Abstract
Beta cell dysfunction is central to the development of type 2 diabetes (T2D). In T2D, environmental and genetic influences can manifest beta cell dysfunction in many ways, including impaired glucose-sensing and secretion coupling mechanisms, insufficient adaptative responses to stress, and aberrant beta cell loss through increased cell death and/or beta cell de-differentiation. In recent years, circadian disruption has emerged as an important environmental risk factor for T2D. In support of this, genetic disruption of the circadian timing system in rodents impairs insulin secretion and triggers diabetes development, lending important evidence that the circadian timing system is intimately connected to, and essential for the regulation of pancreatic beta cell function; however, the role of the circadian timing system in the regulation of beta cell biology is only beginning to be unraveled. Here, we review the recent literature that explores the importance of the pancreatic islet/beta cell circadian clock in the regulation of various aspects of beta cell biology, including transcriptional and functional control of daily cycles of insulin secretion capacity, regulation of postnatal beta cell maturation, and control of the adaptive responses of the beta cell to metabolic stress and acute injury.
Keywords: circadian clock, glucose homeostasis, pancreatic islet, insulin secretion, glucose-secretion coupling, circadian transcriptome, beta cell maturation, beta cell proliferation
Insulin secretion by the pancreatic beta cell is essential for the regulation of various aspects of energy metabolism, including and most notably, glucose homeostasis. In the fed state, rising blood glucose levels drive glucose entry into the beta cell (via GLUT1/2) where glucokinase activity commits glucose to glycolysis. Coupled aerobic glucose oxidation produces adenosine 5′-triphosphate (ATP), accumulation of which gates plasma membrane K+ATP channels, triggering plasma membrane depolarization, calcium influx, and exocytosis of insulin stored in secretory vesicles. This glucose-stimulated insulin secretion (GSIS) rapidly directs glucose uptake into peripheral tissues (muscle, adipose) and controls the oxidation and/or storage of glucose to meet the energy demands of the active phase of the day. Loss of or insufficient GSIS manifests as postprandial hyperglycemia and is often an early beta cell defect contributing to diabetes development. While GSIS is essential for the regulation of postprandial energy metabolism, in the fasted state (ie, in between meals, overnight fast), beta cells secrete a constant, lower, basal level of insulin, which is critical for directing the metabolic response to fasting and maintaining fasted blood glucose levels within a narrow range while ensuring adequate glucose supply to resting insulin-independent tissues. For example, basal insulin has a suppressive effect on glucagon-directed hepatic glucose production such that insufficient basal insulin results in excessive endogenous glucose production, manifesting as fasting hyperglycemia. Conversely, excessive basal insulin secretion during the fasted phase has as suppressive effect on hepatic glucose production and can cause hypoglycemia, which can be deadly. Therefore, it is critical that insulin secretion, both basal and glucose-stimulated, is tightly and dynamically regulated across the cycles of fasting/feeding and activity/rest in a time-of-day-dependent manner to not only ensure that blood glucose levels are maintained within a narrow and appropriate range, but also to ensure that the disparate metabolic needs associated with cycles of fasting/feeding and activity/rest are met.
Almost 50 years ago, it was demonstrated that the stimulated insulin secretion capacity (ie, the amount of insulin that can be secreted when stimulated) is highly regulated in a temporal manner in humans, which ultimately dictates time-of-day glucose tolerance and whole-body glucose metabolism. In healthy human subjects, the amount of insulin measured in the blood in response to an isocaloric meal or a glucose bolus was found to be greater in the morning at the onset of the fed/active phase compared to the evening, when fasting and inactivity are anticipated (1-4). Infusion of tolbutamide, a K+ATP channel blocker and therefore stimulator of insulin secretion, alongside a glucose challenge in healthy human subjects, caused a greater increase in plasma insulin when infused at 7 am compared to 7 pm, consequently increasing the rate of glucose clearance from the blood in the morning (4). These early studies elegantly demonstrated that the beta cell is “programmed” to secrete more insulin via GSIS during the active/fed phase of the day and that these pathways are actively suppressed in the inactive/fasted phase.
Since these early in vivo human studies, more direct assessments of the temporal pattern of insulin secretion capacity have been made using isolated islets from rodent models and human cadaveric donors. Rat and mouse islets isolated at various times of the day and immediately assessed for GSIS capacity show greater stimulation of insulin secretion when isolated from animals in the dark/active phase versus animals in the light/inactive phase (5-8). Similarly, isolated mouse (9, 10) and human islets (11), synchronized ex vivo with forskolin, show a similar pattern of rhythmic GSIS capacity, suggesting that the mechanism of rhythmic insulin secretion regulation is conserved across species. In the next sections, we will discuss some of the emerging mechanisms and pathways that control daily rhythms of insulin secretion, including circadian-regulated transcriptional programming of the beta cell and metabolic function, as well as discuss some recent evidence that supports a role for the circadian clock in functional beta cell maturation, regeneration, and adaptation to metabolic stress.
The Beta Cell Circadian Oscillator, Insulin Secretion, and Glucose Homeostasis
The circadian timing system is a complex series of cellular circadian oscillators that function in every tissue and cell of the body to translate environmental time into biological time and to align our behavior and physiology with light/dark cycles of our environment. The circadian system is arranged in a hierarchical manner, with the “primary” circadian clock located in the suprachiasmatic nucleus (SCN) within the hypothalamus, which presides over the “secondary” oscillators located in every peripheral tissue of the body (12). Notably, the primary clock in the SCN is entrained by light signals via direct input from photosensitive ganglion cells from the retina and directs a number of our circadian behaviors, including the sleep/wake cycle, fasting/feeding behaviors, and cycles of body temperature (13). Unlike the SCN, the peripheral clocks are not entrained by light directly, but rather, are entrained by a number of hormonal, autonomic, and physiological inputs, including critical signals generated by the fasting and feeding cycles (14, 15).
Regardless of body location, the cellular circadian oscillator comprises a highly conserved series of autoregulatory transcriptional and translational feedback loops that drive various clock-controlled output pathways and are also entrained by a series of input pathways that influence the period and amplitude of circadian rhythms. A detailed description of the core circadian clock machinery is reviewed elsewhere (16). Briefly, CLOCK and BMAL1, 2 core circadian clock proteins, comprise the main components of the positive arm of the circadian oscillator. Notably, Bmal1 is the only nonredundant core clock gene (17) and as such, is often targeted in transgenic studies to explore the role of the circadian machinery in cellular and physiological function, as you will see in this review. Once expressed, CLOCK and BMAL1 heterodimerize in the cytoplasm, translocate to the nucleus and activate the expression of numerous “clock-controlled genes,” which include many output genes involved in various biological pathways, and also a set of circadian repressors, Period (Per1, Per2 and Per3) and Cryptochrome (Cry1 and Cry2). Accumulation, heterodimerization, and translocation of PER and CRY proteins to the nucleus inhibits CLOCK:BMAL1 binding to target genes, silencing their expression. Turnover and degradation of PER and CRY proteins ultimately dictate the timing of CLOCK:BMAL1 repression and the negative arm of circadian transcriptional cycle, with the entire cycle taking approximately 24 hours to complete. In addition, the CLOCK:BMAL1 dimer also initiates an E-Box-mediated activation of orphan nuclear-receptor genes, such as RORα/β and Rev-erbα/β. RORα/β inhibits Bmal1 while Rev-erbα/β initiates Bmal1 transcription by competitive binding to RORE binding sites located within the Bmal1 promoter region, together forming an additional feedback loop, which operates in parallel.
In 2010, Marcheva et al demonstrated that pancreatic islets possess a cell-autonomous circadian oscillator. Using Per2:luciferase (Per2:Luc) knock-in reporter mice, high-amplitude rhythmic oscillations of Per2 were observed in isolated islets synchronized ex vivo with forskolin (18). Since this initial study, several groups have demonstrated robust circadian rhythms in Per2 and other core clock genes in rodent (10, 19-22) and cultured human islets (23, 24). To explore the role of the circadian oscillator in islet function and metabolic health, several genetic circadian mutant and transgenic models have been created and characterized. Whole-body clock mutant (ClockΔ19/Δ19) and Bmal1 knockout (Bmal1-/-) mice, as well as pancreas- and beta cell–specific Bmal1 knockout mice (PdxCre:Bmal1fl/fl and Ins2Cre:Bmal1fl/f, respectively) spontaneously develop glucose intolerance, hyperglycemia, and hypoinsulinemia, characterized by reduced plasma insulin levels as well as impaired GSIS after in vivo or ex vivo glucose challenges (18, 19, 25, 26). Notably, glucose intolerance and insulin secretion impairments were more severe and developed at a younger age (2-4 months vs 8 months) in the pancreas- and beta cell–specific Bmal1 knockout models compared with the whole-body ClockΔ19/Δ19 mutant, indicating that circadian disruption of the beta cell alone can autonomously drive diabetes development, largely via an insulin secretion defect. Additionally, specific ablation of the beta cell circadian clock during adulthood using tamoxifen-inducible CreER models (Pdx:CreERT and Ins2:CreERT), triggered a similar rapid impairment of GSIS within 2 weeks of tamoxifen injection (9, 26, 27), indicating that circadian disruption during embryogenesis/development is not a prerequisite for beta cell dysfunction in these models. Rather, acquired circadian disruption in the adult beta cell can sufficiently drive insulin secretion impairment and diabetes development.
In line with rodent circadian disruption models, siRNA knockdown of CLOCK in cultured human islets impaired the overall capacity of islets to secrete insulin in response to a glucose challenge and also disrupted the circadian rhythm of insulin secretion at basal glucose over a 48-hour period (11). Moreover, human cadaveric donor islets from T2D patients show substantial signs of circadian dysfunction, including reduced expression of the core clock genes, Per1 to 3, Cry2, Rev-erbα, Clock, and Dbp in T2D islets (24, 28). Of these genes, PER2 and CRY2 levels showed a negative association with donor HbA1c (ie, poorer glucose control was associated with lower circadian clock gene expression) (28) while PER2, PER3, and CRY showed a positive association with insulin mRNA and protein expression (28). Introduction of Bmal1:Luc and Per2:Luc reporter constructs into human islet cells revealed significantly reduced circadian amplitude of the core oscillator and compromised circadian insulin secretion profiles in a synchronized population of mixed islet cells from human T2D donors (24). Together, these studies demonstrate that, like rodent islets, cultured human islets (both whole islets and dispersed islet cells) display high-amplitude rhythms of the core circadian oscillator when synchronized ex vivo and that perturbations in the human islet circadian oscillator impact insulin secretion capacity and dynamics, potentially contributing to islet pathophysiology in T2D development. Importantly, there is a paucity of experiments in this area of circadian human islet research, mainly due to limited donor tissue availability. Clearly, much more research is needed in this area to elucidate the cause-and-effect relationship between circadian disruption and beta cell dysfunction in the pathophysiology of diabetes.
The Islet and Beta Cell Circadian Transcriptomes
Pancreatic islets comprise a heterogenous population of endocrine cell types and the assessment of whole islet clocks includes input from circadian oscillators in all endocrine cell types. Studies using a triple transgenic mouse line that expresses beta cell– (RIP-Cherry) and alpha cell– (Gcg-Venus) specific fluorescent markers along with the Per2:Luc reporter system not only demonstrate that the individual islet cell types express the highly conserved core circadian machinery, but that the circadian properties of the beta cells are unique relative to other cell islet types (10). Notably, alpha and beta cells displayed circadian oscillations with the same circadian period; however, unique circadian properties were observed, including distinct expression patterns of the core clock transcripts (same amplitude and period but 4 hours phase-advanced in the beta cell) as well as different phases of hormone secretion (the glucagon secretion peak lagged 2 hours behind insulin) (10).
In whole isolated islets, ~27% of transcripts show high-amplitude oscillatory transcriptional profiles (9); however, in sorted populations of alpha and beta cells, it was determined that closer to ~60% of transcripts are oscillatory in one or both cell types (10), likely reflecting different circadian phases in the individual islet cell types that are masked at the whole islet level, but nonetheless demonstrating that beta cells have a high ratio of cycling to non-cycling genes typically seen in metabolic tissues (29). Using healthy male C57BL6 isolated islets synchronized ex vivo and sampled for RNA-seq analyses over 2 circadian cycles, Perelis et al (2015) found significant enrichment of genes critically involved in insulin secretion among the cycling islet gene set, including glucose-sensing and secretion coupling factors (eg, Slc2a2, Gnaq, Creb1, Creb3, Cacna1c) as well as vesicle maturation, trafficking, and exocytosis (eg, Stx1a and Vamp8) (9). Similar results were reported using synchronized human islets from healthy male cadaveric donors where 1800 cycling genes were identified; 481 of which were orthologous to those identified as cycling in mouse, including several similar factors involved in insulin vesicle exocytosis, trafficking, and fusion (9).
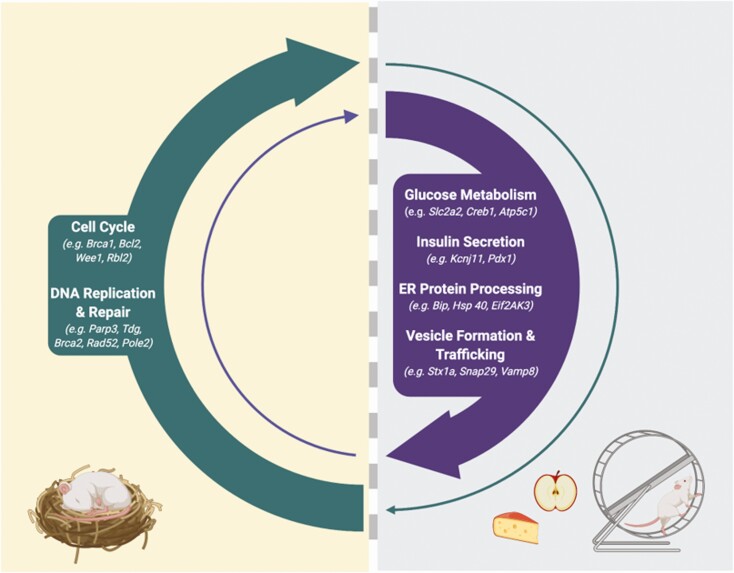
In 2016, Rakshit et al performed a similar transcriptional profiling study, but rather than isolating islets in batch and synchronizing ex vivo, they isolated islets from C57BL6 mice every 4 hours over the light/dark cycle and determined the detailed diurnal expression pattern of key transcripts and pathways in relation to the light (inactive)/dark (active) cycle (30). Nineteen distinct time-dependent cycling profiles were identified that showed significant enrichment of important islet genes in a time-of-day-dependent manner. KEGG analysis of the profiles that peaked in the dark or at the onset of the dark phase (a time when food intake is anticipated and insulin demand and secretion capacity are greatest) revealed enrichment of a number of pathways that are critical for insulin secretion, including “protein processing in the endoplasmic reticulum” (eg, Bip, Hsp40, Hsp70, Grp94, Erolβ, Eif2AK3, Dsk2); “insulin secretion” (eg, Slc2a2, Gck, Creb3L1, Pdx1, KcnJ11); “insulin signaling” (eg, InsR, Ins1), “SNARE interactions in vesicular transport” (eg, Bet1, Snap29, Vamp4); and “oxidative phosphorylation” (eg, Atp5c1, Cox4l1, Ndufa2). Conversely, in the light/inactive phase (or at the end of the dark phase), the most enriched pathways were related to positive regulation of the cell cycle and DNA repair. The antiphase expression of these pathways in the islet suggests a role for the circadian clock in temporally gating these important and diverse biological pathways, which may represent a critical mechanism that allows the beta cell to optimally direct energy and resources for maximal insulin synthesis and secretion in a time-limited manner when food is anticipated, while alternately allowing for defined periods of beta cell rest, repair, and growth, when insulin demand is low (Fig. 1).
Figure 1:
Temporal transcriptional profiling of rodent islets suggests that the circadian clock transcriptionally gates important and diverse biological pathways. During the active phase, when food is anticipated, the circadian clock drives the expression of key insulin secretory genes, including genes involved in glucose metabolism (eg, Slc2a2 (9, 30), Gnaq (9), Creb1 (9), Creb3 (9), Cacna1c (9), Atp5c1 (30), Cox4L1 (30), Ndufa2 (30)), insulin secretion (Slc2a2 (9, 30), Creb3L1 (30), Pdx1 (30), KcnJ11 (30)) ER function and protein processing (eg, Bip, Hsp40, Hsp70, Grp94, Erolβ, Eif2Ak3 (Perk), Dsk2) (30), and vesicle maturation, trafficking and exocytosis (eg, Stx1a (9), Vamp8 (9), Vamp4 (30), Bet1 (30), Snap29 (30)). During the inactive phase of the day, these pathways are suppressed, and pathways involved in growth and repair, including cell cycle control genes (eg, Bcl2, Brca1, Ccnb2, Cdk6, Cdc20, Wee1, Cdkn2b, Rbl2, Hus1, Gadd45a) (27), and DNA replication and repair (eg, Parp3, Tdg, Brca2, Rad52, Pole2) (30) are activated. From these transcriptional programs, we speculate that the beta cell circadian oscillator drives periods of insulin secretion “priming” when food is anticipated with alternating periods of rest and recovery. This hypothesis is based on rodent studies and notably, rodents are nocturnal in nature. Similar temporal transcriptional profiling studies in human islets are currently lacking but are needed to understand how the circadian clock may transcriptionally gate such pathways in diurnal humans. Created with BioRender.com.
In support of this notion, disruption of the islet/beta cell circadian oscillator in adult islets has been shown to alter the transcriptional profile of genes/pathways not only essential for insulin secretion, but also cell cycle control and DNA repair. Pancreas-specific Bmal1 silencing altered the expression of a large number of islet genes (1757); many of which were identified as oscillating in the C57BL6 islet profiling studies (9). KEGG pathway annotation of the altered cycling gene set revealed that highly impacted pathways included vesicle trafficking, tethering, and fusion. Similarly, RNA-seq transcriptional profiling after siRNA knockdown of CLOCK in adult human islets revealed altered expression of transcripts involved in insulin granule formation and secretion (SLC30A8, VAMP3, STX6) as well as altered expression of genes involved in glucose-sensing and metabolism (GNAQ, ATP1A1, ATP5G2, KCNJ11) (11), collectively suggesting that the circadian clock machinery not only converges on and transcriptionally regulates pathways involved in insulin exocytosis, but also upstream glucose-secretion coupling pathways. Furthermore, beta cell–specific Bmal1 deletion attenuated the expression of various cell cycle activators (Bcl2, Brca1, Ccnb2, Cdk6, Cdc20) while also increasing the expression of a number of cell cycle inhibitors (Wee1, Cdkn2b, Rbl2, Hus1, Gadd45a), which was associated with reduced compensatory beta cell proliferation and increased apoptosis when challenged with a high-fat diet (27). Collectively, these findings illustrate a potentially critical role for the beta cell circadian oscillator in coordinating cycles of insulin secretion priming (within a time-limited window) followed by periods of insulin secretion suppression that are associated with recovery and growth. As such, circadian disruption, in addition to driving GSIS impairment, may also impact the ability of the beta cell to recover from or respond to stress-induced DNA damage, leading to greater susceptibility to cellular dysfunction and apoptosis. This concept is discussed in more detail in the following sections.
Beyond Circadian Transcriptional Regulation of the Beta Cell
In addition to transcriptional regulation, many circadian clock output genes are also mediators of posttranscriptional (ie, mRNA polyadenylation (31) and m6A methylation (32)) and posttranslational modifications (ie, phosphorylation, acetylation, sumoylation, methylation, and ubiquitination) (33), adding layers of complexity to circadian regulation and underscoring the importance of looking beyond circadian transcriptional control and evaluating the functional impact of the circadian clock on key regulatory pathways of insulin secretion, in a temporal manner.
Several studies have examined the insulin content of whole isolated islets from various circadian-deficient models and none have reported an impact (9, 18, 26), suggesting that insulin biosynthesis per se is not directly impacted by the circadian clock. Rather, circadian-deficient models consistently display impaired GSIS, likely stemming from disruption somewhere in the secretion coupling and/or exocytotic pathways. Potassium chloride (KCl), an insulin secretagogue that directly depolarizes the cell membrane, is often used as a tool to determine where (ie, upstream in glucose metabolism or downstream of depolarization) a specific defect in the insulin secretory pathway might lie. Notably, application of KCl to Bmal1-/- islets has yielded conflicting reports. Marcheva et al (2010) demonstrated impaired KCl-induced insulin secretion in Bmal1-/- islets, indicative of defective insulin exocytosis (18); however, Lee et al (2011) did not observe such an impairment (25), suggesting a primary defect in glucose metabolism caused by genetically induced circadian dysfunction. While these discrepant results remain unclarified, functional assessment of insulin exocytosis in human islet cells with siRNA-mediated CLOCK knockdown have demonstrated aberrant exocytotic events. Using human islets where the secretory granules are labeled with NPY-cherry and individual islet cell types are labeled with cell-type–specific fluorescent markers, Petrenko et al (2020) elegantly demonstrated that the density of plasma membrane-docked granules was reduced by ~34% in beta cells when CLOCK was knocked down using siRNA (24). Additionally, stimulation of exocytosis with K+ after siRNA-mediated CLOCK knockdown in human islets showed significantly reduced exocytotic events (24). Nearly identical functional results were observed in islets isolated from Bmal1-/- mice (24), demonstrating that disruption of the islet circadian oscillator functionally impacts insulin exocytosis, in both mouse and human islets.
In addition to the functional defects observed at the level of insulin exocytosis, circadian disruption has also been shown to negatively impact oxidative glucose metabolism. Using whole-body Bmal1 knockout mice (Bmal1-/-), Lee et al (2011) demonstrated that Bmal1 deficiency abrogates glucose-induced mitochondrial membrane hyperpolarization with a concomitant reduction in the ATP/ADP ratio, without affecting GLUT-2 or glucokinase expression, mitochondrial number, or expression of electron transport chain complex subunits (25). While glucose oxidation pathways remained largely intact at the transcriptional level, these findings suggest that Bmal1 deficiency causes uncoupling of the electron transport chain function from ATP production, which may be responsible for reduction in glucose-stimulated mitochondrial membrane hyperpolarization, as well as the reduced ATP production and impaired GSIS observed in Bmal1-/- islets. Indeed, uncoupling protein 2 (UCP2), a mitochondrial uncoupling protein that is expressed in beta cells as well as the other endocrine islet cell types, was significantly elevated in Bmal1-/- islets (25). Furthermore, treatment of Bmal1-/- islets with genipin, a pharmacological UCP2 inhibitor, restored glucose-induced ATP production and rescued GSIS deficiency, uncovering an important functional relationship between UCP2, the beta cell circadian oscillator, and control of GSIS.(25) These studies have been confirmed using a beta cell–specific Bmal1 knockout mouse, which demonstrates that this effect is not the consequence of systemic Bmal1 deletion, but rather is an intrinsic beta cell–specific effect (26).
UCP2 is a transmembrane inner mitochondrial membrane protein that mildly uncouples fuel oxidation from ATP production in pancreatic beta cells (34). Beta cell UCP2 negatively regulates insulin secretion (35), and this has been observed in rodent models of type 2 diabetes and obesity (36, 37). Additionally, UCP2-mediated suppression of GSIS has also been observed during long-term fasting (>24 hours), revealing a potentially important physiological role for UCP2 in the beta cell whereby UCP2-mediated suppression of GSIS provides a mechanism to prevent life-threatening hypoglycemia during long-term fasting/starvation (38). While early studies on UCP2 function in the beta cell focused on chronic deletion and overexpression models to extrapolate a physiological function for this uncoupling protein in beta cells, we (8) and others (26) measured the temporal expression of UCP2 in control rodent islets and clonal beta cells over 24 hours and showed that Ucp2 displays a rhythmic expression profile such that Ucp2 expression is low during the dark/active phase, a time when the islets maximally secrete insulin in response to glucose stimulation, but significantly elevated in the light/inactive phase, when glucose-stimulated insulin secretion capacity was suppressed. Using beta cell–specific UCP2 knockout mice and clonal MIN6 cells treated with a genipin, we further demonstrated that daily rhythms of GSIS capacity are dependent on rhythmic UCP2 expression/activity via modulation of glucose-induced ATP production in a time of day-dependent manner (8). Importantly, loss of rhythmic Ucp2 expression impaired glucose tolerance, but only in the light/inactive phase of the day, when Ucp2 expression was upregulated (8). Together, these studies demonstrate that regulation of rhythmic insulin secretion capacity and ultimately temporal glucose tolerance are dependent on the rhythmic expression and activation of key proteins (UCP2, in this case) that regulate metabolic secretion coupling signals.
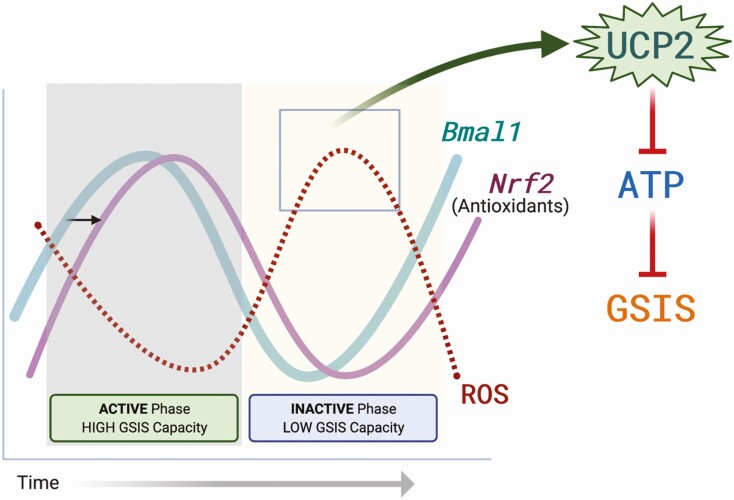
To determine if the circadian clock directly regulates Ucp2 expression (via binding of Bmal1 to E-box elements in the Ucp2 promoter), Lee et al (2013) performed chromatin immunoprecipitation (ChIP) assays using INS-1 cells (unsynchronized, at a single time point) (26). No enrichment of BMAL1 at the Ucp2 promoter was observed, suggesting that the circadian oscillator does not directly drive the temporal expression of Ucp2 but rather, it is likely that secondary signals or other transcription factors that are themselves circadian clock-driven are the driving force behind rhythmic Ucp2 expression. Notably, several known regulators of Ucp2, including SIRT1, PGC-1α, and SREBP1c, are expressed in a circadian fashion in other metabolic tissues (39), but this level of regulation has not been further explored in a beta cell context. Additionally, Lee et al (2013) demonstrated direct binding of BMAL1 to E-box elements in the cis-promoter region of Nrf2, an antioxidant response element (ARE) transcription factor that critically regulates antioxidant gene expression (26). In this same study, impaired GSIS in Ins2Cre:Bmal1fl/fl (β-Bmal1-/-) islets was accompanied by increased reactive oxygen species (ROS) production, scavenging of which (with N-acetylcysteine) fully restored GSIS. Together, these functional investigations reveal a circadian-regulated ROS-UCP2-OXPHOS axis which dictates time-of-day ATP production efficiency and ultimately diurnal rhythms of GSIS (Fig. 2). Additionally, the circadian regulation of antioxidant expression in the beta cell fits with the previously postulated role for the clock in controlling cycles of beta cell stress responses and recovery periods (Fig. 1). While UCP2 appears to be a key player linking circadian-regulated ROS signals to metabolic function in the healthy beta cell, it is not clear if disruption of this axis contributes to the pathophysiology of diabetes development in human T2D islets.
Figure 2:
The ROS-UCP2-OXPHOS axis in the beta cell. Bmal1 has been shown to directly bind and regulate the expression of Nrf2, which is considered the master regulator of antioxidant gene expression. Cyclical expression of Nrf2 in turn drives circadian cycles of reactive oxygen species (ROS) signals. In the inactive phase of the day, ROS-mediated activation of UCP2 uncouples the beta cell mitochondrial electron transport chain, dampening oxidative phosphorylation of ADP to ATP. Reduced ATP production prevents insulin secretion in the inactive phase of the day. As Nrf2 expression is activated by Bmal1, ROS signals are reduced, preventing UCP2 activation and allowing for maximal GSIS capacity in the active phase of the day, when food is anticipated. Created with BioRender.com.
A Role for the Circadian Clock in Postnatal Beta Cell Functional Maturation?
While beta cells emerge from endocrine progenitors during early- to mid-embryogenesis, beta cell development continues postnatally. At birth, amino acid infusion, but not glucose infusion, increases plasma insulin levels in newborn infants (40, 41), demonstrating a lack of glucose-responsiveness in immature neonatal beta cells, which is also seen in rodent models, despite normal insulin content and ion channel activity (42-45). While the intrinsic and extrinsic cellular factors and mechanisms that control postnatal beta cell maturation remain incompletely understood, emerging evidence suggests that establishment of global circadian rhythms and the beta cell circadian oscillator may be critical. Using Per1:LUC isolated rat islets, Rakshit et al (2018) recently demonstrated that rhythmic islet circadian oscillations emerge in the early perinatal period (prior to weaning), with robust circadian cycles observed by postnatal day 30, when daily variations in plasma insulin and robust GSIS are also evident (46). Notably, a similar timing of circadian establishment is seen in the liver (47), which corresponds with the development of circadian feeding rhythms in rodents. Importantly, embryonic deletion of Bmal1 in the beta cell (β-Bmal1-/-) impeded beta cell functional maturation in rat islets, defined by a lack of glucose-responsiveness at postnatal day 25 (when control cells showed functional maturity) and downregulation of pathways critical for beta cell function, including GO terms for oxidation-reduction processes, responses to zinc ions, and regulation of glucose metabolic processes (46). Surprisingly, the lack of beta cell functional maturation in β-Bmal1-/- islets was not associated with changes in the expression of known developmental maturation markers (Ucn3, Pdx-1, and Mafa) or genes typically associated with beta cell immaturity (Ldha) (46). Postnatal beta cell maturation is a highly complex process involving transcriptional reprogramming and major metabolic transitions, which was recently shown to involve nutrient-responsive changes in the microRNA landscape of the islet (48). Interestingly, microRNAs were also shown to bind to and alter the expression of the core components of the circadian oscillator (49), suggesting a contributory role for nutrient-responsive microRNAs in establishing islet circadian rhythms and functional maturity; however, this remains debatable as it was also shown that failure to wean pups from maternal lipid-rich milk to a high-carbohydrate chow diet did not affect the establishment of islet circadian rhythms (46). While studies of circadian ontology and its role in human islets are lacking, these initial preclinical rodent studies suggest that postnatal induction of Bmal1 and establishment of circadian rhythms contributes to the complexity of postnatal beta cell functional maturation and warrants further evaluation.
Further evidence for the importance of the circadian clock in beta cell functional maturation comes from research in the area of stem cell replacement therapy. Cell replacement therapies hold strong therapeutic potential for people living with diabetes. Islet transplant procedures are limited by donor supply and host immunogenicity issues, which has made autologous stem cell-derived beta cell replacement an attractive option. Unraveling and defining the physiological and molecular mechanisms that drive beta cell maturation is essential for the successful generation of functionally mature beta cells from human pluripotent stem cells for use in such therapies. Recently, Alvarez-Dominguez et al (2020) demonstrated that in vitro circadian entrainment of late-stage (Ins+/Gcg-) stem cell-derived islets promoted enhanced functional maturity using an optimized entrainment protocol that mimics fasting and feeding cycles. The enhanced functional maturity was characterized by robust and rhythmic patterns of GSIS along with stable and persistent oscillatory expression of genes involved in glucose metabolism, insulin synthesis and secretion, and maturity-linked factors (PDX-1, NKX6.1, NEUROD1, IAPP, MAFA). Additionally, enhanced functional maturity was linked to newly opened but stable chromatin changes, where core clock transcription factors were found enriched at critical insulin regulatory genes (eg, CADPS, SYT4, STX2). Interestingly, core clock oscillators (ARNTL, PER1/2, and CRY1/2) were not expressed at detectable levels in unentrained stem-cell-derived islets, but rather were only induced upon entrainment, suggesting that fasting and feeding cycles are not only important for synchronization of the islet clock, but also for its activation during development.
A Role for the Circadian Clock in Beta Cell Proliferation and Adaptation to Stress?
Regulation of beta cell proliferation is a dynamic and complex process that requires different factors at different developmental stages, leading to varied proliferative capacities throughout the lifespan. In the perinatal period, functionally immature beta cells are highly proliferative; however, the rate of beta cell proliferation rapidly declines in early life such that, in adulthood, functionally mature beta cells are quiescent with very little proliferative capacity (reviewed in (50)). It has been postulated that the neonatal wave of beta cell mass expansion is essential for the establishment of sufficient adult beta cell mass; lack of which is thought to increase susceptibility to T2D (51). While not yet explored directly, evaluation of beta cell mass and proliferation in embryonic circadian-deficient models suggest that the islet clock does not impact perinatal beta cell proliferation. Notably, circadian mutants and circadian-deficient rodent models are viable and show little impact, if any, on islet architecture and structure. Whole-body ClockΔ19/Δ19 mutants and Bmal1-/- mice have overall normal islet architecture, but generally smaller islets with reduced Ki67+ staining (18); however, evaluation of islet structure in beta cell- and pancreas-specific Bmal1 knockout models reveals no change in beta cell mass or islet size (26, 46), suggesting that the formation of smaller islets in the whole-body circadian mutants is likely secondary to other factors associated with whole-body arrythmia and not specific to the islet oscillator. Additionally, beta cell mass and proliferation were unchanged in the early postnatal period in β-Bmal1-/- mice compared with controls, suggesting that the islet circadian oscillator likely does not have a contributory role in regulation of postnatal beta cell mass expansion.
In the healthy adult, beta cell mass is maintained by a balance of apoptosis and relatively slow rates of beta cell replication in mice, with less clarity of these processes in humans. Although beta cell mass is relatively stable in adulthood, there are conditions where significant beta cell mass expansion occurs, including during pregnancy and in the obese state. Failed adaptive beta cell mass expansion in these states is associated with the development of gestational diabetes and T2D, respectively. Emerging evidence implicates the beta cell circadian clock in the physiological adaptation of the beta cell to diet-induced obesity (27). In control animals, high-fat diet consumption stimulated a 50% increase in beta cell mass with a 3-fold increase in beta cell proliferation as expected; however, conditional deletion of Bmal1 in adult mouse beta cells prevented high-fat diet–induced beta cell mass expansion (characterized by increased apoptosis and reduced proliferation), which was associated with fasting and diurnal hyperglycemia, exacerbated glucose intolerance, and loss of in vivo and in vitro GSIS (27). Interestingly, Bmal1 deletion altered the expression of important cell cycle genes in this model (increased expression of cell cycles inhibitors and reduced expression of cell cycle repressors) and increased apoptosis, suggesting that the circadian clock likely contributes to the regulation of adaptive beta cell mass by shifting the balance between proliferation and apoptosis. Further, this study suggests that maintenance and/or stimulation of circadian rhythms in the beta cell may provide an important strategy to increase beta cell resilience and adaptation capacity to obesity-driven metabolic stress.
Beta cell loss contributes significantly to the pathophysiology of both T1D and T2D and current research aims to develop strategies to promote beta cell regeneration as a means to overcome beta cell loss and restore metabolic health in diabetes. While other metabolic tissues, including the intestine and liver, have a high regenerative capacity after acute cell loss, beta cell regeneration is comparatively limited and is a slow process (taking weeks to months) (52) and our understanding of the regenerative process in the beta cell is incomplete. Using dox-inducible expression of diphtheria toxin A in beta cells (Ins-rtTA/TET-DTA mice) to induce massive beta cell ablation, Petrenko et al (2020) recently demonstrated that beta cell regeneration (ie, proliferation of residual beta cell) is regulated in a circadian manner with peak proliferation (BrdU incorporation, indicative of entering S-phase) occurring during the dark/active phase of the daily cycle (53). Importantly, arrhythmic Bmal1-/- mice were unable to mount a proliferative response to beta cell ablation, leading to elevated hyperglycemia and fatal diabetes (53). Single cell transcriptional profiling of residual, regenerating beta cells revealed that beta cell ablation triggered a modified transcriptional landscape, which included 1635 transcripts that lost rhythmic expression and 2208 genes that acquired circadian rhythmicity after ablation compared with control beta cells (53). Transcripts that lost rhythmicity in residual beta cells were related to mitochondrial function, oxidative phosphorylation, and xenobiotic metabolism; whereas the top 3 canonical pathways that were enriched in the genes that gained circadian rhythmicity included genes related to mitosis, cell cycle control of chromosome replication, and estrogen-mediated S-phase re-entry signaling. The circadian clock–mediated transcriptional reprogramming of the regenerating beta cell provides an interesting perspective and suggests that circadian-regulated transcriptional pathways are malleable and adaptive to environmental and/or stress-related signals, further highlighting the importance of developing strategies to promote or augment circadian function, particularly in high-risk individuals.
Conclusions and Perspectives
Evidence is rapidly accumulating that depicts a critical role for the islet/beta cell circadian clock in the regulation of many aspects of beta cell biology, including control over daily rhythms of insulin secretion, postnatal functional maturation, and adaptation to beta cell stress and injury. With environmental circadian disruption being an inevitability of modern life and the rapidly rising rates of obesity and diabetes worldwide, understanding the mechanistic underpinnings of circadian regulation of beta cell biology has become an essential task. Current studies have begun to define the circadian-regulated transcriptional profiles in healthy and metabolic stress states, but delineating the functional impact of these circadian-regulated programs will be critical to our understanding of beta cell biology in both health and disease and along the path to improved beta cell replacement therapies. Additionally, recent preclinical studies provide evidence that augmentation of beta cell circadian rhythms can improve beta cell function in metabolically distressed states and that exploring therapeutic strategies to achieve circadian augmentation in humans, either pharmacologically or environmentally, has merit as a potential therapeutic option for diabetes treatment or for intervention in people at high-risk for diabetes. For example, Nobiletin, a known ROR agonist, not only augments both the circadian amplitude of core clock gene expression, but treatment of human T2D islets with Nobiletin recovered insulin secretion impairments (24). Additionally, beta cell–specific Bmal1 overexpression (b-Bmal1OV) augments beta cell circadian amplitude and confers protection against beta cell dysfunction and glucose intolerance in response to high-fat diet stress, providing strong rationale that augmentation of circadian rhythms, at least in islets, can be protective against metabolic dysfunction (54). In addition, it should also be noted that with few exceptions, the majority of studies that have been performed to date in this field have almost exclusively used male rodent and human cadaveric islets. Going forward, studies should be designed to include an exploration of the sex-specific effects of the circadian clock on beta cell function and metabolic health.
Acknowledgments
The authors would like to thank Prof. Jim Johnson (University of British Columbia) for insightful discussion and comments on the manuscript. C.A.D. is funded by a Canadian Institutes of Health Research (CIHR) Project Grant (159632), an National Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN/006551-2015) and holds the Dr. John A. Moorhouse Diabetes Fellowship from the Diabetes Foundation of Manitoba.
Glossary
Abbreviations
- ATP
adenosine 5′-triphosphate
- GSIS
glucose-stimulated insulin secretion
- ROS
reactive oxygen species
- SCN
suprachiasmatic nucleus
- T2D
type 2 diabetes
- UCP2
uncoupling protein 2
Additional Information
Disclosures: N.S., nothing to declare; C.A.D., nothing to declare.
Data Availability
No new data were created or analyzed in this review article. Therefore, data sharing is not applicable.
References
- 1. Rigas AN, Bittles AH, Hadden DR, Montgomery DA. Circadian variation of glucose, insulin, and free fatty acids during long-term use of oral hypoglycaemic agents in diabetes mellitus. Br Med J. 1968;4(5622):25-28. [PMC free article] [PubMed] [Google Scholar]
- 2. Malherbe C, De Gasparo M, De Hertogh R, Hoet JJ. Circadian variations of blood sugar and plasma insulin levels in man. Diabetologia. 1969;5(6):397-404. [DOI] [PubMed] [Google Scholar]
- 3. Barter PJ, Carroll KF, Nestel PJ. Diurnal fluctuations in triglyceride, free fatty acids, and insulin during sucrose consumption and insulin infusion in man. J Clin Invest. 1971;50(3):583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll KF, Nestel PJ. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes. 1973;22(5):333-348. [DOI] [PubMed] [Google Scholar]
- 5. Peschke E, Peschke D. Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia. 1998;41(9):1085-1092. [DOI] [PubMed] [Google Scholar]
- 6. Delattre E, Cipolla-Neto J, Boschero AC. Diurnal variations in insulin secretion and K+ permeability in isolated rat islets. Clin Exp Pharmacol Physiol. 1999;26(7):505-510. [DOI] [PubMed] [Google Scholar]
- 7. Picinato MC, Haber EP, Carpinelli AR, Cipolla-Neto J. Daily rhythm of glucose-induced insulin secretion by isolated islets from intact and pinealectomized rat. J Pineal Res. 2002;33(3):172-177. [DOI] [PubMed] [Google Scholar]
- 8. Seshadri N, Jonasson ME, Hunt KL, et al. Uncoupling protein 2 regulates daily rhythms of insulin secretion capacity in MIN6 cells and isolated islets from male mice. Mol Metab. 2017;6(7):760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perelis M, Marcheva B, Ramsey KM, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350(6261):aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrenko V, Saini C, Giovannoni L, et al. Pancreatic α- and β-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev. 2017;31(4):383-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saini C, Petrenko V, Pulimeno P, et al. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes Metab. 2016;18(4):355-365. [DOI] [PubMed] [Google Scholar]
- 12. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517-549. [DOI] [PubMed] [Google Scholar]
- 13. Sinturel F, Petrenko V, Dibner C. Circadian clocks make metabolism run. J Mol Biol. 2020;432(12):3680-3699. [DOI] [PubMed] [Google Scholar]
- 14. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344-2347. [DOI] [PubMed] [Google Scholar]
- 15. Hirao A, Tahara Y, Kimura I, Shibata S. A balanced diet is necessary for proper entrainment signals of the mouse liver clock. Plos One. 2009;4(9):e6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271-R277. [DOI] [PubMed] [Google Scholar]
- 17. Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54(1):120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62(10):3469-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qian J, Yeh B, Rakshit K, Colwell CS, Matveyenko AV. Circadian disruption and diet-induced obesity synergize to promote development of β-cell failure and diabetes in male rats. Endocrinology. 2015;156(12):4426-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petrenko V, Dibner C. Cell-specific resetting of mouse islet cellular clocks by glucagon, glucagon-like peptide 1 and somatostatin. Acta Physiol (Oxf). 2018;222(4):e13021. [DOI] [PubMed] [Google Scholar]
- 23. Pulimeno P, Mannic T, Sage D, et al. Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia. 2013;56(3):497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petrenko V, Gandasi NR, Sage D, Tengholm A, Barg S, Dibner C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc Natl Acad Sci U S A. 2020;117(5):2484-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J, Kim MS, Li R, et al. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in β-cells. Islets. 2011;3(6):381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee J, Moulik M, Fang Z, et al. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol Cell Biol. 2013;33(11):2327-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rakshit K, Hsu TW, Matveyenko AV. Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia. 2016;59(4):734-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stamenkovic JA, Olsson AH, Nagorny CL, et al. Regulation of core clock genes in human islets. Metabolism. 2012;61(7):978-985. [DOI] [PubMed] [Google Scholar]
- 29. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219-16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rakshit K, Qian J, Ernst J, Matveyenko AV. Circadian variation of the pancreatic islet transcriptome. Physiol Genomics. 2016;48(9):677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kojima S, Sher-Chen EL, Green CB. Circadian control of mRNA polyadenylation dynamics regulates rhythmic protein expression. Genes Dev. 2012;26(24):2724-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fustin JM, Doi M, Yamaguchi Y, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155(4):793-806. [DOI] [PubMed] [Google Scholar]
- 33. Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8(2):139-148. [DOI] [PubMed] [Google Scholar]
- 34. Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2(2):85-93. [DOI] [PubMed] [Google Scholar]
- 35. Robson-Doucette CA, Sultan S, Allister EM, et al. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes. 2011;60(11):2710-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105(6):745-755. [DOI] [PubMed] [Google Scholar]
- 37. Lee SC, Robson-Doucette CA, Wheeler MB. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. J Endocrinol. 2009;203(1):33-43. [DOI] [PubMed] [Google Scholar]
- 38. Sheets AR, Fülöp P, Derdák Z, et al. Uncoupling protein-2 modulates the lipid metabolic response to fasting in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294(4): G1017-G1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13(2):125-137. [DOI] [PubMed] [Google Scholar]
- 40. Grasso S, Messina A, Saporito N, Reitano G. Serum-insulin response to glucose and aminoacids in the premature infant. Lancet. 1968;2(7571):755-756. [DOI] [PubMed] [Google Scholar]
- 41. Pildes RS, Hart RJ, Warrner R, Cornblath M. Plasma insulin response during oral glucose tolerance tests in newborns of normal and gestational diabetic mothers. Pediatrics. 1969;44(1):76-83. [PubMed] [Google Scholar]
- 42. Rozzo A, Meneghel-Rozzo T, Delakorda SL, Yang SB, Rupnik M. Exocytosis of insulin: in vivo maturation of mouse endocrine pancreas. Ann N Y Acad Sci. 2009;1152:53-62. [DOI] [PubMed] [Google Scholar]
- 43. Rorsman P, Arkhammar P, Bokvist K, et al. Failure of glucose to elicit a normal secretory response in fetal pancreatic beta cells results from glucose insensitivity of the ATP-regulated K+ channels. Proc Natl Acad Sci U S A. 1989;86(12):4505-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blum B, Hrvatin S, Schuetz C, Bonal C, Rezania A, Melton DA. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol. 2012;30(3):261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aguayo-Mazzucato C, Sanchez-Soto C, Godinez-Puig V, Gutiérrez-Ospina G, Hiriart M. Restructuring of pancreatic islets and insulin secretion in a postnatal critical window. PLoS One. 2006;1:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rakshit K, Qian J, Gaonkar KS, Dhawan S, Colwell CS, Matveyenko AV. Postnatal Ontogenesis of the Islet Circadian Clock Plays a Contributory Role in β-Cell Maturation Process. Diabetes. 2018;67(5):911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sládek M, Jindráková Z, Bendová Z, Sumová A. Postnatal ontogenesis of the circadian clock within the rat liver. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1224-R1229. [DOI] [PubMed] [Google Scholar]
- 48. Jacovetti C, Matkovich SJ, Rodriguez-Trejo A, Guay C, Regazzi R. Postnatal β-cell maturation is associated with islet-specific microRNA changes induced by nutrient shifts at weaning. Nat Commun. 2015;6:8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jacovetti C, Rodriguez-Trejo A, Guay C, et al. MicroRNAs modulate core-clock gene expression in pancreatic islets during early postnatal life in rats. Diabetologia. 2017;60(10):2011-2020. [DOI] [PubMed] [Google Scholar]
- 50. Gunasekaran U, Hudgens CW, Wright BT, Maulis MF, Gannon M. Differential regulation of embryonic and adult β cell replication. Cell Cycle. 2012;11(13):2431-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3(11):758-768. [DOI] [PubMed] [Google Scholar]
- 52. Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117(9):2553-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petrenko V, Stolovich-Rain M, Vandereycken B, et al. The core clock transcription factor BMAL1 drives circadian β-cell proliferation during compensatory regeneration of the endocrine pancreas. Genes Dev. 2020;34(23-24):1650-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rakshit K, Matveyenko AV. Induction of core circadian clock transcription factor bmal1 enhances β-cell function and protects against obesity-induced glucose intolerance. Diabetes. 2021;70(1):143-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this review article. Therefore, data sharing is not applicable.