Abstract
Objectives:
To evaluate the utility of perfusion defects on dual-energy CT angiograms (DECTA) in assessing the clinical severity of pulmonary embolism (PE).
Methods:
We retrospectively reviewed 1136 consecutive diagnostic DECTA exams performed on patients with suspected PE between January 2014 and September 2014. Presence and location of obstructive and non-obstructive PE, right ventricular to left ventricular ratio (RV/LV ratio) and Inferior Vena Cava (IVC) backflow were recorded. Iodine maps were reviewed to establish the presence of perfusion defect and its extent was determined through a score based segmental impaired perfusion was created. Subsequently, the perfusion defect scores were correlated with clinical parameters including vital signs, electrocardiogram (ECG) abnormalities, echocardiogram findings, troponin and brain natriuretic peptide (bnp) levels. Clinical information regarding primary cancer diagnosis, oncologic stage and date of death if applicable were also recorded.
Results:
Of the 1136 diagnostic iodine maps, 96 of these patients had perfusion defects on iodine maps. After uni- and multivariate analysis, significant correlation was found between the presence of a perfusion defect and RV/LV ratio (p=0.05), IVC backflow (p=0.03), elevated troponin (p=0.03) and right heart dysfunction as determined on echocardiogram (p=0.05). The greater the perfusion defect score, the higher the likelihood of IVC backflow (p=0.005) and obstructive PE (p=0.002). When adjusted for oncologic stage, patients with a perfusion defect and a higher perfusion defect score had a higher mortality rate (p=0.005).
Conclusion:
The presence of a perfusion defect correlates with several parameters evaluating PE severity. A perfusion defect and higher perfusion defect score were associated with a lower survival.
Keywords: Pulmonary embolism, survival, computed tomography angiography
Introduction
Early diagnosis and treatment of pulmonary embolism (PE) is essential for optimizing clinical outcomes. Multidetector computed tomography angiography (CTA) is the current reference standard for the detection of PE, demonstrating a sensitivity of 83% and specificity of 96% [1].
Dual energy CTA (DECTA) allows differentiation of materials based on their energy absorption characteristics and has been proposed as a method to aid in the detection of PE. In the lungs, the pattern of iodine enhancement on DECTA has been shown to correspond to lung perfusion on planar scintigraphy and single photon-emission computed tomography [2; 3]. Thus, DECTA allows the simultaneous assessment of pulmonary vasculature and parenchymal iodine distribution, without excess radiation dose [4]. A recent study demonstrated that in a small percentage of patients, DECTA increased the detection of pulmonary emboli over conventional CTA [5].
Most studies emphasize the diagnostic role of CTA; however, recently some have focused on assessing the severity of the PE in order to determine the optimal treatment. Patients with right ventricular (RV) dysfunction at time of PE diagnosis have a worse prognosis than those with normal RV function; therefore, early recognition of RV dysfunction at the time of PE diagnosis is essential for clinical management [6].The two main methods of assessing the severity of PE on CT are CTA obstruction score (which takes into consideration the number of segmental arteries involved and the degree of vascular obstruction) and right ventricular-to-left ventricular ratio (RV/LV), the latter being the most important prognostic factor [7–9].
The purpose of this study was to investigate whether the presence and degree of perfusion defects on DECTA can be used to assess the severity of PE (as assessed by clinical and radiologic features) and whether this is associated with overall survival. For this purpose, we developed a perfusion score to assess the severity of the impaired perfusion and to evaluate the relationship between the perfusion score and clinical parameters of PE severity.
Materials and Methods
Study Population and Imaging Data
Our retrospective study was institutional review board-approved and Health Insurance Portability and Accountability Act-compliant. Informed consent was waived.
All patients who underwent DECTA at a tertiary oncology center from January 1, 2014, to September 11, 2014, were included. DECTA exams were performed at our institution as part of standard clinical care for all consecutive patients with suspected PE. A picture archiving and communication system (PACS) inquiry was performed by a study investigator to capture all DECTA exams performed during our study period, generating 1144 consecutive studies in 1062 patients. Nineteen patients were excluded due to poor quality of the perfusion map, leaving a total of 1136 diagnostic studies.
All DECTA exams were performed on a single-tube DECT scanner (Discovery CT750 HD, GE Healthcare) with rapid kVp switching with tube voltage 130 and 80 kVp, tube current 600 mA, pitch 0.984, and rotation time 0.8 sec.
Patients who underwent CTA of the chest alone received 120 mL iohexol intravenous contrast, while those who underwent CTA of the chest with concurrent abdominopelvic CT imaging received 150 mL iohexol. Contrast was infused at 3.0–4.0 mL/second. An ROI was placed over the pulmonary artery at the level of the carina when infused through a central venous catheter or proximal abdominal aorta at the level of the celiac artery when infused via a peripheral intravenous catheter injection. Iodine maps were generated from DECT acquisition using commercially-available dedicated image post-processing software Gemstone Spectral Imaging (GSI HD 2.0) GE Healthcare.
Imaging Assessment
Consensus reads were performed on all studies. Each DECTA was retrospectively reviewed by two of four thoracic radiologists (1–4 years of attending thoracic radiology experience) and then in consensus with a senior thoracic radiologist (over 20 years of attending thoracic radiology experience) who adjugated any disagreements among the initial readers. All radiologists in our study were blinded to the clinical report. The CTAs were first reviewed for presence of PE (cutoff of contrast opacification in a pulmonary artery, visualization of thromboembolic clot). If a PE was present, the location, level (main, lobar, segmental, or subsegmental pulmonary artery,) and type occlusive (OPE) vs. non-occlusive PE (NPE) were recorded. PEs were classified as occlusive if no contrast was visualized distal to the clot. PE related findings including Inferior Vena Cava (IVC) contrast backflow and right ventricle/left ventricle (RV/LV) ratio were recorded. Measurements of RV/LV ratio were performed on axial CTA images. The short axis of the RV was measured from inner wall to inner wall at the widest point of the RV at the level of the tricuspid valve (Figure 1A). Similarly, the short axis of the left ventricle was measured from inner wall to inner wall at the widest point at the level of the mitral valve (normal cut off value for RV/LV ratio ≤0.9). IVC backflow was determined as the presence of contrast within the IVC and hepatic veins (Figure 1B). Pulmonary findings including airspace opacities, nodules, emphysema, and fibrosis were also recorded.
Figure. 1.
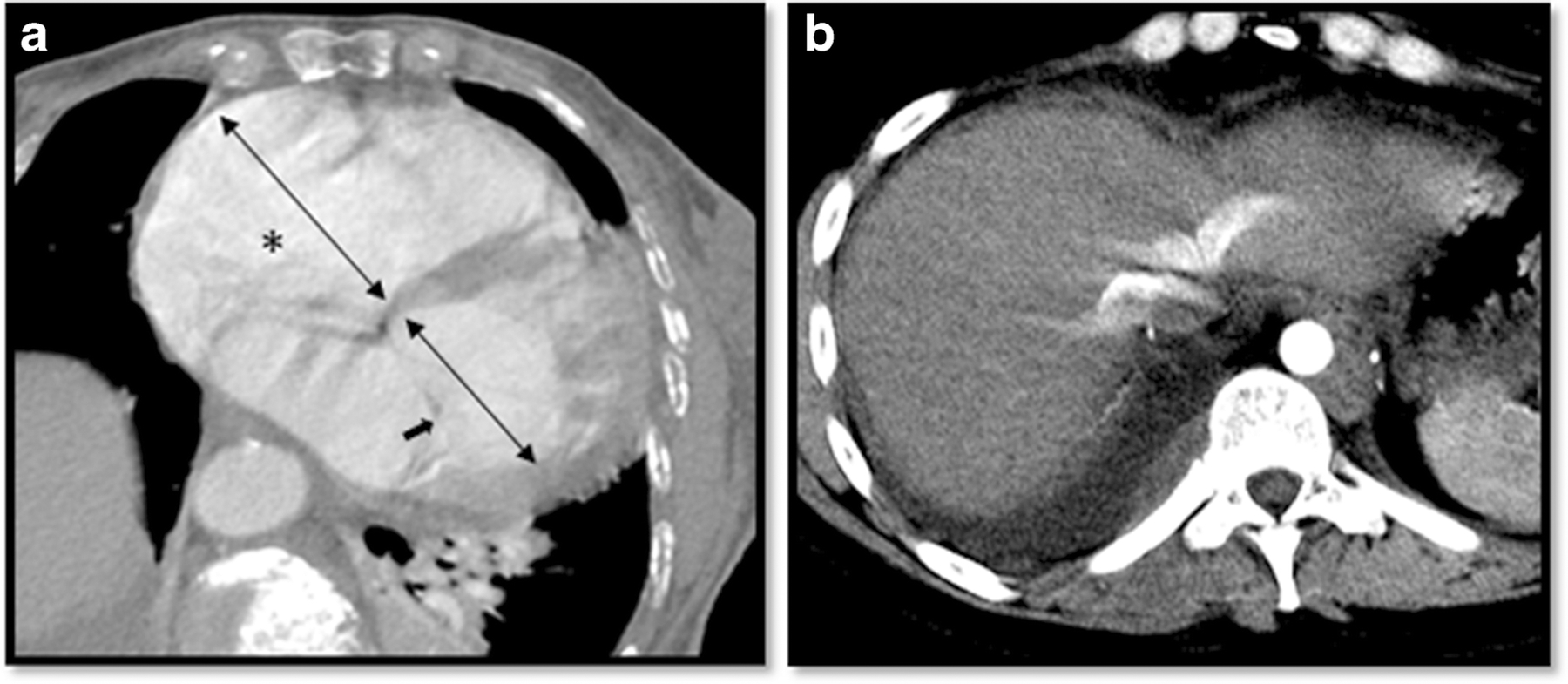
a) RV/LV ratio: the widest short axis of the RV and LV were measured from inner wall to inner wall at the level of the tricuspid (*) and the mitral valve (➔) respectively. b) IVC backflow was determined by the presence of IV contrast within the inferior vena cava and hepatic veins.
For each exam, the iodine map was reviewed after the CTA was interpreted. Image quality on the iodine maps was recorded as either excellent (demonstrating no artifacts); good (minor artifacts); moderate (able to assess iodine distribution); or poor (impossible to assess iodine distribution). The iodine map images were then reviewed for presence of any defect. Iodine map defects were characterized as either consistent with PE if peripheral and wedge shaped (Figure 2) determined as “positive perfusion defect group” or as not consistent with PE determined as control group if they appeared to be caused by tumors or consolidations, or band-like defects consistent with artifact, which were often due to cardiac motion or beam hardening from contrast within the superior vena cava or brachiocephalic vein.
Figure 2.
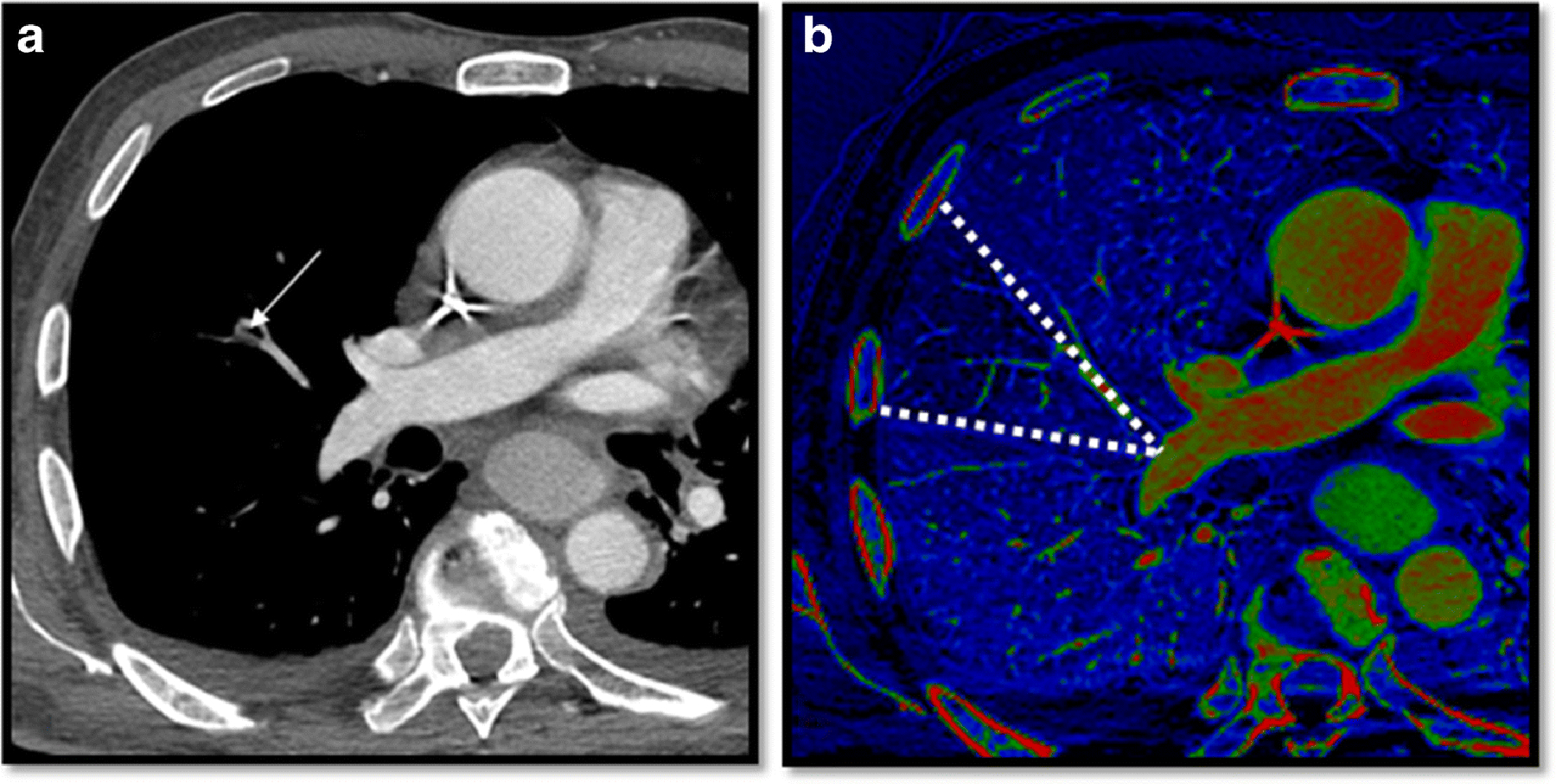
32-year-old patient with metastatic sarcoma who presented with shortness of breath. a) Computed Tomography Angiography demonstrates a subsegmental pulmonary embolism in the right upper lobe (arrow). b) Peripheral wedged shaped perfusion defect is noted (dotted lines) consistent with pulmonary embolism.
For exams with iodine map defects consistent with PE, the color-coded perfusion maps were then reanalyzed on axial images by one reader. Five anatomical pulmonary lobes were assessed with their respective segments. Each segment of each lobe was analyzed separately for the presence and extent of the wedge-shaped defect within the color map. The extent of the perfusion defect was graded according to the percentage of each segment that was not perfused, creating a score by assigning 1 point when <50% of the area of the segment exhibited perfusion defect and 2 points when over 50% of the area of the segment exhibited perfusion defect. The segmental scores were then added to obtain a total iodine score with a maximal score of 36 (max score for the right upper lobe 6, middle lobe 4, right lower lobe 10, left upper lobe 8, left lower lobe 8). In patients with both perfusion defects consistent with PE and defects secondary to other parenchymal abnormalities as consolidations or masses, only the wedged shaped defects were graded for scoring.
Clinical Parameters and laboratory measurements
Patient’s electronic medical records were reviewed to assess age, gender, type of malignancy, stage of malignancy. The date of death if applicable was collected for all patients, establishing an estimate of survival from the date of study to the day of death in days. Clinical parameters such as vital signs, electrocardiogram (ECG) changes and laboratory markers linked to PE severity were also recorded when performed within 24 hours of the DECTA. Vital signs including heart rate (bpm), respiratory rate (rpm), blood pressure (systolic and diastolic in mmHg), O2 saturation (%), significant acute ECG changes (ST depression, T wave inversion, right bundle branch block, R axis deviation, right conduction delay, and non-specific ST and T abnormalities, available in 800 patients), troponin (reference < 0.04 ng/ml, available in 500 patients) and B-type natriuretic peptide (BNP reference 0–100 pg/ml available in 269 patients) levels were recorded. Echocardiogram reports performed within 48 hours of the DECTA scan were assessed for findings related to right heart dilatation, right heart dysfunction (based on RV size and diastolic/systolic ventricular function), pulmonary hypertension both categoric (mild, moderate and severe) and numeric (in mmHg) and tricuspid regurgitation (moderate and severe considered significant). In patients with PE on the CTA, the electronic medical record was also reviewed to record anticoagulation treatment prior to the CTA and after CTA.
Statistical analysis
Clinical and radiological features of PE severity were analyzed as outcome variables while perfusion defects status (binary: positive vs control group) and perfusion defect score (continuous) were treated as predictors. Associations between perfusion defects status or score and different PE features were assessed using generalized estimating equations (GEE) with an exchangeable correlation structure to take into account multiple scans per patient. Continuous features that were not normally distributed were log-transformed to achieve normality. The normal distribution was used for the GEE analyses involving continuous PE features while the binomial distribution was used for binary features. Regression coefficients from GEE analyses were reported for the continuous outcomes while odds ratios were reported from GEE analyses for binary features. Among patients with positive perfusion defects we investigated the association between obstructive PE (predictor) with clinical variables, echocardiogram findings, CT findings, and perfusion defect score (outcomes) using GEE analyses as described above. The Kaplan–Meier method and the log-rank test were employed to examine differences in survival between patients with positive iodine map versus those without, while stratified log-rank test (for categorical variables) and Cox proportional hazards model (for continuous variables) were used to adjust these associations by cancer stage. No multivariable model was built, just adjustment for clinical stage was performed to control for confounding. Associations between categorical factors and perfusion defect status (positive vs control group) were assessed using Fisher’s exact test, while Wilcoxon rank sum test was used to evaluate these associations for continuous factors. All statistical analyses were performed using SAS 9.3 (SAS Institute) and p-values <0.05 were considered to indicate significance.
Results
Out of the 1136 diagnostic iodine maps, 96 patients had positive perfusion defects, 83 of whom had a positive PE on CTA and 74 had OPE (89%). Of the 1040 patients in the control group, 70 had PE on CTA and no perfusion defect of whom 20 had OPE (28%). No significant differences were found between the control group and patients with positive perfusion defect with respect to age, gender, type of cancer diagnosis, or presence of lung parenchymal abnormality. The only significant clinical difference between the groups was that the distribution of cancer stages (p=0.03) (Table 1).
Table 1.
Clinical and demographic characteristics of the study group (positive perfusion defect) and control group.
| Total (n = 1136) |
Positive perfusion defect (n = 96) | Controls (n = 1040) |
P-value | |
|---|---|---|---|---|
| Age | 58.6 ± 15.14 | 58.8 ± 15.50 | 58.6 ± 15.11 | 0.72 |
| Gender | 0.75 | |||
| Female | 665 | 58 | 607 | |
| Male | 471 | 38 | 433 | |
| Cancer diagnosis | 0.43 | |||
| Lung | 17% (195) | 20% (19) | 17% (176) | |
| Breast | 17% (193) | 15% (14) | 17% (179) | |
| Gastrointestinal | 17% (197) | 16% (15) | 18% (182) | |
| Genitourinary | 8% (96) | 8% (8) | 8% (88) | |
| Hematologic | 12% (138) | 7% (7) | 13% (131) | |
| Gynecologic | 9% (97) | 9% (9) | 8% (88) | |
| Sarcoma | 5% (54) | 7% (7) | 5% (47) | |
| Other | 13% (147) | 18% (17) | 13% (130) | |
| Multiple | 2% (19) | 0 | 2% (19) | |
| Cancer staging | (n = 997) | (n = 89) | (n = 908) | 0.03 |
| I | 8% (80) | 4.5% (4) | 8% (76) | |
| II | 9% (91) | 4.5% (4) | 10% (87) | |
| III | 15% (149) | 9% (8) | 16% (141) | |
| IV | 68% (677) | 82% (73) | 66% (604) | |
| Underlying lung disease | ||||
| Airspace opacity | 233 | 18 | 215 | 0.79 |
| Emphysema | 103 | 5 | 98 | 0.20 |
| Fibrosis | 35 | 3 | 32 | 1.0 |
| Mass | 180 | 20 | 160 | 0.19 |
When comparing the control group versus patients with positive perfusion defects, those with positive perfusion defects had higher odds of having an abnormal RV/LV ratio, IVC backflow, elevated troponin and right heart dysfunction on echocardiogram (Table 2, Figure 3). When associating the iodine score with all the variables, the higher the iodine score, the greater the likelihood of presenting with IVC backflow (Table 3). In addition, among patients with positive perfusion defects, having an obstructive PE was significantly associated with having a higher iodine score when compared to patients with non-obstructive PE (p=0.007) (Table 4).
Table 2.
Association of clinical parameters and echocardiogram findings with perfusion defects status (positive versus control group) (n = 1136).
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| IVC backflow | 1.92 (1.24, 2.96) | 0.0032 |
| Elevated BNP | 0.70 (0.32, 1.52) | 0.37 |
| Elevated troponin | 4.16 (1.08, 15.99) | 0.0378 |
| Hypoxemia | 1.43 (0.78, 2.60) | 0.23 |
| ECG significant changes | 1.46 (0.95, 2.25) | 0.08 |
| Echocardiogram (n = 294) | ||
| Right heart dysfunction | 2.11 (1.00, 4.48) | 0.049 |
| Pulmonary hypertension | 1.64 (0.79, 3.41) | 0.17 |
| Tricuspid regurgitation | 0.61 (0.17, 2.13) | 0.441 |
| Regression coefficients (95% CI) | p-value | |
| Abnormal RV/LV (continuous) | 0.02 (0.00, 0.04) | 0.05 |
| Vital signs | ||
| Systolic BP | −1.3 (−5.9, 3.32) | 0.57 |
| Heart rate | 4.35 (−0.62, 9.33) | 0.08 |
| Oxygen saturation | 0.04 (−0.01, 0.098) | 0.14 |
BNP, brain natriuretic peptide; BP, blood pressure; CI, confidence interval; ECG, electrocardiogram; IVC, inferior vena cava; RV, right ventricular; LV, left ventricular
Figure 3.
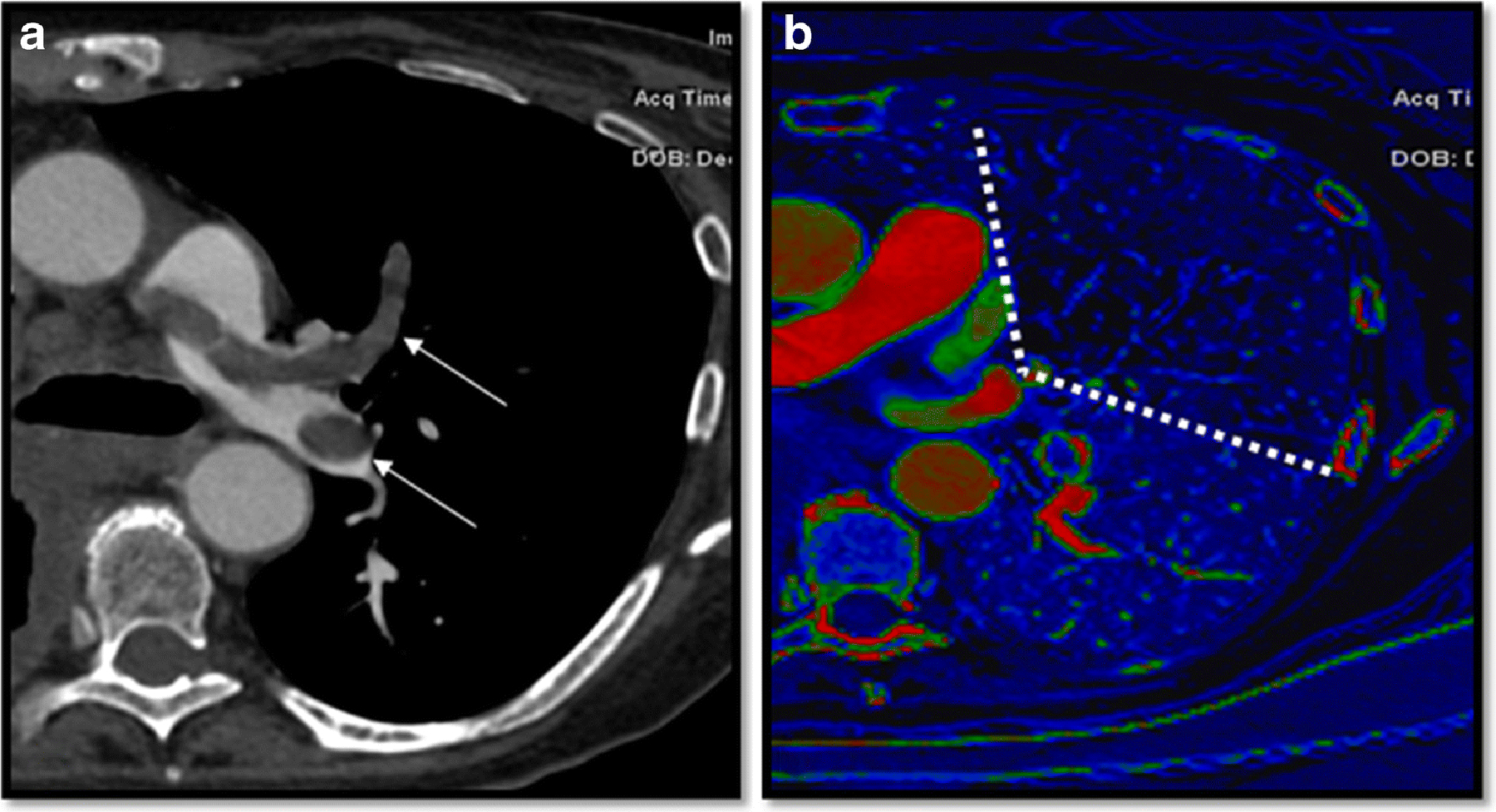
74-year-old patient with gastric cancer, presented with shortness of breath. a) On Computed Tomography Angiography, main pulmonary artery, lobar and segmental pulmonary embolisms were noted (arrows). b) Multiple perfusion defects were noted, with a perfusion defect score was 12 (dotted lines). The right ventricle/left ventricle ratio was 1.7. His laboratory results were significant for elevated brain natriuretic peptide (BNP) 182 pg/mL and elevated troponin of 0.13 ng/mL. On echocardiogram, both right heart dysfunction and pulmonary hypertension were diagnosed with a pulmonary artery pressure of 42 mmHg. The patient passed away within 30 days of the diagnosis of pulmonary embolism.
Table 3.
Association of clinical, CT and echocardiogram findings (outcomes) with perfusion defect score (continuous predictor).
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| IVC backflow | 1.10 (1.03, 1.17) | 0.0054 |
| Abnormal RV/LV ratio (1 vs 0) | 1.06 (0.96, 1.17) | 0.06 |
| Right heart dysfunction | 1.08 (0.98, 1.18) | 0.10 |
| Elevated troponin | 1.10 (0.99, 1.23) | 0.07 |
IVC, inferior vena cava; RV, right ventricular; LV, left ventricular
Table 4.
Association of obstructive PE status with clinical variables, echocardiogram findings, and CT finding in patients with positive perfusion defects (n = 96).
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| IVC backflow | 1.23 (0.58, 2.60) | 0.59 |
| Abnormal RV/LV ratio (1 vs 0) | 1.81 (0.72, 4.54) | 0.20 |
| Regression coefficients (95% CI) | p-value | |
| Pulmonary pressure | −0.10 (−0.29, 0.09) | 0.31 |
| Oxygen saturation | 0.08 (−0.08, 0.23) | 0.34 |
| Iodine map score | 0.22 (0.06, 0.39) | 0.007 |
IVC, inferior vena cava; RV, right ventricular; LV, left ventricular
We examined the associations between the clinical variables of interest and PE differed based on whether the patients had perfusion defects or not and found two significant interactions: heart rate (p=0.02) was associated with PE among patients with perfusion defect but not among those that did not have a perfusion defect. Similarly, significant changes in the ECG (p=0.01), were associated with PE among patients having a perfusion defect but not among those that did not have a perfusion defect.
In our study, 13 patients with perfusion defects did not have detectable PE on CTA. Their perfusion scores were lower when compared to the patients that had detectable PE (2.84 vs 4.74; p=0.04). None of the patients had significant ECG changes or echocardiogram findings. Only one patient had an elevated BNP. Two patients had a history of detectable PE on prior scans and had a persistent defect despite resolution of the thrombus. One patient was first diagnosed with multiple obstructive PE, with several perfusion defects (defect score of 16); on follow-up study 3 months later, no PE was detected and only 2 small perfusion defects were noted, with a score of 3. The second patient was initially diagnosed with a segmental obstructive PE; on his follow-up scan 2 months later, the PE was not detected; however, the perfusion defect persisted with the same score.
When comparing survival between positive perfusion defect and control group, when adjusted by cancer stage, there was a statistically significant difference in survival, those having a positive iodine map having significantly worse survival rates than those without (overall log-rank test p<0.0001, stratified by stage log-rank test p=0.0005); note that there were only 4 patients with perfusion defect and stage I cancer and none had an event so interpreting the results separately for Stage I is questionable (Figure 4). There were also 4 patients with perfusion defects and stage II cancer, however 3 of the 4 patients died, and thus allowing more reliable comparison with the control group.
Figure 4.
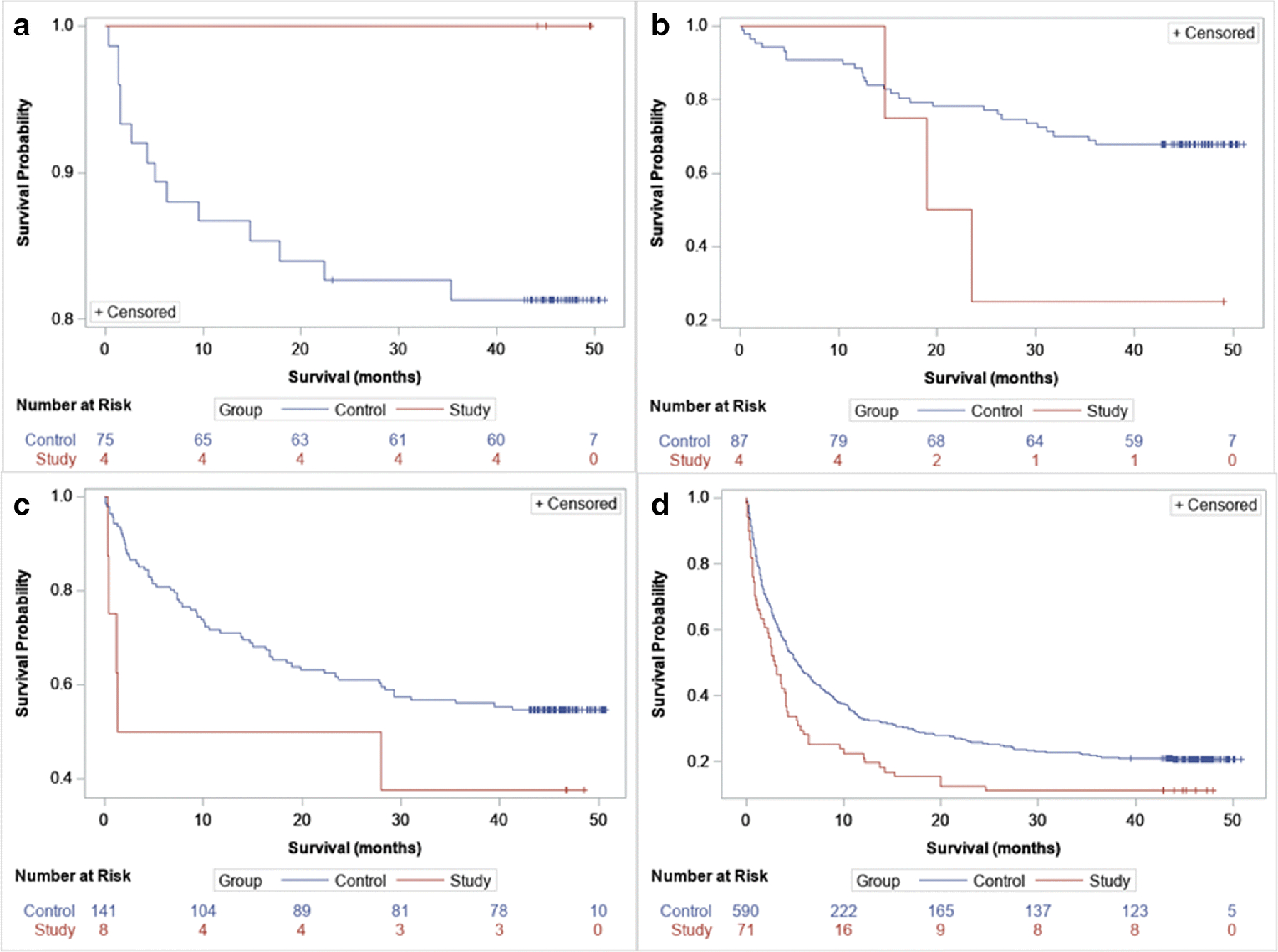
Kaplan–Meier curves comparing control versus study group comparing survival. a) Stage I, b) Stage II, c) stage 3, d) stage 4 overall log-rank test p- < .0001 (comparing control vs study group regardless of stage), log-rank test stratified by stage p = 0.0005.
Similarly, after adjusting by cancer stage, within the entire study group the higher the iodine map score, the higher the mortality (p<0.001) (hazard ratio 1.1; 95% CI: 1.06, 1.14). In addition, when comparing patients with detectable PE on CTA and positive perfusion defect versus patients with detectable PE without perfusion defect, those having a perfusion defect had lower survival rates (log-rank test p=0.039) (Figure 5). In patients with a detectable PE on CTA, the higher the iodine map score, the higher their mortality rate (p=0.02) (hazard ratio 1.06; 95% CI: 1.0, 1.1).
Figure 5.
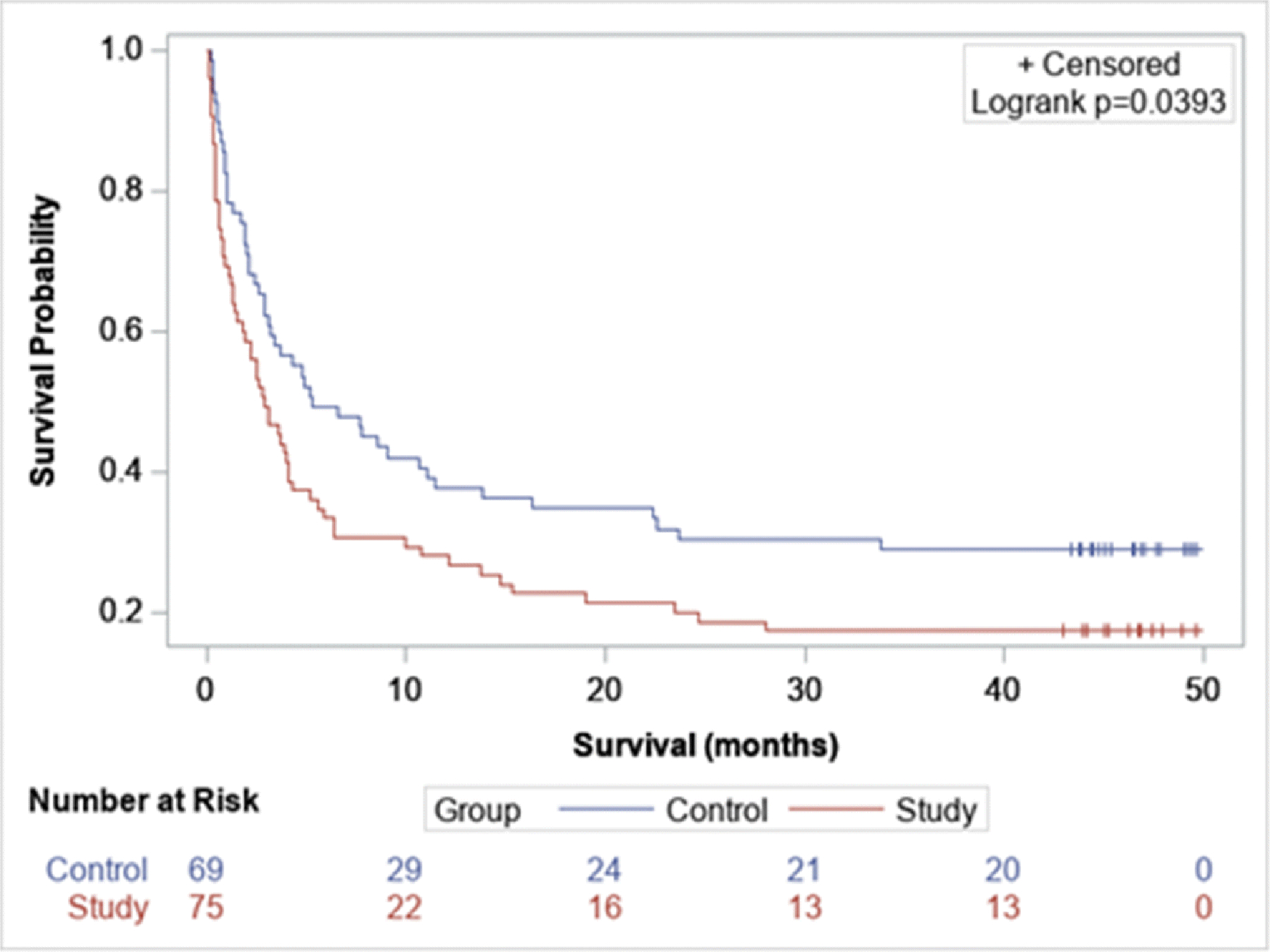
Kaplan–Meier curve comparing survival in patient with detectable PE on CTA and perfusion defect versus patient with PE without perfusion defect (log-rank test p= 0.039)
Discussion
Our study demonstrated that the presence of perfusion defects on DECTA corelates with several parameters used to evaluate the severity of PE. In addition, when adjusted by cancer stage, its presence and extent were associated with lower survival.
Several studies in the literature have shown the feasibility of DECTA in the evaluation of acute PE, with perfusion defects found to correspond to the area of occluded vessel [10]. DECTA has been found to correlate with scintigraphy, with a sensitive of 83% and specificity of 99% [2].
In our study, several internal validators for the clinical severity of PE were used, which have been determined by the American College of Cardiology as features suggesting a worse prognosis, such as: electrocardiographic changes, elevated troponin and BNP, CT increased RV/LV ratio and echocardiographic findings of right heart strain [11]. The cardiovascular effects of acute PE must be considered not only as the result of varying degrees of pulmonary vascular obstruction, but also as the effects of the right ventricle becoming a high-pressure system [12]. In our study we demonstrated that the detection of a perfusion defect on DECTA correlated with physiologic changes related to RV overload such as CT morphologic changes (RV/LV ratio and IVC backflow), troponin levels and right heart dysfunction on echocardiogram.
Previous studies have shown that the extent of perfusion defect correlated with clinical outcome [13–15]. However, prior studies were only based on small number of patients and most of them did not take into consideration echocardiographic findings. Chae et al. reviewed DECTA in 30 patients with PE establishing a perfusion defect score which demonstrated good correlation with RV/LV ratio and CTA obstruction score [13]. Thieme et al. found in 63 patients a significant correlation between the extent of the perfusion defect and CT signs of PE severity such as RV/LV ratio, width of the pulmonary artery and troponin levels [14]. Bauer et al. demonstrated in 53 patients that the size of the perfusion defect correlated with CT and echocardiogram markers of right heart strain [15].
The presence of any perfusion defect on our study correlated with right heart strain; however, our proposed perfusion score did not correlate well, only demonstrating positive association with IVC backflow and obstructive PE. To date, there is no standardized analytical method of assessing lung perfusion defect on DECTA. Multiple prior studies have demonstrated positive correlation between different clinical parameters and perfusion defect scores, calculated in different ways. Thieme et al. proposed a lobar perfusion defect score, with 5 different grades of perfusion defect per lobe [14]. Similar to our score, Chae et al. proposed a score based on segmental perfusion defect, with a 3-point scale based on moderate perfusion reduction or profoundly reduced/absent perfusion [13]. Takagi et al. scored the perfusion defect as we did in our study, by assessing the degree of segmental involvement: 0 points for no defect, 1 point for < 50% involvement and 2 point for > 50% [16]. The lack of proven correlation between perfusion defect score and right heart strain on our study is possibly due to the fact that we only used the axial plane for the assessment of the extend of the perfusion defect, as opposed to previously published studies which used multiplanar assessment. By only using a uniplanar assessment, the extent of the defect might have been underestimated; however, our results are more applicable to routine clinical interpretation. To improve three dimensional measurements of perfusion defects, few studies have investigated volumetric assessment. Afaltrer and Bauer et al. did volumetric assessment of the perfusion defect using a volume analysis software, finding correlation between the perfusion defect volume and RV/LV ratio [15; 17].
Within our group of patients with positive perfusion defects, patients who had obstructive PE presented with a higher perfusion score, as compared to patients with non-obstructive PE. Similarly, both Thieme and Chae et al. have previously demonstrated good agreement between perfusion defect score and CT obstruction score, which takes into account not only the degree of obstruction (partial versus complete obstruction) but also the extent of segmental thrombi [13; 14].
A small group of patients in our study had a perfusion defect on the iodine map; but had no detectable PE on CTA. An earlier study by Chung et al. acquired CT perfusion image data in an experimental PE model in six pigs by injecting two types of emboli into the pulmonary arteries. They found that the CT perfusion image was more sensitive than CTA for detecting small emboli, especially in the peripheral pulmonary artery coursing parallel to the axial plane [18]. Areas in which a perfusion defect was more severe than the vascular obstruction might represent micro-occlusion. Interestingly, we had two studies that were follow-ups of patients with initially diagnosed obstructive PEs, which on 2–3 months follow-up scans had no visible PE on the CTA but had smaller or persistent perfusion defects on the iodine map. Similar findings were noted by Thieme et al. of perfusion defects on DECT and scintigraphy, without evidence of intravascular clot [2]. These findings suggest that the perfusion defect might be secondary to residual peripheral micro occlusion, persistent hypoperfusion secondary to vasoconstrictive mediators or to peripheral emboli that are below the resolution of CTA.
Having a perfusion defect and increased perfusion score did correlate, when adjusted by cancer stage, with overall survival. We found that the higher the perfusion score, the lower the survival rate, except in Stage I group which had a very small sample size of patients with perfusion defects. A few other studies with smaller cohorts have demonstrated that quantitative CT parameters such as perfusion defect, RV/LV ratio and pulmonary obstruction score correlated with adverse clinical outcome and death [15; 17]. Apfaltrer et al. demonstrated in 60 patients that a larger perfusion defect was a predictor of adverse clinical outcome, which included death within 60 days or intensive care treatment [17]. Bauer et al. demonstrated that the relative hazard ratio for death increased by a factor of up to 10 when comparing patients with perfusion defect > 5% relative to lung volume and patients with lower perfusion defects [15]. In addition, when comparing the presence or absence of perfusion defect within all patients with detectable PE, those with perfusion defect had lower survival, suggesting that impaired perfusion to the lung parenchyma might contribute to patient’s clinical deterioration. These findings are in concordance with previously described outcome predictors for ventilation/perfusion scintigraphy, emphasizing the importance of the integrity of the pulmonary vasculature in a patient’s outcome [19; 20].
There are several limitations to our study. The data collection was retrospective, and the patient population was heterogenous in terms of disease. No information on underlying cardiac disease, cardiac function or right heart dysfunction prior to the CTA was included in our study, which may impact the presence of right heart strain and pulmonary hypertension on echocardiogram as well as the presence of RV/LV ratio and IVC backflow on CTA. No record of the cause of death, either related to cancer or cardiovascular compromise was recorded. Another limitation is that the injection rate was not taken into consideration of the presence or absence of IVC backflow.
We did not correlate the perfusion score with the results of other techniques such as perfusion scintigraphy. In addition, the perfusion map obtained on DECTA represented the degree of enhancement of the lung parenchyma at a particular time point and would depend on blood flow, blood volume and transit time of flow, which were not taken into consideration. Our perfusion score was limited by assessment in the axial plane. In areas where there were artifacts such as beam hardening; a true perfusion defect might have been masked and the score may have been underestimated. In patients with both perfusion defects consistent with PE and other defects secondary to airspace opacities or masses, the latter might mask underlying wedged shaped defects, also contributing to the potential underscoring of the impaired perfusion.
In conclusion, we consider that our data based on a large cohort validates prior reports that suggest that DECTA detection of perfusion defects in patients with PE correlate with right heart strain. In addition, the presence and extent of a perfusion defect is a negative predictor for survival in cancer patients.
Key Points:
Detection of perfusion defects on dual energy CT angiograms and its extent corelates with right heart strain in the setting of pulmonary embolism.
The presence and extent of a perfusion defect in patients with pulmonary embolism is associated with lower survival.
Acknowledgements
The authors would like to thank Joanne Chin, MFA, ELS, for assisting with the editing of the manuscript.
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations list:
- CTA
Computed tomography angiography
- DECTA
Dual energy CTA
- PE
Pulmonary embolism
- RV/LV
Right ventricular-to-left ventricular ratio
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Standards
Guarantor:
The scientific guarantor of this publication is Rocio Perez-Johnston, MD.
Conflict of Interest:
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry:
One of the authors (Marinela Capanu, PhD) has significant statistical expertise.
Informed Consent:
Written informed consent was waived by the Institutional Review Board.
Ethical Approval:
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
References
- 1.Stein PD, Fowler SE, Goodman LR et al. (2006) Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 354:2317–2327 [DOI] [PubMed] [Google Scholar]
- 2.Thieme SF, Becker CR, Hacker M, Nikolaou K, Reiser MF, Johnson TR (2008) Dual energy CT for the assessment of lung perfusion--correlation to scintigraphy. Eur J Radiol 68:369–374 [DOI] [PubMed] [Google Scholar]
- 3.Thieme SF, Graute V, Nikolaou K et al. (2012) Dual Energy CT lung perfusion imaging--correlation with SPECT/CT. Eur J Radiol 81:360–365 [DOI] [PubMed] [Google Scholar]
- 4.Thieme SF, Johnson TR, Lee C et al. (2009) Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am J Roentgenol 193:144–149 [DOI] [PubMed] [Google Scholar]
- 5.Weidman EK, Plodkowski AJ, Halpenny DF et al. (2018) Dual-Energy CT Angiography for Detection of Pulmonary Emboli: Incremental Benefit of Iodine Maps. Radiology 289:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torbicki A, Perrier A, Konstantinides S et al. (2008) Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 29:2276–2315 [DOI] [PubMed] [Google Scholar]
- 7.Park JR, Chang SA, Jang SY et al. (2012) Evaluation of right ventricular dysfunction and prediction of clinical outcomes in acute pulmonary embolism by chest computed tomography: comparisons with echocardiography. Int J Cardiovasc Imaging 28:979–987 [DOI] [PubMed] [Google Scholar]
- 8.Qanadli SD, El Hajjam M, Vieillard-Baron A et al. (2001) New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 176:1415–1420 [DOI] [PubMed] [Google Scholar]
- 9.Meinel FG, Nance JW Jr., Schoepf UJ et al. (2015) Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am J Med 128:747–759.e742 [DOI] [PubMed] [Google Scholar]
- 10.Pontana F, Faivre JB, Remy-Jardin M et al. (2008) Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol 15:1494–1504 [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal V, Nicolais CD, Lee A, Bashir R (2017) Acute Management of Pulmonary Embolism. American College of Cardiology Available via https://www.acc.org/latest-incardiology/articles/2017/10/23/12/12/acute-management-of-pulmonary-embolism. Accessed August 1, 2019 [Google Scholar]
- 12.Bryce YC, Perez-Johnston R, Bryce EB, Homayoon B, Santos-Martin EG (2019) Pathophysiology of right ventricular failure in acute pulmonary embolism and chronic thromboembolic pulmonary hypertension: a pictorial essay for the interventional radiologist. Insights Imaging 10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chae EJ, Seo JB, Jang YM et al. (2010) Dual-energy CT for assessment of the severity of acute pulmonary embolism: pulmonary perfusion defect score compared with CT angiographic obstruction score and right ventricular/left ventricular diameter ratio. AJR Am J Roentgenol 194:604–610 [DOI] [PubMed] [Google Scholar]
- 14.Thieme SF, Ashoori N, Bamberg F et al. (2012) Severity assessment of pulmonary embolism using dual energy CT - correlation of a pulmonary perfusion defect score with clinical and morphological parameters of blood oxygenation and right ventricular failure. Eur Radiol 22:269–278 [DOI] [PubMed] [Google Scholar]
- 15.Bauer RW, Frellesen C, Renker M et al. (2011) Dual energy CT pulmonary blood volume assessment in acute pulmonary embolism - correlation with D-dimer level, right heart strain and clinical outcome. Eur Radiol 21:1914–1921 [DOI] [PubMed] [Google Scholar]
- 16.Takagi H, Ota H, Sugimura K et al. (2016) Dual-energy CT to estimate clinical severity of chronic thromboembolic pulmonary hypertension: Comparison with invasive right heart catheterization. Eur J Radiol 85:1574–1580 [DOI] [PubMed] [Google Scholar]
- 17.Apfaltrer P, Bachmann V, Meyer M et al. (2012) Prognostic value of perfusion defect volume at dual energy CTA in patients with pulmonary embolism: correlation with CTA obstruction scores, CT parameters of right ventricular dysfunction and adverse clinical outcome. Eur J Radiol 81:3592–3597 [DOI] [PubMed] [Google Scholar]
- 18.Chung MJ, Goo JM, Im JG, Lee KS, Kim KG, Park JH (2004) CT perfusion image of the lung: value in the detection of pulmonary embolism in a porcine model. Invest Radiol 39:633–640 [DOI] [PubMed] [Google Scholar]
- 19.Nauffal Manzur D, Menendez Villanueva R, Cremades Romero MJ (1997) [The prognostic factors for early mortality and for total or partial gammagraphic resolution in venous thromboembolic disease]. Arch Bronconeumol 33:220–224 [PubMed] [Google Scholar]
- 20.Prediletto R, Paoletti P, Fornai E et al. (1990) Natural course of treated pulmonary embolism. Evaluation by perfusion lung scintigraphy, gas exchange, and chest roentgenogram. Chest 97:554–561 [DOI] [PubMed] [Google Scholar]


