Abstract
Purpose
This study aims to evaluate the beneficial effects of anti-epileptic mechanisms of baicalin (BA) on cognitive dysfunction and neurodegeneration in pentylenetetrazol (PTZ)-induced epileptic rats.
Methods
First, PTZ-induced epileptic rats were administered intraperitoneally a sub-convulsive dose of PTZ (40 mg/kg) daily, and the seizure susceptibility (the degree of seizures and latency) was evaluated using Racine’s criterion. Then, classical behavioral experiments were performed to test whether BA ameliorated cognitive dysfunction. Neurodegeneration was assessed using Fluoro Jade-B (FJB), and NeuN staining was used to determine whether BA offered a neuroprotective role. After BA had been proven to possess anti-epileptic effects, its possible mechanisms were analyzed through network pharmacology. Finally, the key targets for predictive mechanisms were experimentally verified.
Results
The epileptic model was successfully established, and BA had anti-epileptic effects. Epileptic rats displayed significant cognitive dysfunction, and BA markedly ameliorated cognitive dysfunction. Further, we also discovered that BA treatment mitigated neurodegeneration of the hippocampus CA3 regions, thereby ameliorated cognitive dysfunction of epileptic rats. Subsequent network pharmacology analysis was implemented to reveal a possible mechanism of BA in the anti-epileptic process and the TLR4/MYD88/Caspase-3 pathway was predicted. Finally, experimental studies showed that BA exerted an anti-epileptic effect by activating the TLR4/MYD88/Caspase-3 pathway in PTZ-induced epileptic rats.
Conclusion
In conclusion, BA had a protective effect against PTZ-induced seizures. BA improved cognitive dysfunction and exerted a neuroprotective action. The anti-epileptic effects of BA may be potentially through activation of the TLR4/MYD88/Caspase-3 pathway.
Keywords: baicalin, pentylenetetrazol, epilepsy, cognitive dysfunction, network pharmacology, TLR4/MYD88/Caspase-3
Introduction
Epilepsy, a global public health problem, is mainly characterized by sudden sensory, motive, psychic, or autonomic symptoms.1 Psychiatric and behavioural disorders are highly prevalent among people with epilepsy, such as depression, cognitive dysfunction, and anxiety.2 Such unprovoked seizures affect 1% of the global population and all ages.3 At present, drug therapy is the first choice to treat this disease. Patients with epilepsy often required lifelong medication, but some patients are resistant to currently available anti-epileptic drugs.4 Moreover, plenty of clinical reports imply that anti-epileptic drugs have specific side effects, such as teratogenicity, glaucoma, agranulocytosis, and paraesthesia.5 Therefore, exploring safe and effective anti-epileptic methods has become a medical research hotspot.
Epilepsy is a complex disease characterized by multiple clinical features that cannot be explained by a single pathogenic mechanism.6 Diverse studies have confirmed that the pathological features are abnormal discharge of nerve cells, loss of neurons, activation of glial cells, and the eventual occurrence of convulsions, but its entire pathology remains unknown.7 Therefore, establishing a suitable epilepsy model is crucial for epilepsy research. The kindling model, a method of studying the mechanism of epilepsy and evaluating the efficacy of new anti-epileptic drugs, is a process of inducing gradual intensity of convulsive activity as a result of repetitive administration of chemical or electrical sub-convulsive stimulation. Pentylenetetrazol (PTZ) is a selective antagonist of the GABAA receptor.8 The PTZ-induced kindling rat model is a conventional epileptic model with recurrent features and typical pathophysiology.9 This model is a gold standard to study epilepsy and evaluate epileptic drugs.
Pharmacology is an emerging discipline that focuses on drug design based on the theory of biology, backed up with a Bioinformatics database.10,11 It reveals the mystery of multi-molecular drugs in the cooperative treatment against diseases by integrating abundant pharmacokinetics assessment, target prediction, pathway enrichment, and network pharmacology analysis.12 In addition, pharmacology unifies with the Traditional Chinese Medicine (TCM) syndrome, emphasizing the etiologic mechanisms. Network pharmacology provides a workable and powerful tool for elucidating the complicated interrelationships among compounds, targets, pathways, and diseases.13
Baicalin (BA) is a type of flavonoid compound isolated from the dry root of Scutellaria baicalensis Georgi, with continuous in-depth research, it has been attracting broader attention in the treatment of dozens of diseases, such as neurodegenerative disorders, gastrointestinal infections, cardio-cerebrovascular diseases, cancer, and inflammation.14,15 No side effects are reported at a conventional dose of BA.16 However, BA is removed quickly from the body and does not easily reach the brain.17 BA also has a low aqueous solubility.18 The neuroprotective effect of BA could be dependent on the continuous presence of the drug.19,20
In this study, we evaluated the anti-epileptic potential of continuous administration of BA in a PTZ-induced epileptic model and investigated whether the repeated seizures were associated with kindling induce cognitive function and BA ameliorated cognitive dysfunction. Furthermore, to continue exploring and explaining the mechanism of BA in epilepsy, this study used network pharmacology analysis, gene ontology (GO) analysis, and Kyoto encyclopedia of genes, and genomes (KEGG) pathway to predict underlying molecular mechanisms of the anti-epileptic potential of BA. Finally, the key targets were verified in the epileptic model. Information grasped in the current study will assist in developing BA as adjuvant therapy for epilepsy.
Materials and Methods
Materials
BA (purity ≥ 98%, batch number: K198628) was received from the Xian YunYue Company (Xian, China) as a therapeutic drug. Valproate tablets (VPA) were purchased from Hunan Xiang Zhong Pharmaceutical Company (Hunan, China) as a controlled drug. Pentylenetetrazol (PTZ) was obtained from Sigma Chemical Company (St. Louis, MO, USA) to induce seizures. All other analytical reagents were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Animals
To avoid the effects of estrogen on epilepsy, only male rats were used in this experimental research. Male Sprague Dawley rats (4 weeks old, 100 ± 20 g) were purchased from the Animal Experiment Center at Hebei Medical University (certificate number: SCXK(JI) 2018–004, Hebei, China). Two rats were housed per cage in a pathogen-free room (22 ± 2°C) under a 12-h light/dark cycle with free access to food and water. All rats were habituated for 7 days to adapt to the cage environment before the experiment. The study was approved by Hebei Key Laboratory of Turbidity, Hebei Academy of Chinese Medicine Sciences. The rats’ study protocol was conducted following the National Institute of Health Guide for the Care and Use of Laboratory Animals. 56 rats were randomly divided into two groups (8 for the control group and 48 for the epileptic group). All efforts were made to decrease the number of animals used and their suffering.
PTZ Kindling
Figure 1A shows the animal experimental procedure. The model for the kindling procedure followed a previous method with minor modifications.21 Briefly, the model group was intraperitoneally injected (i.p.) with a dose of the subcontinent (40 mg/kg) of PTZ, and the control group was administered an equal volume of saline for 28 days. All rats were weighed before each injection and monitored for signs of seizure over 30 min after each PTZ treatment. According to Racine’s criterion (Table 1), stage 4 or 5 limbic motor seizures or higher on the Racine scale for 3 consecutive days indicated the successful ignition of the model.22 If there were a stage 5 seizure without remission that could intervene in death caused by convulsive symptoms during epileptic seizures in epileptic rats, an immediate injection of 10% chloral hydrate (5 mL/kg) should be used for sedation and anticonvulsion.21,23 Animals were excluded from the study if they did not kindle (animals score less than stage 4 according to the Racine) after 28 days.24,25
Figure 1.
(A) Representative diagram for the experimental process. Epilepsy was induced by intraperitoneal injection of PTZ for 28 days. The model was maintained once per week for the next 21 days. The drug administration was began on day 29 and continued for 21 days to assess the effect of BA on epilepsy. The MWM and NORT were performed to investigate the effects of BA on cognitive dysfunction. Then further verified the results of network pharmacology. (B) Representative diagram for the experiment group.
Table 1.
Racine’s Criterion
| Racine Score | Behavioural Characteristics |
|---|---|
| Stage 0 | No obvious abnormal behavior |
| Stage 1 | Facial activity, ear and whisker twitching |
| Stage 2 | Facial clonus or head nodding |
| Stage 3 | Unilateral limb spasm |
| Stage 4 | Bilateral limb spasm |
| Stage 5 | Tonic-clonic seizure |
Groups
Figure 1B shows the groups. The epileptic rats that met the Racine’ criteria were randomly divided into four groups: (1) epileptic rats treated with normal saline (NS) (Epilepsy group, n = 11), (2) epileptic rats treated with VPA (300 mg/kg/day, VPA group, n = 8), (3) epileptic rats treated with BA low-dose (50 mg/kg/day, L-BA group, n = 8), and (4) epileptic rats treated with BA high-dose (100 mg/kg/day, H-BA group, n = 8). The control group and epilepsy group were given NS in the same volume.26,27 The dose of BA depends on the previous report.28 The treatments started from day 28 till 49 and were given between 9:00 and 11:00 hours once daily via gastric gavage. During the treatment, the model was maintained once every 5 days and continued up to day 21.
On day 49, 24h after the final drug administration, all rats underwent Morris water maze test to assess spatial learning and memory for 5 days. On day 55, all rats underwent the novel object recognition task to assess object learning and memory. On day 56, all rats were anesthetized intraperitoneally with 2% sodium pentobarbital. 5 rats from each group were subjected to transcardiac perfusion, and their cerebral hemispheres were removed and fixed in 4% paraformaldehyde solution. The remaining 3 rats were decapitated, and their hippocampus was removed and stored in liquid nitrogen for further analysis.
Morris Water Maze Test
The Morris water maze test (MWM) was performed following the previous method.29 In a word, the MWM test was composed of a black circular pool (diameter 62 cm, height 43 cm) filled with water (depth 10 cm, temperature 21 ± 1 °C) and a circular platform (diameter 10 cm) for animals to escape.30 The pool was divided into four quadrants with different marks: quadrants W, N, E, and S. All rats were subjected to four training sessions per day for 4 consecutive days, with the interval between trials was 5 min. Each trial lasted for 60 s to find the circular platform. If the subjects did not find the platform within 60 s, the researchers could manually lead them to the platform to remain for 20 s. On day 5, the circular platform was removed to perform the probe test. Each rat was allowed to swim for 60 s. The trial began with the same quadrant to assess the exploration time in the target area and other quadrants.
The Novel Object Recognition Task
In the process of the novel object recognition task (NORT), we used a 60×60 × 40 cm wooden box.31 Two identical objects were placed in two corners of the box so that the tip of the rat’s nose was able to explore the two objects. One of the initial objects was removed after 60 min and replaced with a new object in its position. All rats were monitored by cameras mounted 5 m above the wooden box, and objects were thoroughly cleaned with 70% ethanol between trials. NORT was exploited for assessing recognition memory. A recognition index (RI) was calculated for a novel object, defined as the amount of time spent to explore a novel object over the total time spent exploring both objects. RI = (Novel Time/(Novel Time + Familiar Time) × 100).
Fluoro-Jade B Staining
The rats were anesthetized intraperitoneally with 2% sodium pentobarbital, and brain tissues were removed on ice and then placed in an EP tube filled with 4% paraformaldehyde for 24 h. Tissues were embedded using paraffin and cut into 4-μm-thick sections. Floating slides (n = 3 animals per group, six sections per animal), collected in 0.01 M phosphate buffer saline (PBS), were mounted on gelatin-coated slides and dried at room temperature overnight.32 Brain sections were washed two times for 15 min in xylene. After immersing two times for 5 min in absolute ethanol, 5 min in 85% ethanol, and 5 min in 75% ethanol and distilled water, they were transferred to a solution of 50% glacial acetic acid and a 0.0004% FJB staining solution. Finally, a fluorescence microscope was used to collect an image.
Immunofluorescent Staining of NeuN
The perfused brain tissues were soaked in 4% paraformaldehyde for at least 24 h, and tissues were embedded using paraffin and cut into 4-μm-thick sections (n = 3 animals per group, 6 sections per animal). After conventional dewaxing and hydration, tissue sections were stained with Immunofluorescent staining of NeuN.33 The tissue sections were placed in a box filled with citric acid antigenic repair buffer (pH = 6.0) for antigen retrieval. After natural cooling, the samples were washed thrice with PBS, for 3 min, dripped and sealed with goat serum for 30 min. Then, the primary antibody of rabbit anti-NeuN (Servicebio, Wuhan, China; Catalog: GB11138, diluted at 1:500) was added to the sections and incubated overnight at 4°C. After washing with PBS, secondary goat anti-rabbit antibody (Servicebio, Wuhan, China; Catalog: GB21303, diluted at 1:300) were incubated for 50 min. Finally, the sections were stained with diamidinophenylindole (DAPI) and examined under a microscope.
Assessment of Absorption, Distribution, Metabolism, and Excretion Properties of BA
The absorption, distribution, metabolism, and excretion (ADME) properties of BA were acquired from the TCMSP database.34 Which was a pharmacology platform designed for TCM or related ingredients.35 Oral bioavailability (OB) and drug-likeness (DL) were used to identify their potential bioactive compounds of BA, OB and DL were set to ≥ 30% and ≥ 0.18.
Predicting Functional Targets of BA and Mining Known Epilepsy-Related Genes
The 2D molecular structure of BA was collected from PubChem (https://pubchem.ncbi.nlm.nih.gov/).36 The compound targets were screened by Similarity Ensemble Approach (SEA) (http://sea.bkslab.org/).37 The Comparative Toxicogenomics Database (CTD) (http://ctdbase.org/about/).38 STITCH Database (http://stitch.embl.de/).39 HERB (http://herb.ac.cn/) and TargetNet (http://targetnet.scbdd.com).40,41
Epileptic targets were predicted and gathered using the GeneCards database (http://www.genecards.org).42 DrukBand platform (https://www.drugbank.ca/).43 Therapeutic Target Database (TTD, https://db.idrblab.org/ttd/) and a Database of Gene-Disease Associations (DisGeNET, http://www.disgenet.org/).44,45 Venny (http://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to build the Venn diagram for common targets.
The above common target proteins were used to construct the protein-protein interaction (PPI) network model via the Search Tool for the Retrieval of Interacting Genes (STRING) (http://string-db.org/). Cytoscape 3.7.1 was used to construct networks.46 The top 10 core PPI networks were obtained by analyzing the corresponding networks by MCODE, a Cytoscape plugin.47
GO and KEGG Pathway Analyses
Metascape (http://metascape.org/) was used for GO and KEGG pathway analyses.48 GO enrichment analysis was exploited to explain and annotate genes by three aspects, including cellular component (CC), molecular function (MF), and biological process (BP) analyses. The KEGG database was utilized for pathway analysis. Apart from this, enriched GO terms and relevant pathways with P-values < 0.05 were selected for better prediction and verification of the biological process and mechanism. More detailed pathway analysis data are provided in Supplementary Table S1 .
Immunohistochemistry
Tissues were embedded using paraffin and cut into 4-μm-thick sections (n = 3 animals per group, 6 sections per animal). Sections were deparaffinized and rehydrated, then placed in a box filled with citric acid antigenic repair buffer (pH 6.0) in a microwave oven for antigen retrieval. Later, they were incubated with 3% H2O2 for 10 min at room temperature. After blocking with 5% BSA for 30 min, the tissue sections were incubated with primary antibody anti-Caspase1 (ABclonal, Wuhan, China; Catalog: A0964, diluted at 1:200), anti-Caspase3 (Servicebio, Wuhan, China; Catalog: GB11009-1, diluted at 1:200), anti-TLR4 (Bioss, Beijing, China; Catalogue: bs-20594R, diluted at 1:200), anti-MYD88 (Bioss, Beijing, China; Catalogue: bs-1047R, diluted at 1:200), anti-BAX (ABclonal, Wuhan, China; Catalog: A0207, diluted at 1:500), and anti-BCL-2 (ABclonal, Wuhan, China; Catalog: A0208, diluted at 1:500). The secondary antibody was incubated at room temperature. After PBS washing, the sections were monitored and captured under a microscope.
Enzyme-Linked Immunosorbent Assay
Serum samples were separated from whole blood using high-speed centrifugation for a minimum of 3 rats per group. The content of inflammatory cytokines IL-1β (Multi Sciences, Hangzhou, China; Catalog: EK301B/3-02) and IL-6 (Multi Sciences, Hangzhou, China; Catalog: EK306/3-01) was measured using their respective enzyme-linked immunosorbent assay enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s protocol.
Western Blotting
Western blotting analysis was performed by proteins extracted from brain tissues (n = 3 rats per group). Briefly, the hippocampus tissues were crushed and frozen at −80°C in a mortar. Radioimmunoprecipitation assay, phenylmethylsulfonyl fluoride, and a small amount of liquid nitrogen were added and ground into a uniform liquid state. The sample was transferred to an Eppendorf tube, incubated on ice for 15 min, and centrifuged by a cold centrifuge. Then, the target protein was separated and stored in the refrigerator at −80°C.49 According to the protein concentration result, 5 × loading buffer was added to the total protein sample, denatured at 95°C for 10 min and stored on ice. During electrophoresis, the protein sample was added to the well, the power was turned on, the voltage adjusted to 80v for approximately 25 min, then increased to 120 v. After gel electrophoresis, the protein bands separated on the gel were transferred to Polyvinylidene fluoride (PVDF) membrane, and poly cloned with anti-Caspase1 (ABclonal, Wuhan, China; Catalogue: A0964, diluted at 1:200). The PVDF membrane was incubated with a horseradish peroxidase-labeled secondary antibody. Finally, the bands were visualized by enhanced chemiluminescence. Image J software was used to analyze the intensity of the bands.
Statistical Analysis
The data were expressed as the standard error of the mean. One-way analysis of variance was exploited for multiple-group statistical analyses. The Student Newman Keuls was used to analyze the equal variance data. SPSS Version 22.0 (SPSS Inc, Chicago, IL, USA) was used for the analyses. P < 0.05 is considered statistically significant.
Results
The General Condition of PTZ-Induced Epileptic Rats
In this section, the PTZ-induced epileptic rats’ behavior was discussed. During the experimental process, 42 of 48 epileptic rats (87.5%) developed epileptic status. 3 of 42 epileptic rats (7.1%) died due to severe generalized clonic tonic seizures, and 4 rats were withdrawn from the experiment (10.25%) due to intraperitoneal injection. During the absence of epileptic seizures, most epileptic rats showed general aggressiveness and irritability. The rat’s body weight was measured during PTZ intraperitoneal injection. No significant changes were noted in the bodyweight of different groups during the ignition, and no significant changes were found among the groups during medication.
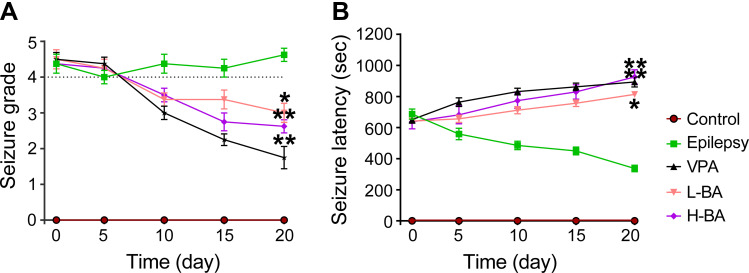
Effects of BA on PTZ-Induced Seizure Susceptibility
Figure 2 shows the effect of BA on the seizure susceptibility of rats. The seizure stage score (stage 4 or greater) and seizure latency (interval from PTZ injection to first seizure) were used to assess seizure susceptibility. A continuous PTZ administration gradually increased the susceptibility of rats to seizures. On the 50th day, 3 animals in the epilepsy group died because of serious generalized seizures. However, no dead animals in other groups. Compared with the epilepsy group, treatment with BA and VPA reduced the seizure stage score and extended seizure latency on the 20th day. Importantly, a significantly longer, but not statistically significant seizure latency for the BA (100 mg/kg) was recorded compared to the VPA group, indicating that BA exhibited anti-epileptic activity in PTZ kindled rats.
Figure 2.
Effects of BA on PTZ-induced seizures. (A) Seizure grade. (B) Seizure latency. The values were presented as the mean ± SEM. (n = 8. * P < 0.05, ** P < 0.01 vs epilepsy group).
Effects of BA on Spatial Learning and Memory
Figure 3 shows the effects of BA on spatial learning and memory, spatial learning, and animals’ memory were evaluated by MWM (Figure 3A). All animals were tested to adapt to the water, and there was no significant difference in swimming speed during the 120 s of habituation in the pool (P > 0.05, Figure 3B), which could eliminate the influence of sensorimotor ability on the results. In the place navigation trial, the escape latency of each group gradually decreased during the 5 days of training (Figure 3C). Compared with the control group, the epilepsy group exhibited significantly longer escape latency (P < 0.01, Figure 3C). However, compared to the epilepsy group, VPA and BA (100 mg/kg) treatment significantly decreased escape latency (P < 0.05 and P < 0.01, respectively). Interestingly, escape latency did not differ significantly between the H-BA and VPA groups (P > 0.05). In the spatial probe test, compared with the control group, crossing-platform time was significantly reduced by PTZ exposure (P < 0.01, Figure 3D), and VPA or BA effectively increased platform crossing (P < 0.05 and P < 0.01, respectively). Notably, no substantial difference between the H-BA and control groups was found (P > 0.05).
Figure 3.
Effects of BA on spatial learning and memory. (A) Diagrams of MWM. (B) Averages swimming speed in target quadrant. (C) The escape latency time. (D) Platform crosses in target quadrant. The values were presented as the mean ± SEM. (n = 8. ## P < 0.01 vs control group; * P < 0.05, ** P < 0.01 vs epilepsy group).
Effects of BA on Object Learning and Memory
Figure 4 shows the effects of BA on object learning and memory. To further probe cognitive ability, NORT was performed following the MWM trial (Figure 4A). Compared with the control group, the resistive index (RI) was significantly reduced based on the PTZ toxicity (P < 0.01, Figure 4C). Compared to the epilepsy group, the BA-treated groups demonstrated a significant increase of RI in a dose-dependent manner (P < 0.05). All study groups showed similar motive ability in the MWM trial, so we tested the movement of all rats again in the NORT, which was shown by the total time of exploring (Figure 4B). Interestingly, the PTZ group decreased total time to explore objects compared to the control group (P < 0.01) and the administration of VPA or BA induced a significant reversal of this effect (P < 0.05). Unexpectedly, no substantial difference between the H-BA and L-BA groups was found.
Figure 4.
Effects of BA on object learning and memory. (A) Diagrams of NORT. (B) Total time of exploring. (C) The percentage of NORT recognition index. The values were presented as the mean ± SEM. (n = 8. ## P < 0.01 vs control group; * P < 0.05, ** P < 0.01 vs epilepsy group).
Effect of BA on Neurodegeneration in the Hippocampal CA3
Figure 5A shows the effect of BA on the expression of degenerating neurons in the hippocampal subfield CA3. FJB staining was evaluated to test whether BA treatment shows neuroprotective effects. The epilepsy group exhibited a significant increase (P < 0.01, Figure 5C) in the number of neuronal injuries compared to the control group. In comparison with the epilepsy group, the number of neuronal injuries was significantly low after the administration of VPA (P < 0.01) or BA (P < 0.01) treatment distinctly prevented this reduction. Also, neuronal injury in the H-BA group was slightly higher than in the L-BA group. However, the difference was not significant.
Figure 5.
Effects of BA on the expression of FJB and NeuN. (A) Expression of degenerating neurons (green) in the CA3 was determined by immunofluorescent staining. (B) Expression of NeuN (green) in the CA3 was determined by immunofluorescent staining. Nuclei were stained with DAPI (blue). Scale bars = 100 μm (200 ×) and scale bars for magnified = 50 μm (400 ×). White arrows in photomicrographs indicated the positive expression. (C and D) The quantitative analysis of positive expression was determined in five groups. The values were presented as the mean ± SEM. (n = 3, ## P < 0.01 vs control group; * P < 0.05, ** P < 0.01 vs epilepsy group).
Figure 5B shows the effect of BA on the expression of surviving neurons in the hippocampus CA3. No NeuN-positive neurons were found in the epilepsy group when compared with the control group (P < 0.01, Figure 5D). Compared with the epilepsy group, the expression levels of NeuN-positive cells in the VPA group and H-BA group were significantly increased (P < 0.01). However, no significant differences were noted between the VPA group and the H-BA group. NeuN-positive cell expression levels in the L-BA group were not significantly different from the epilepsy group (P > 0.05).
ADME Properties of BA
For drug screening and evaluation, Table 2 shows the ADME properties of BA, which involved 12 main key points, like DL and BBB, for drug screening and evaluation. Significantly, OB was the primary feature of oral medications because it played a critical part in assessing the effectiveness of drug distribution for systemic circulation. DL was a criterion for evaluating drug similarity. BA had a relatively small molecular weight and moderate blood-brain barrier permeability with a BBB value of −1.74.
Table 2.
Pharmacological and Molecular Properties of BA by TCMSP
| Name | MW | AlogP | Hdom | Hacc | OB(%) | Caco-2 | BBB | DL | TPSA |
|---|---|---|---|---|---|---|---|---|---|
| Baicalin | 446.39 | 0.64 | 6 | 11 | 40.12 | −0.85 | −1.74 | 0.75 | 187.12 |
The TLR4/MYD88/Caspase-3 Signaling Pathway Was Predicted by Network Pharmacology
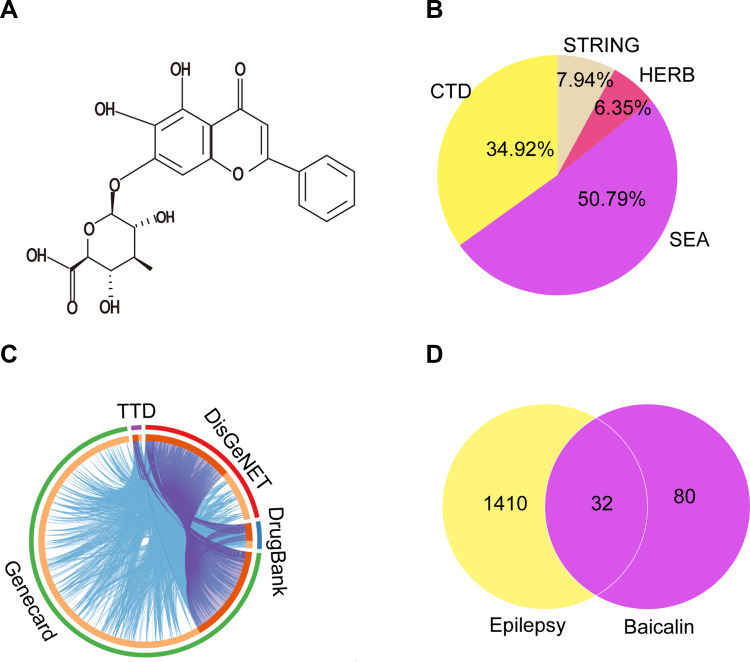
Figure 6 shows the network pharmacology of BA for epilepsy. The molecular formula of BA was C21H18O11, and the molecule structure is shown in Figure 6A. A total of 112 targets of BA were obtained by CTD, SEA, HERB, and STRING. Bioinformatics (http://www.bioinformatics.com.cn/) was exploited to show the distribution of potential targets (Figure 6B).
Figure 6.
The collective targets of BA and epilepsy were identified. (A) Chemical structure formula of BA. (B) Distribution of potential targets of BA. (C) Distribution of potential targets of epilepsy. (D) The Venn diagram of 32 overlapping gene symbols between the epilepsy and BA.
A total of 1442 epilepsy-related targets were obtained from the databases. Among them, DrugBank returned 68 potential human targets; GeneCard returned 1253 potential human targets; TTD returned 35 potential human targets, and DisGeNET returned 282 potential human targets. These epilepsy-related genes with relevance scores > 4.45 were used for subsequent biological network construction and network analysis. Metascape was used to show the distribution of potential targets (Figure 6C).
Combining targets of BA with epilepsy-related targets, all 32 targets of BA were commonly found to be epilepsy-related targets (Figure 6D). A BA-epilepsy-related network was constructed (Figure 7A). The PPI network was constructed, and the top 10 core genes were screened: TP53, MAPK1, VEGFA, AKT1, TNF, TLR4, PTGS2, CASP3, IL-6, and IL-1β (Figure 7B).
Figure 7.
(A) Protein-Protein Interaction (PPI) network analyze. The color and depth of the nodes (blue→ yellow→ orange) represent the size of the degree value, and the thickness of the line reflected the size of betweenness centrality. (B) The top 10 targets of PPI network analysis were TP53, MAPK1, VEGFA, AKT1, TNF, TLR4, PTGS2, CASP3, IL-6 and IL-1β (Oval represents the targets, color from red to yellow indicated decreasing importance).
Figure 8 shows the results of GO term enrichment and KEGG pathway analyses. To elucidate the function and pharmacological mechanism of BA, we conducted GO enrichment and KEGG pathway analyses of the 32 determined targets. GO analysis was determined by the biological process (BP), cell component (CC), and molecular function (MF) terms. The enrichment data were made into the bar diagram (Figure 8A). The top 20 significant pathways, correlated with the targets of BA for epilepsy, were Hepatitis B, human cytomegalovirus infection, Tuberculosis signaling pathway, Salmonella infection, and other major signaling pathways were closely related to the targets of BA for epilepsy (Figure 8B). Based on the enriched pathway of Tuberculosis, and combine the top 10 core genes, the key targets of the TLR4/MYD88/Caspase-3 pathway were obtained through further screening in the epilepsy mechanism. The mechanism for the protection of BA against epilepsy.
Figure 8.
GO analysis and KEGG analysis for the major targets of BA against epilepsy. (A) GO enrichment analysis of target proteins. The number of GO entries in the functional categories of cell biological process (BP), composition (CC) and molecular function (MF), (P < 0.05). The green, orange, and purple color rectangles represent BP, CC, MF respectively. (B) The top 20 KEGG pathways (P < 0.05). The colour dots indicate the different thresholds for the P-values and the sizes of the dots represent the number of genes corresponding to each term.
Effects of BA by Activating TLR4/MYD88/Caspase-3 Signaling Pathway
Figure 9 displays the effect of BA on the expression of key proteins in the TLR4/MYD88/Caspase-3 signaling pathway by using immunohistochemistry. Compared to the control group, the expressions of Bax appreciably increased in the epilepsy group (P < 0.01, Figure 9B), while Bcl-2 expression was markedly reduced in the epilepsy group (P < 0.01, Figure 9C). However, after treatment with BA, Bax was appreciably down-regulated (P < 0.05). Meanwhile, Bcl-2 expression was increased in the VPA and H-BA groups (P < 0.01) compared with the epilepsy group. Furthermore, as illustrated by immunohistochemistry, the hippocampus experienced in the epilepsy group contained an up-regulation of TLR4, MYD88 and Caspase-3 compared with the control group counterparts (P < 0.01, Figure 9E–G, relatively). Nevertheless, the expression of three proteins in VPA and BA groups was markedly decreased versus epilepsy group.
Figure 9.
Effects of BA on the expressions of BAX, Bcl-2, Caspase-3, TLR4 and MYD88. (A and D) Expression of BAX, Bcl-2, Caspase-3, TLR4 and MYD88 in the CA3 was determined by immunohistochemistry of the five groups. (B, C, G and E, F) The quantitative analysis of positive expression was determined in five groups. Scale bar = 100 µm (200×). Black arrows in photomicrographs indicated the positive expression. The values were presented as the mean ± SEM. (n = 3. ## P < 0.01 vs control group; * P < 0.05, ** P < 0.01 vs epilepsy group).
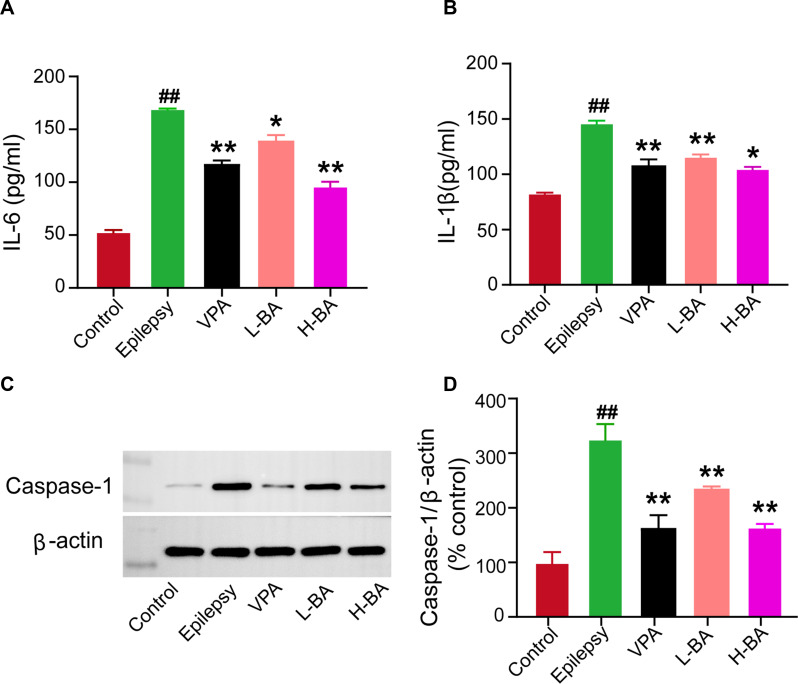
Effects of BA on the Expression of Inflammatory Cytokines in the Hippocampus
Figure 10 shows the effect of BA on the expression of inflammatory cytokines using ELISA in the hippocampus. The IL-1β and IL-6 levels in the hippocampus were elevated in the epilepsy group compared to the control group (P < 0.05, Figure 10A and B). After BA administration, the levels of IL-1β and IL-6 markedly decreased (P < 0.01). Moreover, the H-BA group had significantly reduced the expression of IL-1β compared to the epilepsy group (P < 0.05).
Figure 10.
Effects of BA on the expressions of IL-6, IL-1β, and Caspase-1. (A) Expression of IL-6 in the hippocampus was determined by ELISA of the five groups. (B) Expression of IL-1β in the hippocampus was determined by ELISA of the five groups. (C and D) Representative protein bands and relative protein expressions of Caspase-1 in the hippocampus were determined by Western blotting of the five groups. The band intensities were analyzed by normalization to β-actin. The values were presented as the mean ± SEM. (n = 3. ## P < 0.01 vs control group; * P < 0.05, ** P < 0.01 vs epilepsy group).
Effects of BA on the Expression of Caspase-1 Protein in the Hippocampus
The Caspase-1 protein expression level in the hippocampus was determined to assess the effect of BA against epileptic seizures. Compared with the control group, the Caspase-1 expression level in the epilepsy group was significantly increased (Figure 10C). Compared with the epilepsy group, Caspase-1 expression levels in the VPA group and H-BA group were significantly decreased (P < 0.01). At the same time, the Caspase-1 expression level in the H-BA group was similar to the control group (P > 0.05, Figure 10D).
Discussion
Our findings in the current research demonstrate that sub-convulsive doses of PTZ were first administered, which did not cause seizures. After multiple administration of PTZ, epileptogenic fitness was developed via intraperitoneal injection at 40 mg/kg, and BA treatment reduced the severity of epilepsy at various doses in a progressive manner from day 10 to day 20. These results were consistent with previous study in vivo.28 And these effects were translated into seizure latency and Racine score levels (Figure 2). In this study, BA in its highest dose (100 mg/kg) was able to effectively alleviate epilepsy. Hence, from the findings and the supporting data, BA is a hypothetical compound for anti-epilepsy.
An epileptic seizure is closely linked to socio-economic, psychological, and behavioural changes due to abnormal electrical activity in the brain.50 Cognitive dysfunction is one of the epilepsy complications. These problems are correlated with the disease seizures, neuropathology, and drug side effects. Multiple cognitive domains in these patients could be widely affected, which is becoming a major health threat and bringing a heavy economic burden on society and families.51,52 In recent years, improving cognitive dysfunction is a crucial factor that must be considered when treating epilepsy.53 Therefore, to verify whether BA could improve cognitive impairment in the treatment of epilepsy, MWM and NORT are common behavioural paradigms used to evaluate spatial learning and object cognition in rodents, and the order of different tasks will not have an impact particularly if cognitive tasks are involved.54 We were pleasantly surprised to find that the prominent comorbidity of learning and memory impairment in epilepsy was evidenced by MWM and NORT of epileptic rats, and BA could improve spatial and object memory in PTZ-kindled models (Figures 3 and 4). Our results are consistent with previous research, and these results suggest that BA can attenuate the cognitive impairment.55
In addition to changes in behaviour, the pathological changes that are triggered in the first few hours after seizures, which can cause initial damage and extensive neuronal loss in different brain areas, such as the hippocampus, endopiriform nucleus, and piriform cortex.56 The hippocampus has long been thought to be crucial for learning and memory in rodents, and since it is highly susceptible to injury, these functions can be easily impaired in patients with epilepsy.57,58 Hence, neuronal damage and disorder in this area will cause cognitive deficits. Besides, numerous findings demonstrate that epilepsy, neurodegeneration and inflammatory cytokines are not autonomous, but mutually influence each other. Severe seizures induce neuroinflammation with a rapid increase in the level of protein and gene expression of pro-inflammatory cytokines.59 Neuroinflammation triggered may accelerate neuronal loss and apoptosis. And it is worth noting that neuroinflammation apparently can cause epileptogenesis even in the absence of prominent neuronal death.60,61 Parallel to these observations, we found obvious neuronal damage in the CA3 region of epileptic rats concomitantly with a reduced population of viable neuronal cells relative to control rats (Figure 5). Also, epileptic rats had serious cognitive impairment, but BA treatment reversed these effects. These findings provide further experimental evidence for the essential role of BA in cognitive improvement.
The effectiveness of Traditional Chinese medicine (TCM) has been demonstrated in nervous system disease, for instance, depression, insomnia, stroke, and Alzheimer’s disease.62–65 TCM has shown the better clinical effects in treating epilepsy than conventional treatment, and it is an alternative or supplementary therapy owing to its advantages in syndrome differentiation and safety.66,67 However, it often interacts with multiple protein targets to generate unpredictable reactions, creating significant challenges for drug research.68 From the perspective of macro or global regulation, network pharmacology accounts for the effects of drugs on the biological network and provides new research for the mechanism of drug compounds.69,70 It is an interactive network based on the concept of “disease-gene-target-drug” to reveal the complex mechanism of drugs on the human body.71
In this study, we have adopted a combination of network pharmacology and experimental verification to explore the anti-epileptic possible regulatory mechanism of BA in PTZ-induced epileptic rats. 112 targets of BA were collected from different databases. A total of 1442 epilepsy-related targets and 32 overlapping genes were detected by matching the potential targets of BA with epileptic genes (Figure 6). The PPI network was conducted, and the top ten core genes were identified: TP53, MAPK1, VEGFA, AKT1, TNF, TLR4, PTGS2, CASP3, IL-6 and IL-1β (Figure 7). To obtain a more in-depth understanding of BA on epilepsy, we implemented GO function and KEGG pathway enrichment analyses (Figure 8). Accordingly, TLR4/MYD88/Caspase-3 signaling pathway was obtained through further screening. Based on the ADME properties of BA (Table 2), BA passes through the blood-brain barrier and has broad pharmacological activities, the finding was directly in line with previous finding.72 Thus, BA is a prospect for drug development.
Biological verification is a crucial step to verify that the predicted key targets are the actual key targets and to remove the existence of non-objective signaling pathways.73 For this reason, based on the network pharmacology analysis results and literature reviews, TLR4/MYD88/Caspase-3 signaling pathway was further verified in animals to explore the anti-epileptic mechanism of BA. TLRs, a family of pattern recognition receptors, most of them have been confirmed to be expressed in central nervous diseases.74 And previous studies have revealed that TLR4 plays a major role in various neuropathological processes, including neuroinflammation and neurodegeneration.75 Activated TLR4 signaling upregulates the protein expression levels of TLR4 pathway-associated mediators, including upstream (TLR4 and MyD88) and downstream (IL-1β and IL-6) factors.76,77 MYD88 plays a crucial role in signal transduction in the TLR4 signaling pathway and it was reported that the TLR4/MyD88 signaling pathway plays an important role in neuroinflammation.78,79 IL-1β and IL-6 are typical multifunctional cytokines involved immune responses and inflammation.80 IL-1β has a wide range of effects and may mediate inflammation or be directly involved in the inflammatory process. Likewise, activation of hippocampal IL-1β has been established hallmarks of temporal lobe epilepsy both in experimental and clinical evidences, and also has been implicated in mechanisms of epileptogenesis.81 IL-6 was involved in physiological brain development and several neurological disorders such as schizophrenia, major depression, and Alzheimer’s disease. It also promotes neural growth and provokes neuronal death.82,83 In epileptic rats, we found that the expression of TLR4, MYD88 was significantly increased in the hippocampus CA3, this result ties well previous study wherein epilepsy.84 Bcl-2/Bax/caspase-3 signalling is a crucial regulator in cell survival and apoptosis, which is considered as a potential strategy to reduce the apoptosis of neurons in various neurological diseases.85 The induction of epilepsy regulates the expression of Caspase-3 protein, Bcl-2 and Bax have also changed in parallel with Caspase-3 (Figure 9), a similar pattern of results was obtained in other experimental research.86 Correspondingly, regulating the TLR4/MYD88/Caspase-3 signaling pathway with BA reversed the PTZ-induced seizures and decreased the release of IL-1β and IL-6 inflammatory cytokines (Figure 10).
Conclusion
In conclusion, BA had a protective effect against PTZ-induced seizures. BA improved cognitive dysfunction and exerted a neuroprotective action. The anti-epileptic effects of BA may be effective through activation of the TLR4/MYD88/Caspase-3 signaling pathway.
Acknowledgments
The experiment was supported by the Research Foundation of National Key Research and Development Program (grant number: 2017YFC1701701). Research Foundation of the Hebei Province government funds the clinical medicine outstanding talented person project (grant number: 360601), and scientific research project of Hebei administration of traditional Chinese medicine, China (grant number: 2020176).
Disclosure
The remaining authors declare that they have no relevant conflicts of interest.
References
- 1.Khan AU, Akram M, Daniyal M, et al. Awareness and current knowledge of epilepsy. Metab Brain Dis. 2020;35(1):45–63. doi: 10.1007/s11011-019-00494-1 [DOI] [PubMed] [Google Scholar]
- 2.Sajatovic M, Tatsuoka C, Welter E, et al. Targeted self-management of epilepsy and mental illness for individuals with epilepsy and psychiatric comorbidity. Epilepsy Behav. 2016;64(Pt A):152–159. doi: 10.1016/j.yebeh.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser RM, Henshall DC, Lubin FD. The epigenetics of epilepsy and its progression. Neuroscientist. 2018;24(2):186–200. doi: 10.1177/1073858417705840 [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol. 2010;9(1):27–29. doi: 10.1016/S1474-4422(09)70304-7 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt D. The clinical impact of new antiepileptic drugs after a decade of use in epilepsy. Epilepsy Res. 2002;50(1–2):21–32. doi: 10.1016/s0920-1211(02)00065-7 [DOI] [PubMed] [Google Scholar]
- 6.Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7(4):348–354. doi: 10.1007/s11910-007-0053-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geronzi U, Lotti F, Grosso S. Oxidative stress in epilepsy. Expert Rev Neurother. 2018;18(5):427–434. doi: 10.1080/14737175.2018.1465410 [DOI] [PubMed] [Google Scholar]
- 8.Samokhina E, Samokhin A. Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int J Neurosci. 2018;128(11):1086–1096. doi: 10.1080/00207454.2018.1481064 [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Salam O, Sleem AA, Sayed M, et al. Capsaicin exerts anti-convulsant and neuroprotective effects in pentylenetetrazole-induced seizures. Neurochem Res. 2020;45(5):1045–1061. doi: 10.1007/s11064-020-02979-3 [DOI] [PubMed] [Google Scholar]
- 10.Zhang JX, Chen ZY, Zhang LL, et al. A systems-based analysis to explore the multiple mechanisms of Shan Zha for treating human diseases. Food Funct. 2021;12(3):1176–1191. doi: 10.1039/d0fo02433c [DOI] [PubMed] [Google Scholar]
- 11.Duan H, Khan GJ, Shang LJ, et al. Computational pharmacology and bioinformatics to explore the potential mechanism of Schisandra against atherosclerosis. Food Chem Toxicol. 2021;150:112058. doi: 10.1016/j.fct.2021.112058 [DOI] [PubMed] [Google Scholar]
- 12.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi: 10.1038/nchembio.118 [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Li XM, Duan Y, et al. Mechanism of gypenosides of Gynostemma pentaphyllum inducing apoptosis of renal cell carcinoma by PI3K/AKT/mTOR pathway. J Ethnopharmacol. 2021;271:113907. doi: 10.1016/j.jep.2021.113907 [DOI] [PubMed] [Google Scholar]
- 14.Zhao Q, Chen XY, Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci Bull. 2016;61(18):1391–1398. doi: 10.1007/s11434-016-1136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YY, Song K, Zhang HL, et al. Anti-inflammatory and immunomodulatory effects of baicalin in cerebrovascular and neurological disorders. Brain Res Bull. 2020;164:314–324. doi: 10.1016/j.brainresbull.2020.08.016 [DOI] [PubMed] [Google Scholar]
- 16.Duan XY, Sun Y, Zhao ZF, et al. Baicalin attenuates LPS-induced alveolar type II epithelial cell A549 injury by attenuation of the FSTL1 signaling pathway via increasing miR-200b-3p expression. Innate Immun. 2021;27(4):294–312. doi: 10.1177/17534259211013887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YL, Liu SY, Wan JY, et al. Preparation, characterization and in vivo study of borneol-baicalin-liposomes for treatment of cerebral ischemia-reperfusion injury. Int J Nanomedicine. 2020;15:5977–5989. doi: 10.2147/IJN.S259938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei KC, Shen YJ, He YJ, et al. Baicalin represses C/EBP β via its antioxidative effect in parkinson’s disease. Oxid Med Cell Longev. 2020;2020:8951907. doi: 10.1155/2020/8951907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao ZG, Cao ZQ, Yang JL, et al. Baicalin promotes hippocampal neurogenesis via the Wnt/β-catenin pathway in a chronic unpredictable mild stress-induced mouse model of depression. Biochem Pharmacol. 2021;190:114594. doi: 10.1016/j.bcp.2021.114594 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Liu TT, Li YT, et al. Baicalin ameliorates cognitive impairment and protects microglia from lps-induced neuroinflammation via the SIRT1/HMGB1 pathway. Oxid Med Cell Longev. 2020;2020:4751349. doi: 10.1155/2020/4751349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ping X, Qin SK, Liu SN, et al. Effects of Huazhuo Jiedu Shugan Decoction on cognitive and emotional disorders in a rat model of epilepsy: possible involvement of AC-cAMP-CREB signaling and NPY expression. Evid Based Complement Alternat Med. 2019;2019:4352879. doi: 10.1155/2019/4352879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van EJ, Van DD, De Deyn PP. PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behav. 2019;95:51–55. doi: 10.1016/j.yebeh.2019.02.029 [DOI] [PubMed] [Google Scholar]
- 23.Pranzatelli MR, Tate ED. Chloral hydrate for progressive myoclonus epilepsy: a new look at an old drug. Pediatr Neurol. 2001;25(5):385–389. doi: 10.1016/s0887-8994(01)00350-2 [DOI] [PubMed] [Google Scholar]
- 24.Moradi P, Ganjkhani M, Anarkooli IJ, et al. Neuroprotective effects of lovastatin in the pilocarpine rat model of epilepsy according to the expression of neurotrophic factors. Metab Brain Dis. 2019;34(4):1061–1069. doi: 10.1007/s11011-019-00424-1 [DOI] [PubMed] [Google Scholar]
- 25.Yum M, Ko T, Kim DW. β-Hydroxybutyrate increases the pilocarpine-induced seizure threshold in young mice. Brain Dev. 2012;34(3):181–184. doi: 10.1016/j.braindev.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 26.Shishmanova-Doseva M, Atanasova D, Uzunova Y, et al. Effects of lacosamide treatment on epileptogenesis, neuronal damage and behavioral comorbidities in a rat model of temporal lobe epilepsy. Int J Mol Sci. 2021;22(9):4667. doi: 10.3390/ijms22094667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alachkar A, Lotfy M, Adeghate E, et al. Ameliorating effects of histamine H3 receptor antagonist E177 on acute pentylenetetrazole-induced memory impairments in rats. Behav Brain Res. 2021;405:113193. doi: 10.1016/j.bbr.2021.113193 [DOI] [PubMed] [Google Scholar]
- 28.Liu YF, Gao F, Li XW, et al. The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem Res. 2012;37(8):1670–1680. doi: 10.1007/s11064-012-0771-8 [DOI] [PubMed] [Google Scholar]
- 29.Sumiyoshi E, Hashimoto M, Hossain S, et al. Anredera cordifolia extract enhances learning and memory in senescence-accelerated mouse-prone 8 (SAMP8) mice. Food Funct. 2021;12(9):3992–4004. doi: 10.1039/d0fo03272g [DOI] [PubMed] [Google Scholar]
- 30.Zhao YN, Cao YF, Zhang YH, et al. Nelumbo nucifera Gaertn Stems (Hegeng) improved depression behavior in CUMS mice by regulating NCAM and GAP-43 expression. Evid Based Complement Alternat Med. 2020;2020:3056954. doi: 10.1155/2020/3056954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denninger JK, Smith BM, Kirby ED, Novel object recognition and object location behavioral testing in mice on a budget. J Vis Exp. 2018;141:3791–58593. doi: 10.3791/58593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker BS, Spampanato J, McCarren HS, et al. The K7 modulator, retigabine, is an efficacious antiseizure drug for delayed treatment of organophosphate-induced status epilepticus. Neuroscience. 2021;463:143–158. doi: 10.1016/j.neuroscience.2021.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Cheng Y, Zhang W, et al. Neuroprotective effect of a new free radical scavenger HL-008 in an ischemia-reperfusion injury rat model. Neuroscience. 2021;465:105–115. doi: 10.1016/j.neuroscience.2021.04.012 [DOI] [PubMed] [Google Scholar]
- 34.Ru JL, Li P, Wang JN, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminformatics. 2014;6:13. doi: 10.1186/1758-2946-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang WJ, Wang SZ, Liu TL, et al. Resveratrol: multi-targets mechanism on neurodegenerative diseases based on network pharmacology. Front Pharmacol. 2020;11:694. doi: 10.3389/fphar.2020.00694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SH, Chen J, Cheng TJ, et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019;47(D1):D1102–D1109. doi: 10.1093/nar/gky1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keiser MJ, Roth BL, Armbruster BN, et al. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25(2):197–206. doi: 10.1038/nbt1284 [DOI] [PubMed] [Google Scholar]
- 38.Davis AP, Grondin CJ, Johnson RJ, et al. The Comparative Toxicogenomics Database: update 2019. Nucleic Acids Res. 2019;47:D948–D954. doi: 10.1093/nar/gky868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn M, Szklarczyk D, Pletscher-Frankild S, et al. STITCH 4: integration of protein-chemical interactions with user data. Nucleic Acids Res. 2014;42:D401–D407. doi: 10.1093/nar/gkt1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang SS, Dong L, Liu L, et al. HERB: a high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 2021;49(D1):D1197–D1206. doi: 10.1093/nar/gkaa1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao ZJ, Dong J, Che YJ, et al. TargetNet: a web service for predicting potential drug-target interaction profiling via multi-target SAR models. J Comput Aided Mol Des. 2016;30(5):413–424. doi: 10.1007/s10822-016-9915-2 [DOI] [PubMed] [Google Scholar]
- 42.Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1–1.30.33. doi: 10.1002/cpbi.5 [DOI] [PubMed] [Google Scholar]
- 43.Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YX, Zhang S, Li FC, et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020;48:D1031–D1041. doi: 10.1093/nar/gkz981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piñero J, Bravo A, Queralt-Rosinach N, et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017;45:D833–D839. doi: 10.1093/nar/gkw943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003;4:2. doi: 10.1186/1471-2105-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou YY, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi: 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun ZY, Gao CH, Gao DD, et al. Reduction in pericyte coverage leads to blood-brain barrier dysfunction via endothelial transcytosis following chronic cerebral hypoperfusion. Fluids Barriers CNS. 2021;18(1):21. doi: 10.1186/s12987-021-00255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jennum P, Sabers A, Christensen J, et al. Socioeconomic outcome of epilepsy surgery: a controlled national study. Seizure. 2016;42:52–56. doi: 10.1016/j.seizure.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 51.Hattiangady B, Kuruba R, Shuai B, et al. Hippocampal neural stem cell grafting after status epilepticus alleviates chronic epilepsy and abnormal plasticity, and maintains better memory and mood function. Aging Dis. 2020;11(6):1374–1394. doi: 10.14336/AD.2020.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoda U, Jain S, Samim M, et al. Embelin ameliorates cognitive dysfunction and progression of kindling in pentylenetetrazol-induced kindling in mice by attenuating brain inflammation. Epilepsy Behav. 2021;116:107788. doi: 10.1016/j.yebeh.2021.107788 [DOI] [PubMed] [Google Scholar]
- 53.Willment K, Hill M, Baslet G, et al. Cognitive impairment and evaluation in psychogenic nonepileptic seizures: an integrated cognitive-emotional approach. Clin EEG Neurosci. 2015;46(1):42–53. doi: 10.1177/1550059414566881 [DOI] [PubMed] [Google Scholar]
- 54.Schleicher EM, Ott FW, Müller M, et al. Prolonged cannabidiol treatment lacks on detrimental effects on memory, motor performance and anxiety in C57BL/6J mice. Front Behav Neurosci. 2019;13:94. doi: 10.3389/fnbeh.2019.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin X, Liu MY, Zhang DF, et al. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci Ther. 2019;25(5):575–590. doi: 10.1111/cns.13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartolomei F, Khalil M, Wendling F, et al. Entorhinal cortex involvement in human mesial temporal lobe epilepsy: an electrophysiologic and volumetric study. Epilepsia. 2005;46(5):677–687. doi: 10.1111/j.1528-1167.2005.43804.x [DOI] [PubMed] [Google Scholar]
- 57.Shetty AK. Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: can early neural stem cell grafting intervention provide protection? Epilepsy Behav. 2014;38:117–124. doi: 10.1016/j.yebeh.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramandi D, Elahdadi Salmani M, Moghimi A, et al. Pharmacological upregulation of GLT-1 alleviates the cognitive impairments in the animal model of temporal lobe epilepsy. PLoS One. 2021;16(1):e0246068. doi: 10.1371/journal.pone.0246068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vezzani A. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr. 2014;14(1 Suppl):3–7. doi: 10.5698/1535-7511-14.s2.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vezzani A, Aronica E, Mazarati A, et al. Epilepsy and brain inflammation. Exp Neurol. 2013;244:11–21. doi: 10.1016/j.expneurol.2011.09.033 [DOI] [PubMed] [Google Scholar]
- 61.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2(10):734–744. doi: 10.1038/35094583 [DOI] [PubMed] [Google Scholar]
- 62.Zhu Y, Liu M, Qu S, et al. Elaphuri davidiani cornu improves depressive-like behavior in mice and increases neurotrophic factor expression in mouse primary astrocytes via cAMP and ERK-dependent pathways. Front Pharmacol. 2020;11:593993. doi: 10.3389/fphar.2020.593993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li YP, Liang WY, Guo CJ, et al. Renshen Shouwu extract enhances neurogenesis and angiogenesis via inhibition of TLR4/NF-κB/NLRP3 signaling pathway following ischemic stroke in rats. J Ethnopharmacol. 2020;253:112616. doi: 10.1016/j.jep.2020.112616 [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Pei J, Fu QH, et al. The prognostic value of traditional chinese medicine symptoms in acute ischemic stroke: a pilot study. Evid Based Complement Alternat Med. 2020;2020:1520851. doi: 10.1155/2020/1520851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang SW, Li LL, Hu JT, et al. Polysaccharide of Taxus chinensis var. mairei Cheng et L.K.Fu attenuates neurotoxicity and cognitive dysfunction in mice with Alzheimer’s disease. Pharm Biol. 2020;58(1):959–968. doi: 10.1080/13880209.2020.1817102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu XX, Guan QB, Wang YP, et al. Anticonvulsant and anti-apoptosis effects of salvianolic acid B on pentylenetetrazole-kindled rats via AKT/CREB/BDNF signaling. Epilepsy Res. 2019;154:90–96. doi: 10.1016/j.eplepsyres.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 67.Yang P, Qin Y, Zhu Y, et al. Chaihu-Longgu-Muli decoction relieves epileptic symptoms by improving autophagy in hippocampal neurons. J Ethnopharmacol. 2020;259:112990. doi: 10.1016/j.jep.2020.112990 [DOI] [PubMed] [Google Scholar]
- 68.Tang CP, Ye Y, Feng YJ, et al. TCM, brain function and drug space. Nat Prod Rep. 2016;33(1):6–25. doi: 10.1039/c5np00049a [DOI] [PubMed] [Google Scholar]
- 69.Muhammad J, Khan A, Ali A, et al. Network pharmacology: exploring the resources and methodologies. Curr Top Med Chem. 2018;18(12):949–964. doi: 10.2174/1568026618666180330141351 [DOI] [PubMed] [Google Scholar]
- 70.Huang XJ, He CJ, Liang S, et al. Veratrilla baillonii franch could alleviate lipid accumulation in LO2 cells by regulating oxidative, inflammatory, and lipid metabolic signaling pathways. Front Pharmacol. 2020;11:575772. doi: 10.3389/fphar.2020.575772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang JS, Feng JL, Dai HH, et al. Achyranthis bidentatae radixPotential mechanism of plus semen vaccariae granules in the treatment of diabetes mellitus-induced erectile dysfunction in rats utilizing combined experimental model and network pharmacology. Pharm Biol. 2021;59(1):547–556. doi: 10.1080/13880209.2021.1920621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang ZY, Jiang ZM, Xiao PT, et al. The mechanisms of baicalin ameliorate obesity and hyperlipidemia through a network pharmacology approach. Eur J Pharmacol. 2020;878:173103. doi: 10.1016/j.ejphar.2020.173103 [DOI] [PubMed] [Google Scholar]
- 73.Huang Q, Liu R, Liu J, et al. Integrated network pharmacology analysis and experimental validation to reveal the mechanism of anti-insulin resistance effects of Moringa oleifera seeds. Drug Des Devel Ther. 2020;14:4069–4084. doi: 10.2147/DDDT.S265198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126 [DOI] [PubMed] [Google Scholar]
- 75.Rahimifard M, Maqbool F, Moeini-Nodeh S, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 2017;36:11–19. doi: 10.1016/j.arr.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 76.Cheng ZK, Zhang M, Ling CL, et al. Neuroprotective effects of ginsenosides against cerebral ischemia. Molecules. 2019;24(6):1102. doi: 10.3390/molecules24061102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cong L, Yang S, Zhang Y, et al. DFMG attenuates the activation of macrophages induced by co-culture with LPC-injured HUVE-12 cells via the TLR4/MyD88/NF-κB signaling pathway. Int J Mol Med. 2018;41(5):2619–2628. doi: 10.3892/ijmm.2018.3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang H, Cheng X, Yang YL, et al. Ramulus Cinnamomi extract attenuates neuroinflammatory responses via downregulating TLR4/MyD88 signaling pathway in BV2 cells. Neural Regen Res. 2017;12(11):1860–1864. doi: 10.4103/1673-5374.219048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bajo M, Patel RR, Hedges DM, et al. Role of MyD88 in IL-1β and ethanol modulation of GABAergic transmission in the central amygdala. Brain Sci. 2019;9(12):361. doi: 10.3390/brainsci9120361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou LJ, Liu ZJ, Wang ZX, et al. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci Rep. 2017;7:44822. doi: 10.1038/srep44822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazarati AM, Pineda E, Shin D, et al. Comorbidity between epilepsy and depression: role of hippocampal interleukin-1beta. Neurobiol Dis. 2010;37(2):461–467. doi: 10.1016/j.nbd.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei H, Alberts I, Brain LX. IL-6 and autism. Neuroscience. 2013;252:320–325. doi: 10.1016/j.neuroscience.2013.08.025 [DOI] [PubMed] [Google Scholar]
- 83.Conroy SM, Nguyen V, Quina LA, et al. Interleukin-6 produces neuronal loss in developing cerebellar granule neuron cultures. J Neuroimmunol. 2004;155(1–2):43–54. doi: 10.1016/j.jneuroim.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 84.Zhu X, Liu J, Chen O, et al. Neuroprotective and anti-inflammatory effects of isoliquiritigenin in kainic acid-induced epileptic rats via the TLR4/MYD88 signaling pathway. Inflammopharmacology. 2019;27(6):1143–1153. doi: 10.1007/s10787-019-00592-7 [DOI] [PubMed] [Google Scholar]
- 85.Cui N, Li SX, Zhao XL, et al. Expression of Bcl-2, Bax and Caspase-3 in nerve tissues of rats chronically exposed to 2,5-hexanedione. Neurochem Res. 2007;32(9):1566–1572. doi: 10.1007/s11064-007-9359-0 [DOI] [PubMed] [Google Scholar]
- 86.Cao H, Zhang L, Qu Z, et al. The protective effect of hydroxylated fullerene pretreatment on pilocarpine-induced status epilepticus. Brain Res. 2021;1764:147468. doi: 10.1016/j.brainres.2021.147468 [DOI] [PubMed] [Google Scholar]