Supplemental Digital Content is available in the text.
Keywords: cardiovascular diseases, ischemic stroke, menopause, risk, women
Abstract
Background and Purpose:
The few epidemiological studies that addressed the association between age at menopause and ischemic and hemorrhagic stroke risk in women had conflicting findings. We aimed to investigate whether age at (natural and surgical) menopause is a risk factor for total, ischemic, and hemorrhagic stroke in women.
Methods:
We analyzed data from 16 244 postmenopausal women, aged 26 to 70 years at recruitment who were enrolled in the European Prospective Investigation into Cancer and Nutrition–Netherlands cohort between 1993 and 1997. Participants were followed for the occurrence of stroke until January 1, 2011. At baseline, participants filled in questionnaires about health, reproductive history including age at menopause, diet, and lifestyle. Cox regression was used to investigate the association between age at menopause and stroke. All analyses were adjusted for age, smoking, systolic blood pressure, and body mass index.
Results:
Mean age of menopause was 46.4 (7.0) years. A total of 830 strokes (571 ischemic, 162 hemorrhagic, 97 unclassified) were identified. Earlier menopause was associated with an increased risk of total stroke. Compared with women who experienced menopause between 50 and 54 years old, women who underwent menopause before age 40 years had 1.48× higher risk (95% CI, 1.19–1.85) of total stroke. In continuous analyses, we observed a 2% lower total stroke risk for each year menopause was delayed (hazard ratio, 0.98 [95% CI, 0.97–0.99]). The risk between earlier menopause and stroke was confined to ischemic stroke, earlier menopause was not associated with hemorrhagic stroke. The association with age at menopause was stronger for natural menopause (hazard ratio <40 versus 50–54 years, 1.74 [95% CI, 1.12–2.70]) than for surgical menopause (hazard ratio <40 versus 50–54 years, 1.26 [95% CI, 0.84–1.89]).
Conclusions:
The risk of total and ischemic stroke decreased with an increase in age at menopause. Whether this should have clinical consequences such as intensified risk factor control should be subject of further studies.
See related article, p 2592
Stroke is the second leading cause of death worldwide affecting 2.6 million women in 2016.1 Over the past years, the lifetime stroke risk has increased significantly.2 Women have around 4% higher lifetime stroke risk than men,3–5 which might be attributable to female-specific risk factors like the age at menopause.
Some studies have reported that early menopause increases the risk of cardiovascular diseases,6 which is generally ascribed to the postmenopausal decrease in endogenous estrogens.7–9 In the past decade, an increasing number of epidemiological studies have investigated the relationship between age at menopause and (ischemic and hemorrhagic) stroke risk, with conflicting results. Recently, a meta-analysis concluded that women who were older at the onset of natural menopause (≥55 years) had a higher risk of hemorrhagic stroke (pooled relative risk, 2.24 [95% CI, 1.19–4.21]). Conversely, they found no statistically significant associations between earlier natural menopause and total stroke (pooled relative risk, 0.86 [95% CI, 0.42–1.74]) and ischemic stroke (pooled relative risk, 0.90 [95% CI, 0.22–3.74]) in comparison with women with menopause between 50 and 54 years. However, only a small number of studies could be pooled together due to diversity in reference categories and the use of different outcome definitions. In addition, there was a high degree of heterogeneity, which makes it challenging to interpret these results.10
Effects of menopause may also differ between type of menopause mainly due to other underlying pathophysiology. After surgical menopause, women experience an abrupt loss of ovarian hormones, whereas natural menopausal is considered to be a gradual process over several years.11,12 Currently, only a limited number of studies investigated both natural and surgical menopause and found results in different directions. One study found a nonsignificant association between an earlier age at natural menopause and stroke risk (hazard ratio [HR], 0.51 [95% CI, 0.07–3.78]), whereas another study showed a positive nonsignificant association (HR, 1.48 [95% CI, 0.81–2.69]).13,14
In sum, the reported associations on menopausal age and the risk of stroke are difficult to interpret. This is due to differences in adjustments, sample sizes, study designs, and study end points. Here, we investigated whether the age at menopause is a risk factor for total, ischemic, and hemorrhagic stroke and assessed if this association is similar for natural and surgical menopause, in a Dutch cohort of 16 244 postmenopausal women who were followed for an average of 15 years.
Methods
Study Population
The EPIC-NL (European Prospective Investigation into Cancer and Nutrition–Netherlands) prospective cohort consists of the 2 Dutch contributions to the EPIC study: The Prospect cohort and the MORGEN (Monitoring Project on Risk Factors for Chronic Diseases) cohort.
Prospect comprises 17 357 women aged 49 to 70 years, living in the city of Utrecht or its vicinity, and were recruited between 1993 and 1997 through a breast cancer screening program. The MORGEN cohort includes 22 654 men and women aged 20 to 64 years, recruited through random sampling from the general population in the cities of Doetinchem, Maastricht, and Amsterdam, between 1993 and 1997. At baseline, EPIC-NL participants filled in a food frequency questionnaire and a general questionnaire containing questions about demographic characteristics, lifestyle habits, chronic diseases, and risk factors. In addition, they underwent a physical examination, including body weight, waist and hip circumference, and blood pressure measurements. Height and weight were measured by trained examiners. Finally, blood samples were drawn and stored in liquid nitrogen for future studies. All participants signed informed consent before study inclusion. Both studies complied with the declaration of Helsinki.15 The data that support the findings of this study are available on request via the EPIC-NL steering committee through an application process.
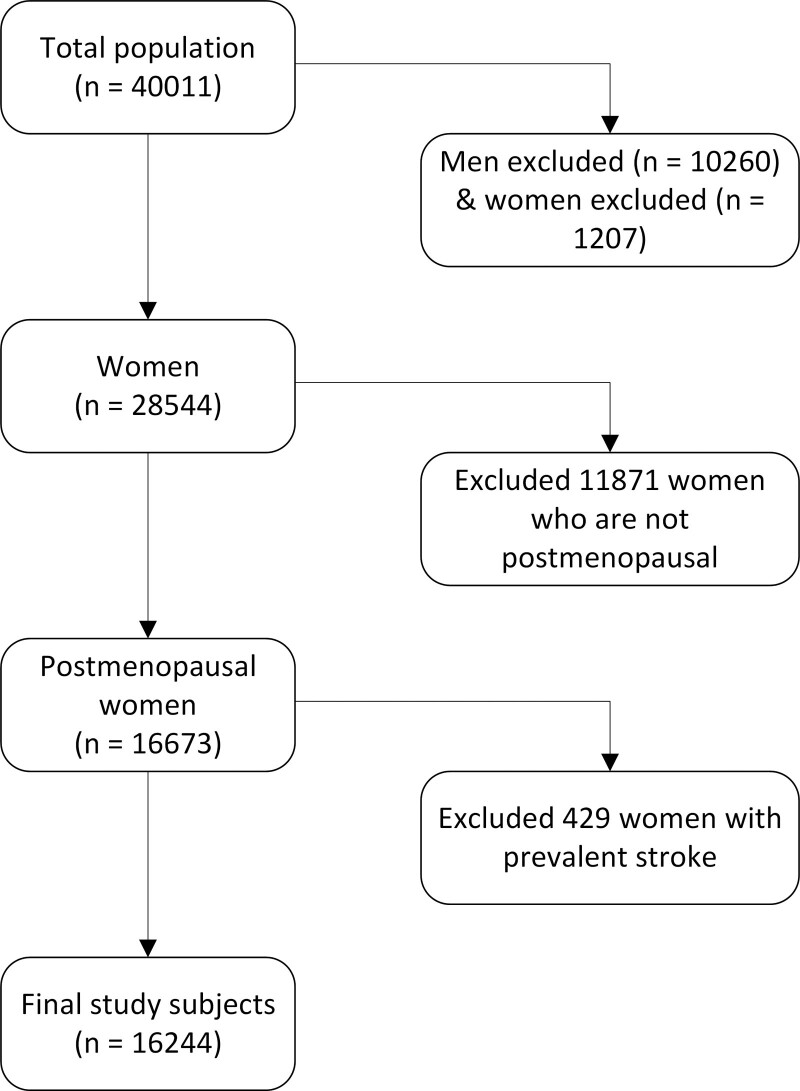
For the present study, we excluded participants who have withdrawn their consent for study inclusion (n=1); men (n=10 259); women who were not postmenopausal (11 871); participants who gave no informed consent for linkage with vital status and causes of death registries (n=1161); participants with missing information on cause of death (n=46); and women with missing data on a prevalent stroke before study entry (n=429). In the final analysis, we included 16 244 women (Figure).
Figure.
Study population flow chart, EPIC-NL (European Prospective Investigation into Cancer and Nutrition–Netherlands).
Age at Menopause
At baseline, information on reproductive factors was collected using self-administered questionnaires. Participants reported age at first and last menstrual period, current and past use of oral contraceptives and hormone replacement therapy, and whether they had undergone a hysterectomy or oophorectomy. Prospect participants provided information on the number of full-term pregnancies (the sum of live births and stillbirths), and for MORGEN participants, the number of children was used as a proxy for the number of full-term pregnancies. Women were considered postmenopausal when they reported not having experienced menstrual bleedings for at least 12 months. In addition, women who underwent a hysterectomy or oophorectomy were considered surgical postmenopausal. We categorized age at menopause into 5 groups, younger than 40, 40 to 44 years, 45 to 49 years, 50 to 54 years, and ≥55 years. The 50 to 54 years age group was taken as the reference category.
Other Measurements
The baseline questionnaire also comprised demographic characteristics, the presence of chronic diseases, and other risk factors for cardiovascular diseases. Also, information about medical history and lifestyle was collected. Educational level was coded as low (lower vocational training or primary school), medium (intermediate vocational training or secondary school), or high (higher vocational training or university). Smoking was categorized as current, former, and never smoker. Physical activity was assessed with a validated questionnaire and classified according to the Cambridge Physical Activity Index with single-imputed data for missing values (n=4930).16 The Cambridge Physical Activity Index was categorized into inactive, moderately inactive, moderately active, and active. All physical measurements were performed by trained examiners. Systolic and diastolic blood pressure was measured twice in supine position using a Boso Oscillomat (Bosch & Son, Jungingen, Germany) in the Prospect cohort and using a random sphygmomanometer in the MORGEN cohort. Hypertension was classified according to a physician-diagnosed self-reported questionnaire, the use of antihypertensive medication, or a systolic blood pressure >140 mm Hg or diastolic blood pressure of >90 mm Hg. Body weight was measured in light indoor clothing without shoes to the nearest 0.5 kg with a floor scale (Seca, Atlanta, GA). Height was measured to the nearest 5 mm in standing position without shoes. Body mass index (BMI) was calculated as weight divided by the square of height in meters. Non-HDL (high-density lipoprotein) and HDL-cholesterol were measured using homogeneous assays with enzymatic end points, as described previously.15 Diabetes and previous cardiovascular disease (International Classification of Diseases, Ninth Revision [ICD-9] codes 410–414, 427.5, 428, 415.1, 443.9, 430–438, 440–442, 444, 798.1,798.2, 798.9 or International Classification of Diseases, Tenth Revision [ICD-10] codes I20–I26, I46, R96, G45, I60–I67, I69, I70–I74, I50) were classified through self-reported questionnaires and by linkage with the National Medical Registry between 1990 and 1997. Hyperlipidemia was defined according to self-reported questionnaires.15
Stroke Ascertainment
Participants are followed for vital status, causes of death, and the occurrence of cancer and cardiovascular diseases through linkage to several registries. Information on vital status was obtained through digital linkage with municipal registries. In contrast, causes of death were obtained through linkage with Statistics Netherlands and coded according to the ICD-10. Data on nonfatal stroke events were further obtained through linkage with the Dutch Hospital Association and Order of Medical Specialists providing hospital discharge diagnoses and coded according to ICD-9 by medical staff. Since 1990, this linkage is possible because data on admission and discharge are archived.15 In this study, the primary outcome was total stroke defined as the first hospitalization for or death of stroke (ICD-9 430–438/ICD-10 I60–I67, I69, G45). Secondary outcomes were ischemic stroke (ICD-9 433–435/ICD-10 I63–I65, G45) and hemorrhagic stroke (ICD-9 430–432/ICD-10 I60–I62).
Statistical Analysis
Baseline characteristics were calculated using means with SD for normally distributed continuous variables and percentages for categorical variables. Non-normally distributed variables were presented as median and interquartile range. Missing data in this study were missing at random; therefore, missing data were multiple imputed with 10 imputations and combined according to Rubin rules using the package mice in R.
We analyzed the association between the age at menopause and the risk of (ischemic and hemorrhagic) stroke using Cox proportional hazard models with age from birth until censoring as the underlying time scale. SEs were used to calculate the 95% CI. Age at menopause was entered in the predefined categories and also as a continuous variable. Results are presented as HRs with their corresponding 95% CIs.
We controlled for potential confounding by entering covariates one by one in multivariable Cox proportional hazards models. If a covariate changed the association between the age at menopause and stroke by ≥10%, we considered this variable as a potential confounder and adjusted for it. We also decided to consider known stroke risk factors as potential confounders. In addition to the crude model (model 1) we adjusted for age at enrollment (model 2), and we further adjusted for smoking (never, former, current), systolic blood pressure, and BMI (model 3). Smoking was modeled categorically, whereas systolic blood pressure and BMI were modeled continuously. All analyses were stratified for the cohort to account for cohort effects such as differences in covariate measurements. Physical activity, education level, marital status, waist to hip ratio, alcohol consumption, hormone replacement use, oral contraceptive use, diabetes, full-term pregnancy, hysterectomy, ovariectomy, and number of children were also tested as confounders but did not satisfy the predefined criteria. We examined the proportional hazards assumption through inspection of the Schoenfeld residuals in three randomly picked imputation sets.
Because the association between age at menopause and stroke may vary according to the type of menopause, we also repeated the analyses for naturally and surgically postmenopausal women separately. As for excluding stroke cases that occurred after menopause, but before baseline may introduce immortal time bias, we performed sensitivity analyses where we included these stroke cases in the study. All analyses were performed with the statistical package R and P<0.05 were considered statistically significant.
Results
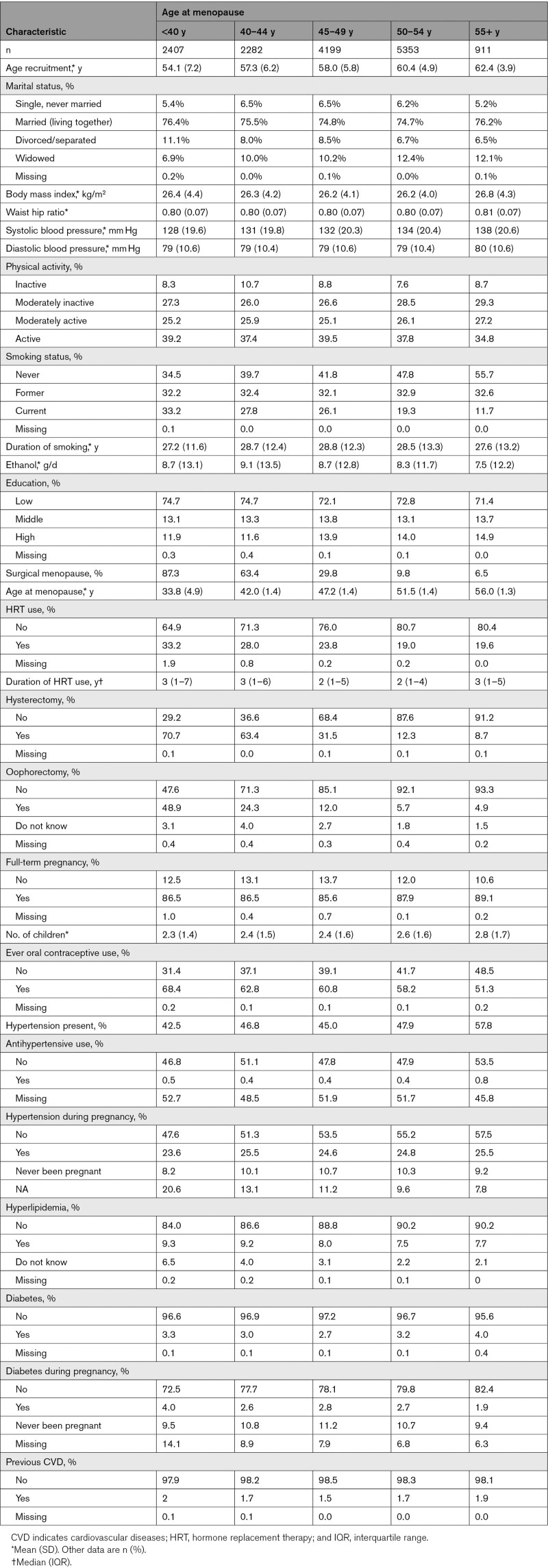
The baseline characteristics across categories of menopausal age are presented in Table 1. Mean age at baseline was 58.2 years with an SD of 6.1. We identified a total of 830 incident strokes during a mean follow-up of 15 years. Of these, 571 (68.8%) were ischemic strokes, and 162 (19.5%) were cerebral hemorrhages; for 97 (11.7%) strokes, the type could not be determined. Mean age at natural menopause was 49.4 (SD, 4.6), whereas the mean age at surgical menopause was 40.9 (SD, 7.2) years. Women with the earliest menopause were more likely to experience surgical menopause, smoked more often, and had a lower level of education. Women who were older at the onset of menopause had higher blood pressure, a higher BMI, and were consuming less alcohol.
Table 1.
Baseline Characteristics of the Study Population
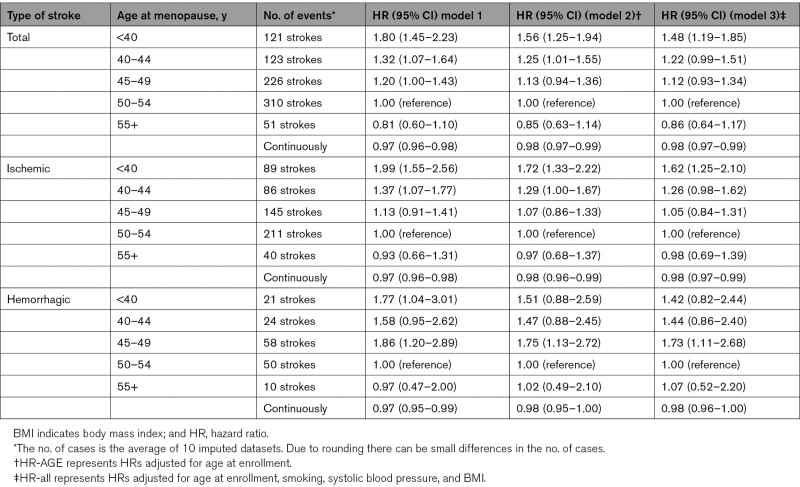
Table 2 shows the associations between age at menopause and total, ischemic and hemorrhagic stroke. After adjusting for age at enrollment, BMI, smoking, and systolic blood pressure, the hazard ratios for total stroke were 1.48 (95% CI, 1.19–1.85), 1.22 (95% CI, 0.99–1.51), 1.12 (95% CI, 0.93–1.34), and 0.86 (95% CI, 0.64–1.17) for menopause at age <40, 40 to 44, 45 to 49, and ≥55 years respectively, compared with menopause at age 50 to 54 years (Table 2). When entering the age at menopause as a continuous variable, a later age of menopause was associated with a lower risk of total stroke (HR, 0.98 per year [95% CI, 0.97–0.99]), meaning a 2% risk reduction for each year menopause is later.
Table 2.
Association Between Age at Menopause and Total Stroke, Hemorrhagic and Ischemic Strokes, Crude and Adjusted Hazard Ratios
For ischemic stroke, in particular, the youngest age at menopause categories were associated with disease risk. Women who underwent menopause before age 40 years had 1.62× higher risk (95% CI, 1.25–2.10) of an ischemic stroke compared to the reference category. In addition, the HRs showed a decreasing trend with increasing age at menopause for ischemic stroke (HR, 0.98 per year [95% CI, 0.97–0.99]). This trend means a 2% risk reduction for each year menopause is later.
For hemorrhagic stroke, we found a nonsignificant association for the youngest age at menopause category (HR, 1.42 [95% CI, 0.82–2.44]) in comparison with the reference category. Continuous analyses showed that an earlier age at menopause was not statistically significant associated with hemorrhagic stroke (HR, 0.98 [95% CI, 0.96–1.00]).
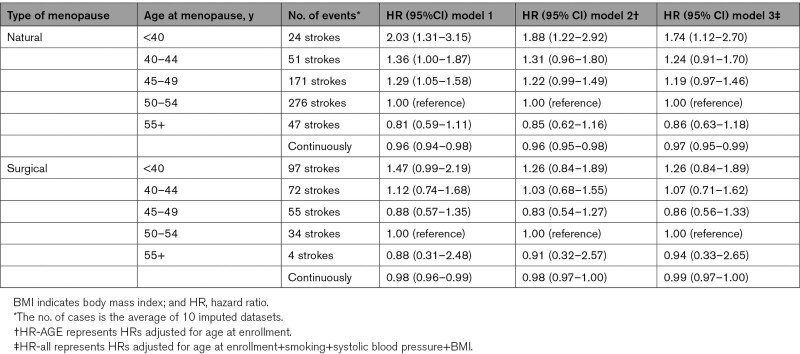
The associations between age at menopause and total stroke risk by type of menopause are shown in Table 3. The risk of early menopause on total stroke seemed similar for naturally and surgically postmenopausal women. In the natural menopause group, women with a menopausal age before 40 years had a 1.74× higher risk (95% CI, 1.12–2.70), whereas women who experienced menopause after 55 years of age had a nonsignificant lower risk (HR, 0.86 [95% CI, 0.63–1.18]). Naturally postmenopausal women experience a 3% risk reduction for each year menopause is later (HR, 0.97 per year [95% CI, 0.95–0.99]).
Table 3.
Association Between Age at Menopause and Total Stroke Risk by Type of Menopause, Crude and Adjusted Hazard Ratios
In the surgical postmenopausal group, we found nonsignificant associations with total stroke risk. Continuous analyses showed that an earlier age at surgical menopause was not statistically significantly associated with total stroke (HR, 0.99 per year [95% CI, 0.97–1.00]).
In a sensitivity analysis, where we included stroke cases that occurred between menopause and enrollment in the study, we observed similar results. A menopausal age before 40 years increased the risk of stroke (multivariable-adjusted HR, 1.51 [95% CI, 1.25–1.84]), whereas for age at menopause >55 years, it was 0.81 (95% CI, 0.61–1.08; Table I in the Data Supplement).
Visual inspection of the Schoenfeld residuals showed that the residuals were independent of time, suggesting that the proportional hazards assumptions hold.
Discussion
In this study, we investigated the association between age at menopause and total, ischemic and hemorrhagic stroke risk. In summary, earlier age at menopause was statistically significantly associated with a higher risk of total and ischemic stroke, but not with hemorrhagic stroke. Total stroke and ischemic stroke risk decreased with older ages at menopause.
Changes in endogenous hormones may explain the association between earlier age at menopause and increased stroke risk. An earlier menopause results in an early decline in estradiol and this might either have a direct adverse effect on the blood vessels or harmfully affect stroke risk factors that in turn, increase the risk of stroke.17 The fact that postmenopausal hormone therapy, either estrogen plus progestin or estrogen alone taken orally, increases stroke risk in healthy postmenopausal women contradicts a protective role of estrogens in the occurrence of stroke.18,19
Before further interpreting our findings, some strengths and limitations need to be addressed. Advantages of our study include the long follow-up of a mean of 15 years together with the large sample size; this enables us to identify a large number of ischemic stroke events. In addition, we used age as the underlying time scale in our cox regression models, which reduces the effect of immortal time bias. Immortal time bias refers to the time between birth and the age at menopause in this study.20,21 Women must have survived the time between birth and study inclusion to participate in the study. In that specific time, the event cannot occur. This may lead to an overestimation because women with an older age at menopause are more likely to experience a stroke before the menopause. However, the potential impact of immortal time bias is relatively minor, because few women experience a stroke at a young age. In addition, in sensitivity analyses, we also included the prevalent stroke cases to investigate the impact of this bias and we found similar results.
Our study has some limitations. First, in our study, the age at menopause is lower compared to the general population due to the age at enrollment in our cohort. Women were aged 49 to 70 years at inclusion. Because not all women were postmenopausal at inclusion, those women that already went through menopause will by chance have had an earlier menopause. The age at menopause was self-reported, which is prone to recall bias. However, earlier studies reported that the validity of self-reported menopause is good.22–25 One previous study showed that seven years after menopause, ≈76% of the women could accurately report their menopausal age,26 although precision decreased with increasing time since menopause. The recall bias in our study is similar for women with and without a stroke but uncertainty remains whether this leads to underestimation or overestimation of the true effect. In addition, several studies have reported that ≈95% of the women can accurately report whether they experienced natural or surgical menopause.22
Second, we considered women who experienced hysterectomy as surgically postmenopausal, which may have led to misclassification bias. However, it has been reported that in women above 50 years of age, 78% of the hysterectomies include bilateral oophorectomy.27 Also women with unilateral oophorectomy were classified as postmenopausal, which could affect the reported association.
Third, in this study, 97 strokes were unclassified because the medical specialists in the hospital did not specify the information on the type of stroke. This may have affected our statistical power in the subgroup analyses for ischemic and hemorrhagic stroke. In addition, follow-up on stroke events were obtained through registries collected by medical professionals upon discharge of the patient (one main diagnosis and up to 10 secondary diagnoses, reflecting current medical practice). However, we do not have information on the diagnostic work-up. Finally, a relatively small number of identified strokes were hemorrhagic, and after dividing them into menopausal age categories, only limited cases were available for analyses. The lower power for hemorrhagic strokes possibly explains why similar hazard ratios were not statistically significant.
In this study, we found that an earlier age at menopause is significantly associated with an increased risk of total and ischemic stroke but not significantly with hemorrhagic stroke. This is not in line with a recent systematic review and meta-analysis that found no significant associations between earlier age at natural menopause and ischemic and total stroke. They concluded that women who were older at the onset of natural menopause (≥55 years) had a higher risk of hemorrhagic stroke (pooled relative risk, 2.24 [95% CI, 1.19–4.21]) in comparison with women with a menopausal age between 50 and 54 years. However, although in this systematic review, 78 studies were included, in the analysis they only pooled a limited number of studies together due to a variety of reference categories. Furthermore, the included studies observed a limited number of events.13,28,29 All other observational studies that we are aware of were included in this systematic review, and most of them were not in line with our findings.14,30–36 However, most of the studies assessed stroke mortality14,30–36 or looked at natural menopause only,14,30,33–36 and 4 of the studies did not adjust for smoking,31–34 although this is a strong risk factor for both ages at menopause and stroke. We found that early age at menopause is significantly associated with a higher risk of stroke when menopause at 50 to 54 years was used in the reference category, this is in line with the results of the Framingham cohort study.4
Very recently, a pooled analysis of individual patient data of 301 438 women among 15 observational studies showed that women with an earlier age at natural menopause had an increased risk of stroke (HR, 1.72 [95% CI, 1.43–2.07]) in comparison with women with a menopausal age of 50 to 51 years.37 This finding is in line with our results (HR, 1.74 [95% CI, 1.12–2.70]). Although, in this study, they only focused on natural menopause.
We did not observe a significant association between surgical menopause and total stroke risk. This is in line with another study that investigated the relationship between earlier age at surgical menopause and total and hemorrhagic stroke risk.13 In contrast, a Swedish study among patients who underwent an oophorectomy between 18 and 49 years of age found significantly higher risks of stroke (HR, 1.47 [95% CI, 1.16–1.87]) compared with women without a hysterectomy or oophorectomy.38 The reason for this difference in results could be attributed to the categorization of menopausal age categories. In our study, we looked specifically to an earlier age at menopause, that is, under the age of 40 years, whereas in this study, an age between 18 and 49 years was used. In addition, information on smoking status was lacking in that study, so it is possible that the observed association was confounded by smoking.
Conclusions
In summary, in this Dutch population, we showed that early menopause is associated with an increased risk of total and ischemic stroke. Whether this should have clinical consequences such as intensified risk factor control should be subject of further studies.
Acknowledgments
We thank Statistics Netherlands and the PHARMO Institute for follow-up data on causes of death and cardiovascular disease.
Sources of Funding
The EPIC-NL study (European Prospective Investigation Into Cancer and Nutrition–Netherlands) was funded by Europe against Cancer program of the European Commission (until 2014 the Directorate-General for Health and Consumers, now Directorate-General for Health and Food Safety [DG SANTE]; DG SANCO), the Dutch Ministry of Health, Welfare and Sports (Volksgezondheid, Welzijn en Sport [VWS]), the Dutch cancer Society, the Netherlands Organisation for Health Research and Development (ZonMW) and the World Cancer Research Fund (WCRF).
Disclosures
None.
Supplemental Materials
Online Table
Supplementary Material
Nonstandard Abbreviations and Acronyms
- EPIC-NL
- European Prospective Investigation into Cancer and Nutrition–Netherlands
- HDL
- high-density lipoprotein
- HR
- hazard ratio
- ICD-9
- International Classification of Diseases, Ninth Revision
- ICD-10
- International Classification of Diseases, Tenth Revision
- MORGEN
- Monitoring Project on Risk Factors for Chronic Diseases
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.030558.
For Sources of Funding and Disclosures, see page 2590.
Contributor Information
Sabrina J.G.C. Welten, Email: s.welten@amsterdamumc.nl.
Jolanda M.A. Boer, Email: jolanda.boer@rivm.nl.
W.M. Monique Verschuren, Email: monique.verschuren@rivm.nl.
References
- 1.Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019; 18:417–418. doi: 10.1016/S1474-4422(19)30030-4 [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd-Allah F, Abejie; GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018; 379:2429–2437. doi: 10.1056/NEJMoa1804492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007; 6:1106–1114. doi: 10.1016/S1474-4422(07)70291-0 [DOI] [PubMed] [Google Scholar]
- 4.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009; 40:1044–1049. doi: 10.1161/STROKEAHA.108.542993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushnell CD. Stroke in women: risk and prevention throughout the lifespan. Neurol Clin. 2008; 26:1161–76, xi. doi: 10.1016/j.ncl.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006; 13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea [DOI] [PubMed] [Google Scholar]
- 7.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993; 15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115 [DOI] [PubMed] [Google Scholar]
- 8.van der Graaf Y, de Kleijn MJ, van der Schouw YT. Menopause and cardiovascular disease. J Psychosom Obstet Gynaecol. 1997; 18:113–120. doi: 10.3109/01674829709085577 [DOI] [PubMed] [Google Scholar]
- 9.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999; 84:4025–4030. doi: 10.1210/jcem.84.11.6158 [DOI] [PubMed] [Google Scholar]
- 10.Poorthuis MH, Algra AM, Algra A, Kappelle LJ, Klijn CJ. Female- and male-specific risk factors for stroke: a systematic review and meta-analysis. JAMA Neurol. 2017; 74:75–81. doi: 10.1001/jamaneurol.2016.3482 [DOI] [PubMed] [Google Scholar]
- 11.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010; 65:161–166. doi: 10.1016/j.maturitas.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ, III. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond). 2009; 5:39–48. doi: 10.2217/17455057.5.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010; 17:506–510. doi: 10.1097/gme.0b013e3181c7dd41 [DOI] [PubMed] [Google Scholar]
- 14.Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005; 16:556–562. doi: 10.1097/01.ede.0000165392.35273.d4 [DOI] [PubMed] [Google Scholar]
- 15.Beulens JW, Monninkhof EM, Verschuren WM, van der Schouw YT, Smit J, Ocke MC, Jansen EH, van Dieren S, Grobbee DE, Peeters PH, et al. Cohort profile: the EPIC-NL study. Int J Epidemiol. 2010; 39:1170–1178. doi: 10.1093/ije/dyp217 [DOI] [PubMed] [Google Scholar]
- 16.Haftenberger M, Schuit AJ, Tormo MJ, Boeing H, Wareham N, Bueno-de-Mesquita HB, Kumle M, Hjartåker A, Chirlaque MD, Ardanaz E, et al. Physical activity of subjects aged 50-64 years involved in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2002; 5(6B):1163–1176. doi: 10.1079/PHN2002397 [DOI] [PubMed] [Google Scholar]
- 17.Lisabeth L, Bushnell C. Menopause and stroke: an epidemiologic review. Lancet Neurol. 2012; 11:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, et al. ; WHI Investigators. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006; 113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077 [DOI] [PubMed] [Google Scholar]
- 19.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, et al. ; WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003; 289:2673–2684. doi: 10.1001/jama.289.20.2673 [DOI] [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S. Modern epidemiology. 1998, Lippincott-Raven [Google Scholar]
- 21.van Walraven C, Davis D, Forster AJ, Wells GA. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol. 2004; 57:672–682. doi: 10.1016/j.jclinepi.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 22.den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas. 1997; 27:117–123. doi: 10.1016/s0378-5122(97)01122-5 [DOI] [PubMed] [Google Scholar]
- 23.Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987; 126:319–325. doi: 10.1093/aje/126.2.319 [DOI] [PubMed] [Google Scholar]
- 24.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, Rand WM. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002; 155:672–679. doi: 10.1093/aje/155.7.672 [DOI] [PubMed] [Google Scholar]
- 25.Cairns BJ, Liu B, Clennell S, Cooper R, Reeves GK, Beral V, Kuh D. Lifetime body size and reproductive factors: comparisons of data recorded prospectively with self reports in middle age. BMC Med Res Methodol. 2011; 11:7. doi: 10.1186/1471-2288-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn RA, Eaker E, Rolka H. Reliability of reported age at menopause. Am J Epidemiol. 1997; 146:771–775. doi: 10.1093/oxfordjournals.aje.a009353 [DOI] [PubMed] [Google Scholar]
- 27.Parker WH. Bilateral oophorectomy: Solving the risk/benefit equation - Choosing candidates, monitoring outcomes. Contemporary OB/GYN. 2011. Accessed March 11 2021. https://www.contemporaryobgyn.net/view/bilateral-oophorectomy-solving-riskbenefit-equation--choosing-candidates-monitoring-outcomes. July 1 2011.
- 28.Choi SH, Lee SM, Kim Y, Choi NK, Cho YJ, Park BJ. Natural menopause and risk of stroke in elderly women. J Korean Med Sci. 2005; 20:1053–1058. doi: 10.3346/jkms.2005.20.6.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999; 159:1061–1066. doi: 10.1001/archinte.159.10.1061 [DOI] [PubMed] [Google Scholar]
- 30.Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Inaba Y, et al. ; JACC Study Group. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J Epidemiol. 2006; 16:177–184. doi: 10.2188/jea.16.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002; 155:339–345. doi: 10.1093/aje/155.4.339 [DOI] [PubMed] [Google Scholar]
- 32.Hong JS, Yi SW, Kang HC, Jee SH, Kang HG, Bayasgalan G, Ohrr H. Age at menopause and cause-specific mortality in South Korean women: Kangwha Cohort Study. Maturitas. 2007; 56:411–419. doi: 10.1016/j.maturitas.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen BK, Heuch I, Kvåle G. Age at natural menopause and stroke mortality: cohort study with 3561 stroke deaths during 37-year follow-up. Stroke. 2004; 35:1548–1551. doi: 10.1161/01.STR.0000131746.49082.5c [DOI] [PubMed] [Google Scholar]
- 34.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005; 162:1089–1097. doi: 10.1093/aje/kwi324 [DOI] [PubMed] [Google Scholar]
- 35.Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, Iso H, Jacobs DR, Jr, Phillips RL. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989; 79:709–714. doi: 10.2105/ajph.79.6.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998; 8:229–235. doi: 10.1016/s1047-2797(97)00207-x [DOI] [PubMed] [Google Scholar]
- 37.Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019; 4:e553–e564. doi: 10.1016/S2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011; 32:745–750. doi: 10.1093/eurheartj/ehq477 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.