Abstract
OBJECTIVES
The purpose of this study was to analyze cumulative Medicare expenditures at index admission and after discharge by race or ethnicity.
BACKGROUND
Heart failure with preserved ejection fraction (HFpEF) is a growing proportion of heart failure (HF) admissions. Research on health care expenditures for patients with HFpEF is limited.
METHODS
Records of patients discharged from the Get With The Guidelines-Heart Failure registry between 2006 and 2014 were linked to Medicare data. The primary outcome was unadjusted payments for acute care services. Comparisons between race/ethnic groups were made using generalized linear mixed models. Cost ratios were reported by race/ethnicity, and adjustments were made sequentially for patient characteristics, hospital factors, and regional socioeconomic status.
RESULTS
Median Medicare costs for index hospitalizations were $7,241 for the entire cohort, $7,049 for whites, $8,269 for blacks, $8,808 for Hispanics, $8,477 for Asians, and $8,963 for other races. Median costs at 30 days for readmitted patients were $9,803 and $17,456 for the entire cohort at 1-year. No significant differences were seen in index admission cost ratios by race/ethnicity. At 30 days among readmitted patients, costs were 9% higher (95% confidence interval [CI]: 1% to 17%; p = 0.020) for blacks in the fully adjusted model than whites. At 1 year, costs were 14% higher (95% CI: 9% to 18%; p < 0.001) for blacks, 7% higher (95% CI: 0% to 14%; p = 0.041) for Hispanics, and 24% higher (95% CI: 8% to 42%; p = 0.003) for patients of other races. No significant differences between white and Asian expenditures were noted.
CONCLUSIONS
Minority patients with HFpEF have greater acute care service costs. Further research of improving care delivery is needed to reduce acute care use for vulnerable populations.
Keywords: BMI, CMS, diastolic heart failure, health care costs, health care disparities, heart failure with preserved ejection fraction, hospital readmissions, hospitalization
Direct medical costs for heart failure (HF) were estimated at $28.5 billion with an average growth rate of 1.1% per year in the United States for 2013 (1). An estimated 6.5 million American adults had HF between 2011 and 2014, based on self-reported data from the National Health and Nutrition Examination Survey (2). By 2030, the prevalence of HF was expected to increase to over 8 million people secondary to shifting age demographics (3). HF prevalence among Medicare beneficiaries was 13.5% in 2015 (4). Medicare Parts A and B combined spending averaged $28,963 per HF beneficiary and was the second most expensive chronic condition behind stroke in 2015 (5). Although improvements in the prevention and treatment of ischemic heart disease have lowered the age-standardized rates of HF with reduced ejection fraction (HFrEF), the proportion of patients with HF with preserved ejection fraction (HFpEF) continues to grow (6,7).
Relatively few studies have been peformed which have evaluated health care use and expenditures associated with HFpEF. Patients with HFpEF have an observed lower mortality rate but higher readmission rate than HFrEF patients (8). With respect to race/ethnicity, black patients with HFpEF have a higher risk of readmission at 30 days and 1 year when adjusting for patient characteristics, socioeconomic status (SES), and hospital factors (9). Furthermore, SES may also influence health care use. How the uses of health care for patients with HFpEF may differ based on race/ethnicity and SES status are not well described. This study reports differences in Medicare inpatient expenditures by race/ethnicity, using the Get With The Guidelines-Heart Failure (GWTG-HF) registry linked to the Centers for Medicare and Medicaid Services administrative data.
METHODS
COHORT.
Patients discharged from the GWTG-HF registry between January 1, 2006, and December 1, 2014, were screened. All patients included in GWTG-HF registry were identified by medical providers based on clinically diagnosed HF. Inclusion in the final cohort required age ≥65, eligibility for Medicare Parts A and B fee-for-service benefit during the discharge month, and left ventricular ejection fraction (LVEF) ≥50% on quantitative assessment; or if quantitative LVEF was not available, qualitative assessment of normal or mild dysfunction was included. LVEF criteria were consistent with society guideline definitions (10,11). Patients were excluded if disposition indicated transfer to a hospice facility, or they left against medical advice, or disposition was unknown. Patients were also excluded if regional SES variables were not available. The GWTG-HF registry was linked to Centers for Medicare and Medicaid Services administrative claims data providing use of services, expenditures, and outcomes at index admission and post-discharge.
STATISTICAL ANALYSIS.
Baseline patient and hospital characteristics were described by race/ethnic groups. Patient factors included age, sex, medical history, vital signs, body mass index, laboratory test values (i.e., blood urea nitrogen, serum creatinine, serum sodium, hemoglobin, hemoglobin A1c, and lipid panel). Hospital characteristics included region, rural location, teaching status, and size (number of beds). Percentages and median interquartile ranges were reported for categorical and continuous variables, respectively. The Pearson chi-squared test was used to compare categorical variables, and the Wilcoxon rank-sum test was used to compare ordinal categorical variables or continuous variables. Standardized differences were used to describe significant differences between groups. SES was linked using patients’ zip codes, geocoding to the 2015 Area Health Resource File provided through the Health Resources and Services Administration (12). SES variables included median household income, median home values, percentages of patients with high school diplomas, and percentage with ≥4 years’ of college (13). Patients’ SES variables were assigned values from census data of the year closest to the patients’ year of admission.
Primary outcomes included unadjusted Medicare (Part A) inpatient payments at index hospitalization, at 30 days, and at 1 year. Costs were standardized to 2014 dollars by using the Personal Health Care Hospital Care Index (14). Comparisons of health care use were modeled using generalized linear mixed models with a log link function and robust Poisson error distribution to allow for over-dispersion. Hospital-level random intercepts were used to account for clustering of patients within hospitals. Cost ratios were reported by race/ethnic classification controlling for relevant covariates. Secondary outcomes included the proportional change in variance for 1-year Medicare health care expenditures within the mixed models (15). Models were adjusted sequentially for patient and hospital characteristics, followed by patient SES based on zip code (Online Tables 1 to 3). Control variables were selected based on a review of published reports and prior established models used in GWTG-HF (8,13,16). Cumulative costs at 1 year were averaged over the number of patients at risk and accounting for the competing risk of death and differential length of time observed. Medical conditions coded as missing were imput to not present. Patient covariates with missingness were imputed for generalized linear mixed models (Online Table 4). Multiple imputations with 25 datasets were used to impute other patient covariates. Hospital characteristics were not imputed. Analyses were performed in SAS version version 9.4 software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
The final cohort included 53,065 beneficiaries (Online Tables 5 to 8). The median age of hospitalization was 83 years for whites, 77 years for blacks, 79 years for Hispanics, and 81 years for Asians (Table 1). A higher proportion of women was observed among blacks than among other race/ethnic groups. Additional stratified analyses by sex were made available (Online Tables 9 to 11). Black and Hispanic patients had lower rates of atrial fibrillation than the other race/ethnic groups. Black and Hispanic patients had higher rates of hypertension, diabetes, and median body mass index. Among black, Hispanic, and Asian patients, chronic renal disease and dialysis were more common. Systolic blood pressure was highest among black patients, followed by Hispanic and Asian patients. LVEF rates were similar among the racial/ethnic groups. More black patients were admitted to teaching hospitals.
TABLE 1.
Baseline Patient and Hospital Characteristics for Overall Patients With HFpEF and by Race/Ethnic Groups
| White (n = 44,871) |
Black (n = 4,767) |
Hispanic (n = 2,260) |
Asian (n = 842) |
Other (n = 325) |
Overall (N = 53,065) |
Standardized Difference White vs. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p Value | Black | Hispanic | Asian | Other | |||||||
| Demographics | |||||||||||
| Age, yrs | 83 (76-88) | 77 (71-84) | 79 (72-85) | 81 (75-87) | 77 (71-83) | 82 (75-88) | <0.0001 | 52.1 | 34.6 | 13.4 | 51.7 |
| Categorical age, yrs | <0.0001 | ||||||||||
| 65-69 | 9.26 | 20.54 | 16.02 | 11.64 | 17.54 | 10.65 | 32.1 | 20.4 | 7.8 | 24.5 | |
| 70-74 | 11.79 | 19.93 | 16.46 | 11.28 | 19.69 | 12.76 | 22.4 | 13.4 | 1.6 | 21.8 | |
| 75-79 | 15.56 | 19.13 | 18.58 | 19.95 | 18.77 | 16.10 | 9.4 | 8.0 | 11.5 | 8.5 | |
| ≥80 | 63.39 | 40.40 | 48.94 | 57.13 | 44.00 | 60.49 | 47.3 | 29.4 | 12.8 | 39.6 | |
| Women | 65.75 | 70.40 | 68.32 | 62.47 | 68.31 | 66.24 | <0.0001 | 10.0 | 5.5 | 6.8 | 5.5 |
| Medical history | |||||||||||
| Hypertension | 80.22 | 89.76 | 84.73 | 82.74 | 78.86 | 81.29 | <0.0001 | 27.0 | 11.9 | 6.5 | 3.4 |
| CAD | 45.33 | 37.26 | 44.44 | 41.24 | 45.30 | 44.51 | <0.0001 | 16.5 | 1.8 | 8.3 | 0.1 |
| Prior MI | 13.41 | 11.29 | 11.30 | 9.77 | 12.42 | 13.07 | <0.0001 | 6.4 | 6.4 | 11.4 | 3.0 |
| Atrial flutter/fibrillation | 47.33 | 25.33 | 25.44 | 31.85 | 32.55 | 44.12 | <0.0001 | 47.0 | 46.7 | 32.0 | 30.5 |
| Hyperlipidemia | 49.26 | 47.22 | 48.84 | 49.11 | 47.32 | 49.05 | 0.1314 | 4.1 | 0.8 | 0.3 | 3.9 |
| Peripheral vascular disease | 12.75 | 11.70 | 11.87 | 5.46 | 11.07 | 12.49 | <0.0001 | 3.2 | 2.7 | 25.6 | 5.2 |
| Diabetes (insulin or noninsulin treated) | 36.36 | 56.38 | 56.03 | 43.91 | 50.00 | 39.17 | <0.0001 | 41.0 | 40.2 | 15.4 | 27.8 |
| CVA/TIA | 16.70 | 19.82 | 15.18 | 16.12 | 12.42 | 16.88 | <0.0001 | 8.1 | 4.2 | 1.6 | 12.2 |
| COPD or asthma | 31.76 | 32.73 | 28.51 | 22.34 | 28.86 | 31.54 | <0.0001 | 2.1 | 7.1 | 21.3 | 6.3 |
| ICD only | 1.48 | 1.26 | 1.09 | 1.02 | 1.01 | 1.43 | 0.3242 | 1.9 | 3.5 | 4.2 | 4.2 |
| Anemia | 21.93 | 26.25 | 20.57 | 18.78 | 24.16 | 22.22 | <0.0001 | 10.1 | 3.3 | 7.8 | 5.3 |
| Dialysis (chronic) | 2.21 | 8.82 | 8.09 | 9.26 | 8.05 | 3.19 | <0.0001 | 29.3 | 26.8 | 30.7 | 26.7 |
| Chronic renal insufficiency, SCr >2.0 | 19.14 | 30.42 | 24.21 | 24.75 | 25.17 | 20.48 | <0.0001 | 26.3 | 12.3 | 13.6 | 14.6 |
| Depression | 12.73 | 7.02 | 10.26 | 5.20 | 7.38 | 11.97 | <0.0001 | 19.2 | 7.7 | 26.6 | 17.8 |
| Ischemic cause: history of CAD, MI, prior PCI, prior CABG, or prior PCI/CABG | 50.23 | 41.26 | 49.93 | 45.18 | 49.66 | 49.34 | <0.0001 | 18.1 | 0.6 | 10.1 | 1.1 |
| History panel missing | 5.50 | 6.75 | 6.42 | 6.41 | 8.31 | 5.69 | 0.0005 | 5.2 | 3.9 | 3.8 | 11.1 |
| Smoking | 6.64 | 10.05 | 6.75 | 4.32 | 7.17 | 6.92 | <0.0001 | 12.4 | 0.4 | 10.2 | 2.1 |
| Medications on admission | |||||||||||
| ACE-I | 29.41 | 30.61 | 29.81 | 21.50 | 33.16 | 29.43 | 0.0007 | 2.6 | 0.9 | 18.3 | 8.1 |
| ARB | 16.23 | 19.32 | 22.57 | 26.49 | 23.32 | 16.98 | <0.0001 | 8.1 | 16.1 | 25.2 | 17.9 |
| Aldosterone antagonist | 5.60 | 5.61 | 3.80 | 4.99 | 4.66 | 5.51 | 0.0551 | 0.0 | 8.6 | 2.7 | 4.3 |
| Aspirin | 45.14 | 40.84 | 40.10 | 32.82 | 53.37 | 44.40 | <0.0001 | 8.7 | 10.2 | 25.5 | 16.5 |
| Beta-blocker | 55.05 | 54.41 | 55.21 | 50.10 | 54.92 | 54.93 | 0.2437 | 1.3 | 0.3 | 9.9 | 0.3 |
| Diabetic medications (any) | 21.56 | 34.00 | 36.92 | 31.36 | 35.38 | 23.56 | <0.0001 | 28.1 | 34.3 | 22.4 | 31.0 |
| Anticoagulation therapy | 29.61 | 16.98 | 14.98 | 15.36 | 15.54 | 27.57 | <0.0001 | 30.2 | 35.7 | 34.7 | 34.1 |
| Diuretic agent | 61.87 | 56.96 | 52.86 | 44.15 | 57.51 | 60.75 | <0.0001 | 10.0 | 18.3 | 36.1 | 8.9 |
| Hydralazine | 5.80 | 15.01 | 9.73 | 6.91 | 4.66 | 6.78 | <0.0001 | 30.5 | 14.7 | 4.6 | 5.1 |
| Lipid-lowering agent (any) | 54.71 | 56.89 | 57.00 | 55.09 | 62.69 | 55.05 | 0.0166 | 4.4 | 4.6 | 0.8 | 16.3 |
| Vitals on admission | |||||||||||
| Heart rate, beats/min | 79 (68-92) | 79 (68-91) | 78 (68-91) | 77 (68-93) | 80 (69-92) | 79 (68-92) | 0.459 | 0.7 | 2.5 | 0.7 | 3.3 |
| SBP, mm Hg | 143 (124-163) | 153 (133-178) | 149 (130-172) | 147 (128-166) | 144 (123-162) | 144 (125-165) | <0.0001 | 34.5 | 21.8 | 11.8 | 0.0 |
| DBP, mm Hg | 72 (62-83) | 76 (66-88) | 72 (62-84) | 72 (62-84) | 73 (63-84) | 72 (62-84) | <0.0001 | 27.1 | 5.7 | 2.1 | 7.8 |
| BMI, kg/m2 | 27.46 (23.27-32.95) | 30.07 (25.21-36.33) | 29 (24.61-34.06) | 23.84 (21.03-27.55) | 29.26 (24.47-34.55) | 27.66 (23.43-33.27) | <0.0001 | 32.0 | 15.7 | 56.7 | 10.8 |
| Laboratory values | |||||||||||
| LVEF source | 0.0155 | ||||||||||
| Quantitative LVEF | 91.17 | 92.22 | 91.55 | 93.59 | 90.15 | 91.31 | 3.8 | 1.3 | 9.1 | 3.5 | |
| Qualitative LVEF | 8.83 | 7.78 | 8.45 | 6.41 | 9.85 | 8.69 | 3.8 | 1.3 | 9.1 | 3.5 | |
| EF, % | 60 (55-64) | 60 (55-65) | 60 (55-65) | 60 (55-65) | 60 (55-65) | 60 (55-64) | <0.0001 | 8.2 | 2.6 | 13.0 | 10.0 |
| Serum creatinine, mg/dl | 1.2 (0.9-1.7) | 1.5 (1.1-2.4) | 1.3 (0.9-2) | 1.3 (0.9-2.1) | 1.3 (0.9-2.1) | 1.2 (0.9-1.7) | <0.0001 | 12.7 | 9.0 | 10.9 | 10.2 |
| Serum sodium, mEq/l | 138 (135-141) | 139 (137-142) | 138 (135-140) | 137 (134-140) | 138 (135-140) | 138 (135-141) | <0.0001 | 10.7 | 14.1 | 13.0 | 5.4 |
| BUN, mg/dl (IQR) | 25 (18-36) | 25 (17-39) | 25 (18-40) | 26 (18-39.5) | 25 (17.5-40.5) | 25 (18-36) | 0.0086 | 5.7 | 10.6 | 13.6 | 11.1 |
| BNP on admission, pg/ml | 560 (304-1,020) | 571 (234.5-1,199.5) | 564 (270-1,120) | 627 (316-1,140) | 598 (275.5-1,120.5) | 562 (297-1,038.8) | 0.4905 | 13.0 | 8.5 | 7.9 | 2.9 |
| Hemoglobin, g/dl | 11.5 (10.2-12.8) | 10.9 (9.6-12.2) | 11.3 (9.9-12.5) | 11.3 (10-12.7) | 10.9 (9.6-12.2) | 11.4 (10.1-12.8) | <0.0001 | 16.8 | 6.9 | 0.7 | 27.4 |
| HbA1C (0-20), % | 6.5 (5.9-7.3) | 6.6 (5.9-7.6) | 6.8 (6-7.7) | 6.6 (6-7.3) | 6.5 (6-8.4) | 6.5 (5.9-7.4) | 0.0102 | 7.8 | 18.8 | 1.7 | 16.6 |
| Total cholesterol (10-1,000), mg/dl | 134 (111-161) | 142 (118-172) | 134 (114-166) | 139 (116-166) | 121 (102-147) | 135 (112-163) | <0.0001 | 21.4 | 9.9 | 15.2 | 27.9 |
| HDL (0-120), mg/dl | 40 (32-51) | 46 (36-56) | 41 (32-52) | 42 (35-52) | 40 (32-47) | 41 (33-51) | <0.0001 | 29.9 | 3.9 | 14.1 | 3.3 |
| LDL (30-500), mg/d | 72 (55-94) | 78 (58-101) | 72 (57-97) | 74 (59-92) | 64 (50-89) | 73 (56-94) | <0.0001 | 18.8 | 7.4 | 4.5 | 20.0 |
| Triglycerides (5-2,000), mg/dl | 89 (66-123) | 78 (59-110) | 95 (70-132) | 93 (64-127) | 94 (69.5-126) | 88 (65-122) | <0.0001 | 22.3 | 12.3 | 2.7 | 3.6 |
| Hospital characteristics | |||||||||||
| Hospital size, number of beds | 348 (222-481) | 438 (292-610) | 296 (243-438) | 330 (217-400) | 358 (194-368) | 348 (227-527) | <0.0001 | 44.4 | 0.8 | 4.7 | 18.6 |
| Geographic region | <0.0001 | ||||||||||
| West | 9.73 | 3.29 | 17.88 | 60.81 | 35.38 | 10.47 | 26.3 | 23.8 | 126.5 | 64.5 | |
| South | 30.40 | 43.36 | 41.19 | 15.20 | 16.92 | 31.70 | 27.1 | 22.7 | 36.8 | 32.1 | |
| Midwest | 24.13 | 22.99 | 8.41 | 8.79 | 28.62 | 23.14 | 2.7 | 43.6 | 42.3 | 10.2 | |
| Northeast | 35.74 | 30.35 | 32.52 | 15.20 | 19.08 | 34.70 | 11.5 | 6.8 | 48.5 | 38.0 | |
| Rural location | 7.42 | 4.95 | 1.15 | 7.15 | 16.36 | 6.98 | <0.0001 | 10.3 | 31.3 | 1.0 | 27.9 |
| Teaching status | 55.18 | 72.00 | 40.53 | 51.37 | 74.77 | 56.13 | <0.0001 | 35.5 | 29.6 | 7.6 | 42.0 |
| Heart transplants performed at site | 9.11 | 12.72 | 5.16 | 7.71 | 5.56 | 9.22 | <0.0001 | 11.6 | 15.4 | 5.0 | 13.7 |
Values are median (interquartile range) or %. Standardized differences are references to those in whites. Standardized differences of ≥10 are clinically meaningful.
ACE-I = angiotensin-converting enzyme inhibitors; ARB = angiotensin II receptor blocker; BMI = body mass index; BNP = B-type natriuretic peptide; BUN = blood urea nitrogen; CABG = coronary artery bypass grafting; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; DBP = diastolic blood pressure; HbA1C = hemoglobin A1C; HDL = high-density lipoprotein; ICD = implantable cardioverter defibrillator; LDL = low-density lipoprotein; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; SBP = systolic blood pressure; SCr = serum creatinine; TIA = transient ischemic attack.
Median Medicare Part A costs for index hospitalization were $7,241 for the entire cohort, $7,049 for whites, $8,269 for blacks, $8,808 for Hispanics, $8,477 for Asians, and $8,963 for other race (Figure 1, Table 2). Minorities overall had higher hospitalization costs for the index admission. Median costs at 30 days for readmitted patients were $9,803 and $17,456 at 1 year for the entire cohort. Medicare costs among minority patients at index, at 30 days, and at 1 year were higher. Unadjusted cumulative average Medicare costs by race/ethnicity are shown in Figure 2.
FIGURE 1. Mean Cumulative Unadjusted Costs at 1 Year for Patients With HFpEF by Race/Ethnicity.
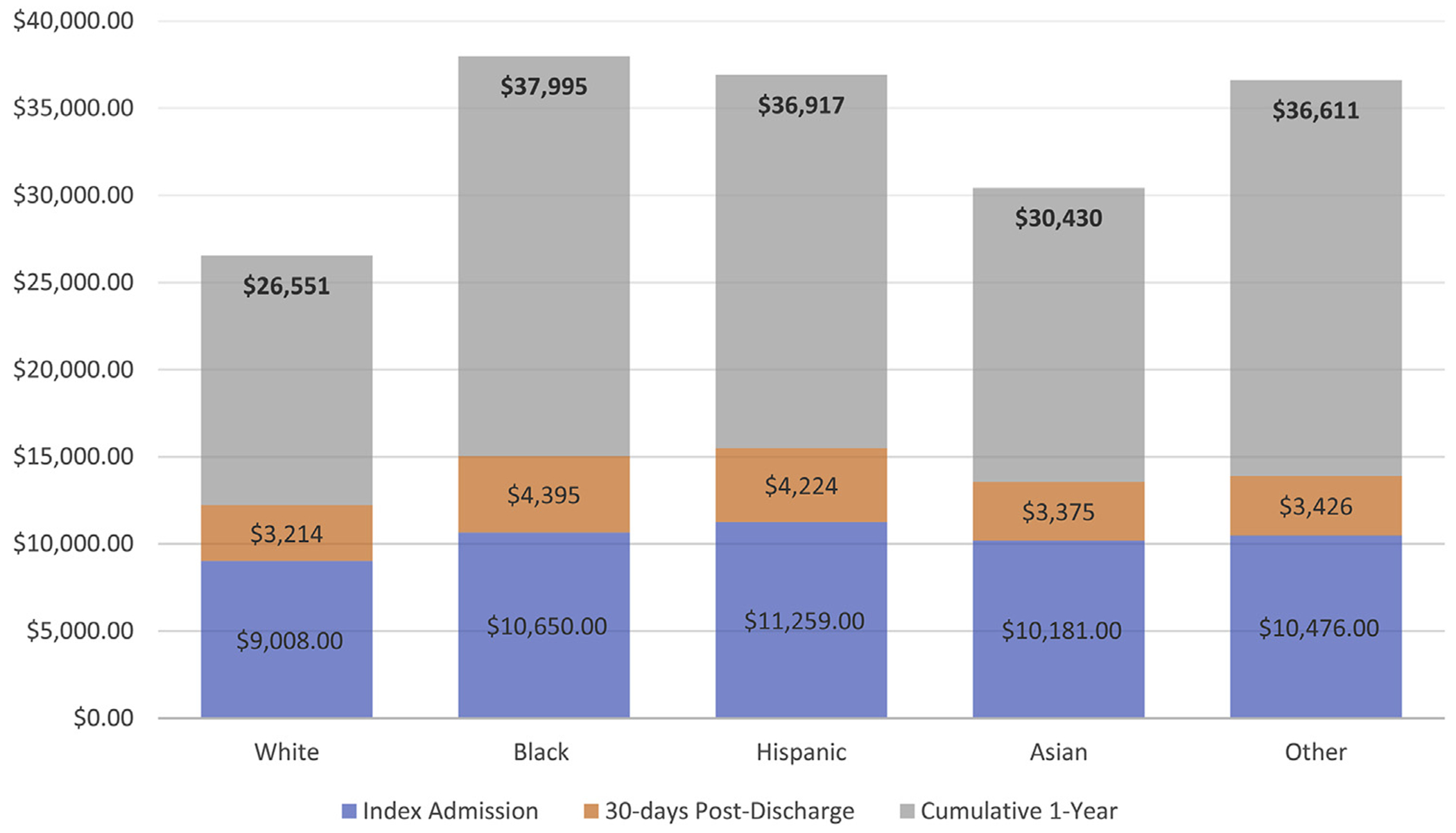
HFpEF = heart failure with preserved ejection fraction.
TABLE 2.
Total Medicare Part A Costs in 2014 Dollars
| Overall (N = 53,065) |
White (n = 44,871) |
Black (n = 4,767) |
Hispanic (n = 2,260) |
Asian (n = 842) |
Other (n = 325) |
% Std. Diff. vs White |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs | p Value | Black | Hispanic | Asian | Other | ||||||
| ALL patients | |||||||||||
| Index admission | <0.0001 | 14.9 | 21.2 | 12.3 | 15.7 | ||||||
| Median | $7,241 | $7,049 | $8,269 | $8,808 | $8,477 | $8,963 | |||||
| Mean | $9,279 | $9,008 | $10,650 | $11,259 | $10,181 | $10,476 | |||||
| ±SD | $10,050 | $9,722 | $12,157 | $11,400 | $9,286 | $8,898 | |||||
| 90th percentile | $14,259 | $13,654 | $17,236 | $17,542 | $14,719 | $15,331 | |||||
| 99th percentile | $47,143 | $45,788 | $52,390 | $56,791 | $47,982 | $51,115 | |||||
| Patients discharged alive and with follow-up data | |||||||||||
| At 30 days | (N = 51,543) | (n = 43,521) | (n = 4,686) | (n = 2,208) | (n = 813) | (n = 315) | <0.0001 | 9.3 | 8.6 | 1.4 | 2.2 |
| Median | $0 | $0 | $0 | $0 | $0 | $0 | |||||
| Mean | $3,368 | $3,214 | $4,395 | $4,224 | $3,375 | $3,426 | |||||
| ±SD | $10,865 | $10,210 | $14,754 | $13,102 | $12,002 | $8,795 | |||||
| 90th percentile | $10,671 | $10,265 | $13,423 | $13,007 | $10,789 | $12,325 | |||||
| 99th percentile | $45,526 | $43,563 | $53,418 | $54,126 | $48,845 | $44,896 | |||||
| At 1 yr | (N = 43,212) | (n = 36,380) | (n = 4,003) | (n = 1,832) | (n = 715) | (n = 282) | <0.0001 | 27.3 | 25.1 | 9.2 | 23.2 |
| Median | $9,065 | $8,544 | $13,105 | $12,250 | $9,055 | $13,418 | |||||
| Mean | $19,048 | $17,683 | $27,529 | $25,948 | $20,635 | $26,030 | |||||
| ±SD | $30,895 | $28,632 | $42,118 | $36,778 | $35,309 | $42,044 | |||||
| 90th percentile | $50,120 | $46,435 | $70,563 | $71,026 | $52,799 | $66,771 | |||||
| 99th percentile | $144,885 | $131,656 | $201,000 | $167,371 | $177,330 | $198,913 | |||||
| Cumulative 1 yr | (N = 43,212) | (n = 36,380) | (n = 4,003) | (n = 1,832) | (n = 715) | (n = 282) | <0.0001 | 29.9 | 29.1 | 11.4 | 26.9 |
| Median | $17,663 | $16,898 | $23,266 | $23,292 | $18,168 | $23,497 | |||||
| Mean | $28,180 | $26,551 | $37,995 | $36,917 | $30,430 | $36,611 | |||||
| ±SD | $33,137 | $30,772 | $44,596 | $39,804 | $36,914 | $43,136 | |||||
| 90th percentile | $62,335 | $57,988 | $84,220 | $85,392 | $66,235 | $77,571 | |||||
| 99th percentile | $163,171 | $150,311 | $226,525 | $182,913 | $193,769 | $209,350 | |||||
| Readmitted patients only | |||||||||||
| At 30 days | (N = 11,401) | (n = 9,500) | (n = 1,140) | (n = 536) | (n = 160) | (n = 65) | <0.0001 | 14.5 | 14.2 | 12.7 | 10.5 |
| Median | $9,803 | $9,545 | $11,333 | $11,485 | $10,876 | $11,619 | |||||
| Mean | $14,936 | $14,443 | $17,603 | $17,246 | $16,994 | $16,035 | |||||
| ±SD | $18,672 | $17,418 | $25,368 | $21,903 | $22,345 | $12,449 | |||||
| 90th percentile | $29,055 | $27,887 | $33,503 | $33,771 | $37,203 | $33,386 | |||||
| 99th percentile | $84,955 | $82,081 | $107,372 | $83,174 | $105,841 | $58,524 | |||||
| At 1 yr | (N = 28,593) | (n = 23,830) | (n = 2,871) | (n = 1,254) | (n = 449) | (n = 189) | <0.0001 | 28.7 | 30.8 | 16.0 | 29.5 |
| Median | $17,456 | $16,589 | $23,179 | $24,890 | $19,659 | $23,865 | |||||
| Mean | $28,636 | $26,859 | $38,114 | $37,801 | $32,607 | $38,578 | |||||
| ±SD | $34,130 | $31,653 | $45,477 | $39,079 | $39,879 | $46,362 | |||||
| 90th percentile | $62,598 | $58,587 | $85,126 | $85,530 | $72,867 | $74,307 | |||||
| 99th percentile | $168,916 | $155,460 | $233,052 | $182,865 | $198,151 | $265,895 | |||||
Standardized differences are referenced to those in whites. Standardized differences of ≥10 are clinically meaningful. Costs at 30 days and 1 yr do not include index admission costs.
FIGURE 2. Cumulative Average Medicare Part A Costs by Race/Ethnic Groups.
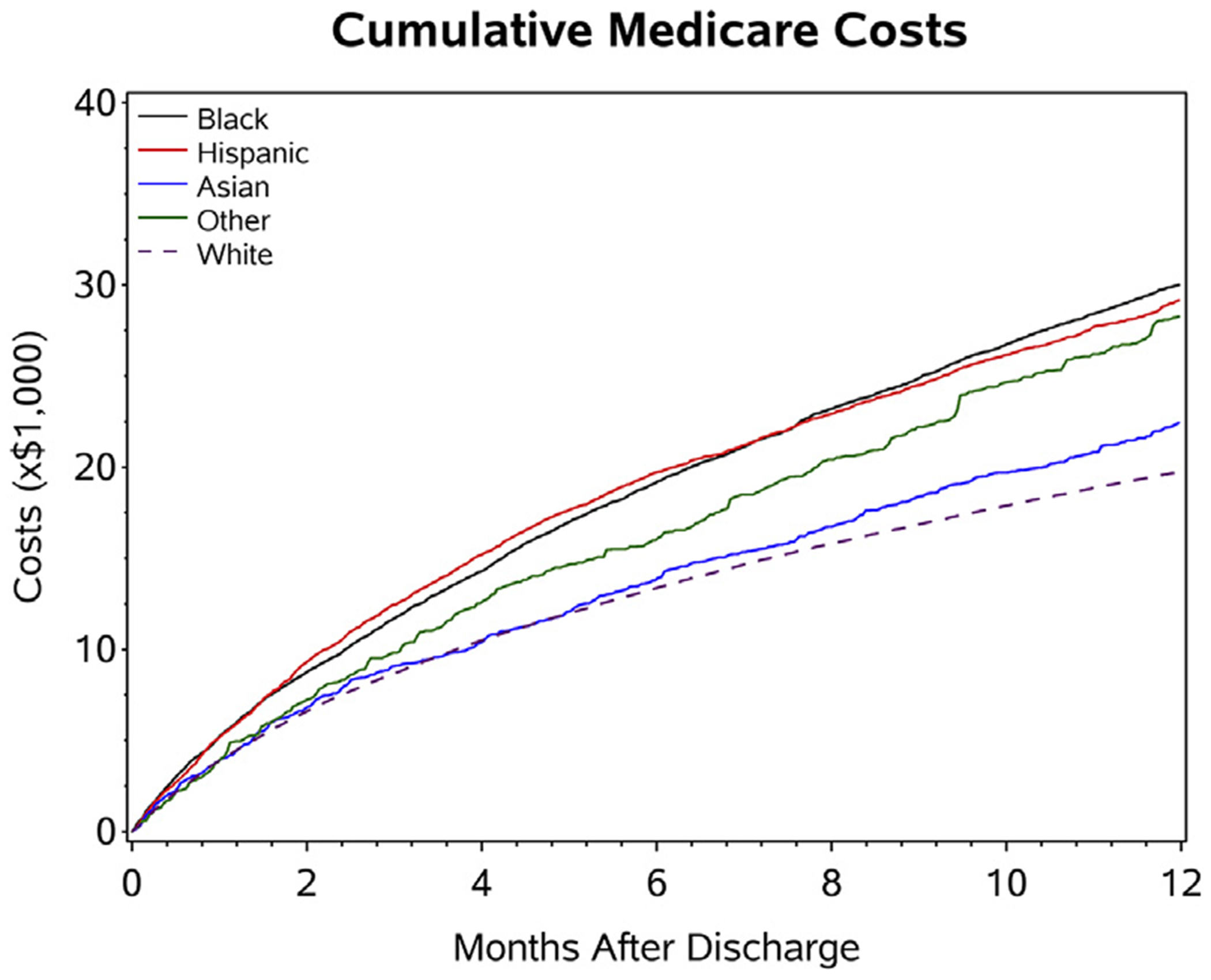
Costs were summed and divided by the number of patients at risk during a period of observation. Therefore, the competing risk of death is adjusted.
After adjustments for patient characteristics, hospital factors, and regional SES, cost ratios for the index admission were not considerably different based on race/ethnicity across all models (Table 3). At 30 days, Medicare costs were greater for blacks with a 9% (95% confidence interval [CI]: 1% to 17%) higher relative cost than for whites after sequential adjustments across all models. At 1 year, Medicare costs were higher for black, Hispanic, and patients of other races than for whites, which was significant across all models. For blacks, unadjusted costs were 27% higher, and adjusted costs (for patient characteristics, hospital factors, and regional SES) were 14% higher than for whites. For Hispanics, unadjusted costs were 19% higher, and costs after sequential adjustment were 7% to 8% higher. For the other race category, unadjusted costs were 40% higher, and sequentially adjusted costs were 24% to 25% higher. Costs among Asians were not significantly different compared with those of whites across any of the models.
TABLE 3.
Ratios of Medicare Part A Costs by Race/Ethnic Groups
| Unadjusted Model |
Cluster Adjusted |
Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Costs | Cost Ratio (95% CI) |
p Value | Cost Ratio (95% CI) |
p Value | Cost Ratio (95% CI) |
p Value | Cost Ratio (95% CI) |
p Value | Cost Ratio (95% CI) |
p Value |
| Among all patients | ||||||||||
| Index admission | ||||||||||
| Black | 1.18 (1.15-1.22) | <0.001 | 1.02 (0.99-1.05) | 0.232 | 0.99 (0.96-1.02) | 0.344 | 0.98 (0.95-1.01) | 0.278 | 0.98 (0.95-1.02) | 0.317 |
| Hispanic | 1.25 (1.20-1.30) | <0.001 | 1.04 (0.99-1.09) | 0.107 | 1.01 (0.96-1.05) | 0.702 | 1.01 (0.97-1.06) | 0.599 | 1.01 (0.96-1.05) | 0.750 |
| Asian | 1.13 (1.05-1.21) | <0.001 | 0.96 (0.90-1.04) | 0.314 | 0.96 (0.89-1.02) | 0.207 | 0.95 (0.89-1.02) | 0.145 | 0.95 (0.88-1.02) | 0.128 |
| Other | 1.16 (1.04-1.30) | 0.008 | 1.04 (0.93-1.15) | 0.492 | 1.00 (0.90-1.11) | 0.978 | 0.99 (0.90-1.10) | 0.912 | 0.99 (0.89-1.10) | 0.864 |
| White | Reference | Reference | Reference | Reference | Reference | |||||
| Among readmitted patients | ||||||||||
| At 30 days | ||||||||||
| Black | 1.22 (1.13-1.31) | <0.001 | 1.14 (1.06-1.23) | <0.001 | 1.10 (1.03-1.18) | 0.006 | 1.10 (1.03-1.18) | 0.007 | 1.09 (1.01-1.17) | 0.020 |
| Hispanic | 1.19 (1.08-1.32) | <0.001 | 1.09 (0.98-1.21) | 0.098 | 1.05 (0.95-1.16) | 0.361 | 1.06 (0.95-1.17) | 0.283 | 1.01 (0.91-1.13) | 0.810 |
| Asian | 1.18 (0.98-1.41) | 0.080 | 1.11 (0.93-1.33) | 0.237 | 1.10 (0.92-1.31) | 0.287 | 1.08 (0.91-1.29) | 0.371 | 1.04 (0.87-1.23) | 0.701 |
| Other | 1.11 (0.83-1.49) | 0.484 | 1.04 (0.79-1.38) | 0.781 | 0.97 (0.74-1.27) | 0.836 | 0.96 (0.73-1.26) | 0.767 | 0.95 (0.72-1.25) | 0.715 |
| White | Reference | Reference | Reference | Reference | Reference | |||||
| At 1 yr | ||||||||||
| Black | 1.42 (1.36-1.48) | <0.001 | 1.27 (1.22-1.33) | <0.001 | 1.14 (1.10-1.19) | <.0001 | 1.14 (1.09-1.19) | <0.001 | 1.14 (1.09-1.18) | <0.001 |
| Hispanic | 1.41 (1.33-1.49) | <0.001 | 1.19 (1.11-1.27) | <0.001 | 1.08 (1.01-1.15) | 0.018 | 1.08 (1.02-1.15) | 0.015 | 1.07 (1.00-1.14) | 0.041 |
| Asian | 1.21 (1.10-1.35) | <0.001 | 1.11 (0.99-1.23) | 0.063 | 1.08 (0.98-1.19) | 0.137 | 1.07 (0.97-1.19) | 0.161 | 1.06 (0.96-1.17) | 0.260 |
| Other | 1.44 (1.24-1.66) | <0.001 | 1.40 (1.21-1.61) | <0.001 | 1.25 (1.08-1.43) | 0.002 | 1.25 (1.08-1.43) | 0.002 | 1.24 (1.08-1.42) | 0.003 |
| White | Reference | Reference | Reference | Reference | Reference | |||||
The unadjusted model did not include hospital random intercepts; all other models included hospital random intercepts. Model 1 was adjusted for patient characteristics only; model 2 was adjusted for patient and hospital characteristics; model 3 was adjusted for patient, hospital characteristics, and regional SES variables based on patient zip code.
CI = confidence interval; p = p value; SES = socioeconomic status.
The proportional changes in variance (PCV) from the mixed models with sequential adjustments were used to explain hospital-level variations in Medicare expenditures. For the overall cohort, patient factors explained 14.7% of the variances among hospitals and hospital factors added 23.5%, and regional SES 30.4% to the incremental PCV (Table 4). When we evaluated these factors by race/ethnicity, we found that black race and hospital factors contributed less to the observed variations in Medicare expenditures with a larger incremental PCV (57.1%) explained by the addition of regional SES. For Hispanics, both hospital factors and regional SES explained a greater portion of Medicare expenditure variation than patient factors alone.
TABLE 4.
Impact of Patient, Hospital, and SES Factors on Hospital Variations in Medicare Expenditures at 1 Year by Race/Ethnic Groups
| Overall |
White |
Black |
Hispanic |
|||||
|---|---|---|---|---|---|---|---|---|
| PCV | Incremental PCV* | PCV | Incremental PCV* | PCV | Incremental PCV* | PCV | Incremental PCV* | |
| Adjusted model 1: patient factors | 14.7 | −10.2 | −1.7 | −14.2 | ||||
| Adjusted model 2: patient and hospital | 34.7 | 23.5 | 19.0 | 26.6 | 10.7 | 12.2 | 39.1 | 46.6 |
| Adjusted model 3: patient, hospital, and SES | 54.6 | 30.4 | 37.9 | 23.3 | 61.7 | 57.1 | 67.4 | 46.5 |
Incremental PCV calculates the PCV from the previous model.
PCV = proportional change in variance; other abbreviation as in Table 3.
Rates of acute care service use was higher among minority patients (Table 5). Cumulative length of stay for patients readmitted at 1 year was higher for black, Hispanic, and other patients. Readmission rates at 30 days and at 1 year were also higher for minority patients, along with medical procedure rates.
TABLE 5.
Acute Care Services by Race/Ethnicity Over 1 Yr From Index Admission
| Overall (N = 53,065) |
White (n = 44,871) |
Black (n = 4,767) |
Hispanic (n = 2,260) |
Asian (n = 842) |
Other (n = 325) |
% Standard Difference vs. Whites |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p Value | Black | Hispanic | Asian | Other | |||||||
| Among all patients | |||||||||||
| Index LOS | 4 (3-7) | 4 (3-7) | 4 (3-7) | 4 (3-7) | 4 (2-7) | 4 (3-7) | <0.0001 | 6.2 | 7.0 | 9.5 | 5.1 |
| Among 1 yr follow-up patients | |||||||||||
| Readmissions | 1 (0-2) | 1 (0-2) | 1 (0-3) | 1 (0-3) | 1 (0-2) | 1 (0-3) | <0.0001 | 22.1 | 19.4 | 9.0 | 14.3 |
| Cumulative LOS | 5 (0-14) | 5 (0-14) | 7 (0-20) | 6 (0-18) | 4 (0-12) | 6 (0-18) | <0.0001 | 24.4 | 17.3 | 4.1 | 17.7 |
| Mean LOS per admission | 2 (0-4.1) | 2 (0-4) | 2.5 (0-5) | 2.3 (0-4.7) | 1.5 (0-4) | 2.3 (0-4.5) | <0.0001 | 21.9 | 11.5 | 3.0 | 13.9 |
| Among readmitted patients | |||||||||||
| Readmissions | 2 (1-3) | 2 (1-3) | 2 (1-4) | 2 (1-4) | 2 (1-3) | 2 (1-4) | <0.0001 | 20.6 | 24.1 | 9.3 | 19.0 |
| Cumulative LOS | 10 (5-20) | 10 (5-20) | 13 (6-26) | 12 (6-26) | 9 (4-18) | 12 (6-25) | <0.0001 | 24.6 | 19.9 | 2.8 | 22.4 |
| Mean LOS per admission | 3.3 (2-5.3) | 3.3 (2-5.2) | 3.7 (2.3-6.2) | 3.7 (2.1-5.6) | 3.3 (2-5.3) | 3.6 (2.2-5.5) | <0.0001 | 20.4 | 12.0 | 0.1 | 18.5 |
| Unadjusted rates | |||||||||||
| 30-day mortality rate | 5.87 | 6.19 | 4.05 | 4.12 | 4.06 | 5.71 | <0.0001 | 9.7 | 9.3 | 9.7 | 2.0 |
| 30-day readmission rate | 22.13 | 21.84 | 24.33 | 24.28 | 19.68 | 20.63 | <0.0001 | 5.9 | 5.8 | 5.3 | 3.0 |
| 1-yr mortality rate | 33.10 | 34.26 | 27.35 | 25.98 | 25.87 | 29.08 | <0.0001 | 15.0 | 18.1 | 18.4 | 11.2 |
| 1-yr readmission rate | 66.21 | 65.54 | 71.77 | 68.50 | 62.80 | 67.38 | <0.0001 | 13.5 | 6.3 | 5.7 | 3.9 |
| 1-yr any procedures | 46.24 | 44.96 | 55.08 | 51.15 | 45.59 | 56.03 | <0.0001 | 20.3 | 12.4 | 1.3 | 22.3 |
| 1-yr cardiac procedures | 24.07 | 22.39 | 35.70 | 29.26 | 27.41 | 34.04 | <0.0001 | 29.6 | 15.7 | 11.6 | 26.1 |
| 1-yr dialysis | 6.76 | 5.22 | 15.74 | 13.70 | 13.57 | 14.54 | <0.0001 | 34.8 | 29.3 | 28.9 | 31.6 |
Values are median (interquartile range) or %. Reported rates are %. Standardized differences are referenced to those in whites. Standardized differences of >10 are clinically meaningful.
LOS = length of stay.
DISCUSSION
This study describes the differential expenditures for HFpEF for Medicare Part A payments based on race/ethnicity. We found health care costs at 1 year after an index admission were higher among black, Hispanic, and other race patients when adjusting for patient characteristics, hospital factors, and regional SES. On average, at 1-year, Medicare Part A paid $9,846 more per black beneficiary and $8,265 more per Hispanic beneficiary. The higher use of health care for acute care services indicates an opportunity to reduce disparities in patient outcomes by investing in cost-effective, high-value interventions that would lower the incidence of HF. Manageable comorbidities such as poorly controlled hypertension, diabetes, chronic kidney disease, and elevated atherosclerotic risk are prevalent among race/ethnic minority populations, and improved treatment may help reduce acute care service use among Medicare beneficiaries (17,18).
Overall health care expenditures are concentrated among patients with chronic conditions. A study of Medicare beneficiaries in the top decile for costs noted a much higher prevalence of HF (44.4%) and a higher representation of black and Hispanic beneficiaries compared with patients in the lower deciles (19). The top decile patients in Medicare consume 73.0% of all the acute care service spending (19). Many of these hospitalizations and emergency department visits may be preventable with improvements in outpatient management. Vulnerable populations such as minorities with HFpEF are observed to be high users of acute care services (9,19). The differential expenditures for minority patients we observed at 30 days and 1 year are primarily driven by higher admission and readmission rates for minority patients (9). Interventions and systems of care tailored to vulnerable populations are needed to decrease the observed disparities in outcomes and use of acute care services.
Medicare is known to spend nearly as much on post-acute care services and readmissions within 30 days of discharge as at initial hospitalizations (20). Between 1994 and 2009, post-acute care service spending doubled for HF (21). In a the Olmsted County cohort study, patients with HFpEF were observed to have 23.6% higher lifetime medical costs than HFrEF patients when controlling for other comorbidities and patient factors (22). We describe similarly high cost burdens for patients with HFpEF at the year after an index admission, with marked variation with respect to race/ethnicity.
Using sequential adjustments, we have attempted to demonstrate what factors may be driving differences in Medicare expenditures based on race/ethnic classification. We found that among minority groups, regional SES explained greater differences in Medicare expenditures at 1 year than it did among whites. Differences in expenditures adjusted for patient factors alone did not explain much of the variation in Medicare expenditures. This suggests that the disparities we observed based on race/ethnic categorization may largely be driven at the hospital or regional level. Communities in low-SES regions may lack the resources for high-quality acute care services and may be doubly disadvantaged with poor quality outpatient care, thus increasing the risk of using acute care services repeatedly. Variability in the effectiveness of clinical care likely contributes to differences in acute care use. Patients with HF do better when aware of their disease process and self-management strategies (23,24). Perhaps a less consistent and effective effort is made when engaging race/ethnic minority patients to ensure understanding of the care plan upon discharge or outpatient follow-up. Understanding how regional SES might be influencing strains on care networks and increased use of costly acute care services requires further investigation.
Adequate control of hypertension and volume status are critical to clinical management of HFpEF. Additional therapies have not shown effectiveness in altering the natural disease course or improving survival for patients with HFpEF (10). Despite limited treatment strategies, a combination of known behavioral and conventional cardiovascular risk factors predispose to the risk of developing and exacerbating symptoms of HFpEF (25). Recent research highlights the potential to reduce cardiovascular health care use with improvements in lifestyle related cardiovascular risk factors (26). For race/ethnic minorities and black patients especially, targeting known risk factors such as hypertension, diabetes, and obesity may be critical to both preventing and managing HFpEF (27,28).
STUDY LIMITATIONS.
This analysis used Medicare Part A administrative data to calculate costs. Costs for Medicare Part B used data related to outpatient visits, inpatient physician payments, and testing, and ambulance services were not included. Medicare Part D was also not included to capture costs related to outpatient prescription drug services. If patients switched to Medicare Advantage plans during the period observation, these costs were not captured for the year following index admission. Overall, these limitations underestimate the true total Medicare expenditures on patients with HFpEF. Prior research using Medical Expenditure Panel Survey data has shown that approximately 40% of the total direct health care expenditures on patients with HF relates to outpatient care, prescription medications, and other services (29). Medicare Part A payments were left as unadjusted costs. The Medicare payment calculator includes adjustments based on geographic factors related to regional differences in facility/provider input costs. Although the generalized linear models used for cost ratios included adjustments for regional SES, these do not mirror adjustments made by the Medicare payment formula. If minority patients are predominately seen in urban hospitals with higher average Medicare payments, there are expected to be residual differences in spending related to Medicare’s geographic payment adjustments. The SES factors used decreased the measured disparity in Medicare expenditures. Additional unobserved SES factors not included in the statistical models may further explain differences based on race/ethnicity. There are also potential selection biases because the GWTG-HF registry is based on voluntary hospital participation. However, prior studies have suggested that Medicare beneficiaries enrolled in this registry are representative of the U.S. Medicare population (30).
CONCLUSIONS
Medicare costs for race/ethnic minority patients with HFpEF are greater at index admission and at 1 year after an index admission, suggesting these patients are more vulnerable to use of acute care services. Interventions to prevent readmissions and lower the incidence of HFpEF among all beneficiaries, but especially minority patients, are needed to reduce health care spending on acute care services. A significant portion of the disparity in use relates to differences at the hospital level and regional SES. Further research that investigates the differences in hospital performance in lower SES regions and outpatient care networks are needed to understand differences in the use rate of acute care services.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Vulnerable minority patients with HFpEF are at greater risk for repeat admission after an index hospitalization. Hospital and regional factors increase the risk of acute care service use. Ensuring safe transitions of care out of the hospital and quality outpatient management are important for minimizing the risk of acute hospitalizations and improving patient outcomes.
TRANSLATIONAL OUTLOOK:
Further qualitative work evaluating the delivery of hospital and community care is needed to develop interventions that reduce the hospitalization burden for patients with HFpEF, particularly for minority populations.
Acknowledgments
Dr. Ziaeian was supported by the American College of Cardiology Presidential Career Developmental Award and American Heart Association 2015 Young Investigator Database Research Seed Grant. The Get With The Guidelines-Heart Failure program, provided by the American Heart Association, is sponsored in part by Amgen Cardiovascular and previously by Medtronic, GlaxoSmithKline, Ortho-McNeil, and American Heart Association Pharmaceutical Roundtable. Dr. DeVore has received research support from American Heart Association, Amgen, Novartis; and has consulted for Novartis. Dr. Hernandez has received research support from AstraZeneca, Bayer, Luitpold, GlaxoSmithKline, Merck, Novartis, Portola Pharmaceuticals, and Verily; and has received honoraria from Amgen, Bayer, Boehringer-Ingelheim, Boston Scientific, Myokardia, Novartis, and Sanofi. Dr. Bhatt is a member of the advisory boards of Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; sits on the Board of Directors of Boston VA Research Institute and Society of Cardiovascular Patient Care; is Chair of the American Heart Association Quality Oversight Committee; a member of the Data Monitoring Committees of Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, and Population Health Research Institute; has received honoraria from American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), and VA CART Research and Publications Committee (Chair); research funding from Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Eli Lilly, Medtronic, Pfizer, Roche, Sanofi, and The Medicines Company; royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); is site co-investigator for Biotronik, Boston Scientific, and St. Jude Medical (now Abbott); a trustee of American College of Cardiology; and has performed unfunded research from FlowCo, Merck, PLx Pharma, and Takeda. Dr. Fonarow has received research support from U.S. National Institutes of Health; and has consulted for Amgen, Janssen, Medtronic, Novartis, and St. Jude Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- GWTG-HF
Get With The Guidelines-Heart Failure
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- SES
socioeconomic status
Footnotes
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996-2013. JAMA 2016;316:2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the united states a policy statement from the american heart association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Medicare and Medicaid Services. Medicare Chronic Conditions Dashboard: Region Level. Comparison of Geographic Areas by Chronic Conditions, 2015. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Dashboard/chronic-conditions-region/cc_region_dashboard.html. Accessed July 8, 2017.
- 5.Centers for Medicare and Medicaid Services. Use/Spending State Level: All Beneficiaries, 2007-2015. Chronic Cond. 2017:1. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Downloads/CC_Util_Spend_State.zip. Accessed July 8, 2017.
- 6.Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2,000 to 2010. JAMA Intern Med 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg BA, Zhao X, Heidenreich P a, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 8.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014;168:721–30. [DOI] [PubMed] [Google Scholar]
- 9.Ziaeian B, Heidenreich PA, Xu H, et al. Race/ethnic differences in outcomes among hospitalized medicare patients with heart failure and preserved ejection fraction. J Am Col Cardiol HF 2017;5:483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services. Area Health Resource File. Available at: https://datawarehouse.hrsa.gov/topics/ahrf.aspx. Accessed December 28, 2017.
- 13.Eapen ZJ, McCoy LA, Fonarow GC, et al. Utility of socioeconomic status in predicting 30-day outcomes after heart failure hospitalization. Circ Heart Fail 2015;8:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey: Using Appropriate Price Indices for Analyses of Health Care Expenditures or Income Across Multiple Years. Washington DC: U.S. Department of Health and Human Services. Available at: https://meps.ahrq.gov/about_meps/Price_Index.shtml. Accessed December 14, 2017. [Google Scholar]
- 15.Merlo J, Yang M, Chaix B, Lynch J, Råstam L. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Community Health 2005;59:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vivo RP, Krim SR, Liang L, et al. Short- and long-term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. J Am Heart Assoc 2014;3:e001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnethon MR, Pu J, Howard G, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation 2017;136:e393–423. [DOI] [PubMed] [Google Scholar]
- 18.Daviglus ML, Talavera G a, Avilés-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/latino individuals of diverse backgrounds in the United States. JAMA 2012;308:1775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joynt KE, Gawande a a, Orav EJ, Jha a K. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA 2013;309:2572–8. [DOI] [PubMed] [Google Scholar]
- 20.Mechanic R Post-acute care–the next frontier for controlling Medicare spending. N Engl J Med 2014;370:692–4. [DOI] [PubMed] [Google Scholar]
- 21.Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in medicare point to the potential for cost savings in these settings. Health Aff (Millwood) 2013;32:864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes 2011;4:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evangelista L, Rasmusson K, Laramee A, et al. Health literacy and the patient with heart failure–implications for patient care and research: a consensus statement of the Heart Failure Society of America. J Card Fail 2010;16:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA 2011;305:1695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah SJ. Sedentary lifestyle and the risk for HFpEF. J Am Coll Cardiol 2017;69:1143–6. [DOI] [PubMed] [Google Scholar]
- 26.Aaron KJ, Colantonio LD, Deng L, et al. Cardiovascular health and health care use and expenditures among medicare beneficiaries: the reasons for geographic and racial differences in stroke (REGARDS) study.J Am Heart Assoc 2017;6:e005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation 2005;111:1233–41. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A, Colvin-Adams M, Yancy CW. Heart failure in African Americans: disparities can be overcome. Cleve Clin J Med 2014;81:301–11. [DOI] [PubMed] [Google Scholar]
- 29.Echouffo-Tcheugui JB, Bishu KG, Fonarow GC, Egede LE. Trends in health care expenditure among US adults with heart failure: the Medical Expenditure Panel Survey 2002-2011. Am Heart J 2017;186:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis LH, Greiner MA, Hammill BG, et al. Representativeness of a national heart failure quality-of-care registry: comparison of OPTIMIZE-HF and Non-OPTIMIZE-HF medicare patients. Circ Cardiovasc Qual Outcomes 2009;2:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


