ABSTRACT
Cancer-associated fibroblasts (CAFs) and hypoxia are central players in the complex process of tumor cell-stroma interaction and are involved in the alteration of the anti-tumor immune response by impacting both cancer and immune cell populations. However, even if their independent immunomodulatory properties are now well documented, whether the interaction between these two components of the tumor microenvironment can affect CAFs ability to alter the anti-tumor immune response is still poorly defined. In this study, we provide evidence that hypoxia increases melanoma-associated fibroblasts expression and/or secretion of several immunosuppressive factors (including TGF-β, IL6, IL10, VEGF and PD-L1). Moreover, we demonstrate that hypoxic CAF secretome exerts a more profound effect on T cell-mediated cytotoxicity than its normoxic counterpart. Together, our data suggest that the crosstalk between hypoxia and CAFs is probably an important determinant in the complex immunosuppressive tumor microenvironment.
KEYWORDS: Cancer-associated fibroblasts, hypoxia, immunosuppression, CTL
Introduction
Within the tumor microenvironment (TME), activated fibroblasts that share similarities with fibroblasts stimulated by inflammatory signals, activated during a wound healing process or observed during tissue fibrosis, termed cancer-associated fibroblasts (CAFs), have emerged as important regulators of tumor progression.1–3 CAFs are spindle-shaped cells who represent the prominent stromal component in many types of human tumors, and who differentiate in the TME in a transforming growth factor-β (TGF-β)-, platelet-derived growth factor (PDGF)- and fibroblast growth factor (FGF)-dependent manner from other cell types such as resident fibroblasts, endothelial, epithelial or mesenchymal stem cells.4–8 Phenotypically, these cells can be identified by the expression of common fibroblastic markers such as fibroblast-specific protein-1 (FSP-1/S100A4) or PDGF receptors (PDGFR) α/β, and by the presence of activation markers including, but not limited to, α-smooth muscle actin (α-SMA), fibroblast-activation protein (FAP), tenascin-C (TNC), periostin (POSTN) or podoplanin (PDPN),9 even if none of these markers is entirely specific for normal or activated fibroblasts. By directly interacting with tumor cells and by secreting extracellular matrix components, matrix metalloproteinases (MMPs), chemokines, vascularization inducing proteins or other factors, CAFs play a crucial role in tumor growth, angiogenesis, extracellular matrix remodeling and tissue invasion/metastasis formation.10,11 Importantly, CAFs have also emerged as important regulators of the anti-tumor immune response and several studies have shown that CAFs exhibit a particular immunomodulatory proteome and secretome that can potentially regulate both innate and adaptive anti-tumor immune responses.12–14 For example, CAFs can increase the recruitment and differentiation of myeloid-derived suppressive cells (MDSC),15 regulatory T cells (Tregs)16 or type 2 tumor-associated macrophages (TAM) within the TME,17,18 can affect dendritic cell (DC) maturation and antigen presentation functions19 or can alter proliferation, survival and cytolytic functions of cytotoxic T cells (CTL)20 and natural killer (NK) cells.21–23
Another component of the TME is hypoxia. Indeed, solid tumor tissues are often poorly oxygenated, with a limited number of fully functional blood vessels, resulting in the presence of zones with a low O2 pressure called “hypoxic zones”.24,25 Hypoxia influences several biological parameters (angiogenesis, metabolism, cell proliferation, apoptosis …) that can modify the malignant potential of the tumor and its susceptibility to anti-tumor therapies.26,27 Furthermore, hypoxia also contributes to the immunosuppressive network within the TME.28–30 For example, hypoxic stress within the stroma alters NK and T cell activation and effector functions,31 induces PD-L1 expression on MDSC32 and attracts TAM or Tregs to the tumor bed.33 Importantly, hypoxia also promotes tumor cell secretion of TGF-β and PDGF that can potentially promote differentiation of precursor cells into CAFs34,35 and favors their PDPN expression.36 Similarly, it was shown that hypoxic stress, especially through hypoxia-driven reactive oxygen species (ROS) production leading to dermal fibroblast activation, enhances melanoma aggressiveness.37 Furthermore, in colorectal cancers, hypoxic CAFs hyper-secretion of TGF-β2 can induce strong expression of GLI2 (GLI family zinc finger 2) by cancer stem cells, and consequently promotes resistance to therapy.38 In mammary tumors, hypoxia also induces a pronounced remodeling of the CAF proteome, favoring endothelial sprouting and angiogenesis.39 Thus, these studies, and others, indicate a role of hypoxia in the process of CAFs activation, and on their functionality within the TME. Furthermore, one may consider that hypoxia might also modulate CAF immunosuppressive properties, even if this particular point needs to be further explored.
In the present study, we thus used fibroblasts isolated from melanoma tumor biopsies to evaluate in vitro the effect of hypoxia on CAF immunosuppressive capabilities. Using this model, we validated the effect of hypoxia on CAF activation and most importantly we observed that hypoxia increases the expression and/or secretion of several immune suppressive factors (including, but not limited to, TGF-β, IL6, IL10, VEGF, MMPs and PD-L1). Furthermore, we provide evidence that these hypoxia-mediated changes in the CAFs immune-suppressive proteome potentiate the inhibitory effect of CAFs on T cell-mediated killing of melanoma tumor cells.
Materials and methods
Isolation and culture of melanoma-associated fibroblasts
A panel of five pathology-confirmed melanoma tumor resections was obtained in accordance with consent procedures approved by the Gustave Roussy Institute. CAF were obtained by mechanical dissociation of these biopsies, serial passage and low trypsin detachment, as previously described.21 Cells population homogeneously displaying a fibroblast-like morphology were phenotypically characterized and assessed for purity by the analysis of the indicated markers. The obtained cell populations were maintained in culture in DMEM-F12, 10% FCS, 100 U/ml penicillin and 100 mg/ml streptomycin.
Hypoxic conditioning of CAFs
Hypoxic treatment was conducted during 48 hrs in a hypoxia workstation (Invivo2 400, Ruskinn) in a humidified atmosphere containing 5% CO2, 1% O2 and 94% N2 at 37°C. All RNAs and proteins were prepared by lysing cells directly in the hypoxia workstation to avoid reoxygenation.
Preparation of fibroblast-derived conditioned media
CAFs were seeded at equal density (2 × 105 cells/well in 12 well plates) and maintained in culture under normoxic or hypoxic conditions during 48 hrs in DMEM/F12 medium supplemented with 1% FCS. Culture supernatants were then collected and centrifuged 15 min at 2,000 rpm to remove cell debris. These supernatants, considered as conditioned media (CM), were then immediately used or frozen at -20°C for subsequent use.
Tumor cell line and CTL culture
T1 tumor cells, established from the primary lesion of a patient suffering from a melanoma,40 were grown in RPMI1640/Glutamax™ supplemented with 10% FCS, 100 U/ml penicillin, 100 mg/ml streptomycin and 1% sodium pyruvate (Life Technologies). The LT12 CTL clone was isolated from T1 autologous tumor infiltrating lymphocytes, recognizes a peptide derived from the MelanA/MART-1 Ag and was maintained in culture as described40,41 in RPMI1640/Glutamax™ supplemented with 5% human serum and 50 U/mL recombinant human IL2.
Viability and proliferation assays
CAFs viability under normoxic and hypoxic conditions was measured after trypan blue dye staining using a TC20 automated cell counter (Biorad) to determine the live/dead cell ratio. CAF proliferation was evaluated with MTT assay (Roche) following the manufacturer’s instructions.
Flow cytometry analysis
Briefly, 0.2 × 106 fibroblasts were stained at 4°C with the following Abs: anti-FSP-1/S100A4 rabbit mAb (clone EPR2761, Abcam); anti-vimentin rabbit mAb (clone SP20, Abcam); PE-conjugated anti-E Cadherin/CD324 mouse mAb (clone 67A4, Biolegend); Alexa Fluor® 488-conjugated anti-CD31 mouse mAb (clone M89D3, BD Pharmingen); FITC-conjugated anti-CD34 mouse mAb (clone AC136, Miltenyi Biotec.); PE-conjugated anti-CD45RO mouse mAb (clone UCHL1, DakoCytomation); anti-FAP mouse mAb (ab54651, Abcam); PE-conjugated anti-PD-L1 mouse mAb (clone 29E.2A3, BioLegend). For LT12 CTL clones, cells were fixed and permeabilized with Cytofix/Cytoperm kit (BD Pharmingen), followed by intracellular staining with APC-conjugated anti-GzmB mouse mAb (clone GR05, Invitrogen). Acquisitions were performed using a BD Accuri™ C6 flow cytometer (BD Biosciences) and data were processed using the FlowJo program.
Fluorescence microscopy
Cells were grown on rat collagen-coated glass coverslips (Sigma) and fixed for 15 min in PBS/2% PFA, washed and incubated 10 min in PBS/50 mM NH4Cl. Cells were then washed with PBS, permeabilized for 5 min in PBS/0.2% Triton X-100. After 2 washes in PBS, coverslips were placed in blocking solution (PBS/10% FCS) for 30 min, washed once in PBS and incubated for 1 hr at RT with anti-α-SMA mouse mAb (clone 4A4, Abcam) and anti-Vimentin rabbit mAb (clone SP20, Abcam) in incubation buffer (PBS/0.05% Triton X-100). Cells were then washed 3 times with incubation buffer and incubated 1 hr at RT with Alexa-Fluor 488 and Alexa-Fluor 647-conjugated secondary antibody (Life Technologies) in incubation buffer containing 5% FCS. Cells were then washed 3 times in PBS and mounted in Vectashield mounting medium containing DAPI (Vector Laboratories) before imaging (IX83 microscope, Olympus) and analysis (CellSens Dimension software, Olympus).
Western Blot
Total cellular extracts were prepared by lysing cells in RIPA buffer (Thermo Scientific) containing a cocktail of protease inhibitors (Roche). Protein concentration was determined using PierceTM BCA protein assay. Protein denaturation was performed by boiling samples in Laemmli buffer before SDS-PAGE separation on 4–20% precast gels (Biorad). Blots were probed with the following Ab: anti-α-SMA mouse mAb (clone 4A4, Abcam), anti-HIF-1 mouse mAb (clone 54/HIF1α, BD Biosciences), HIF-2 rabbit mAb (clone D9E3, Cell Signaling), anti-Vimentin rabbit mAb (clone SP20, Abcam), anti-PD-L1 rabbit mAb (clone E1L3N, Cell Signaling), HRP-conjugated anti-actin mouse mAb (clone AC-74, Sigma), HRP-conjugated goat anti-mouse or anti-rabbit Ab (Santa Cruz Bio.). Images were captured using a ChemiDoc Imaging System (Biorad). CAF CMs were analyzed by silver staining using PierceTM silver stain kit. Western blot quantifications were performed using Image-J densitometry software.
RT-qPCR analysis
Total RNA extraction was performed with TRIzol TriReagent (Sigma). cDNAs were synthesized using a Maxima first strand cDNA synthesis kit (Thermo-Fischer Scientific). Gene expression was quantified by SYBR Green qPCR method using SYBR Select Master Mix on a StepOnePlus Real Time PCR system (Thermo Scientific). Relative expression was calculated using the comparative Ct method (2-ΔΔCt). Transcript level of 18S was used as endogenous control. Primers were purchased from Sigma-Aldrich and their sequences are listed in supplementary table 1.
Multiplex and ELISA analysis
Human TGF-β concentration was measured in normoxic and hypoxic CAF CMs using a TGF-β1 ELISA kit (eBiosciences). Human VEGF, IL6, IL10, CCL2, CCL5 and IFN-γ concentrations were simultaneously measured in normoxic or hypoxic CAF CMs using a Bio-Plex ProTM human cytokine Multiplex Immunoassay, according to the manufacturer protocol. Data were analyzed using a Bio-Plex 200 instrument and Bio-Plex software (Biorad).
Measurement of MMPs activity in conditioned media
The metalloproteinases activity in CAFs CMs was measured using the fluorometric SensoLyte® 520 Generic MMP Activity Kit (AnaSpec) according to a modified manufacturer protocol. This assay is based on a 5-FAM/QXL520 fluorescence resonance energy transfer (FRET) peptide as an MMP substrate. In the intact FRET peptide, the fluorescence of 5-FAM is quenched by QXL520. Upon cleavage into two separate fragments by MMPs, the fluorescence of 5-FAM is recovered and can be monitored. Briefly, the supernatants of normoxic or hypoxic CAFs were collected and centrifuged for 10–15 min at 1,000 g at 4°C. MMPs substrate and MMPs diluents were then mixed together in a 96 wells plate before adding freshly prepared CAFs CMs to initiate the enzymatic reaction. Fluorescence was then immediately measured and monitored every 10 min at Ex/Em = 490/520 nm during 8 hrs using a FLUOstar Optima microplate reader (BMG Labtech).
Chromium release assay
Cryopreserved LT12 CTL clones were thawed and pre-treated during 16 hrs with normoxic or hypoxic CAF CMs (diluted 3:2 (v/v) in regular CTL culture medium containing IL-2) before measuring their lytic activity against T1 tumor target cells. CAF culture medium was used as control. Cytotoxicity was measured by a 4 hrs chromium release assay as previously described.42 Experiments were performed in triplicate. Data are expressed as the percentage of specific 51Cr release from target cells, calculated as (experimental release-spontaneous release)/(maximum release-spontaneous release) x 100.
Statistical analysis
Data are expressed as mean ± standard deviation (s.d.). P values were determined by unpaired two-tailed student’s t tests using Prism – GraphPad.
Results
Phenotypic characterization of human fibroblasts isolated from melanoma
In order to investigate the impact of hypoxia on melanoma-associated fibroblast immunosuppressive properties, we established five fibroblast primary cell cultures from melanoma patient’s tumor resection, named CAF1-5 (Figure 1(a)). The adherent cells displaying a fibroblast-like morphology (i.e. elongated cells with cytoplasmic extensions) (Figure 1(b)) were carefully characterized by flow cytometry and/or fluorescence microscopy. These cell populations express the fibroblastic marker FSP-1 and the mesenchymal marker vimentin, but not the epithelial marker E-cadherin, the endothelial marker CD31, the hematopoietic marker CD34 or the leukocyte marker CD45 (Figure 1(c)). Furthermore, the melanoma-derived fibroblasts exhibit an activated phenotype as shown by α-SMA (Figure 1(d-e)) and FAP (figure 1(f)) expression. Together, these observations indicate that these cell populations are mainly fibroblasts, with minimal contamination with epithelial, endothelial or hematopoietic cells, and confirm their activated status, as expected.
Figure 1.
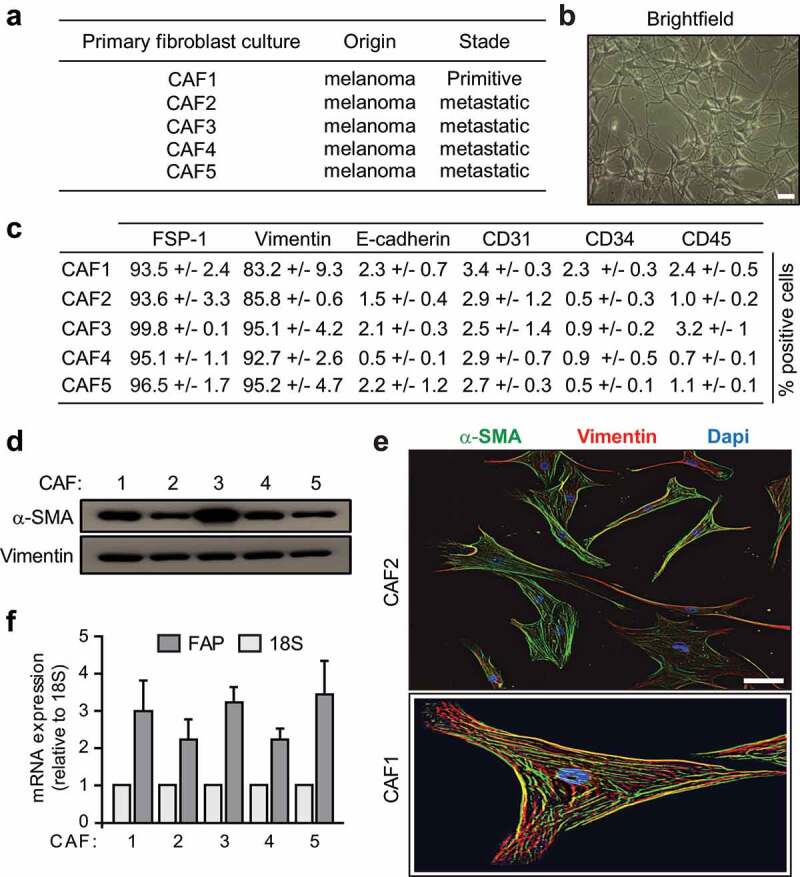
Phenotypes of fibroblasts derived from melanoma . (a) List of the different fibroblast populations isolated from melanoma primitive or metastatic tumor biopsies. (b) Representative morphology of the different fibroblastic primary cultures by brightfield microscopy (scale bar: 50 μm). (c) Expression of the fibroblastic, mesenchymal epithelial, endothelial, hematopoietic or leukocytic markers FSP-1, Vimentin, E-cadherin, CD31, CD34 and CD45 by the different isolated fibroblast populations. Data are expressed as the percentage positive cells ± s.d. from three independent flow cytometry experiments. (d-f) CAFs isolated from melanoma patient’s tumor biopsies display an activated phenotype. Expression of the activation marker α-SMA, together with the mesenchymal marker Vimentin, was evaluated by western blot (d) and fluorescence microscopy (e). FAP mRNA expression was measured by RT-qPCR and compared to 18S (f). Data are representative of two independent immunoblots (d) or the mean ± s.d. from three independent qPCR (f). Two representative fluorescence microscopy images (CAF2 and CAF1; scale bar: 10 μm) are shown in (e)
Hypoxia induces HIF-1α expression in melanoma-derived fibroblasts and increases their expression of activation markers
In order to validate that our CAFs are able to respond to hypoxic stress, CAF1-5 were cultured during 48 hrs under hypoxic condition (1% O2) to mimic the median 1.5% of O2 in melanoma compared to 5.3% in the related normal tissue, before evaluating expression of HIF-1α (Hypoxia-Inducible Factor 1-alpha), a subunit of the heterodimeric transcription factor HIF-1, considered as the master regulator of cellular response to hypoxia,25 and of HIF-2. As compared to normoxia, a strong expression of HIF-1αwas observed in CAF1-5 following hypoxia exposure (Figure 2(a)). Of note, HIF-2αexpression was not detected in either normoxic or hypoxic CAFs. Consequently, expression of VEGF (Vascular Endothelial Growth Factor), CA-IX (Carbonic Anhydrase IX), SLC2A1/GLUT1 (Solute Carrier Family 2 Member 1/Glucose transporter 1) and PDK1 (Pyruvate Dehydrogenase Kinase 1), four well-known HIF-1 target genes, is also strongly upregulated in CAF1-5 in response to hypoxia (Figure 2(b)). Furthermore, CAFs viability (Figure 3(a)) and CAFs proliferation (Figure 3(b)) are not affected by hypoxia. However, we observed that hypoxic conditions increase FAP, but not vimentin, mRNA expression (Figure 3(c)), αSMA protein level (Figure 3(d-e)), FAP expression at the cell surface (figure 3(f-g)), as well as the overall CAFs secretion of soluble factors (Figure 3(h)). Together, these data demonstrate that melanoma-derived fibroblasts are able to respond to hypoxic stress by inducing HIF-1α expression, confirm that hypoxia participates and/or improves their activation, as previously suggested,43–46 and improves their global secretion of soluble proteins.
Figure 2.
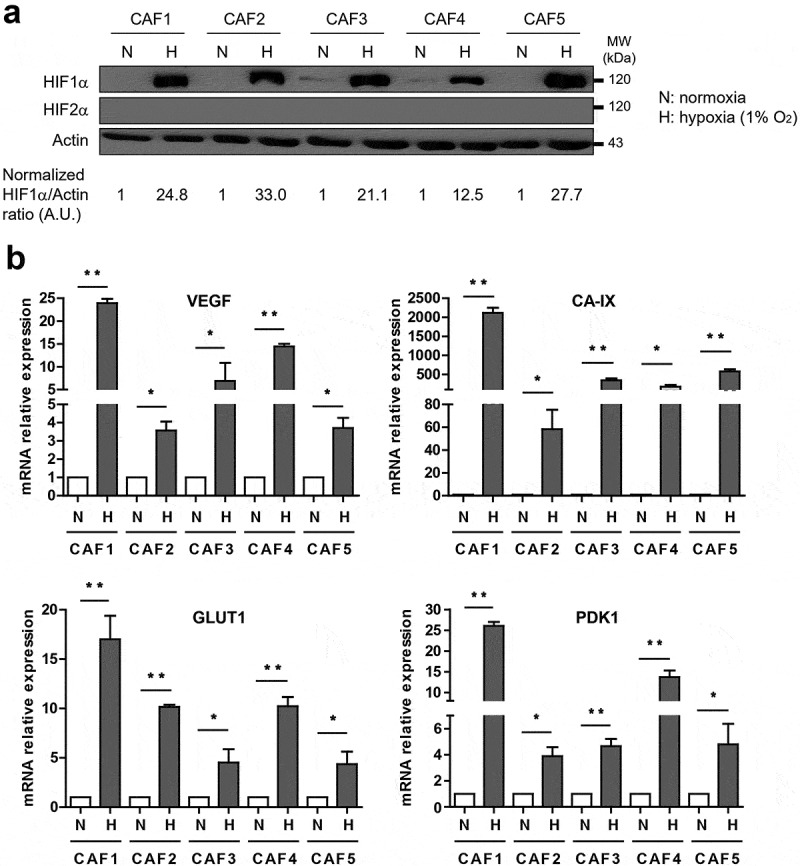
Hypoxia induces HIF-1α and HIF-1-target genes expression in CAFs. (a) CAFs cultured in hypoxia (1% pO2) for 48 hrs express more HIF-1α (but not HIF-2) compared to their normoxic counterpart. The HIF-1α/Actin ratio was calculated by densitometry and normalized to “1” for each normoxic CAF (A.U: arbitrary units). (b) Expression of HIF-1α target genes (VEGF, CA-IX, GLUT1, PDK1), tested by RT-qPCR, is increased in hypoxic CAFs. Results are expressed as the mean ± s.d. of three independent experiments, normalized to “1” in normoxic conditions (N: Normoxia; H: Hypoxia). P values were determined by unpaired two-tailed student’s t-test. (*p < .05; ** p < .01)
Figure 3.

Hypoxia increases CAF activation level. (a-b) CAFs viability (a) and proliferation (b) are not affected by 48 hrs culture under hypoxic conditions (1% pO2). Viability and proliferation percentages (mean ± s.d. of three independent experiments) were normalized to “100” for normoxic conditions. (c-g) Expression of the CAF activation markers FAP, tested by RT-qPCR (c) and flow cytometry (f-g), and αSMA, evaluated by fluorescence microscopy (d) or western blot (e), is strongly increased in hypoxic condition. Vimentin expression was used as control. Results are expressed as the mean ± s.d. of three independent experiments, normalized to “1” in normoxic condition (c). A representative fluorescence microscopy image for CAF2 (D; scale bar: 10 μm) or western blot (E) and a representative flow cytometry histogram for CAF1 (f) together with the mean ± s.d. of three independent experiments (g) are shown. (h) Protein secretion level was measured in CAF culture supernatants (conditioned medium; CM) under normoxic and hypoxic conditions using SDS-Page and silver staining. The intensity level of each profile is indicated. A representative image (CAF2) is shown. P values (a-c, g) were determined by unpaired two-tailed student’s t-test. (NS: non-significant; *p < .05; ** p < .01). N: Normoxia; H: Hypoxia
Hypoxia increases melanoma-derived fibroblasts expression of several immune-suppressive factors
As mentioned above, several studies have shown that CAFs exhibit a particular immunomodulatory “secretome”. In particular, CAF secretion of TGF-β, arginase (ARG), indoleamine-2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), interleukin (IL)-6 or -10, MMP-2 or -9, C-X-C motif chemokine ligand-12 (CXCL12/SDF1), chemokine ligand-2 (CCL2/MCP-1) or -5 (CCL5/Rantes) and/or VEGF profoundly affects both the innate and adaptive antitumor immune response.12,13 Similarly, CAF can potentially express ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1/CD39),47 Programmed death-ligand 1 (PD-L1)48–50 or Fas ligand (FasL/CD95-L)51 at their surface, further altering the efficacy of the immune system within the TME. In order to elucidate the impact of hypoxia on CAF immunosuppressive secretome, we thus used RT-qPCR to evaluate the impact of hypoxic stress on the expression of all these factors. As mentioned earlier (Figure 2(b)), VEGF expression is strongly increased in CAF1-5 after hypoxia exposure. Similarly, TGF-β1or TGF-β2, ARG1 or ARG2, IDO, CXCL12, Cox2 (involved in PGE2 formation) and MMP2 expression is consistently increased following CAF1-5 exposure to hypoxic stress, while IL6, IL10 and MMP9 expression is significantly up-regulated in three out of the five hypoxic fibroblast populations (Figure 4(a)). In parallel, we observed a strong up-regulation of CD39, but not CD73, in hypoxic CAF1-5 (Figure 4(b)), an increase of PD-L1 (but not PD-L2) expression in hypoxic CAF2-5 (Figure 4(c)) and a more pronounced expression of FasL by hypoxic CAF1-3 (Figure 4(d)). In addition, we observed a significant induction of CD47 (a cellular receptor known for its immunoregulatory functions that can be induced in tumor cells by hypoxic stress) in three out of the five hypoxic CAFs (Figure 4(e)). On the opposite, no significant changes regarding CCL2 and CCL5 expression was observed after exposure of CAF1-5 to hypoxia, compared to normoxia (figure 4(f)). Thus, these data suggest that hypoxia profoundly affects the expression of several immune suppressive factors by melanoma-derived fibroblasts although some variability between cell cultures was observed.
Figure 4.
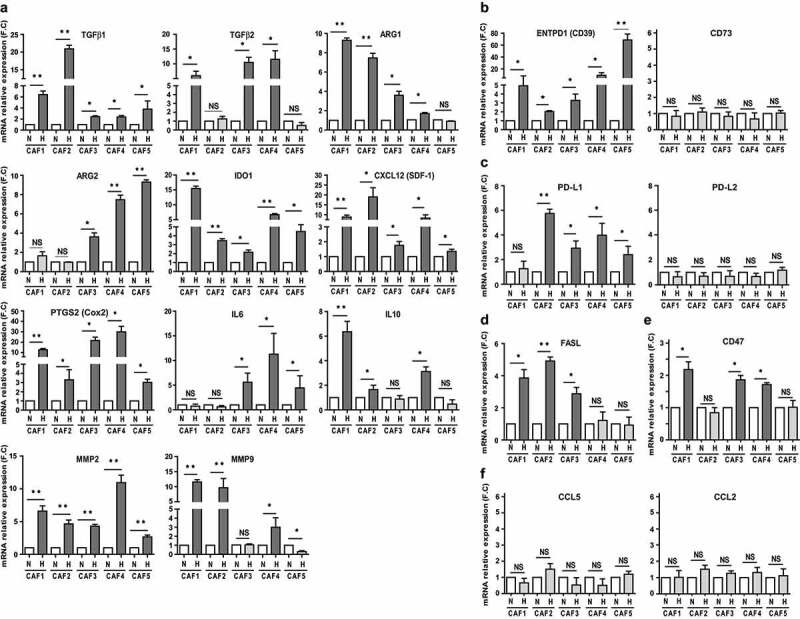
Hypoxic conditioning of CAFs improves their expression of several immune-suppressive factors. RT-qPCR analysis of (a) TGF-β1, TGF-β2 ARG1, ARG2, IDO1, CXCL12 (SDF-1), PTGS2 (Cox2), IL6, IL10, MMP2, MMP9, (b) ENTPD1 (CD39), CD73, (c) PD-L1, PD-L2, (d) FasL, (e) CD47, (f) CCL2 and CCL5 expression by hypoxic versus normoxic CAFs. Results are expressed as the mean ± s.d. of three independent experiments, normalized to “1” in normoxic conditions (N: Normoxia; H: Hypoxia). P values were determined by unpaired two-tailed student’s t-test. (NS: non-significant; *p < .05; ** p < .01)
Hypoxia increases the secretion of several immune-suppressive proteins by melanoma-derived fibroblasts and improves their expression of PD-L1
To validate our data on the hypoxic CAF secretome at the protein level, we used conditioned media (CMs) obtained from the cell culture supernatants of either normoxic or hypoxic CAFs and used them to randomly validate the secretion of the chosen target. Using multiplex immunoassays, we quantified the concentration of VEGF, IL6, IL10, CCL2, CCL5 and IFN-γ (used as control) in the CMs of CAFs. Consistently with the RT-qPCR data, and compared to normoxia, VEGF concentration is significantly increased in hypoxic CAF1-5 CMs, IL6 and IL10 concentration are significantly increased in respectively hypoxic CAF3-5 and CAF1-2,4 CMs, while hypoxia does not affect the secretion of CCL2/5 by CAFs (Figure 5(a)) and IFN-γ was not detected in either normoxic or hypoxic conditions (data not shown). Furthermore, we evaluated by ELISA the concentration of TGF-β in the CMs of CAFs, and observed that hypoxic conditions strongly increase its secretion compared to normoxia (Figure 5(b)). We also evaluated MMPs activity in the normoxic and hypoxic CAFs CMs. We used a fluorescence-based assay to measure the activity of MMP-1, 2, 3, 7, 8, 9, 12, 13, and 14. Using this approach, we showed that hypoxic CAFs CMs contain higher levels of active MMPs compared to the CMs of normoxic CAFs (Figure 5(c-d)). Finally, we evaluated PD-L1 and PD-L2 expression by normoxic and hypoxic CAFs. Flow cytometry experiments demonstrated that PD-L1 expression is significantly increased at the surface of hypoxic CAFs, except for CAF1 (Figure 5(e-f)). These data were also validated by western blot for the PD-L1+ CAF2-4 (Figure 5(g)). Of note, PD-L2 expression was almost undetectable at the surface of CAFs, regardless of the normoxic or hypoxic conditions (Figure 5(e) and data not shown). Together, these data show that hypoxia increases the CAF-dependent secretion or expression at their surface of several immuno-suppressive molecules.
Figure 5.

Hypoxia increases the secretion of TGF-β, IL6, IL10, VEGF and MMPs by CAFs and improves their expression of PD-L1. (a) VEGF, IL-6, IL-10, CCL2/MCP1, and CCL5/Rantes concentration simultaneously measured in normoxic or hypoxic CAF conditioned media (CM) by multiplex analysis. (b) TGF-β1 concentration measured in normoxic or hypoxic CAF conditioned media by ELISA. Results (a-b) are expressed as the mean ± s.d. of two independent experiments performed in duplicate. (c-d) The activity of MMPs was evaluated in the conditioned media of normoxic or hypoxic CAFs by measuring the fluorescence emission of 5-FAM/QXL520 FRET peptide as MMP substrate. Fluorescence was measured every 10 min. A representative experiment performed in triplicate for CAF2 CM is displayed in (c). The relative mean fluorescence intensity ± s.d of two independent experiments using CAF1-5 CMs, measured at 8 hrs, is represented in (d). (A.U.: arbitrary units). Regular culture medium was used as background control. (e-g) PD-L1 and PD-L2 expression at the surface of normoxic or hypoxic CAFs was measured by flow cytometry (e-f). A representative flow cytometry histogram for CAF2 is shown in (e) and the percentage PD-L1+ CAF1-5 (mean ± s.d. of three independent experiments) in (f) (not shown for PD-L2). (g) PD-L1 expression measured by western blot for PD-L1High CAF2-4. P values (a-b, f) were determined by unpaired two-tailed student’s t-test. (NS: non-significant; *p < .05). N: Normoxia; H: Hypoxia
Hypoxia improves the CAF-dependent alteration of CTL-mediated lysis
As previously mentioned, several studies have pointed out that CAFs can regulate T cell activity and function in the TME by the secretion of several factors,20 including TGF-β, ARG or IDO. Thus, to validate the physiological relevance of our data pointing out improved immunosuppressive capabilities of hypoxic CAFs, we used T1 melanoma tumor cells and the autologous LT12 CTL clone in order to test the differential effect of normoxic versus hypoxic CAF CMs on T cell cytotoxic activity. LT12 CTLs were cultured during 16 hrs in the presence of either normoxic or hypoxic CAFs CM before testing their ability to kill their autologous tumor target cells (T1). As expected, normoxic CAF1-5 CMs decrease the lytic potential of LT12 CTL (Figure 6(a)). Importantly, hypoxic CAF1-5 CMs also decrease LT12 killing activity (Figure 6(b)), but to a greater level compared to normoxic CAF CMs-treated CTL (Figure 6(c)). Interestingly, these data correlate with an enhanced decrease of the cytotoxic serine protease granzyme B (GzmB) expression by LT12 CTL following hypoxic CAF1-5 CMs treatment, compare to normoxic CAF CMs (Figure 6(d-e)). Together, these data demonstrate that hypoxic CAFs possess an enhanced capability to alter CTL killing activity than normoxic CAFs.
Figure 6.

Hypoxia potentiates CAF-dependent alteration of CTL-mediated cytotoxicity (a-c) The lysis of T1 melanoma cell line by LT12 CTL clone, pre-treated with normoxic or hypoxic CAF1-5 conditioned medium (CM) during 16 hrs, was evaluated by 51Cr release assay at different effector:target (E:T) ratios. Regular culture medium was used as control. Data (a-b) are the mean ± s.d. from two independent experiments performed in triplicate. Experiments in (a-b) were performed at the same time but separated in two different panels. (c) represents the mean ± s.d. of all T cell-mediated lysis experiments from (a-b) using the normoxic or hypoxic CAF CMs pre-treatment of the LT12 CTL clone. (d-e) Granzyme B expression by LT12 CTL clone after 16 hrs incubation with normoxic or hypoxic CAF CMs, measured by flow cytometry. Regular culture medium was used as control. A representative flow cytometry histogram using CAF2 CM treatment is shown in (d) and the percentage GzmB+ LT12 T cell after treatment with hypoxic or normoxic CAF1-5 CMs (mean ± s.d. of three independent experiments) in (e). N: Normoxia; H: Hypoxia. P values (a-e) were determined by unpaired two-tailed student’s t-test. (*p < .05)
Discussion
Within the TME, the complex immunosuppressive network is still not fully decrypted but is of major importance for the understanding of the antitumor immune response global regulation and for the development of more integrated immunotherapeutic therapies. This immunosuppressive network is not only related to tumor cells or to the different individual components of the tumor stroma, but is also the consequence of the crosstalk between these different components. In this regard, solid tumors create a hostile hypoxic microenvironment which can interfere with the effectiveness of the immune response.
We sought to understand the influence of this metabolic component and to “mimic” in vitro a hypoxic TME in which are the CAFs. In particular, we focus our attention on the effect of hypoxia on the immunomodulatory capacities of melanoma-associated fibroblasts and on an eventual additional or synergetic effect between these two components of the TME. Here, we provide evidence that fibroblasts isolated from melanoma tumor biopsies respond to hypoxia by inducing HIF-1α (but not HIF-2) expression. Interestingly, using different tumor models, several studies have suggested that HIF-1 activation in fibroblasts participates to their acquisition of a CAF phenotype.52 Consistently, we observed in our model that HIF-1 induction in hypoxic CAFs correlates with a greater activation level compared to normoxia, as shown by the increased expression of activation markers FAP and α-SMA.
It is now well established that different soluble factors released by tumor cells in the TME locally activate fibroblasts which acquire phenotypic and functional properties different from their normal counterparts. In particular, when exposed to these stimuli, CAFs express and/or secrete different proteins involved in the modulation of the anti-tumor immune response. Hypoxia seems to participate and to improve the expression/secretion of these immunosuppressive factors by melanoma-associated fibroblasts. Indeed, the mRNA expression of most of the major proteins previously described as CAF-related important regulators of anti-tumor immunity (TGF-β, ARG, IDO, VEGF, CXCL12, Cox2, IL6, IL10, MMP2, MMP9, PD-L1, CD39, CD47 or FasL) is significantly increased under hypoxic conditions. Consequently, the increased secretion or membrane expression of VEGF, IL-6, IL-10, TGF-β, MMPs or PD-L1 by hypoxic CAFs were successfully validated in our model. Thus, hypoxia-dependent HIF-1 induction correlates with a high activation level of CAF and an improved immunosuppressive potential of these cells. Of note, some of the genes encoding these immunosuppressive factors have been described as direct target of HIF-1α transcription factor, including VEGF,53 TGF-β,54,55 IL-10,56 MMP-957 or PD-L1.32 Moreover, studies revealed that CD39 is transcriptionally induced by hypoxia through an SP1-dependent transcriptional pathway in cardiac ischemia models.58,59 ARG activity is also increased in MDSC by a HIF-1α-dependent miR210 induction mechanism,60 and hypoxia enhances IDO production in dendritic cells61 or MMP-2 expression by endothelial cells.62 Thus, hypoxia probably induces the expression of several immunosuppressive factors by CAF in a HIF-2-independent but HIF-1-dependent manner, even if this particular point clearly needs further investigations.
Of note, CCL2/5 or CD73 expression is not increased following hypoxic exposure of CAFs. This point is perhaps surprising since CD73, CCL2/ and CCL5 are known as two direct HIF-1α targets, but might reflect a particularly regulation of these proteins in the context of CAFs and melanoma. Moreover, it is also important to note that a degree of variability between the CAF populations used in our study has been observed regarding the expression level of some of these immunosuppressive factors. For example, ARG1 seems to be mostly expressed by hypoxic CAF1-2, while ARG2 expression is mostly increased in CAF4-5. This particular point might be the reflection of the heterogeneity of CAF population between patients, but might also be the reflection of the presence within the same tumor of distinct subsets of functional CAFs, with possible diverse functions, as suggested.63
Finally, we validated the improved immunosuppressive potential of hypoxic CAFs by studying their impact on T cell-mediated cytotoxicity. We observed that hypoxia potentiates the inhibitory effect of CAF CMs on CTL-mediated lysis of melanoma tumor cells. Thus, the increased secretion of one or several immunosuppressive factors by hypoxic CAFs is probably responsible for the observed effect. For example, hypoxia might potentially increase the alteration of cytotoxic CD8+ T cell function by increasing the CAF-dependent secretion of TGF-β, and consequently its negative effect on the expression of key genes involved in their cytotoxic activity, including perforin, or granzymes.64,65 As such, we observed that hypoxic CAFs possess a greater capability to decrease GzmB expression by LT12 CTL, compare to normoxic CAF. However, and even if this question is beyond the main point of this study and will require further validation, other factors than TGF-β and other targets than GzmB are probably involved in the enhanced alteration of T cell functionality triggered by hypoxic CAFs. For example, pancreatic cancer suffering patients with CAFs expressing high levels of Arg2 in HIF-1α+hypoxic zones, demonstrate a poor clinical outcome,66 which to our point of view might be directly related to the important inhibition of T cell functions with the TME. Furthermore, we believe that the hypoxic CAF secretome also influences through different mechanisms other immune cell populations within the TME, and potentiate their immune suppressive capabilities. As such, targeting hypoxia and hypoxic signaling,67 for example using HIF-1 inhibitors like Acriflavine or PX-478, might be a relevant approach to indirectly but broadly target CAF activation level with the TME and to decrease, at least partly, the hypoxic CAF secretion/expression of immunosuppressive factors. In other words, lowering the hypoxic CAF immunosuppressive secretome by targeting tumor hypoxia or for example HIF-1 expression by CAFs,68 may help to reduce immune effector cell dysfunctions as well as the recruitment of immunosuppressive cells in hypoxic zones, thus releasing the “brake” for a more effective immune response in combination with therapies targeting immune checkpoints or other mechanisms impairing the anti-tumor immune response in patients.
Supplementary Material
Acknowledgments
We thank A. Cavalcanti and C. Robert (Gustave Roussy, Villejuif, France) for the melanoma tumor biopsies and G. Favre (Cancer Research Center, Toulouse, France) for helpful discussion and technical advices.
Funding Statement
This work was supported by INSERM and the French “Ligue Nationale Contre Le Cancer” (LNCC-Equipe Labéllisée). L.Z was supported by a PhD training fellowship from the French “Ligue Nationale Contre Le Cancer” and by the “taxe d’apprentissage” program (Direction de la Recherche-Gustave Roussy).
Disclosure statement
The authors declare no conflict of interest.
Author contributions
L.Z. performed experiments and analyzed data. S.B. helped with some experiments. S.C. participates to helpful discussion and help to edit the manuscript. J.T. conceived and supervised the project, helped designing experiments, performed some experiments, analyzed data and wrote the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–13. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115. [DOI] [PubMed] [Google Scholar]
- 3.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. [DOI] [PubMed] [Google Scholar]
- 5.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. [DOI] [PubMed] [Google Scholar]
- 6.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, Carpenter AE, Jirstrom K, Magnusson K, Ebert BL, et al. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest. 2011;121:784–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valcz G, Sipos F, Tulassay Z, Molnar B, Yagi Y. Importance of carcinoma-associated fibroblast-derived proteins in clinical oncology. J Clin Pathol. 2014;67:1026–1031. [DOI] [PubMed] [Google Scholar]
- 11.Linares J, Marin-Jimenez JA, Badia-Ramentol J, Calon A. Determinants and functions of CAFs secretome during cancer progression and therapy. Front Cell Dev Biol. 2020;8:621070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziani L, Chouaib S, Thiery J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front Immunol. 2018;9:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteran L, Erez N. The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol. 2019;10:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mhaidly R, Mechta-Grigoriou F. Fibroblast heterogeneity in tumor micro-environment: role in immunosuppression and new therapies. Semin Immunol. 2020;48:101417. [DOI] [PubMed] [Google Scholar]
- 15.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–479. [DOI] [PubMed] [Google Scholar]
- 17.Kuen J, Darowski D, Kluge T, Majety M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS One. 2017;12:e0182039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33:2423–2431. [DOI] [PubMed] [Google Scholar]
- 19.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman P, Mielgo A. Cancer-associated fibroblast mediated inhibition of CD8+ cytotoxic T cell accumulation in tumours: mechanisms and therapeutic opportunities. Cancers (Basel). 2020;12:2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziani L, Safta-Saadoun TB, Gourbeix J, Cavalcanti A, Robert C, Favre G, Chouaib S, Thiery J. Melanoma-associated fibroblasts decrease tumor cell susceptibility to NK cell-mediated killing through matrix-metalloproteinases secretion. Oncotarget. 2017;8:19780–19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:20847–20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154–161. [DOI] [PubMed] [Google Scholar]
- 24.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. [DOI] [PubMed] [Google Scholar]
- 27.Terry S, Faouzi Zaarour R, Hassan Venkatesh G, Francis A, El-Sayed W, Buart S, Bravo P, Thiery J, Chouaib S. Role of hypoxic stress in regulating tumor immunogenicity, resistance and plasticity. Int J Mol Sci. 2018;19:3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis A, Venkatesh GH, Zaarour RF, Zeinelabdin NA, Nawafleh HH, Prasad P, Buart S, Terry S, Chouaib S. Tumor hypoxia: a key determinant of microenvironment hostility and a major checkpoint during the antitumor response. Crit Rev Immunol. 2018;38:505–524. [DOI] [PubMed] [Google Scholar]
- 29.Noman MZ, Hasmim M, Lequeux A, Xiao M, Duhem C, Chouaib S, Berchem G, Janji B. Improving cancer immunotherapy by targeting the hypoxic tumor microenvironment: new opportunities and challenges. Cells. 2019;8:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chouaib S. The antitumor cytotoxic response: if the killer cells play the music, the microenvironmental hypoxia plays the tune. Crit Rev Immunol. 2020;40:157–166. [DOI] [PubMed] [Google Scholar]
- 31.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, Chouaib S. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126:3672–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giaccia AJ, Schipani E. Role of carcinoma-associated fibroblasts and hypoxia in tumor progression. Curr Top Microbiol Immunol. 2010;345:31–45. [DOI] [PubMed] [Google Scholar]
- 35.Zhu G, Cao B, Liang X, Li L, Hao Y, Meng W, He C, Wang L, Li L. Small extracellular vesicles containing miR-192/215 mediate hypoxia-induced cancer-associated fibroblast development in head and neck squamous cell carcinoma. Cancer Lett. 2021;506:11–22. [DOI] [PubMed] [Google Scholar]
- 36.Tejchman A, Lamerant-Fayel N, Jacquinet JC, Bielawska-Pohl A, Mleczko-Sanecka K, Grillon C, Chouaib S, Ugorski M, Kieda C. Tumor hypoxia modulates podoplanin/CCL21 interactions in CCR7+ NK cell recruitment and CCR7+ tumor cell mobilization. Oncotarget. 2017;8:31876–31887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comito G, Giannoni E, Di Gennaro P, Segura CP, Gerlini G, Chiarugi P. Stromal fibroblasts synergize with hypoxic oxidative stress to enhance melanoma aggressiveness. Cancer Lett. 2012;324:31–41. [DOI] [PubMed] [Google Scholar]
- 38.Tang YA, Chen YF, Bao Y, Mahara S, Yatim S, Oguz G, Lee PL, Feng M, Cai Y, Tan EY, et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc Natl Acad Sci U S A. 2018;115:E5990–E5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kugeratski FG, Atkinson SJ, Neilson LJ, Lilla S, Knight JRP, Serneels J, Juin A, Ismail S, Bryant DM, Markert EK, et al. Hypoxic cancer-associated fibroblasts increase NCBP2-AS2/HIAR to promote endothelial sprouting through enhanced VEGF signaling. Sci Signal. 2019;12:eaan8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dufour E, Carcelain G, Gaudin C, Flament C, Avril MF, Faure F. Diversity of the cytotoxic melanoma-specific immune response: some CTL clones recognize autologous fresh tumor cells and not tumor cell lines. J Immunol. 1997;158:3787–3795. [PubMed] [Google Scholar]
- 41.Meslin F, Thiery J, Richon C, Jalil A, Chouaib S. Granzyme B-induced cell death involves induction of p53 tumor suppressor gene and its activation in tumor target cells. J Biol Chem. 2007;282:32991–32999. [DOI] [PubMed] [Google Scholar]
- 42.Ben Safta T, Ziani L, Favre L, Lamendour L, Gros G, Mami-Chouaib F, Martinvalet D, Chouaib S, Thiery J. Granzyme B-activated p53 interacts with Bcl-2 to promote cytotoxic lymphocyte-mediated apoptosis. J Immunol. 2015;194:418–428. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9:3515–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, Richardson M, Rigaill G, Parrini MC, Lucchesi C, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiavarina B, Whitaker-Menezes D, Migneco G, Martinez-Outschoorn UE, Pavlides S, Howell A, Tanowitz HB, Casimiro MC, Wang C, Pestell RG, et al. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: autophagy drives compartment-specific oncogenesis. Cell Cycle. 2010;9:3534–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren J, Guo H, Wu H, Tian T, Dong D, Zhang Y, Sui Y, Zhang Y, Zhao D, Wang S, et al. GPER in CAFs regulates hypoxia-driven breast cancer invasion in a CTGF-dependent manner. Oncol Rep. 2015;33:1929–1937. [DOI] [PubMed] [Google Scholar]
- 47.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inoue C, Miki Y, Saito R, Hata S, Abe J, Sato I, Okada Y, Sasano H. PD-L1 induction by cancer-associated fibroblast-derived factors in lung adenocarcinoma cells. Cancers (Basel). 2019;11:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshikawa K, Ishida M, Yanai H, Tsuta K, Sekimoto M, Sugie T. Prognostic significance of PD-L1-positive cancer-associated fibroblasts in patients with triple-negative breast cancer. BMC Cancer. 2021;21:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC, Raju GS, Reyes VE, Powell DW. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology. 2008;135:1228–1237, 1237 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat Commun. 2018;9:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–19260. [DOI] [PubMed] [Google Scholar]
- 54.Deng B, Zhu JM, Wang Y, Liu TT, Ding YB, Xiao WM, Lu GT, Bo P, Shen XZ. Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-beta1 in gastric cancer. PLoS One. 2013;8:e63777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hung SP, Yang MH, Tseng KF, Lee OK. Hypoxia-induced secretion of TGF-beta1 in mesenchymal stem cell promotes breast cancer cell progression. Cell Transplant. 2013;22:1869–1882. [DOI] [PubMed] [Google Scholar]
- 56.Meng X, Grotsch B, Luo Y, Knaup KX, Wiesener MS, Chen XX, Jantsch J, Fillatreau S, Schett G, Bozec A. Hypoxia-inducible factor-1alpha is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat Commun. 2018;9:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai H, Ma Y, Jiang L, Mu Z, Jiang Z, Chen X, Wang Y, Yang GY, Zhang Z. Hypoxia response element-regulated MMP-9 promotes neurological recovery via glial scar degradation and angiogenesis in delayed stroke. Mol Ther. 2017;25:1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poth JM, Brodsky K, Ehrentraut H, Grenz A, Eltzschig HK. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J Mol Med (Berl). 2013;91:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noman MZ, Janji B, Hu S, Wu JC, Martelli F, Bronte V, Chouaib S. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res. 2015;75:3771–3787. [DOI] [PubMed] [Google Scholar]
- 61.Song X, Zhang Y, Zhang L, Song W, Shi L. Hypoxia enhances indoleamine 2,3-dioxygenase production in dendritic cells. Oncotarget. 2018;9:11572–11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-Yosef Y, Miller A, Shapiro S, Lahat N. Hypoxia of endothelial cells leads to MMP-2-dependent survival and death. Am J Physiol Cell Physiol. 2005;289:C1321–31. [DOI] [PubMed] [Google Scholar]
- 63.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. 2014;4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmadzadeh M, Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215–5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. [DOI] [PubMed] [Google Scholar]
- 66.Ino Y, Yamazaki-Itoh R, Oguro S, Shimada K, Kosuge T, Zavada J, Kanai Y, Hiraoka N. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One. 2013;8:e55146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–169. [DOI] [PubMed] [Google Scholar]
- 68.Shao S, Zhao L, An G, Zhang L, Jing X, Luo M, Li W, Meng D, Ning Q, Zhao X, et al. Metformin suppresses HIF-1alpha expression in cancer-associated fibroblasts to prevent tumor-stromal cross talk in breast cancer. FASEB J. 2020;34:10860–10870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


