Abstract
Introduction:
We determined if actigraphy-derived sleep patterns led to 7-year cognitive decline in middle-aged to older Hispanic/Latino adults.
Methods:
We examined 1035 adults, 45 to 64 years of age, from the Hispanic Community Health Study/Study of Latinos. Participants had repeated measures of cognitive function 7 years apart, home sleep apnea studies, and 1 week of actigraphy. Survey linear regression evaluated prospective associations between sleep and cognitive change, adjusting for main covariates.
Results:
Longer sleep-onset latency was associated with declines in global cognitive function, verbal learning, and verbal memory. Longer sleep-onset latency was also cross-sectionally associated with verbal learning, verbal memory, and word fluency. Sleep fragmentation was not associated with cognitive change.
Conclusion:
In a cohort of mostly middle-aged Hispanic/Latinos, actigraphy-derived sleep-onset latency predicted 7-year cognitive change. These findings may serve as targets for sleep interventions of cognitive decline.
Keywords: actigraphy, cognitive decline, cohort studies, Hispanic/Latinos, risk factors in epidemiology, sleep
1 |. INTRODUCTION
More than 50 million people worldwide have cognitive decline and dementia, with an annual global economic burden of disease that exceeded US $1 trillion in 2018.1 An increasing number of studies have shown associations between objective measures of poor sleep and cognitive decline. However, most of these studies have limited information on ethnic/racial minorities. Furthermore, U.S. Hispanics/Latinos, the largest U.S. minority group, are at increased risk for early cognitive decline and dementia compared to U.S. non-Hispanic/Latino White adults.2,3 Of importance, sleep disturbances are highly prevalent among Hispanic/Latino adults, as seen in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL).4,5 In HCHS/SOL, we described cross-sectional curvilinear (inverted U-shaped) associations between self-reports of sleep duration and cognitive function, such that participants with 7.8 hours ± 1.7 hours of sleep had better cognitive scores, compared to the extremes of sleep duration.6 In addition, self-reported long sleep duration (> 9 hours) and insomnia symptoms were associated with more pronounced 7-year cognitive decline.6 We also observed cross-sectional associations between decreased actigraphic sleep duration and sleep continuity with increased hypertension, obesity, and poor blood pressure control.7–9 The current study examined prospective associations between actigraphy-derived sleep patterns with 7-year cognitive decline in HCHS/SOL. We hypothesized that actigraphy-derived sleep duration and decreased sleep continuity would predict 7-year cognitive decline.
2 |. METHODS
2.1 |. Analytical sample: Hispanic Community Health Study/Study of Latinos
The HCHS/SOL is a multicenter community-based cohort study that evaluates the prevalence and risk factors of chronic disease among 16,415 Hispanic/Latino adults. The Visit-1 assessment (2008 to 2011) obtained information on demographics, socioeconomic status, lifestyle habits, medical history, and current health.10 The respiratory event index (REI) was measured as previously described with an unattended home sleep apnea study (ARES Unicorder 5.2; B-Alert, Carlsbad, California) for one night.11 Cognitive function was obtained at baseline (n = 9623) in participants 45 to 75 years of age. The Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA) is the cognitive ancillary study concurrent to HCHS/SOL Visit-2 (2015 to 2018). SOL-INCA evaluated the prevalence and determinants of cognitive decline and cognitive disorders of middle-aged and older Hispanic/Latinos using the same neuropsychological tests from Visit-1, and new neuropsychological tests as described below. SOL-INCA leveraged the HCHS/SOL complex sampling approach to reflect its targeted populations.12
2.2 |. Analytical subpopulation
The Sueño ancillary study aimed to determine the sleep patterns associated with cardiometabolic disease using wrist actigraphy in a sample of mostly middle-aged participants (18 to 64 years). We examined participants with actigraphy data from the Sueño visit and cognitive information at both Visit-1 and Visit-2. The Sueño ancillary study enrolled 2189 eligible HCHS/SOL participants. Sueño eligibility required that participants be part of the HCHS/SOL Visit-1, between 18 and 64 years of age, had their Visit-1 examination <24 months from the Sueño visit, not have self-reported narcolepsy, and not have severe sleep apnea (REI > 50) by home sleep apnea testing obtained at HCHS/SOL Visit-1. For the current analysis, we excluded 907 participants younger than 45 years of age at Visit-1, 159 participants who did not take part in Visit-2 SOL-INCA cognitive tests, and 88 participants with missing values on any of the covariates of interest. The unweighted analytical sample size was 1035. Figure S1 in supporting information provides a schema of the study exclusion criteria and sample size. All participants provided informed consent for all ancillary studies, which were approved by the institutional review board of each institution. Detailed HCHS/SOL sampling procedures are published13 and available at: https://sites.cscc.unc.edu/hchs/.
2.3 |. Outcomes: Cognitive function and score analysis
The cognitive tests were administered in the participants’ preferred language (English or Spanish) at Visit-1 and Visit-2. The Visit-1 tests included the: (1) Six-Item Screener (SIS; mental status); (2) Brief-Spanish English Verbal Learning Test (B-SEVLT; verbal episodic learning and memory); (3) phonemic verbal fluency test (or Word Fluency, WF; verbal fluency) and (4) Digit Symbol Subtest (DSS; processing speed). To evaluate cognitive decline, SOL-INCA repeated the above cognitive battery at Visit-2 and also administered the Trail Making Test part B (TMT).14,15 For the cognitive measures that were administered at both visits, a change score indicator was generated using regression-based techniques.16 Briefly, these change scores were calculated using survey linear regression to predict cognitive performance at Visit-2 as a function of Visit-1 cognitive performance, adjusting for age, education, and lapsed time (in days) between cognitive assessments. Test-specific standardized measures of change (Δ) were subsequently calculated using T2–T2pred/SEE. T2 represents a respondent’s score on a cognitive test at Visit-2, T2pred is the predicted value for that respondent on the test derived from the regression model specified above, and SEE is root mean squared error of the fit model. Additional information about the SOL-INCA tests and approach has been previously published.17
2.4 |. Main exposure: actigraphy sleep patterns
Sueño derived sleep patterns using seven days of wrist actigraphy with an Actiwatch Spectrum (Philips Respironics, Murrysville, Pennsylvania). A standardized protocol and validated scoring algorithm was used to identify rest and sleep periods on an epoch-by-epoch basis; participants required at least 5 days of valid data.18 Sleep patterns were assessed in the domains of sleep duration (sleep during the primary sleep period) and sleep continuity (sleep efficiency and sleep fragmentation index) as previously defined.9 Sleep variables were calculated as the weighted average of weekday and weekend data across all nights of valid data.18 Sleep duration was modeled continuously and non-linearly by categorizing sleep duration across tertiles. Measures of sleep continuity and sleep fragmentation index were modeled continuously.8,9,18 Sleep-onset latency (time before falling asleep) was measured in minutes, as the average time in bed to sleep onset, across all main rest intervals with sleep.8,9,18 Sleep maintenance efficiency is calculated as the proportion of time from sleep onset to sleep offset scored as sleep. Naps were defined as a self-reported period of sleep (based on event markers or diary) outside the main rest period containing at least 15 minutes of actigraphy scored sleep. Sleep duration per nap was assessed using actigraphy. Napping was a dichotomous variable indicating whether there was >1 nap over the recording period. Sueño inquired about sleep symptoms, including daytime sleepiness with the Epworth sleepiness scale (ESS) sleep quality; sleep medications; and19–22 insomnia, using the Insomnia Severity Index (ISI),22,23 defined as an ISI score ≥15.
2.5 |. Covariates
All covariates used in this analysis were from Visit-1. We included sex and continuous age at Sueño ancillary study (years). Sociodemographic indicators included education in three categories (less than high school; high school or equivalent degree; more than high school), background (Cuban, Dominican, Mexican, Puerto Rican, Central American, South American), field center, continuous body mass index (BMI) at Sueño, and self-reported alcohol use (never/formerly drank alcohol; currently drinks alcohol). The analyses also controlled for daytime sleepiness (using the ESS), self-reported physical activity measured using a trichotomous indicator (poor, intermediate, and ideal) based on American Heart Association criteria,24 depressive symptoms’ score (10-item Center for Epidemiological Studies Depression Scale [CESD 10]), anxiety symptoms’ score (10-item State-Trait Anxiety Inventory [STAI-10]),25,26 use of antidepressants, heart failure (self-reported doctor diagnosis of heart failure), and prevalent cerebrovascular accident (self-report of physician made diagnosis of stroke or transient ischemic attack [TIA]). Last, we controlled for REI (3% desaturation), time between sleep assessment and SOL-INCA measured in days, number of weekdays with valid actigraphy data, and a binary indicator for whether a participant had at least 2 weekend days with valid actigraphy data.
2.6 |. Statistical analyses
First, we generated descriptive statistics to characterize the study sample by the covariates, sleep exposure variables, and outcomes of interest (Table 1). All SOL-INCA cognitive outcomes were standardized (generated using [Yi-Mean Yi]/Standard Deviation) for analyses to facilitate comparison of the estimated associations across tests. Second, we fit survey linear regression models to examine the independent associations between each sleep exposure and cognitive performance at SOL-INCA. For each of the six cognitive outcomes we fit three regression models: (1) crude (without covariate adjustments); (2) age, education, sex, ethnicity, and time from sleep assessment to Visit-2 adjusted; and (3) further adjustment for BMI, alcohol consumption, physical activity, heart failure, stroke/TIA, CESD-10, STAI-10, antidepressant use, field center, REI, ESS, number of weekdays with valid actigraphy data, and a binary indicator for whether a participant had at least two weekend days with valid actigraphy data (Table 2 and Table S1 in supporting information). Third, we used survey linear regression to examine associations between sleep exposures, independently, and change in cognitive performance as detailed above (Table 3 and Table S2 in supporting information). Two regression models were fit for each of the change outcomes. Model 1 adjusted for sex and time lapse between sleep assessment and SOL-INCA; and model 2 additionally adjusted for age, Latino background, education, BMI, alcohol, physical activity, heart failure, stroke/TIA, CESD-10, STAI-10, antidepressants use, field center, REI, ESS, number of weekdays with valid actigraphy data, and a binary indicator for whether a participant had at least two weekend days with valid actigraphy data. Fourth, to facilitate the interpretation of the results generated from the regression models, as described in steps 2 and 3 above, for each of the examined cognitive performance and cognitive change outcomes that were statistically associated with a sleep exposure, we estimated and plotted crude and adjusted average marginal means across the sleep exposures continua along with 95% confidence intervals (Figures 1 and 2). Of note, models with ESS as an exposure did not adjust for ESS.
TABLE 1.
Sociodemographic and sleep characteristics of target population (unweighted n = 1035)
| Sexb | |
| Female | 53.82 (1.82) |
| Male | 46.18 (1.82) |
| Ethnicityb | |
| Dominican | 11.82 (1.42) |
| Central American | 6.57 (1.22) |
| Cuban | 25.21 (3.09) |
| Mexican | 32.52 (2.86) |
| Puerto Rican | 19.87 (1.95) |
| South American | 4.02 (0.66) |
| Educationb | |
| Less than high school | 33.81 (2.13) |
| High school | 23.16 (1.76) |
| Greater than high school | 43.03 (2.13) |
| Alcoholb | |
| No | 52.23 (2.18) |
| Yes | 47.77 (2.18) |
| Physical activityb | |
| Poor | 25.33 (1.79) |
| Intermediate | 13.59 (1.36) |
| Ideal | 61.09 (1.94) |
| Heart failureb | |
| No heart failure | 97.55 (0.60) |
| Heart failure | 2.45 (0.60) |
| Stroke/TIAb | |
| No previous stroke/TIA | 96.72 (1.06) |
| Yes previous stroke/TIA | 3.28 (1.06) |
| Antidepressant medicationb | |
| No | 90.46 (1.14) |
| Yes | 9.54 (1.14) |
| Centerb | |
| Bronx | 28.61 (2.41) |
| Chicago | 13.61 (1.45) |
| Miami | 33.91 (3.48) |
| San Diego | 23.88 (2.74) |
| Age at Sueño clinic visit (years)a | 55.22 (2.52) |
| Time from INCA to Sueño (days)a | 1548.50 (174.55) |
| BMI @ Sueño (kg/m2)a | 30.08 (2.77) |
| CESD-10 @ Sueñoa | 7.32 (3.04) |
| STAI-10 @ Sueñoa | 16.60 (2.71) |
| REI at baseline (events/hour)a | 7.23 (4.60) |
| Number of good actigraphy recording days | 7.24 (0.42) |
| Good weekend actigraphy recording days | |
| <2 days | 3.91 (1.16) |
| ≥2 days | 96.09 (1.16) |
| Primary exposures | |
| Average sleep time (hours)a | 6.73 (0.53) |
| Average WASO (minutes)a | 53.79 (12.85) |
| Secondary exposures | |
| Average sleep onset
latency (minutes)a |
11.34 (11.46) |
| Average sleep fragmentation in main sleep intervals (%)a | 22.38 (4.08) |
| Average sleep efficiency in main rest intervals (%)a | 85.57 (3.34) |
| Average wake bouts in sleep intervalsa | 40.08 (6.93) |
| Average sleep maintenance efficiency (%)a | 88.35 (2.66) |
| Days with naps with sleep >= 15a | 1.11 (0.80) |
| Average sleep time per
nap (minutes)a |
49.71 (17.48) |
| Epworth sleepiness scalea | 5.72 (2.14) |
| Insomnia severity indexb | |
| <15 | 78.60 (1.86) |
| ≥15 | 21.40 (1.86) |
Notes.
SOL-INCA was conducted between 2015 and 2018; on average 7 years after Visit-1 cognitive testing.
Abbreviations: BMI, body mass index; CESD-10, Center for Epidemiologic Studies Depression Scale; INCA, Investigation of Neurocognitive Aging; REI, respiratory invent index; STAI-10, State-Trait Anxiety Inventory; TIA, transient ischemic attack; WASO, wake after sleep onset.
Mean (standard deviation).
% (standard error).
TABLE 2.
Associations between Sueño-derived sleep characteristics and cognitive performance at SOL-INCA
| B-SEVLT-Sum |
B-SEVLT-Recall |
WF |
DSS |
Trails A |
Trails B |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 β/(SE) | M2 β/(SE) | M3 β/(SE) | M1 β/(SE) | M2 β/(SE) | M3 β/(SE) | M1 β/(SE) | M2 β/(SE) | M3 β/(SE) | M1 β/(SE) | M2 β/(SE) | M3 β/(SE) | M1 β/(SE) | M2 β/(SE) | M3 β/(SE) | M1 β/(SE) | M2 β/(SE) | M3 β/(SE) |
| Average sleep time | |||||||||||||||||
| −0.0062 | −0.0014 | −0.0047 | −0.0078 | −0.0137 | −0.0106 | −0.0788* | −0.0545 | −0.0569 | −0.0558* | −0.0201 | −0.0267 | 0.100*** | 0.0635* | 0.0634* | 0.0452 | 0.0147 | 0.0151 |
| (0.0326) | (0.0308) | (0.0313) | (0.0356) | (0.0329) | (0.0348) | (0.0361) | (0.0340) | (0.0317) | (0.0284) | (0.0242) | (0.0246) | (0.0292) | (0.0278) | (0.0283) | (0.0333) | (0.0315) | (0.0287) |
| Average WASO | |||||||||||||||||
| −0.0039* | −0.0011 | 0.0007 | −0.0056** | −0.0030 | −0.0007 | −0.0001 | 0.0012 | 0.0005 | −0.0022 | −0.0000 | −0.0004 | −0.0000 | −0.0008 | −0.0012 | 0.0029 | 0.0012 | 0.0018 |
| (0.0016) | (0.0016) | (0.0015) | (0.0018) | (0.0016) | (0.0015) | (0.0014) | (0.0014) | (0.0013) | (0.0015) | (0.0015) | (0.0013) | (0.0012) | (0.0012) | (0.0011) | (0.0016) | (0.0015) | (0.0013) |
| Average sleep onset latency | |||||||||||||||||
| −0.0038*** | −0.0032*** | −0.0030*** | −0.0042*** | −0.0035** | −0.0032** | −0.0020** | −0.0021*** | −0.0021** | −0.0022** | −0.0019* | −0.0018 | 0.0007 | 0.0008 | 0.0006 | 0.0030** | 0.0028** | 0.0027** |
| (0.0008) | (0.0008) | (0.0007) | (0.0013) | (0.0013) | (0.0012) | (0.0007) | (0.0006) | (0.0007) | (0.0007) | (0.0008) | (0.0010) | (0.0006) | (0.0005) | (0.0004) | (0.0009) | (0.0008) | (0.0010) |
| Average sleep fragmentation in main sleep intervals | |||||||||||||||||
| −0.0138* | −0.0030 | 0.0027 | −0.0174** | −0.0064 | 0.0002 | −0.0028 | −0.0002 | −0.0007 | −0.0070 | −0.0006 | −0.0005 | 0.0006 | −0.0008 | −0.0024 | 0.0093 | 0.0048 | 0.0054 |
| (0.0055) | (0.0055) | (0.0054) | (0.0058) | (0.0050) | (0.0048) | (0.0046) | (0.0046) | (0.0040) | (0.0053) | (0.0051) | (0.0050) | (0.0036) | (0.0038) | (0.0034) | (0.0054) | (0.0053) | (0.0045) |
| Average sleep efficiency in main rest intervals | |||||||||||||||||
| 0.0163** | 0.0078 | 0.0031 | 0.0213** | 0.0127* | 0.0070 | 0.0026 | −0.0003 | 0.0016 | 0.0093 | 0.0038 | 0.0047 | 0.0027 | 0.0035 | 0.0044 | −0.0131* | −0.0090 | −0.0105* |
| (0.0056) | (0.0055) | (0.0055) | (0.0068) | (0.0058) | (0.0057) | (0.0055) | (0.0053) | (0.0043) | (0.0051) | (0.0045) | (0.0043) | (0.0039) | (0.0038) | (0.0034) | (0.0055) | (0.0051) | (0.0042) |
| Average wake bouts in sleep intervals | |||||||||||||||||
| −0.0047 | 0.0025 | 0.0032 | −0.0080* | −0.0011 | 0.0000 | −0.0015 | 0.0007 | 0.0002 | −0.0033 | 0.0010 | 0.0022 | 0.0002 | −0.0010 | −0.0026 | 0.0042 | 0.0010 | 0.0004 |
| (0.0027) | (0.0028) | (0.0027) | (0.0034) | (0.0029) | (0.0027) | (0.0026) | (0.0027) | (0.0024) | (0.0024) | (0.0025) | (0.0021) | (0.0026) | (0.0027) | (0.0022) | (0.0028) | (0.0028) | (0.0024) |
Notes .
SOL-INCA was conducted between 2015 and 2018; on average 7 years after Visit-1 cognitive testing. M1: exposure; M2: M1 + age, education, sex, Latino background, time from INCA to Sueño; M3: M2 + BMI, alcohol consumption, physical activity, heart failure, stroke/TIA, CESD-10, STAI-10, antidepressants use, center, REI, ESS, number of days in sleep study, and having at least 2 weekend days (yes, no); For Trails A & B higher values indicate worse function.
Abbreviations: β, beta coefficient; BMI, body mass index; CESD, Center for Epidemiologica Studies Depression Scale; B-SEVLT, Brief Spanish English Verbal Fluency Test; DSS, Digit Symbol Substitution; ESS, Epworth sleepiness scale; REI, respiratory event index; SE, standard error; SOL-INCA, Study of Latinos-Investigation of Neurocognitive Aging; STAI, State-Trait Anxiety Inventory; WASO, wake after sleep onset; WF, Word Fluency.
= P < 0.05.
= P < 0.01.
= P < 0.001.
TABLE 3.
Associations between Sueño sleep characteristics and (average) 7-year cognitive change among middle-aged and older Hispanics/Latinos
| Δ B-SEVLT-Sum |
Δ B-SEVLT-Recall |
ΔSIS |
||||
|---|---|---|---|---|---|---|
| Ml β/SE | M2 β/SE | Ml β/SE | M2 β/SE | Ml β/SE | M2 β/SE | |
| Average sleep onset latency | ||||||
| −0.0028** | −0.0028** | −0.0037* | −0.0036* | −0.0025* | −0.0023* | |
| (0.0010) | (0.0009) | (0.0017) | (0.0016) | (0.0011) | (0.0010) | |
Notes: M1: exposure; M2: M1 + age, education, sex, Latino background, time from INCA to Sueño, BMI, alcohol consumption, physical activity, heart failure, stroke/TIA, CESD-10, STAI-10, antidepressants use, center, REI, ESS, number of days in sleep study, and having at least 2 weekend days (yes, no).
Abbreviations: β, beta coefficient; B-SEVLT, Brief Spanish English Verbal Fluency Test; BMI, body mass index; CESD, Center for Epidemiological Studies Depression Scale; ESS, Epworth sleepiness scale; INCA, Investigation of Neurocognitive Aging; REI, respiratory event index; SE, standard error; SIS, Six Item Screener; STAI, State-Trait Anxiety Inventory.
= P < 0.05.
= P < 0.01.
= P < 0.001.
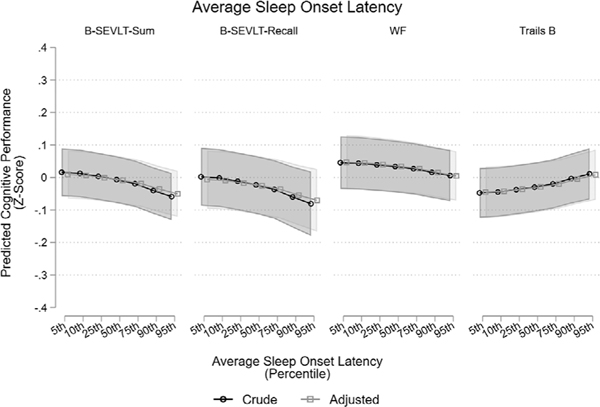
FIGURE 1.
Associations between average sleep-onset latency and neurocognitive performance at SOL-INCA. Notes: SOL-INCA was conducted between 2015 and 2018; on average 7 years after Visit-1 cognitive testing. Adjusted models include age, education, sex, ethnicity, time from INCA to Sueño, BMI, alcohol consumption, physical activity, heart failure, stroke/TIA, CESD-10, STAI-10, antidepressants use, center, REI, ESS, number of days in sleep study, and having at least 2 weekend days (yes, no). For Trails B higher values indicate worse function. BMI, body mass index; B-SEVLT, Brief-Spanish English Verbal Fluency Test; CESD, Center for Epidemiologic Studies Depression Scale; ESS, Epworth sleepiness scale; REI, respiratory event index; SOL-INCA, Study of Latinos-Investigation of Neurocognitive Aging; STAI, State-Trait Anxiety Inventory; TIA, transient ischemic attack; WF, Word Fluency
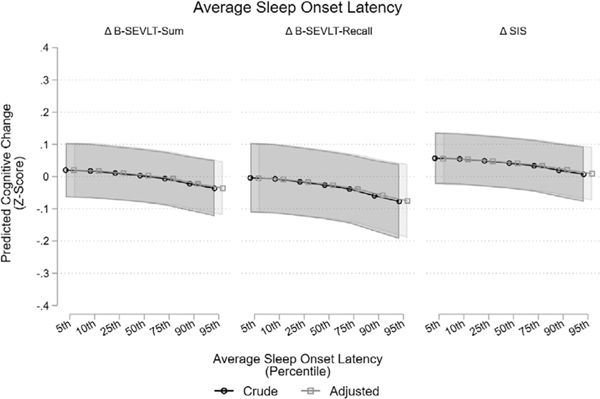
FIGURE 2.
Associations between average sleep-onset latency and change in cognitive function. Notes: Adjusted models include age, education, sex, ethnicity, time from INCA to Sueño, BMI, alcohol consumption, physical activity, heart failure, stroke/TIA, CESD-10, STAI-10, antidepressants use, center, REI, ESS, number of days in sleep study, and having at least 2 weekend days (yes, no). BMI, body mass index; B-SEVLT, Brief-Spanish English Verbal Fluency Test; CESD, Center for Epidemiologic Studies Depression Scale; ESS, Epworth sleepiness scale; INCA, Investigation of Neurocognitive Aging; REI, respiratory event index; STAI, State-Trait Anxiety Inventory; TIA, transient ischemic attack
2.7 |. Sensitivity analyses
To check the consistency of our findings to additional covariate adjustments and target population we conducted three additional sets of sensitivity analyses. First, we refit our models with an additional covariate adjustment for sleep medication use. Second, all analyses were repeated excluding participants with moderate to severe sleep apnea (REI ≥15 events/hour; Tables S3 and S4 in supporting information). Third, we repeated all primary analyses additionally excluding participants (unweighted n = 40) with <2 weekend days of actigraphy data (Tables S5 to S9 in supporting information).
3 |. RESULTS
Demographic and clinical characteristics of the analytical population are presented in Table 1. The mean age at Sueño visit was 55.2 ± 2.5 years. More than half of the target population were females (53.8%) and almost a third had less than high school education (33.8%). The mean BMI at Sueño was 30.1 ± 2.8 kg/m2, and close to a quarter of the target population satisfied AHA criteria for poor physical activity. A detailed characterization of other covariates for the target population is included in Table 1. Average sleep onset latency was 11.3 ± 11.5 (standard deviation [SD]) minutes and average sleep time per nap was 49.7 ± 17.5 (SD) minutes. Mean REI at baseline was 7.2 ± 4.6 (SD) events/hour, average sleep duration was 6.7 ± 0.5 (SD) hours, average ESS was 5.7± 2.1, and close to one in five satisfied criteria for excessive sleepiness (ESS ≥10, 17.5%) and insomnia (ISI ≥15, 21.4%). A detailed characterization of the sleep measures for the target population is included in Table 1.
3.1 |. Cognitive performance (Table 2 and Table S1)
After full covariate adjustment, increased average sleep-onset latency was associated with worse performance (z-score units) on B-SEVLT-Sum (β = –0.003 [0.0007]; P < 0.001), B-SEVLT-Recall (β = –0.0032 [0.0012]; P < 0.01), Word Fluency (β = –0.0021 [0.0007]; P < 0.01), and TMT B (β = 0.003 [0.0010]; P < 0.01). The magnitude of the reported associations with B-SEVLT-Sum, B-SEVLT-Recall, and TMT B were attenuated by 21.1%, 23.8%, and 10.0%, respectively, from the crude models after adjusting for covariates. Several other measures were associated with worse performance on learning and memory measures. In particular, higher average wake after sleep onset (WASO) and average sleep fragmentation index in main sleep intervals were associated with worse performance on B-SEVLT-Sum (β= –0.004 [0.0016]; P < 0.05 and β= –0.014 [0.0055]; P < 0.05, respectively) and B-SEVLT-Recall (β= –0.006 [0.0018]; P < 0.01 and β= –0.017 [0.0058]; P < 0.01, respectively), but the associations were explained by covariate adjustment. Higher average sleep efficiency in main rest intervals was associated with better performance on B-SEVLT-Sum (β = 0.016 [0.0056]; P < 0.01) and B-SEVLT-Recall (β= 0.021 [0.0068]; P < 0.01), but was no longer statistically significant after adjusting for covariates. There was an association between average sleep time and TMT A (β= –0.063 [0.0283]; P < 0.05) after adjusting for covariates, with an attenuation of a 37.1% in the magnitude of the association relative to the crude model. Finally, higher average wake bouts in sleep intervals had an inverse association with B-SEVLT-Recall (β = –0.008 [0.0034]; P < 0.05), but the association was explained by covariate adjustment. These associations are summarized in Table 2, and nonsignificant associations between other sleep exposures and cognitive outcomes are summarized in Table S1. These results were unchanged in sensitivity analysis with additional adjustment for sleep medication usage, as well as in the subpopulation with an REI < 15 events/hour (Table S3), and after excluding participants with <2 weekend days of actigraphy data (Table S6).
3.2 |. Cognitive change (Table 3 and Table S2)
Average sleep-onset latency was the only considered sleep exposure that was significantly associated with cognitive change (z-score units) in both crude and adjusted models. Higher average sleep-onset latency was inversely associated to the B-SEVLT-Sum (β = –0.003 [0.0009]; P < 0.001), B-SEVLT-Recall (β = –0.004 [0.0016]; P < 0.05), and SIS (β = –0.002 [0.0010]; P < 0.05) after adjusting for covariates. Higher average sleep duration (minutes) per nap had positive association with B-SEVLT-Recall (β = 0.004 [0.0016]; P < 0.05) in a crude model that only adjusted for sex and time from INCA to Sueño, but the association was no longer significant after full covariate adjustment. Longer average sleep time (hours) had a negative association with change in the six-item-screener (a global measure of mental status) score (β= – 0.070 [0.0324]; P < 0.05) in a fully adjusted model, but the association was not significant in a crude model that only adjusted for sex and time from INCA to Sueño. No other objective sleep measures were consistently associated with change in cognitive outcomes between Visit-1 and Visit-2. These associations are summarized in Table 3, and nonsignificant associations between other sleep exposures and cognitive outcomes are summarized in Table S2. These results were unchanged in sensitivity analysis additionally adjusting for sleep medication usage. The association between average sleep-onset latency and verbal learning, verbal memory, and mental status were consistent in sensitivity analyses focusing on participants without evidence for moderate/severe sleep apnea (REI < 15 events/hour; Table S4), and after excluding participants with <2 weekend days of actigraphy data (Table S7).
4 |. DISCUSSION
In this observational study of mostly middle-aged community-dwelling U.S. Hispanic/Latino adults, actigraphy-derived sleep patterns predicted 7-year cognitive change. Longer sleep-onset latency was associated with declines in global cognitive function, verbal learning, and verbal memory. Longer sleep-onset latency was also cross-sectionally associated with verbal learning, verbal memory, and word fluency. We did not observe prospective associations between actigraphy-derived sleep duration and sleep continuity (eg, sleep fragmentation) with cognitive change. In addition, self-reports of daytime sleepiness and insomnia symptoms were not associated with cognitive change. Similar to our study, a sample of middle-aged and older adults from the Rotterdam study found that actigraphy-derived greater sleep-onset latency had a cross-sectional association with worse global cognitive function, verbal memory, and verbal fluency. In particular, participants ≥70 years with longer sleep-onset latency performed worse in measures of executive function (Stroop interference test).27 Epidemiological studies of predominantly older adults describe cross-sectional associations between sleep continuity with worse global cognitive function, processing speed, semantic memory, verbal learning and memory, and executive function.27–36
Of interest, the Osteoporotic Fractures in Men Study described prospective associations between actigraphy-derived sleep continuity (sleep efficiency, WASO) with declines in global cognition and executive functions.37 In the Rush Memory and Aging Project, decreased sleep fragmentation attenuated the effect of the apolipoprotein E (APOE) ε4 allele on cognitive decline.38 Covariate adjustment explained the associations among measures of sleep continuity, fragmentation, and cognitive change in our study. Our divergent findings can be explained by differences in our methodology and target populations. First, unlike a majority of actigraphy sleep studies, our analyses controlled for sleep apnea, which is a source of significant sleep fragmentation. Second, unlike the aforementioned studies, our target population were exclusively Hispanic/Latino. Third, our target populations were younger and predominantly in middle age. Most cohort studies evaluated older adults, in whom the coexistence of poor sleep and neurodegenerative cognitive decline introduce the likelihood of reverse causation, whereas sleep patterns in middle age are more likely the result of behavior or lifestyle demands. Furthermore, middle age may be an inflection point at which sleep disturbances begin to affect longitudinal cognitive function. Sleep fragmentation might be a consequence of neurodegeneration in older adults,28 not yet observed in our middle-aged sample. For example, cognitive impairment or dementia status mostly explained the associations between actigraphy-derived sleep fragmentation and cognitive function in a cohort of older women.28
Prolonged sleep-onset latency could affect cognitive function through decreased slow wave sleep, a measure of homeostatic sleep drive important for synaptic plasticity, brain amyloid beta (Aβ) clearance, redox homeostasis, and memory consolidation.39–41 Prolongation of sleep-onset latency can be associated with attention deficits and subsequent difficulties on performing attention-dependent cognitive tasks.27,42 It is plausible that prolonged sleep onset-latency represents circadian misalignment. However, measures of inter-day stability, a behavioral measure of circadian rhythmicity, were not associated with our cognitive measures. While the specifics of the interplay between sleep and neurodegeneration merit further study, actigraphy-defined sleep-onset latency may be a marker or harbinger of cognitive decline starting in middle age.
Increased sleep time per nap was associated with less decline in verbal memory. Findings attenuated when adjusting for daytime sleepiness. Sleep time per nap and daytime sleepiness could share variance that explains these null findings. Our results stand in contrast with the results from the Osteoporotic Fractures in Men Study. In that study of older males with normal cognitive function at baseline, participants within the quartile of longest nap duration had the highest risk of developing cognitive impairment at 12 years of follow-up.30 While the cause for the discrepancy is unclear, longer naps in older adults could be the consequence of either poor sleep during the main rest interval, circadian misalignment,43 or a symptom of neurodegeneration.44 In contrast, longer naps in middle-aged individuals could aid in sleep-related memory consolidation by mitigating the effects of either curtailed or non-restorative sleep. However, we had a low frequency of adults that engage in naps. In comparison, we observed a greater decline in global cognitive function among participants with longer average sleep time, which could be a symptom of non-restorative sleep, hypersomnia, or circadian misalignment leading to increased homeostatic sleep drive that manifest as prolonged periods in bed. This finding is consistent with our prior analysis of HCHS/SOL that demonstrated greater longitudinal cognitive decline among those with self-reported long sleep duration.6
Although increased actigraphy-defined sleep-onset latency predicted cognitive decline, self-reported sleep measures did not. For example, the insomnia-severity index was not found to have cross-sectional or prospective associations with cognitive function in our study. This may be explained by subjective-objective sleep discrepancies that can differ by age, ethnicity, and cognitive function.45 Alternatively, the measure of sleep-onset latency obtained in our study could represent subclinical insomnia not yet perceived as a sleep-related clinical complaint. Actigraphy and self-reported sleep measures may each provide valid information on different aspects and domains of sleep health that merit further investigations.
4.1 |. Study strengths and limitations
There are several strengths of this study. First, our study was prospective and examined a large cohort of mostly middle-aged individuals from diverse Hispanic/Latino backgrounds. Second, our study contained a wealth of data beyond actigraphy and cognitive function, from which a robust model could account for a number of major sleep confounders. However, we have a few methodological limitations. First, actigraphy is an incomplete objective measure of sleep. In the absence of polysomnography (PSG) with electroencephalography, we did not assess sleep stages, precluding assessment of sleep macro- or micro-architecture. In addition, home sleep apnea studies can underestimate the REI.46 Second, PSG, compared to actigraphy, provides better estimates of WASO,47 sleep-onset latency,48 sleep duration, and sleep efficiency.49 The decreased precision of actigraphy-derived sleep measures, compared to PSG, could limit the effect size and significant associations with cognitive function. Of importance, longer actigraphy-defined sleep-onset latency predicted more than a 50% increase in incident dementia and Alzheimer’s disease in the Rotterdam study.50 Actigraphy affords an objective evaluation of multiple nights in the participant’s sleep environment, providing a better measure of sleep patterns than a single night of PSG. Third, we are unable to address what processes explain the associations between sleep and each of the specific cognitive domains tested. Fourth, we cannot address causality with our study design, and results may not be generalizable to other ethnic groups. Last, there may have been unmeasured confounders not included in our statistical models.50
4.2 |. Sleep and neurocognitive decline: future directions
This study establishes that measures of actigraphy-defined sleep-onset latency, sleep duration, and nap duration predict cognitive change in a cohort mainly of middle-aged Hispanic/Latino adults. Further study is needed to understand the etiologies that influence prolonged sleep-onset latency, sleep duration, and napping behaviors to identify potential mechanisms of cognitive decline and preserve cognitive function.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: The authors reviewed the literature using PubMed, where few analyses found prospective associations between actigraphy-defined sleep patterns and decline in cognitive function. These relevant studies are cited. There is a gap of studies that contain mostly middle-aged adults, more so from Hispanic/Latino background. In addition, there is limited information on sleep latency, sleep duration, and napping behavior with cognitive decline in population-based studies that also contain measures of sleep apnea, insomnia, and daytime sleepiness.
Interpretation: We identified the actigraphy-defined sleep patterns of cognitive decline in a diverse sample of Hispanic/Latinos.
Future directions: This study establishes that measures of actigraphy-defined sleep-onset latency, sleep duration, and nap duration predict cognitive change in a cohort mainly of middle-aged Hispanic/Latino adults. Further study is needed to understand the etiologies that influence prolonged sleep-onset latency, sleep duration, and napping behaviors to identify potential mechanisms of cognitive decline and preserve cognitive function.
Acknowledgments
FUNDING INFORMATION
This work is supported by National Institute on Aging (R01AG048642, RF1AG054548, RF1AG061022, and R21AG056952) and by the National Heart Lung Blood Institute (R01HL098297). Dr. Ramos receive additional support from R21HL140437. The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements.
Funding information
National Institute on Aging, Grant/Award Numbers: R01AG048642, RF1AG054548, RF1AG061022, R21AG056952; National Heart Lung Blood Institute, Grant/Award Number: R01HL098297; University of North Carolina, Grant/Award Number: N01HC65233; University of Miami, Grant/Award Number: N01-HC65234; Albert Einstein College of Medicine, Grant/Award Number: N01-HC65235; Northwestern University, Grant/Award Number: N01-HC65236; San Diego State University, Grant/Award Number: N01-HC65237
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1.Patterson C. World Alzheimer Report 2018: the State of the Art of Dementia Research: New Frontiers. London, UK: Alzheimer’s Disease International (ADI); 2018. [Google Scholar]
- 2.Haan MN, Mungas DM, González HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. [DOI] [PubMed] [Google Scholar]
- 3.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in US race/ethnic populations. Alzheimers Dement. 2017;13(1):72–83. [DOI] [PubMed] [Google Scholar]
- 4.Cespedes EM, Dudley KA, Sotres-Alvarez D, et al. Joint associations of insomnia and sleep duration with prevalent diabetes: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). J Diabetes. 2016;8:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SR, Sotres-Alvarez D, Castaneda SF, et al. Social and Health Correlates of Sleep Duration in a US Hispanic Population: results from the Hispanic Community Health Study/Study of Latinos. Sleep. 2015;38:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos AR, Tarraf W, Daviglus M, et al. Sleep duration and neurocognitive function in the Hispanic Community Health Study/Study of Latinos. Sleep. 2016;39:1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott SM, Weng J, Reid KJ, et al. Sleep timing, stability, and BP in the Sueno Ancillary Study of the Hispanic Community Health Study/Study of Latinos. Chest. 2019;155:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos AR, Weng J, Wallace DM, et al. Sleep patterns and hypertension using actigraphy in the Hispanic Community Health Study/Study of Latinos. Chest. 2018;153:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley KA, Weng J, Sotres-Alvarez D, et al. Actigraphic sleep patterns of U.S. Hispanics: the Hispanic Community Health Study/Study of Latinos. Sleep. 2017;40:zsw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez HM, Tarraf W, Gouskova N, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol. 2015;30:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaVange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowe SF. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J Clin Psychol. 1998;54:585–591. [DOI] [PubMed] [Google Scholar]
- 15.Besha XS, Spencer RJ, Bieliauskas LA. PPVT-I administration rules significantly shorten PPVT-III/IV administration. Int J Neurosci. 2017;127:412–416. [DOI] [PubMed] [Google Scholar]
- 16.Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez HM, Tarraf W, Fornage M, et al. A research framework for cognitive aging and Alzheimer’s disease among diverse US Latinos: design and implementation of the Hispanic Community Health Study/Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Alzheimers Dement. 2019;15:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel SR, Weng J, Rueschman M, et al. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino Population. Sleep. 2015;38:1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 20.Veqar Z, Hussain ME. Validity and reliability of insomnia severity index and its correlation with pittsburgh sleep quality index in poor sleepers among Indian university students. Int J Adolesc Med Health. 2017;32. [DOI] [PubMed] [Google Scholar]
- 21.Allen R, Oertel W, Walters A, et al. Relation of the International Restless Legs Syndrome Study Group rating scale with the Clinical Global Impression severity scale, the restless legs syndrome 6-item questionnaire, and the restless legs syndrome-quality of life questionnaire. Sleep Med. 2013;14:1375–1380. [DOI] [PubMed] [Google Scholar]
- 22.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Mendoza J, Rodriguez-Munoz A, Vela-Bueno A, et al. The Spanish version of the Insomnia Severity Index: a confirmatory factor analysis. Sleep Med. 2012;13:207–210. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 25.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CESD (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 26.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 27.Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Ikram MA, Tiemeier H. Associations of the 24-h activity rhythm and sleep with cognition: a population-based study of middle-aged and elderly persons. Sleep Med. 2015;16:850–855. [DOI] [PubMed] [Google Scholar]
- 28.Spira AP, Stone KL, Redline S, et al. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep. 2017;40(8):zsx073. 10.1093/sleep/zsx073. PMID: 28472447; PMCID: PMC5806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–410. [DOI] [PubMed] [Google Scholar]
- 30.Leng Y, Redline S, Stone KL, Ancoli-Israel S, Yaffe K. Objective napping, cognitive decline, and risk of cognitive impairment in older men. Alzheimers Dement. 2019;15:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim AS, Yu L, Costa MD, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein JPK, DeVito A, Calamia M. Subjectively and objectively measured sleep predict differing aspects of cognitive functioning in adults. Arch Clin Neuropsychol. 2019;34:1127–1137. [DOI] [PubMed] [Google Scholar]
- 34.Lambiase MJ, Gabriel KP, Kuller LH, Matthews KA. Sleep and executive function in older women: the moderating effect of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69:1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavuoto MG, Ong B, Pike KE, Nicholas CL, Bei B, Kinsella GJ. Objective but not subjective sleep predicts memory in community-dwelling older adults. J Sleep Res. 2016;25:475–485. [DOI] [PubMed] [Google Scholar]
- 36.Miyata S, Noda A, Iwamoto K, Kawano N, Okuda M, Ozaki N. Poor sleep quality impairs cognitive performance in older adults. J Sleep Res. 2013;22:535–541. [DOI] [PubMed] [Google Scholar]
- 37.Blackwell T, Yaffe K, Laffan A, et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014;37:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musiek ES, Lim MM, Yang G, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging. 2009;1:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shute GE, Fitzgerald SG, Haynes SN. The relationship between internal attentional control and sleep-onset latency in elderly adults. J Gerontol. 1986;41:770–773. [DOI] [PubMed] [Google Scholar]
- 43.Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YS. Circadian rest-activity pattern changes in aging and preclinical Alzheimer Disease. JAMA Neurol. 2018;75:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Donnell D, Silva EJ, Munch M, Ronda JM, Wang W, Duffy JF. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. J Sleep Res. 2009;18:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bianchi MT, Goparaju B. Potential underestimation of sleep apnea severity by at-home kits: rescoring in-laboratory polysomnography without sleep staging. J Clin Sleep Med. 2017;13:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blackwell T, Ancoli-Israel S, Redline S, Stone KL, Osteoporotic Fractures in Men Study G. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J Clin Sleep Med. 2011;7:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–396. [DOI] [PubMed] [Google Scholar]
- 50.Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement. 2020;16(9):1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.