translocation markers in children with MIS-C, children with acute coronavirus disease 2019 (COVID-19) infection, SARS-CoV-2-seropositive children, and controls.
Results
MIS-C children exhibited significantly higher levels of C-reactive protein (CRP), alpha2 macroglobulin (α2M), serum amyloid P (SAP), lipopolysaccharide (LPS), sCD14, and LPS binding protein (LBP) and significantly lower levels of haptoglobin (Hp) in comparison with seropositive, control, and/or COVID-19 children. In addition, COVID-19 children exhibited significantly higher levels of most of the above markers in comparison with seropositive and control children. Principal component analysis using a set of these markers could clearly discriminate MIS-C and COVID-19 from seropositive and control children. MIS-C children requiring pediatric intensive care unit admission and COVID-19 children with severe disease had higher levels of CRP, SAP, and/or sCD14 at admission.
Conclusions
Our study describes the role of systemic inflammation and microbial translocation markers in children with MIS-C and COVID-19 and therefore helps in advancing our understanding of the pathogenesis of different presentations of SARS-CoV-2 infection in children.
Keywords: acute phase proteins, biomarkers, COVID-19, microbial translocation, MIS-C, seropositive
Typically, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children manifests as a mild or asymptomatic infection for the most part [1, 2]. More recently, a hyperinflammatory manifestation of SARS-CoV-2 sequelae known as multisystem inflammatory syndrome in children (MIS-C), or pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection (PIMS-TS), has been described [3, 4]. This syndrome occurs as a late manifestation of SARS-CoV-2 infection (4–6 weeks after), with many children presenting with severe multi-organ dysfunction, an exaggerated inflammatory response, and elevated levels of SARS-CoV-2 immunoglobulin G (IgG) antibodies [1]. The pathogenesis of MIS-C is poorly understood. Most children have evidence of previous SARS-CoV-2 exposure and features resembling Kawasaki disease with cardiac and/or gastrointestinal manifestations [5, 6].
Previous studies have described elevated levels of pro-inflammatory cytokines, activated neutrophils and monocytes, thrombocytopenia, and lymphopenia in MIS-C [7]. However, a detailed examination of the acute phase response or microbial translocation processes has not been performed. Due to the hyperinflammatory nature of the disease and predominant gastrointestinal involvement, we postulated that several acute phase proteins and markers of intestinal dysbiosis would be elevated in MIS-C. We, therefore, performed a comprehensive examination of MIS-C children compared them with children with COVID-19, seropositive children, and healthy control children.
METHODS
Patient Consent
The study was approved by the Kanchi Kamakoti CHILDS Trust Hospital (KKCTH) CHILDS Trust Medical Research Foundation (CTMRF) ethics committee and the National Institute for Research in Tuberculosis ethics committee. The study was also registered at Clinical Trials Registry India (CTRI/2021/01/030605). Written informed consent/assent was obtained from all the children and/or caretakers in this study.
Study Population and Procedures
Blood samples were collected from children admitted to KKCTH, Chennai, India, from June 1, 2020, to September 30, 2020. Inclusion criteria were age 1 to 18 years and willingness of caretaker to give informed consent/assent. Blood collection was performed before any medication. Plasma was isolated and used for measuring multiple parameters. The demographic, epidemiological, medical, and laboratory data are shown in Table 1. Anonymized data were shared by KKCTH with National Institute for Research in tuberculosis for statistical analysis. COVID-19 disease and severity of COVID-19 were defined according to the Ministry of Health and Family Welfare (MOHFW) guidelines [8] issued by the Government of India, and children with MIS-C were diagnosed according to the World Health Organisation (WHO) case definition [9]. SARS-CoV-2 real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed by Indian Council of Medical Research (ICMR)–approved laboratories. Serology was performed using the iFlash SARS-CoV-2 IgG chemiluminescence antibody assay (CLIA; YHLO Biotechnology Corporation, Shenzhen, China) according to the manufacturer’s instructions. An IgG antibody titer of ≥10 AU/mL was considered positive.
Table 1.
Characteristics of COVID-19, MIS-C, and Seropositive (Non-MIS-C) Children
| MIS-C | COVID-19 | Seropositive | P Valuea | |
|---|---|---|---|---|
| n = 44 | n = 33 | n = 47b | ||
| Age, median (IQR), y | 7 (1–14) | 5 (1–17) | 4.4 (1–17) | <.05 |
| Male, No. (%) | 19 (43) | 20 (60) | 35 (74) | <.05 |
| Serology IgG positive, No. (%) | 44 (100) | 0 | 47 (100) | |
| Underlying conditions, No. (%) | 1 (2)c | 6 (19) | 11 (23)d | <.05 |
| Coexisting infections, No. (%) | 5 (11)e | 4 (12) | 6 (13)f | <.05 |
| Median duration since proven or suspected COVID illness or contact (range) | 3 wk (10 d–4 wk) | NA | 3.2 wk (10 d–5 wk)g | .46 |
| Laboratory parameters n = 44 n = 29 n = 31 | ||||
| CRP (<5 mg/L), median (IQR), mg/L | 169 (39–473) | 5 (<5–181) | 5 (<5–181)h | <.00001 |
| Lymphocyte (1500–4000/mm3), median (IQR), /mm3 | 1386 (330–2200) | 2945 (650–12 000) | 3890 (650–12 000) | <.00001 |
| Neutrophils (1500–7000/mm3), median (IQR), /mm3 | 11 658 (9918–14 878) | 3681 (120–13 160) | 6300 (120–13 160) | <.00001 |
| Platelets (200–450×109/L), median (IQR), ×109/L | 110 (62–210) | 278 (180–400) | 327 (100–540) | <.00001 |
| Sodium (135–145 mmol/L), median (IQR), mmol/L | 134 (127–138) | 136 (135–145) | 138 (135–148) | <.00001 |
| D-dimer (100–500 ng/mL FEU), median (IQR), ng/mL FEU | 4890 (2446–10 000) | NA | NA | |
| Ferritin (7–140 ng/mL), median (IQR), ng/mL | 605 (38–2571) | NA | NA | |
| LDH (125–243 U/L), median (IQR), U/L | 494 (200–905) | NA | NA | |
| Median duration of stay, d | 4.5 (3–12) | 3 (1–7) | 3 (1–10) | .0001 |
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; FEU, fibrinogen equivalent units; HHFNC, high-flow nasal cannula oxygen; IgG, immunoglobulin G; IQR, interquartile range; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; MIS-C, multisystem inflammatory syndrome in children; NA, not applicable; PICU, pediatric intensive care unit.
a P value between MIS-C, seropositive, and COVID-19.
bAdmitted for reasons other than COVID-19.
cAtrial septal defect.
dAcute lymphoblastic leukemia, neurodevelopmental delay, medulloblastoma, inborn error of metabolism, osteosarcoma, thalassemia, hemolytic anaemia, seizure disorder.
eUrinary tract infection, scrub typhus, enteric fever.
fScrub typhus, dengue viral fever, tuberculosis, brucellosis.
gNo history of COVID illness or contact in 4 children.
hHigh CRP seen in children with co-infections.
Acute Phase Proteins
Plasma levels of alpha-2 macroglobulin (α 2M), C-reactive protein (CRP), haptoglobin (Hp), and serum amyloid P (SAP), using a Milliplex MAP Human CVD Panel Acute Phase magnetic bead panel 3 from Millipore, were measured using a multiplex platform according to the manufacturer’s instructions. The lowest detection limits for acute phase proteins were as follows: α 2M, 0.49 ng/mL; CRP, 0.05 ng/mL; Hp, 0.06 ng/mL; and SAP, 0.06 ng/mL.
Microbial Translocation Markers
To inactivate plasma proteins, plasma samples were heated to 75°C for 5 minutes. LPS levels were measured using a limulus amoebocytes lysate assay (Cell Sciences Hycult Biotech, Canton, MA, USA) according to the manufacturer’s protocol. Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to measure plasma levels of lipid-binding protein (LBP), endotoxin core antibodies IgG (EndoCAb), intestinal fatty acid binding protein (iFABP; all Cell Sciences Hycult Biotech), and sCD14 (R&D Systems, Minneapolis, MN, USA). The lowest detection limits for microbial translocation markers were as follows: LPS, 0.04 EU/mL; LBP, 4.4 ng/mL; EndoCAb, 0.063 GMU/mL; iFABP, 15.6 pg/mL; and sCD14, 250 pg/mL.
Statistical Analysis
For analysis, children were categorized into 4 groups: COVID-19 (RT-PCR positive), MIS-C, seropositive (IgG-positive non-MIS-C), and control (both serology and RT-PCR negative). Continuous variables are presented as medians and interquartile ranges (IQRs), and categorical variables are reported as numbers and proportions. Comparison between the groups was performed using the Mann-Whitney U test. Geometric means (GMs) were used for measurements of central tendency. Statistically significant differences between MIS-C, COVID-19, seropositive, and control children were analyzed using the Kruskal-Wallis test with Dunn’s multiple comparisons. The Mann-Whitney test was used to compare the levels of acute phase proteins and microbial translocation markers between MIS-C children admitted to pediatric intensive care unit (PICU) care and those who did not require PICU admission, as well as between COVID-19 children with mild disease and those with moderate to severe disease. P ≤ .05 was considered statistically significant, and all tests were 2-sided. Analyses were performed using GraphPad PRISM, version 9.0 (GraphPad Software, San Diego, CA, USA). Principal component analysis (PCA) and correlation matrix analysis were done using JMP 14.0 (SAS, Cary, NC, USA).
RESULTS
Baseline Characteristics
Our study included 145 children, as previously described (33 COVID-19, 44 MIS-C, 47 seropositive, and 21 control children) (Table 1) [8]. The median age (range) was 5 years (1 month–17 years), and 58% (84/145) were male. All COVID-19 children were SARS-CoV-2 RT-PCT positive, and all MIS-C children were seropositive (IgG). Of the 33 COVID-19 children, 22 (67%) presented with mild symptoms, 3 (9%) had moderate symptoms, 2 (6%) had severe symptoms needing PICU care, and 6 (18%) children were asymptomatic. Among the 47 seropositive non-MIS-C children, 30 (64%) had no systemic symptoms and were admitted to the hospital for elective procedures. Control children (n = 21) were both SARS-CoV-2 RT-PCR negative and seronegative. They had similar median (IQR) ages (6 [1–15] years) and sex distributions (male: 52%, 11/21) and presented to the hospital for elective procedures; they therefore had no systemic symptoms, no other comorbid conditions, and no history of COVID-19 contact.
Plasma Levels of Acute Phase Proteins Are Elevated in MIS-C and COVID-19 Children
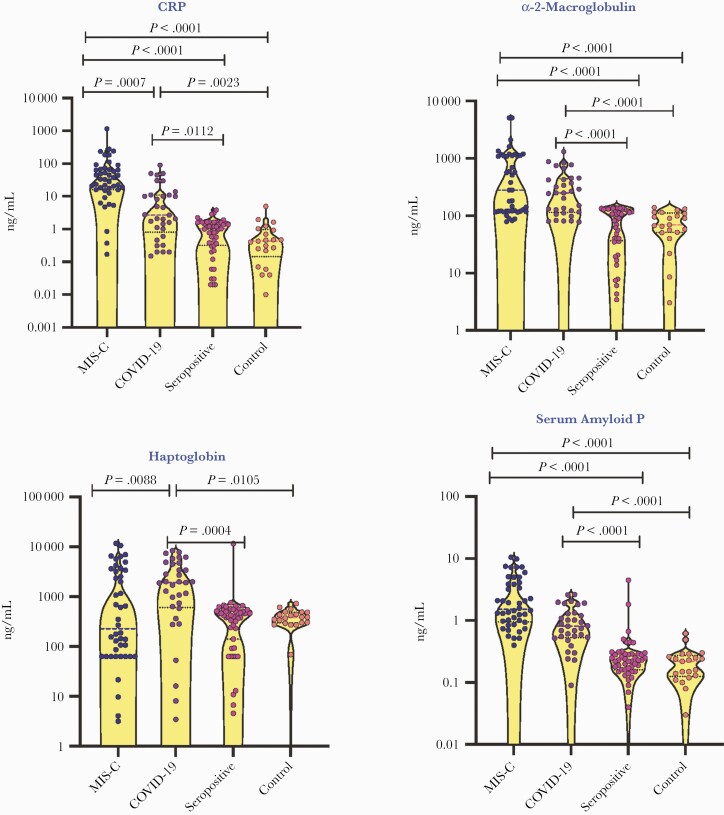
To elucidate if acute phase proteins can distinguish between different clinical manifestations, we measured the plasma levels of CRP, α 2M, SAP, and Hp and found that MIS-C children had significantly higher levels of CRP in comparison with COVID-19 children and higher α 2M and SAP in comparison with seropositive and control children (Figure 1). We also observed lower levels of Hp in MIS-C children when compared with COVID-19 children. Similarly, COVID-19 children had high CRP, α 2M, SAP, and Hp in comparison with seropositive and control children. However, no significant differences were observed in acute phase proteins between seropositive and control children.
Figure 1.
Elevated plasma levels of acute phase proteins in children with MIS-C and COVID-19. A, The plasma levels of CRP, α 2M, haptoglobin, and SAP were measured in MIS-C (n = 44), COVID-19 (n = 33), seropositive (n = 47), and control children (n = 21). The data are presented in scatter violin plots, with each circle representing a single individual. P values were calculated using the Kruskal-Wallis test with Dunn’s post hoc for multiple comparisons. Abbreviations: α 2M, alpha-2 macroglobulin; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; MIS-C, multisystem inflammatory syndrome in children; SAP, serum amyloid P.
Plasma Levels of Microbial Translocation Markers Are Elevated in MIS-C and COVID-19 Children
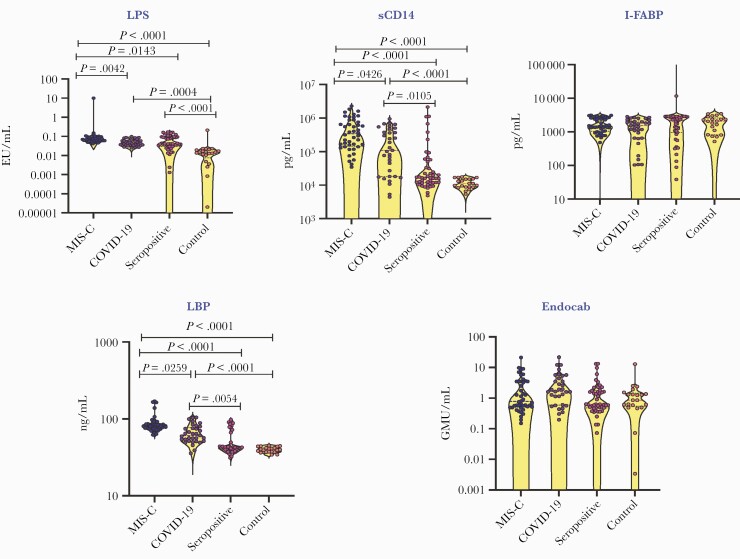
We analyzed the plasma microbial translocation markers to determine whether microbial translocation is a characteristic feature of MIS-C (Figure 2). MIS-C children had significantly higher levels of LPS, sCD14, and LBP in comparison with COVID-19, seropositive, and control children. Similarly, COVID-19 children also showed significantly higher levels of LPS, sCD14, and LBP than seropositive and control children. When comparing the seropositive and control children, only LPS was found to be elevated in seropositive children. Lastly, we did not find any significant differences in the levels of iFABP and EndoCAb between any of the groups.
Figure 2.
Elevated plasma levels of microbial translocation markers in children with MIS-C and COVID-19. A, The plasma levels of LPS, LBP, EndoCAb, iFABP, and sCD14 were measured in MIS-C (n = 44), COVID-19 (n = 33), seropositive (n = 47), and control children (n = 21). The data are presented in scatter violin plots, with each circle representing a single individual. P values were calculated using the Kruskal-Wallis test with Dunn’s post hoc for multiple comparisons. Abbreviations: COVID-19, coronavirus disease 2019; EndoCAb, endotoxin core antibodies; iFABP, intestinal fatty acid binding protein; IgG, immunoglobulin G; LBP, lipid-binding protein; LPS, lipopolysaccharide; MIS-C, multisystem inflammatory syndrome in children.
Plasma acute phase proteins and microbial translocation markers can distinguish MIS-C from COVID-19, seropositive, and control children
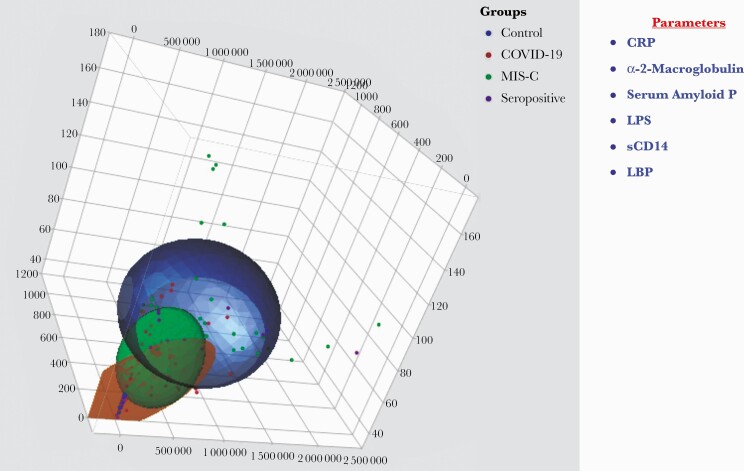
We performed a PCA of CRP, α 2M, SAP, LPS, sCD14, and LBP to determine the discriminatory power of acute phase proteins and microbial translocation markers in distinguishing MIS-C children from COVID-19, seropositive, and control children (Figure 3). Our PCA data clearly demonstrate the ability of these markers to differentiate MIS-C, COVID-19, seropositive, and control children.
Figure 3.
PCA to estimate the discriminatory power of inflammatory markers in MIS-C, COVID-19, seropositive, and control children. The PCA plot presents normalized data from plasma levels of CRP, α 2M, SAP, LPS, sCD14, and LBP in combinations of 4 different experimental groups (MIS-C [colored in green], COVID-19 [colored in red], seropositive [colored in purple], controls [colored in blue]). Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; LBP, lipid-binding protein; LPS, lipopolysaccharide; MIS-C, multisystem inflammatory syndrome in children; PCA, principle component analysis; SAP, serum amyloid P.
Plasma Acute Phase Proteins and Microbial Translocation Markers Are Biomarkers of Disease Severity in MIS-C
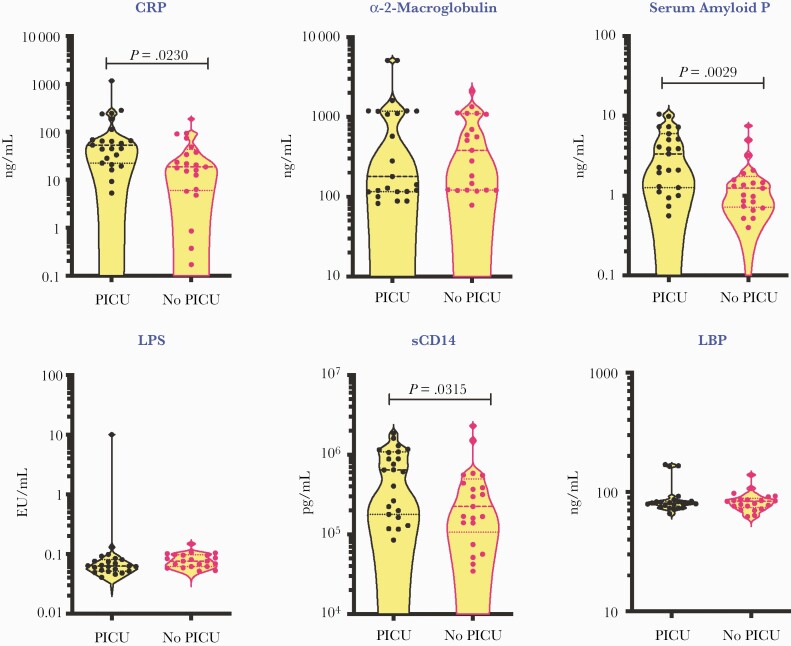
To evaluate the role of acute phase response and microbial translocation in determining disease severity in MIS-C, we compared the levels of CRP, α 2M, SAP, LPS, sCD14, and LBP between MIS-C children needing PICU care (indicating more severe disease) and children who did not require PICU care (indicating less severe disease). The demographics of these children are shown in Supplementary Table 1. As shown in Figure 4, MIS-C children requiring PICU care had significantly elevated levels of CRP, SAP, and sCD14 in comparison with children not requiring PICU, indicating that these parameters may serve as biomarkers of disease severity in MIS-C.
Figure 4.
Elevated plasma levels of inflammatory markers in MIS-C children requiring PICU care. The plasma levels of CRP, α 2M, SAP, LPS, sCD14, and LBP were measured in MIS-C children requiring PICU care (n = 23) and MIS-C children in wards (who did not require PICU care; n = 21). The data are presented in scatter violin plots, with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons. Abbreviations: α 2M, alpha-2 macroglobulin; CRP, C-reactive protein; LBP, lipid-binding protein; LPS, lipopolysaccharide; MIS-C, multisystem inflammatory syndrome in children; PCA, principle component analysis; PICU, pediatric intensive care unit; SAP, serum amyloid P.
Plasma Acute Phase Proteins Are Biomarkers of Disease Severity in COVID-19
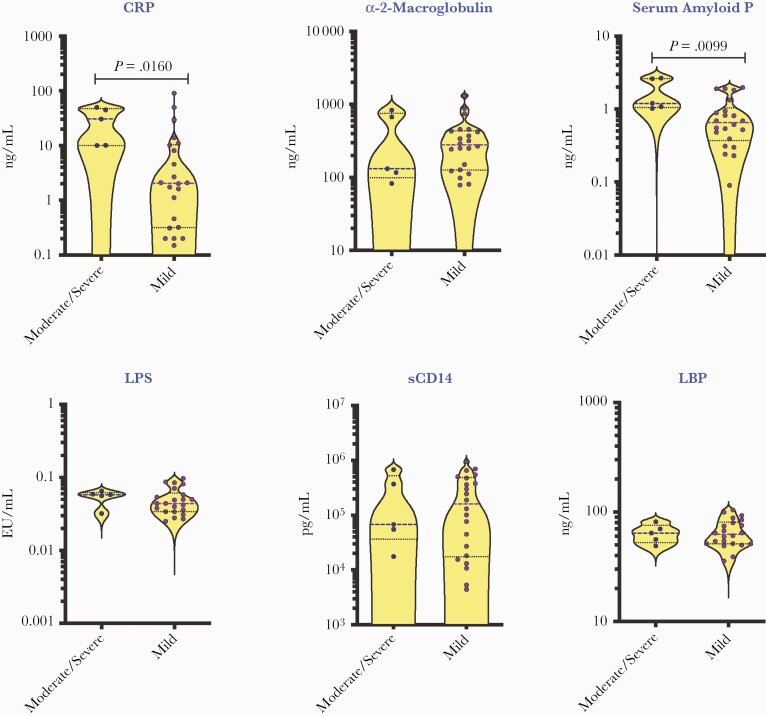
We also evaluated if the baseline levels of acute phase proteins and microbial translocation markers may be used as biomarkers of disease severity in COVID-19 children. These COVID-19 children (n = 22/3/2/6) had mild, moderate, severe, and asymptomatic disease. Among these COVID-19 children, 28 had fever, 10 had a respiratory infection, and 8 had a gastrointestinal infection; also, 6 children had underlying conditions. We compared CRP, α2M, SAP, LPS, sCD14, and LBP between COVID-19 children with moderate/severe disease vs mild disease. As shown in Figure 5, moderate/severe COVID-19 children had significantly elevated levels of CRP and SAP in comparison with mild COVID-19 children, indicating that these parameters may be used as biomarkers of disease severity in COVID-19.
Figure 5.
Elevated plasma levels of inflammatory markers in children with moderate to severe COVID-19. The plasma levels of CRP, α 2M, SAP, LPS, sCD14, LBP, and VEGF, Vascular endothelial growth factor were measured in COVID-19 children with moderate to severe (n = 5) and mild (n = 22) disease. The data are presented in scatter violin plots, with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons. Abbreviations: α 2M, alpha-2 macroglobulin; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; LBP, lipid-binding protein; LPS, lipopolysaccharide; SAP, serum amyloid P.
Associations Between APPs, MTMs, and Other Clinical Parameters
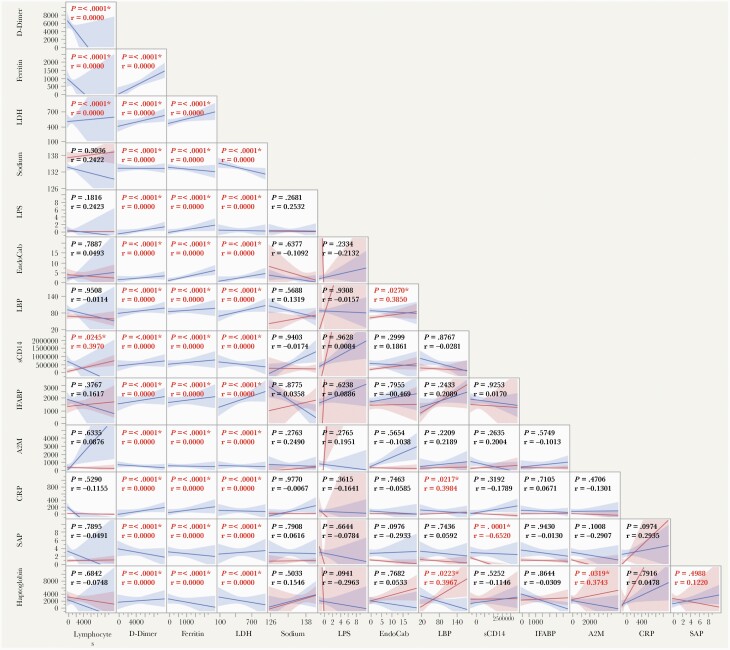
We wanted to identify correlations between acute phase proteins (APPs), microbial translocation markers (MTMs), and other clinical parameters in patients with MISC-C and COVID-19. A multiparametric matrix correlation plot showed strong positive correlations between levels of APPs and MTMs with D-dimer, ferritin, and lactate dehydrogenase (Figure 6). We did not find any significant correlation between APPs/MTMs with lymphocyte count and sodium. Taken together, these data suggest that inflammatory markers and MTMs are very well correlated with the clinical parameters.
Figure 6.
Relationship between immune markers and clinical parameters. Multiparametric matrix correlation plot of APPs, MTMs, lymphocyte count, sodium, D-dimer, ferritin, and LDH in all children with MIS-C and COVID-19. Spearman’s correlation coefficients are visualized. Abbreviations: APPs, acute phase proteins; COVID-19, coronavirus disease 2019; LDH, lactate dehydrogenase; MIS-C, multisystem inflammatory syndrome in children; MTMs, microbial translocation markers.
DISCUSSION
MIS-C is thought to be a postinfectious phenomenon of SARS-CoV-2 infection related to either antibody-dependent enhancement of disease, development of auto-antibodies as a postinfection syndrome, or a delayed cytokine storm that occurs due to the delayed immunological reaction of children to the virus [1, 7]. The characteristic clinical features of MIS-C are fever with gastrointestinal manifestations, cardiac dysfunction with or without coronary artery aneurysms, neurological manifestations, vasculitis and endothelial dysfunction, skin manifestations, and minimal to moderate respiratory manifestations. The majority of the symptoms are similar to Kawasaki disease and macrophage activation syndrome [9–12]. The innate immune response profile consists of elevated pro-inflammatory cytokines and chemokines and enhanced neutrophil and monocyte/macrophage activation, while the adaptive immune response profile consists of T-cell, B-cell, and NK-cell lymphopenias, enhanced antiviral antibody responses, and elevated circulating autoantibodies [13–18].
While CRP levels have always been measured as an indicator of disease pathology in MIS-C and COVID-19 [19], the association of other acute phase proteins has not been examined in detail. Our study examined the association of CRP, α 2M, SAP, and Hp and demonstrated that most of these markers are significantly differentially expressed between MIS-C/COVID and seropositive/control children. Interestingly, CRP levels were elevated in MIS-C children compared with COVID-19, and Hp levels were diminished, indicating a subtle difference in the acute phase response of these 2 distinct disease manifestations of SARS-CoV-2 disease in children. Moreover, acute phase proteins contribute to the distinction of MIS-C from COVID-19 and controls in PCA analysis, indicating a major contribution to the pathogenesis of this disease entity. Children with MIS-C have a predominantly gastrointestinal manifestation of their disease, with few respiratory symptoms in most cases [6]. It is postulated that this could be due to either primary replication of SARS-CoV-2 in the gastrointestinal tract or other antibody-related sequelae affecting the gastrointestinal tract [7].
Microbial translocation is the procedure by which microbial products such as lipopolysaccharide (LPS) and bacterial DNA translocate from the intestinal lumen to the systemic circulation in the absence of overt bacteremia [20]. Stimulation of Toll-like receptors by LPS is then assumed to lead to systemic immune activation [20], while sCD14 and LPS-LBP are used to establish evidence of direct LPS stimulation [20, 21]. EndoCAb is used as a surrogate marker for the quantification of circulating LPS levels [20–22]. iFABP reflects disruption in epithelial integrity associated with chronic intestinal infections [22, 23]. Nevertheless, the presence of microbial translocation, which is an indicator of intestinal dysbiosis and epithelial damage [20], has not been examined in MIS-C or COVID-19 in children. Our study clearly reveals the presence of elevated levels of LPS, LBP, and sCD14, all markers of gut damage and microbial translocation across the gastrointestinal tract, similar to that observed in HIV [20]. Our data also reveal that these markers can further help differentiate MIS-C from COVID-19 and control children in PCA analysis.
Our study also provides a detailed investigation of utilizing markers of acute phase response and microbial translocation as biomarkers of disease severity. Our data show that a combination of CRP, SAP, and sCD14 may help predict disease severity in both MIS-C and COVID-19 children. As these parameters were measured at the time of admission and before any medication, our data also suggest that these markers are potentially good biomarkers of diagnosing disease severity in children. Our study has several limitations including the inclusion of children from 1 hospital and the lack of follow-up samples from the children. Nevertheless, our study innovates by examining a comprehensive set of biomarkers in different groups of children with SARS-CoV-2 infection and providing a greater in-depth analysis of inflammatory parameters.
In conclusion, our study is the first study describing the detailed inflammatory profile of children with MIS-C and COVID-19 from India. MIS-C and COVID-19 are characterized by systemic inflammation and microbial translocation, 2 processes that potentially play a pivotal role in disease pathogenesis, including multi-organ involvement and dysfunction in these children. Further understanding of the mechanism underlying these findings should help in better characterization of this deleterious disease afflicting children.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank our NIRT Director for the constant support thorough the study; we also thank all the Pediatric Consultants and infection control nurses at Kanchi Kamakoti CHILDS Trust Hospital (KKCTH) and CTMRF for their support and contributions. We also sincerely thank the NIRT-ICER staff for technical support.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Availability of data and materials. All the reported data are available within the manuscript.
References
- 1. Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 2020; 20:453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carter MJ, Shankar-Hari M, Tibby SM. Paediatric inflammatory multisystem syndrome temporally-associated with SARS-CoV-2 infection: an overview. Intensive Care Med 2021; 47:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feldstein LR, Rose EB, Randolph AG. Multisystem inflammatory syndrome in children in the United States. Reply. N Engl J Med 2020; 383:1794–5. [DOI] [PubMed] [Google Scholar]
- 5. Panupattanapong S, Brooks EB. New spectrum of COVID-19 manifestations in children: Kawasaki-like syndrome and hyperinflammatory response. Cleve Clin J Med. In press. [DOI] [PubMed] [Google Scholar]
- 6. Miller J, Cantor A, Zachariah P, et al. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: a single center experience of 44 cases. Gastroenterology 2020; 159:1571–4.e1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henderson LA, Yeung RSM. MIS-C: early lessons from immune profiling. Nat Rev Rheumatol 2021; 17:75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venkataraman A, Kumar NP, Hanna LE, et al. Plasma biomarker profiling of PIMS-TS, COVID-19 and SARS-CoV2 seropositive children - a cross-sectional observational study from southern India. EBioMedicine 2021; 66:103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soma VL, Shust GF, Ratner AJ. Multisystem inflammatory syndrome in children. Curr Opin Pediatr 2021; 33:152–8. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JC, May JW, Cunningham MW, Jones OY. Multisystem inflammatory syndrome in children (MIS-C), a post-viral myocarditis and systemic vasculitis—a critical review of its pathogenesis and treatment. Front Pediatr 2020; 8:626182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020; 7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radia T, Williams N, Agrawal P, Harman K, Weale J, Cook J, Gupta A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev 2021; 38:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol 2021; 22:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gruber CN, Patel RS, Trachtman R, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 2020; 183:982–995.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Consiglio CR, Cotugno N, Sardh F, et al. ; CACTUS Study Team. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020; 183:968–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 2020; 26:1701–7. [DOI] [PubMed] [Google Scholar]
- 17. Diorio C, Henrickson SE, Vella LA, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest 2020; 130:5967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee PY, Day-Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest 2020; 130:5942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramcharan T, Nolan O, Lai CY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol 2020; 41:1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol 2012; 30:149–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page EE, Nelson M, Kelleher P. HIV and hepatitis C coinfection: pathogenesis and microbial translocation. Curr Opin HIV AIDS 2011; 6:472–7. [DOI] [PubMed] [Google Scholar]
- 22. Rajamanickam A, Munisankar S, Bhootra Y, et al. Microbial translocation associated with an acute-phase response and elevations in MMP-1, HO-1, and proinflammatory cytokines in Strongyloides stercoralis infection. Infect Immun 2017; 85:e00772-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. George PJ, Anuradha R, Kumar NP, et al. Evidence of microbial translocation associated with perturbations in T cell and antigen-presenting cell homeostasis in hookworm infections. PLoS Negl Trop Dis 2012; 6:e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.