Abstract
Drawing from basic knowledge of stem-cell biology, embryonic development, wound healing, and aging, regenerative medicine seeks to develop therapeutic strategies that complement or replace conventional treatments by actively repairing diseased tissue or generating new organs and tissues. Among the various clinical-translational strategies within the field of regenerative medicine, several can be broadly described as promoting disease resolution indirectly through local or systemic interactions with a patient’s cells, without permanently integrating or directly forming new primary tissue. In this review, we focus on such therapies, which we term disease-modulating regenerative therapies (DMRT), and on the extent to which they have been translated into the clinical arena in four distinct areas of nephrology: renovascular disease (RVD), sepsis-associated AKI (SA-AKI), diabetic kidney disease (DKD), and kidney transplantation (KTx). As we describe, the DMRT that has most consistently progressed to human clinical trials for these indications is mesenchymal stem/stromal cells (MSCs), which potently modulate ischemic, inflammatory, profibrotic, and immune-mediated tissue injury through diverse paracrine mechanisms. In KTx, several early-phase clinical trials have also tested the potential for ex vivo–expanded regulatory immune cell therapies to promote donor-specific tolerance and prevent or resolve allograft injury. Other promising DMRT, including adult stem/progenitor cells, stem cell–derived extracellular vesicles, and implantable hydrogels/biomaterials remain at varying preclinical stages of translation for these renal conditions. To date (2021), no DMRT has gained market approval for use in patients with RVD, SA-AKI, DKD, or KTx, and clinical trials demonstrating definitive, cost-effective patient benefits are needed. Nonetheless, exciting progress in understanding the disease-specific mechanisms of action of MSCs and other DMRT, coupled with increasing knowledge of the pathophysiologic basis for renal-tissue injury and the experience gained from pioneering early-phase clinical trials provide optimism that influential, regenerative treatments for diverse kidney diseases will emerge in the years ahead.
Keywords: chronic kidney disease, basic science, clinical trials, diabetic kidney disease, inflammation, mesenchymal stromal cells, regenerative medicine, regulatory T cells, renovascular disease, senescence, sepsis, stem cells
Introduction
Since it was first introduced into biomedical parlance by William Haseltine, the term regenerative medicine has become broadly recognizable to healthcare providers and the general public alike. After two decades of intense interest and diverse research initiatives, the original concept of “an approach to therapy that…employs human genes, proteins and cells to regrow, restore or provide mechanical replacements for tissues that have been injured by trauma, damaged by disease or worn by time” still conveys a succinct and valid definition of the field (1). Critically, one of the central tenets of regenerative medicine has been the merging of basic insights into organ/tissue development, stem-cell science, and disease pathophysiology with innovative translational concepts and manufacturing procedures to reverse disease more effectively than can currently be achieved by conventional pharmacotherapy and interventional procedures (1,2).
For the nephrologist, the promise of regenerative medicine is compelling. Most acute and chronic kidney diseases remain incurable, life limiting, and are typically managed with drug combinations or procedures that are costly and carry a high burden of adverse effects. Added to this is the nephrologist’s natural affinity for the application of cellular and physiologic science to patient management. In this article, we describe recent progress within a specific aspect of regenerative medicine, which we term “disease-modulating regenerative therapies” (DMRT), in the field of nephrology. In focusing on DMRT, we specifically refer to therapeutic concepts based on systemic or localized administration of cells, subcellular components, biomaterials, or combinations of these that engage in a complex molecular crosstalk with resident cells and tissues of the host to modify or reprogram damaging biologic activity. We distinguish DMRT from other regenerative strategies that are based on harnessing pluripotent/multipotent stem cells and advances in tissue engineering to directly repair or replace damaged organs and tissue. This aspect of regenerative medicine, which might be termed “organ- and tissue-replacing regenerative therapies,” will not be addressed in this article but has been expertly reviewed by others (3–5).
First and foremost among DMRT are mesenchymal stem/stromal cells (MSCs), the subject of two decades of translational research, which have been administered safely to patients in numerous clinical trials (6,7). Critically, MSCs are now considered to mediate their therapeutic benefits predominantly through inducible secretion of paracrine mediators and reprogramming of myeloid and lymphoid immune cells, and can be expanded in culture to large numbers from a range of autologous or allogeneic tissue sources (6). In addition to MSC-based cell therapies, other forms of DMRT have made varying degrees of progress along a similar translational path: (1) regulatory T cells (T-reg) and other immunologic cells, which suppress inflammation or harmful immune responses (8,9); (2) additional types of adult progenitor cells (including those derived from the kidney) with prorepair paracrine effects (10,11); (3) stem/progenitor cell–derived extracellular vesicles (EVs), which may transfer proregenerative biomolecules to target cells (12); and (4) injectable hydrogels and other biomaterials, which may have inherent regenerative properties or serve to enhance the benefits of cell-based therapies (13). As summarized in Figure 1 and described in detail in subsequent sections of the review, we focus on the progress toward clinical translation of MSCs and other DMRT that has occurred to date in four important areas of clinical practice in nephrology: renovascular disease (RVD), sepsis-associated AKI (SA-AKI), diabetic kidney disease (DKD), and kidney transplantation (KTx). For each of these exemplars, we highlight the extent to which early-phase clinical trial experiences with DMRT are being driven by increased understanding of their potential mechanisms of action and are linked to advances in knowledge of the pathophysiologic basis of the targeted condition.
Figure 1.
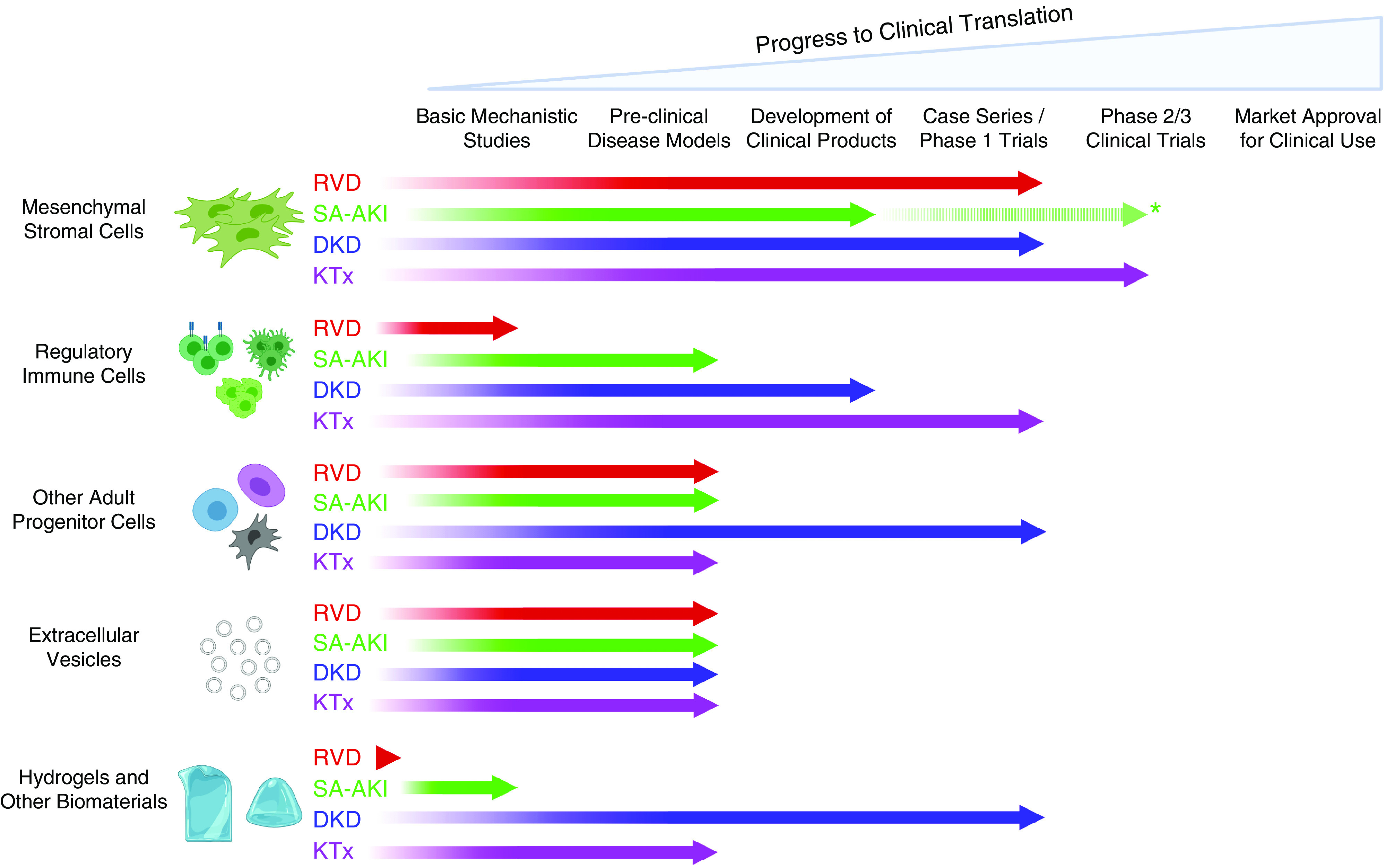
Variable progress has been made toward clinical translation of five categories of disease-modulating regenerative therapies (DMRT) for four different areas of nephrology practice. The figure summarizes current translational status of mesenchymal stromal/stem cells, regulatory immune cells, other adult progenitor cells, extracellular vesicles, and hydrogel/biomaterials as potential DMRT for renovascular disease (RVD), sepsis-associated AKI (SA-AKI), diabetic kidney disease (DKD), and kidney transplantation (KTx). *Broken line indicates that clinical trials of MSCs have been reported in sepsis, including some patients with SA-AKI, but not with kidney function as a primary outcome. Figure created using Biorender.com.
Renovascular Disease
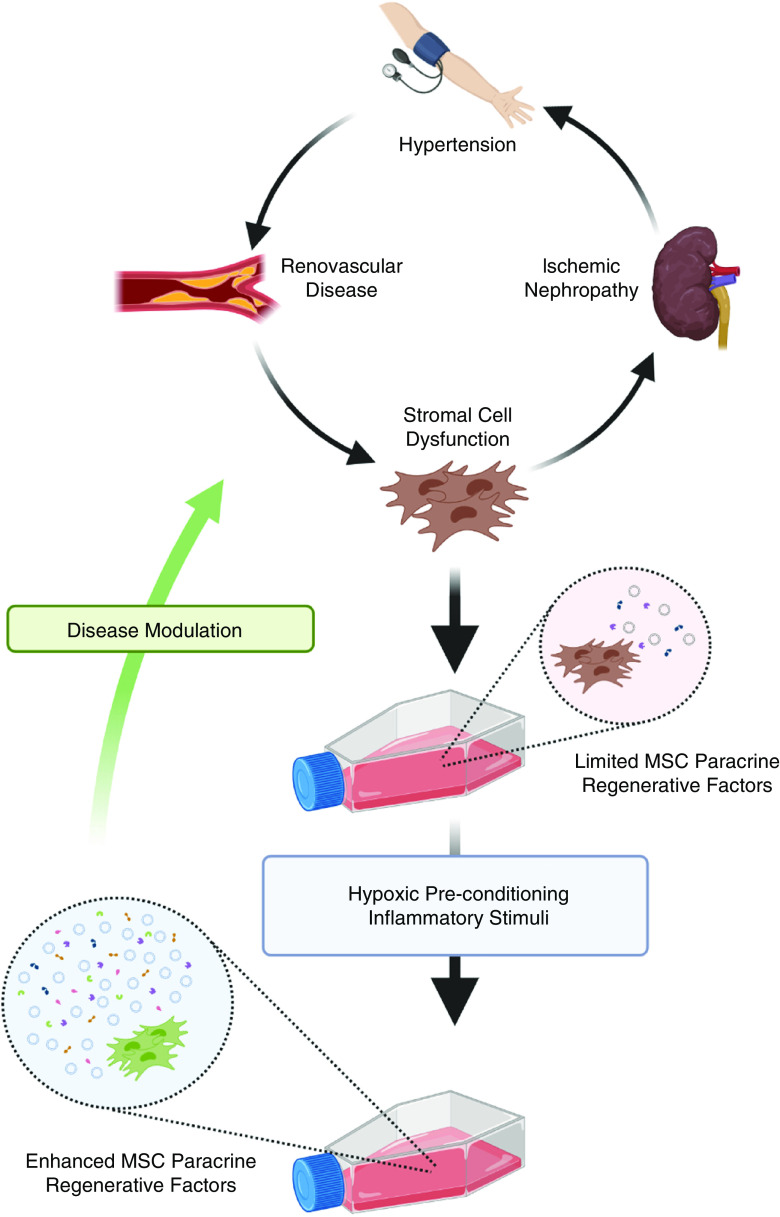
Hypertension is a major risk factor for CKD, with hypertensive kidney disease accounting for approximately 30% of all ESKD cases (14). Hypertensive kidney disease—characterized by vascular damage, endothelial dysfunction, and loss of endogenous vasodilators—results in progressive loss of the renal microvasculature (15). RVD is a common cause for secondary hypertension in individuals aged ≥65 years, and RVD attributed to atherosclerotic plaque development with reduction in renal-artery dimension represents a unique intersection between hypertension and CKD/ESKD leading to progressive renal insufficiency (16). In the setting of significant RVD, further reduction of renal blood flow (RBF) and hypoxia trigger inflammation, oxidative stress, and profibrotic pathways, leading to scarring and further deterioration of renal function (ischemic nephropathy) (17). Importantly, recent clinical trials and experimental studies indicate that restoration of large-vessel patency alone is not enough to regain kidney function in most patients with atherosclerotic RVD (18,19). The limited number of currently available strategies for effectively modulating RVD, and the realization that the natural history of this disease involves transition from a hemodynamic component to a proinflammatory and profibrotic disease, highlight the need for a paradigm shift in therapy (20). Importantly, RVD-associated ischemic nephropathy and hypertensive kidney disease, in the absence of RVD, share common pathophysiologic features that include activated renin-angiotensin-aldosterone system (RAAS), increased sodium retention, and—consequently—increased oxygen consumption (21). In evidence of this, studies in hypertensive rats using oxygen microelectrodes found pronounced medullary and cortical hypoxia in spontaneously hypertensive rats compared with normotensive controls (22). Furthermore, the presence of ischemic, rather than hypertrophic, glomeruli in hypertensive kidney disease suggests that hypoxia and ischemia are the predominant mechanisms (23). Therefore, in subjects with prolonged hypertension and relevant genetic-, environmental-, and lifestyle-related risk factors, limited blood flow and oxygenation to areas of renal parenchyma, where the oxygen tension is <10 mm Hg, makes the kidney vulnerable to ischemic injury which resembles that due to RVD. In this context, implementation of DMRT, in particular the use of MSCs, can be viewed as a novel therapeutic option for RVD and hypertensive kidney disease.
Importantly, MSCs have immunomodulatory, anti-inflammatory, and proangiogenic properties that have been demonstrated in experimental studies in animals and humans with RVD (24–26). Arterial delivery of MSCs in the swine model of RVD was associated with protection of the stenotic kidney and improved RBF and function, with reduction of oxidative stress and inflammation, contributing to tissue repair (24). The strong paracrine effect of MSCs, and not their differentiation capacity, seems to be the principal mechanism of their therapeutic action. In clinically relevant, large-animal models of RVD, MSCs have been shown to release a variety of soluble mediators that act locally within the kidney to ameliorate ischemic nephropathy through proangiogenic, anti-inflammatory, and antioxidative mechanisms (24). Also consistent with a paracrine model, the therapeutic potential of stem/progenitor cell–derived EVs in renal diseases has been highlighted by showing decreased renal inflammation and injury through intrarenal delivery of EVs in pigs with RVD and concomitant metabolic syndrome (27). Other studies have demonstrated their role as carriers of anti-inflammatory genes and proteins and their capacity to be engineered to deliver specific substances or to have enhanced uptake by target cells (28–30). Therefore, MSC-derived EVs may serve as an acellular therapeutic option to attenuate inflammation and fibrosis in RVD and other forms of renal disease. As illustrated in Figure 2 for the clinical target of RVD and associated ischemic nephropathy, the paracrine, regenerative activities of MSCs, including both secreted soluble mediators and released EVs, are now recognized to be “tunable.” Thus, as described later in greater detail, disease-associated dysfunction of ex vivo–expanded, autologous MSCs may be reversed through hypoxic preconditioning and other manipulations (31,32).
Figure 2.
Hypoxic pre-conditioning is a potential strategy for enhancing MSC therapeutic potency in hypertensive kidney disease. As illustrated, chronic hypertension, RVD, and ischemic nephropathy lead to stromal-cell dysfunction, which is associated with limited production of paracrine regenerative factors (released extracellular vesicles and soluble mediators) by patient-derived, culture-expanded, mesenchymal stem/stromal cells (MSCs). Culture under low oxygen tension (hypoxic preconditioning) may restore the production of extracellular vesicles and soluble mediators, resulting in enhanced paracrine regenerative activity and increased potential for disease modulation after localized or systemic delivery of autologous MSCs. Figure created using Biorender.com.
There is now promising evidence that observations of RVD modulation by MSCs in animal models can be translated into clinical benefits. In a phase 1 trial in patients with RVD, we (L.J.H. and S.M.H.) recently demonstrated that intra-arterial infusion of autologous adipose tissue–derived MSCs into poststenotic kidneys (without concomitant renal-artery angioplasty) resulted in increased cortical RBF and GFR compared with the baseline values. These improvements in hemodynamic and functional indices were associated with attenuation of tissue hypoxia, inflammatory cytokines, and angiogenic biomarkers, along with a fall in BP between baseline and 3 month follow-up. These changes were more prominent in the patients treated with a higher MSC dose (26,33). Of further interest, we also observed RBF increases in the (non-MSC-infused) contralateral kidneys (26,33). This phenomenon likely occurred due to “crosstalk” signaling between kidneys and/or wider systemic effects of signaling and homing signals for MSCs. Moreover, beneficial off-target effects, beyond the kidney, are also possible because infusion of MSCs or endothelial progenitor cells into the renal artery has been associated with attenuation of hypertensive cardiomyopathy in experimental models of renovascular hypertension (34). Despite the promising evidence base of preclinical and clinical application of MSCs and MSC-derived EVs in RVD, there has been limited exploration to date of alternative DMRT (such as regulatory immune cells, other adult progenitor cells, and implantable hydrogels) in this area (Figure 1). Nonetheless, experimental evidence in models of renal ischemia-reperfusion injury could provide a basis for the translation of such therapies for chronic ischemic nephropathy in the future (13,35,36).
Sepsis-Associated Acute Kidney Injury
AKI has a prevalence ranging from 1% to 20% of patients who are hospitalized and 50% to 60% of patients in the intensive care unit. Mortality is proportional to the severity of AKI, and 30% of survivors die within the first year after hospital discharge. Sepsis, which is the most frequent cause of AKI in patients who are critically ill (37), is a life-threatening syndrome resulting from a disordered immune response to uncontrolled microbial infection (38). The pathophysiology of sepsis is dominated by dysregulated inflammation and immune suppression, with endothelial and epithelial injury, leukocyte aggregation, mitochondrial dysfunction, apoptosis, and impaired regeneration (37). SA-AKI differs from ischemic and toxic AKI, being characterized by global renal hyperemia with altered RBF distribution and inflammation incited by both infiltrating immune cells and resident parenchymal cells (37). In SA-AKI, there is an abnormal repair process due to prolonged hypoxia, cytokine expression, and defective adaptive immune cell function. Patients who are critically ill with persistent or recurrent AKI are at very high risk for secondary infection and increased mortality, and represent a key target for novel and more-effective therapies (39). In this regard, MSCs have demonstrated benefits in multiple sepsis models, including LPS administration, bacterial pneumonia, and polymicrobial abdominal sepsis (40–42). In animal models of sepsis, MSC administration is reportedly associated with improved survival; reduced organ injury; increased clearance of bacteria, cells, and fluid; and resolution of inflammation (43–45). Some animal-model studies of SA-AKI have demonstrated improvement in tubular-injury scores and kidney function (42,45), whereas others have not (43,44)—an inconsistency that may reflect differences among the models used.
From a mechanistic perspective, it is now clear that MSCs administered intravenously in models of sepsis localize predominantly in the lungs. From this location, they mediate their systemic benefits through mechanisms involving crosstalk with immune cells that result in modulatory effects on cytokine expression, vascular permeability, removal of apoptotic cells, and clearance of bacteria by neutrophils and macrophages (Figure 3) (46). In addition to release of soluble mediators and reprogramming of immune cells by viable MSCs, it has recently been shown that disease modulation may occur as a result of the induction of apoptosis of intravenously infused MSCs by cytotoxic lymphocytes followed by their phagocytosis by resident myeloid cells (monocytes and macrophages). This process, referred to as efferocytosis, results in polarization of myeloid cells toward alternatively activated (M2-like) phenotypes with potent anti-inflammatory effects (47,48). Furthermore, either directly or through their effects on myeloid cells, MSCs also augment tissue-repair processes through promoting expansion of T-reg (49).
Figure 3.

The systemic therapeutic effects of intravenousMSC therapy in SA-AKI are triggered by immune cell interactions in the lungs. As illustrated, intravenous administration of MSCs in the setting of SA-AKI results in MSC trapping in the lungs, where complex interactions (crosstalk and efferocytosis) with resident immune cells (mononuclear phagocytes [macrophages] and lymphocytes) result in beneficial localized effects within the alveolar spaces and systemic effects (LPS neutralization, secretion of anti-inflammatory factors, enhanced phagocytosis) with potential to promote resolution of inflammation, disrupted vascular integrity, and increased cell death in the kidneys. Improved cardiorespiratory function as a result of MSC local and systemic effects may provide further indirect effects to more effectively resolve SA-AKI. Figure created using Biorender.com. HGF, hepatocyte growth factor; KGF, keratinocyte growth factor.
A number of specific soluble factors have been identified as mediating the paracrine effects of MSCs and their immune-cell partners in models of sepsis. These include IL-10, keratinocyte growth factor, PGE2, vascular endothelial growth factor (VEGF), antibacterial peptides LL-37 and hepcidin, and other proresolution factors (50). The role of IL-10 has been most convincingly demonstrated. In the mouse cecal ligation and puncture model of polymicrobial sepsis, Németh et al. (45) first reported that intravenously administered MSCs stimulate IL-10 production by macrophages through PGE2/EP2-receptor interaction, resulting in the prevention of neutrophil extravasation into tissue. This MSC-induced pulse of IL-10 production has since been replicated in several other studies (49). Transfer of specific microRNAs or mitochondria via nanotubes may also underlie some of the effects of MSCs to enhance macrophage phagocytic activity or endogenous stem-cell fitness in the septic environment (51). A further strategy to enhance the immunomodulatory features of MSCs in sepsis is through preconditioning (“licensing”) with proinflammatory cytokines, toll-like receptor ligands, carbon monoxide, and eicosapentaenoic acid (52–54). In the case of carbon-monoxide licensing of MSCs, this was reported by Tsoyi et al. (52) to result in reduced organ damage (including kidney injury) and superior survival in mouse models of sepsis, while also allowing for later MSC administration. Mechanisms by which preconditioning has been reported to enhance MSC activity include activation of the lipoxygenase pathway and enhanced exosome delivery of microRNA to macrophages. As already described in the context of RVD, MSC-derived EVs also have the potential to be developed as a subcellular DMRT for sepsis and SA-AKI through the transfer of a wide range of bioactive molecules (49,55).
Although the clinical application of MSCs in sepsis and SA-AKI is at an early stage, they have shown promising safety profiles in early-phase human trials (49). For example, in a phase 1, dose-escalation trial involving nine patients with sepsis, treatment with MSCs was found to be safe and well tolerated, albeit with no overt effect on sepsis parameters. An analysis of cytokine levels in treated patients demonstrated no increase in known proinflammatory mediators or biomarkers of organ dysfunction after MSC treatment (56,57). In a phase 2, multicenter, randomized, placebo-controlled, clinical trial, Swaminathan et al. examined the effect of allogeneic MSC therapy delivered intra-aortically in patients undergoing cardiac surgery who developed postbypass AKI. More than half of these patients had impaired renal function at baseline (58). Although this trial was carried out in patients with a sterile form of AKI, the design and results have important implications for the future planning of clinical trials of DMRT in SA-AKI. Disappointingly, in this trial, MSC administration resulted in no difference in recovery of renal function, dialysis, or death compared with placebo (58). Although carried out in a relatively homogenous patient population, differences in renal reserve, complexity of cardiac surgical procedure, bypass time, and other postoperative complications may yet have hindered the ability to observe any modest clinical benefit of MSCs. This negative study in sterile AKI does not necessarily blunt interest in the clinical translation of MSCs for SA-AKI. Indeed, trials of MSCs and other DMRT in sepsis may be better suited to detecting their effects on the development or severity of AKI. Because sepsis is a multiorgan disorder, favorable effects of systemically administered MSCs on kidney function may derive from improved function of other organ systems and from reprogramming of immune cells at distant sites. Indeed, preclinical studies indicate that infusion of apoptotic versus viable MSCs within the lung led to greater suppression of inflammation, oxidative stress, cellular markers of immune reactivity, and a less marked kidney injury in a cecal ligation and perforation model of sepsis (59). Given the high prevalence of AKI among patients with sepsis and its implications for morbidity and mortality, it will be important for future clinical trials of DMRT in sepsis to include patients with—or at risk for developing—AKI, for equal numbers of patients with similar stages of AKI to be randomized, and for specific renal end points to be included in the trial design.
Diabetic Kidney Disease
Due to the growth of the aging population, the estimated number of individuals with diabetes mellitus (DM) worldwide increased from 108 million in 1980 to 422 million in 2014 (60). Moreover, the global epidemic of DM has contributed approximately 50% of the increased health burden due to CKD (61). DKD is characterized by vascular damage, resulting from cumulative effects of a wide range of predominantly hyperglycemia-driven maladaptive processes, including chronic inflammation, increased oxidative stress, advanced accumulation of glycation end products, steatosis, insulin resistance, renal hypoxia, apoptosis, cellular dedifferentiation and senescence, and altered RAAS activation (62–64). Intrinsic renal regenerative capacity is limited in DM, exacerbating chronic glomerulosclerosis, tubulointerstitial fibrosis, and chronic inflammation (62,64,65). Although recent clinical trials of sodium-glucose cotransporter-2 inhibitors and other pharmacologic agents have shown that the rate of renal functional loss can be slowed in DKD due to type 2 DM (66–68), successful targeting of multiple injurious pathways—such as those mediating inflammation, oxidative stress, renal hypoxia, and fibrosis—may be necessary to truly halt DKD.
With this goal in mind, DMRT represent novel therapeutic options for the delivery or induction of a wide range of mediators to simultaneously target maladaptive processes that contribute to DKD progression. As with other renal diseases, the most extensively studied DMRT in DKD is the MSC (64). In many preclinical experimental models of DM and diabetic nephropathy (64,68), the paracrine-mediated actions and cell-cell interactions of exogenously administered MSCs have shown potential to modulate a range of pathophysiologic processes that contribute, both locally and systemically, to the progressive renal injury and functional loss that characterize DKD (Figure 4). External to the kidneys, MSCs delivered intravenously or by other routes have been experimentally shown in models of DM to modulate adipose-tissue inflammation, preserve islet function, and enhance insulin sensitivity, leading indirectly to beneficial renal effects through reducing glycemia and the proinflammatory systemic environment (64,69,70). Concomitantly, MSCs themselves, their released mediators, and regulatory immune cells induced as a result of MSC administration may transfer to the kidneys to mediate beneficial effects within distinct renal compartments, including the glomerulus, microvasculature, tubules, and interstitium. Reductions in glomerular size, podocyte apoptosis, glomerular matrix expansion/sclerosis, peritubular interstitial fibrosis, renal tubular epithelial cell death and dedifferentiation, tubulointerstitial fibrosis, and microvascular rarefaction have been observed in association with reduced albuminuria and stabilization of GFR (64,69,70).
Figure 4.

Multiple potential therapeutic effects have been identified for systemically administered MSCs in DKD. Extensive preclinical and limited clinical trial data indicate that MSCs (center) may exert both extrarenal and intrarenal modulatory effects, through a range of key mediators, after intravenous administration in diabetes and DKD. Upper left: Extrarenal effects which diminish adipose-tissue inflammation, enhance insulin sensitivity, and preserve islet function can stabilize or reverse the course of DKD by improving glycemic control and reducing systemic inflammation and oxidative stress. Lower right: Intrarenal effects by which key MSC-generated and -induced mediators have been shown experimentally to modulate multiple aspects of DKD pathophysiology within the glomerulus, the tubulointerstitial compartment, and the microvasculature. Figure created using Biorender.com.
Preclinical, MSC-based, experimental studies have demonstrated benefits derived from a variety of therapeutically relevant mediators (Figure 4). These include soluble factors with antifibrotic (hepatocyte growth factor [HGF]), proangiogenic (VEGF), antiapoptotic/homeostasis (HGF, VEGF, stromal cell–derived factor [SDF-1/CXCL-12]), and immunomodulatory (indoleamine 2,3-dioxygenase, PGE2, and IL-10) activity. Such soluble factors may be secreted inherently by MSCs, triggered in MSCs by signaling from proinflammatory cytokines and immune cells, or secondarily produced by alternatively activated (M2) macrophages and T-reg induced by interactions with, or uptake of, exogenously administered MSCs. One of the most important growth factors, HGF, reduces kidney fibrosis by blocking tubular epithelial cell dedifferentiation and inhibiting intrarenal expression of monocyte chemoattractant protein-1 and macrophage infiltration (70,71). Other key mediators associated with the direct and induced paracrine effects of MSCs in DKD include indoleamine 2,3-dioxygenase, a potent immunomodulatory enzyme; PGE2, a likely mediator of T-reg differentiation; and IL-10, an anti-inflammatory cytokine released by macrophages after phagocytosis of apoptotic MSCs (64,72,73). The many observations from experimental models of DM and DKD that key soluble and released factors mediate the disease-modulatory effects of MSCs have also stimulated interest in the use of MSC-derived conditioned medium and EVs as alternative DMRT (74,75). Despite the focus on paracrine mechanisms in many preclinical studies, however, it remains unclear whether soluble factors released by MSCs after systemic delivery can explain all of the beneficial effects reported in experimental DKD. Specifically, the transient survival of intravenously administered MSCs, and reports in other disease models of therapeutic effects mediated by apoptotic or heat-inactivated MSCs, suggest the existence of other mechanisms (47,48,72,76). Although transmigration and prolonged engraftment of a minority of administered cells to the kidneys remains theoretically possible, the phenomena of MSC apoptosis and efferocytosis by macrophages (48) and MSC-induced expansion of T-reg (77) represent more compelling mechanisms by which their anti-inflammatory, prorepair effects within the kidneys could be augmented and prolonged beyond the initial release of soluble mediators.
In addition to MSCs from various tissue sources, similar renal regenerative effects have been observed for a variety of other stem/progenitor-like cells and their secreted trophic factors or EVs (36,68). Primary cells derived from the kidney may also exert paracrine-mediated, disease-modulating effects in a similar fashion to MSCs, and are being actively pursued as potential DMRT. For example, selected renal cells (SRC), composed of isolated tubular and aquaporin 2–positive collecting duct cells, have advanced to the clinical-translation phase (78). These primary cells induce tubular cell proliferation while attenuating TNF-α–induced NF-κB and TGF-β1–mediated plasminogen activator inhibitor-1 signaling pathways that contribute to inflammation and fibrosis in experimental DKD (79). Given the transient period in which exogenously administered cells reside in the diseased microenvironment, use of biomaterials, such as hydrogels, to enrich cell delivery and duration of action have been pursued (13,80). As discussed below, this has since been translated to a locally delivered therapeutic strategy for DKD in which a gelatin-based hydrogel containing expanded autologous SRC is implanted into the kidneys (78).
Despite numerous studies in experimental DKD, clinical translation of DMRT has been limited. In 2016, Packham et al. reported the results of a randomized, placebo-controlled, dose-escalation study that tested the safety and feasibility of intravenous infusion of allogeneic mesenchymal precursor cells (rexlemestrocel-L) in adults with type 2 DM and CKD stages 3/4. The cell infusions were well tolerated, and trends in kidney function during a 12-week follow-up period favored stabilization or improvement in 20 patients treated with cell infusions compared with ten patients treated with placebo (81). Other early-phase clinical trials are now investigating allogeneic bone marrow–derived MSCs (M.D.G.; Italy, Ireland, United Kingdom; ClinicalTrials.gov, NCT02585622), autologous adipose-derived MSCs (L.J.H., S.M.H.; NCT03840343), and allogeneic umbilical cord–derived MSCs (Japan, NCT04125329; China, NCT04216849) in DKD. As mentioned above, the therapeutic combination of primary kidney cells (SRC) in hydrogels (named Neo-Kidney Augment) is also being investigated as a DMRT for DKD in phase 1 and 2 clinical trials (NCT02008851, NCT03270956, NCT02836574). Of note, a report of the phase 1 trial involving laparoscopically assisted intracortical implantation of SRC, in seven male patients with type 2 DM and CKD stages 3/4, indicated an unacceptable number of postprocedural complications, prompting changes in implantation methodology. Nonetheless, renal function and urine albumin-creatinine ratio remained relatively stable for 12 months, whereas eGFR tended to decline from months 12 to 24 after SRC administration (78). Although also promising, injection of DRMT-derived EVs is not yet underway in human DKD studies.
Kidney Transplantation
Beyond the “holy grail” of donor-specific tolerance, steady advances in understanding pathologic processes that underlie the common causes of early and late renal allograft failure have revealed other important new therapeutic targets that are not adequately addressed by conventional immunosuppressive drugs and clinical practices (82). These include inflammatory and metabolic pathways that mediate donor-organ injury before and early after transplantation, immunologic processes that drive the formation of donor-specific antibodies and antidonor memory T cells, effector mechanisms responsible for subsequent acute or chronic immune-mediated rejection, and maladaptive cellular processes such as fibrosis and senescence. Against this backdrop, the potential for DMRT, such as MSCs and regulatory immune cells, to address some or all of these major unmet needs for improved long-term KTx survival is being robustly pursued. In the following paragraphs and illustrated in Figure 5, we provide overviews of recent progress in the translation of these DMRT to the field of KTx, and how they may address key mechanisms of graft injury.
Figure 5.
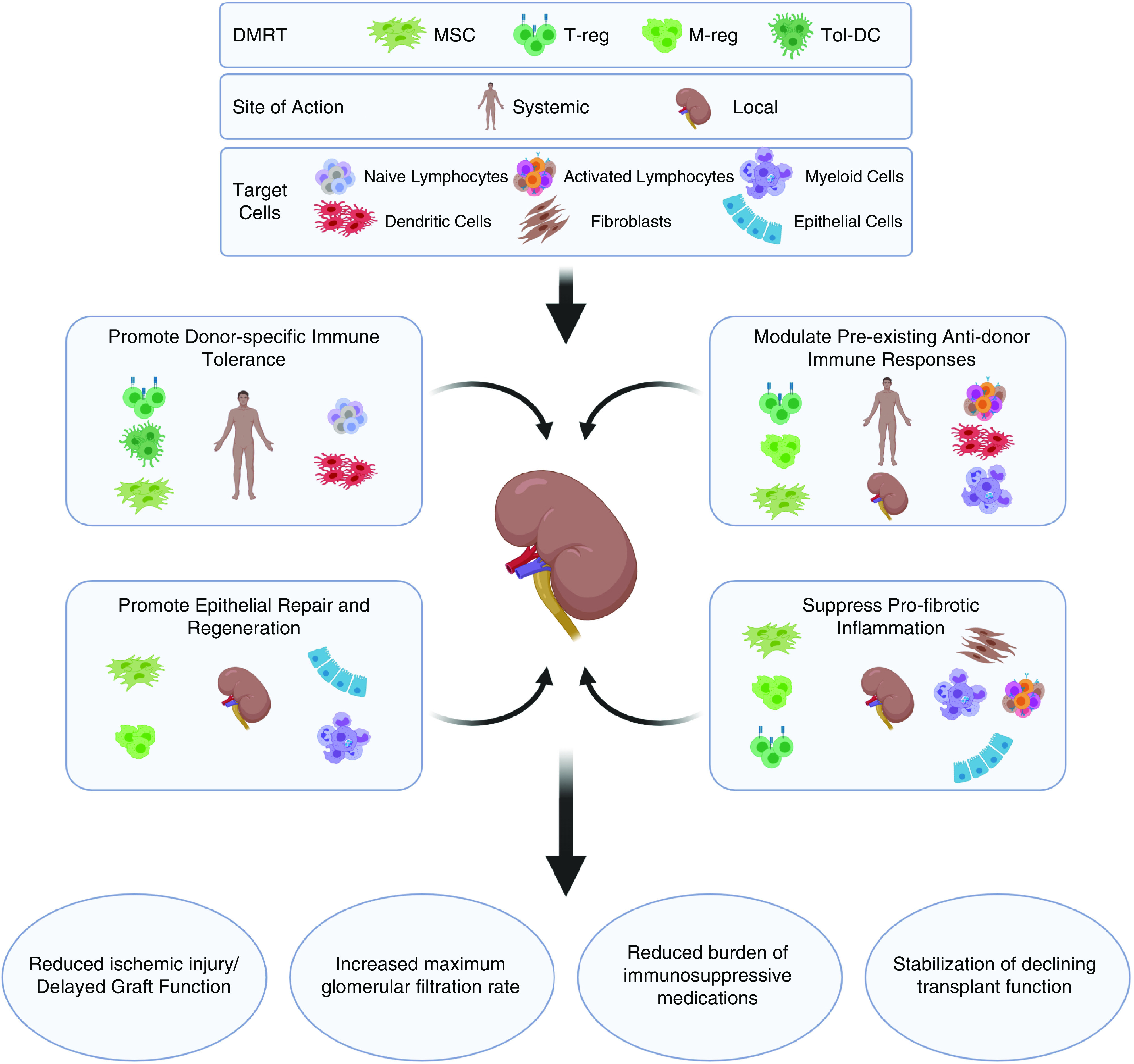
Disease modulating regenerative therapies for kidney transplantation address diverse mechanisms and potential clinical benefits. Upper panel: Diverse types of modulatory cellular therapies that have been the subject of early-phase clinical trials in KTx recipients along with their potential sites of action and target cells. Middle panel: Four major mechanistic goals of DMRT applied to KTx with illustration of the most relevant cellular therapies for each, along with their predicted sites of action and most significant cell targets for each (based on preclinical studies and profiling/monitoring analyses of subjects from clinical trial). Lower panel: Significant clinical benefits that represent the most immediate goals for the clinical translation of DMRT in KTx. Figure created using Biorender.com. M-reg, regulatory macrophages; Tol-DC, tolerogenic dendritic cells; T-reg, regulatory T cell.
Mesenchymal Stem/Stromal Cells
Extensive preclinical evidence that MSCs modulate harmful antidonor immune responses and maladaptive inflammation associated with allogeneic organ transplants and may promote immune tolerance has accumulated over the past 18 years (83). In 2012, Tan et al. (84) reported the results of a phase 2 clinical trial in which 104 recipients of living-related-donor KTx received a novel induction regimen consisting of two intravenous infusions of autologous bone marrow–derived MSCs at the time of transplantation and 2 weeks later, followed by maintenance therapy with conventional- or low-dose cyclosporine. For MSC-induced recipients, early recovery of renal function and frequency of acute rejection and opportunistic infection during the first post-transplant year were comparable or superior to those of a control group induced with anti–IL-2 receptor antibody followed by conventional-dose cyclosporine (84). Although the trial provided an encouraging demonstration of the safety and potential efficacy of peritransplant MSC infusions, the lack of mechanistic studies and of a measurable indicator of the in vivo activities of the infused cells precluded any immediate progress toward wider clinical practice. For this reason, several other centers have focused on evaluating both autologous and allogeneic MSC therapies in smaller numbers of KTx recipients, along with longitudinal immunologic and, in some cases, histologic monitoring of the grafts. Details of the designs, major outcomes, and documented immunologic consequences of MSC administration to KTx recipients in such early-phase trials carried out to date have been summarized and expertly reviewed by Podestà et al. (83). Results from one further phase 1 trial have also been very recently reported (85). In addition to determining safety profiles, these trials have begun to address whether autologous or allogeneic MSC infusions can: (1) promote T-reg and/or donor-specific T-cell hypo-responsiveness (86–88), (2) reverse or stabilize subclinical tubulointerstitial inflammation and fibrosis/tubular atrophy (89), and (3) allow for early or delayed reduction (or even eventual withdrawal) of calcineurin inhibitor–based immunosuppression (85,90,91). A further interesting question, currently being addressed in preclinical studies (92–94) and an early-phase clinical trial (NCT04388761), is whether ex vivo perfusion of kidneys procured for transplantation with MSCs can modulate ischemic tissue injury and ameliorate subsequent delayed graft function.
Taken together, the trial reports to date support the conclusion that intravenous or intrarenal infusion of MSCs in KTx recipients is safe and associated with comparable early-to-midterm patient/graft outcomes and potentially superior renal function compared with conventional therapeutic regimens. Where examined, they also provide evidence that pre- or early post-transplant MSC infusions may be associated with favorable immunologic effects, such as increases in circulating T-reg or T-reg/effector T cell ratios. Nonetheless, it should be acknowledged that overall patient numbers remain small and the possibility of more subtle adverse effects—such as localized proinflammatory response, reduced antiviral immunity, or (in the case of allogeneic MSCs) stimulation of anti-HLA antibodies—should be carefully addressed by larger trials and longer follow-up (83). Development of clinically applicable assays of potency and in vivo effects in the context of KTx will also likely be required for optimal translation of MSCs and other DMRT into routine clinical practice. For example, for the clinical target of acute graft versus host disease after allogeneic hematopoietic stem cell transplantation, Cheung et al. (95) recently demonstrated that an increase in serum PGE2 1–4 days after MSC infusion distinguished patients with clinical response to cell therapy from nonresponders; this finding being consistent with in vitro cellular assays and mechanistic animal model studies carried out by the same group (48).
Regulatory Immune Cells
Because the recognition that forkhead box P3 (FoxP3)–positive T-reg are essential for maintaining peripheral immune tolerance to autoantigens and nonthreatening foreign antigens, the concept that treatment with ex vivo–expanded regulatory immune cells could prevent allogeneic transplant rejection and foster donor-specific tolerance in organ allograft recipients has been energetically pursued (96,97). In addition to T cells, it has also become clear that other major types of immune effector cells, including macrophages, dendritic cells, and B cells, incorporate subpopulations or alternative functional states that mediate counter-regulatory/suppressive effects and may be amenable to clinical exploitation (97). In keeping with the concept of DMRT, the potential therapeutic actions of regulatory immune cell therapies can be broadly viewed as modulating (as opposed to blocking) the interactions between donor-derived (allo-)antigens or proinflammatory stimuli and recipient immune effectors to prevent or reverse acute and chronic organ allograft injury. On the basis of evidence from almost two decades of basic and preclinical research, early-phase clinical trials of ex vivo–expanded autologous T-reg have been recently completed in recipients of KTx and liver transplant (8,96,98–101). Those carried out in KTx recipients have, to date, consistently demonstrated safety in combination with various conventional immunosuppressive regimens, preliminary evidence for persistence of infused T-reg in the blood for 1–3 months (99), and of an increase in total circulating T-reg numbers for at least 12 months after administration (98). Ongoing early-phase trials and planned phase 2 trials are likely to further clarify whether polyclonal, autologous T-reg therapies robustly modulate post-transplant immune responses toward donor-specific tolerance (96). Other regulatory immune cell types—specifically regulatory macrophages and tolerogenic dendritic cells—have also been developed to the point of early-phase clinical trials in KTx recipients (102,103). Very recently, a report of the post-transplant outcomes and immunologic profiling results for recipients of living-donor KTx enrolled into a suite of early-phase regulatory cell therapy trials has been published by the ONE Study consortium (100). In this unique study, the observed results for 38 recipients of living-donor KTx receiving one of four different T-reg, one regulatory macrophage, or one tolerogenic dendritic cell therapeutic product, in combination with a tapered conventional immunosuppressive drug regimen, were collated and compared with results for a group of 66 recipients that were managed by a standard-of-care regimen across eight sites in Europe and the United States. Although no conclusions about the protolerogenic efficacy for any single regulatory cell therapy can be made from this report, the authors convincingly show a good safety profile for such therapies in KTx recipients. In addition, the combined cell-therapy cohort had no increase in graft rejection or reduction in graft survival, experienced a strikingly reduced rate of viral infections, and appeared to revert to a more favorable peripheral blood immune cell profile compared with recipients who were treated conventionally (100). Subsequently, Roemhild et al. (101) published a very detailed report of the ONEnTreg13 Trial—a component of the ONE Study. In this phase 1/2a trial, autologous, ex vivo–expanded, natural T-reg (nTreg) were administered 7 days after transplantation to 11 recipients of living-donor KTx at Charité – Berlin University of Medicine (Berlin, Germany) and the results were compared with those of nine patients who were previously transplanted and managed with the standard-of-care regimen at the same center. These results demonstrated excellent safety and 3-year graft outcomes for the KTx recipients treated with nTreg, along with successful tapering of immunosuppression to tacrolimus monotherapy in eight of 11 subjects and evidence of oligoclonal (presumably alloantigen-driven) expansion of nTreg at 60 weeks post-transplant (101). Finally, in another very recent, early-phase trial report, Morath et al. (104) describe favorable safety and graft outcomes after pretransplant administration of donor-derived modified immune cell (mitomycin C–treated PBMC) infusions to ten recipients of living-donor KTx. Post-transplant immunologic studies also provided evidence for prolonged donor-specific T-cell hypo-responsiveness and increased numbers of circulating IL-10–producing regulatory B cells (which have been associated with immune tolerance) (104).
Overall, recently reported and ongoing early-phase clinical trials of several cell-based DMRT in the area of KTx provide important reassurances regarding the safety and feasibility of such therapies, both before and at early or later times after transplantation. The generally favorable short-to-midterm patient and graft outcomes, and promising immune profiling and histologic studies, should be interpreted with caution in the absence of larger, randomized controlled trials. Nonetheless, cohesion among some of the observations made in patients participating in these trials and the growing scientific knowledge regarding immunologic tolerance and of the pathobiology of KTx complications should provide a strong impetus for further progress. As illustrated in Figure 5, individual DMRT have the potential to target specific clinical and immunologic challenges associated with current limitations to the long-term health of KTx recipients.
Autologous versus Allogeneic Cell Therapies: The Influence of Patient-Specific Factors
As we move toward clinical application of DMRT, optimization of cell product remains vital to successful translation of preclinical findings. Allogeneic cell–based products offer a readily available “off-the-shelf” treatment option. Yet, patient-derived (autologous) cells may be preferable for individualized therapy or for repeated dosing, given the lower risk for allosensitization. In this regard, the influence of patient- or disease-specific factors on the growth and functionality of culture-expanded stromal and other primary cell therapies represents a key research area—particularly in settings of high relevance to kidney disease, such as older age, DM, vascular disease, and reduced renal function (105). In DM and kidney disease, oxidative stress, autophagy, and cellular senescence induce dysfunction of autologous cells (106). In metabolic syndrome and DM, stem-cell mobilization and therapeutic effect are diminished (65,74). In older individuals with atherosclerotic RVD, we identified altered MSC functional capacity (migration, angiogenesis) and increased cellular-senescence burden compared with controls (32). Despite this, our clinical trial results, described above in RVD, confirm that intrarenal administration of autologous MSC was associated with improved RBF and preserved GFR (26,33). Similarly, in MSCs harvested from adults with DKD and healthy controls, we have observed transcriptome alterations and reduced in vitro MSC migration but preserved or increased immunomodulatory and renal reparative activities in vitro (L.J.H., under review). In ESKD and KTx recipients, autologous MSCs have been shown to undergo comparable culture-expansion characteristics to those from individuals with normal kidney function, and to maintain the capacity to inhibit antidonor HLA immune response when compared with control MSCs (83,107).
Despite these encouraging results, counteracting biologic processes that potentially limit the regenerative functions of manufactured cell products may prove to be important for maximizing the benefits of autologous cell therapies. Recent developments in the understanding of cellular senescence may offer exciting opportunities for the application of DMRT to kidney disease. Premature senescence reduces MSC replicative capacity and limits cell expansion in manufacturing protocols. The abundance of senescent cells also fuels proinflammatory pathways in the pathogenesis of disease processes, such as DM and DKD (108,109). Furthermore, the microenvironmental stressors of kidney diseases (uremia, hyperglycemia, kidney aging, RAAS alteration, oxidative stress, inflammation) contribute to senescent-cell accumulation, potentially diminishing endogenous and exogenous MSC regenerative capacity (105,110). Emerging therapeutic strategies offer the possibility of modulating the microenvironments from which primary stromal cells are extracted through senescent-cell clearance in vivo. In a pilot clinical trial, we recently observed that a 3-day oral senolytic regimen of dasatinib and quercetin can diminish senescent-cell abundance in adipose and epithelial tissue and improve MSC proliferation in subjects with DKD (111). We and others are also pursuing other preconditioning methods to optimize autologous MSC functionality. Interventions such as exposure to hypoxia or melatonin during culture expansion may enhance the prorepair properties of MSCs (32,112). Taken together, these insights suggest that further integration of in vivo or ex vivo conditioning regimens could improve the success of autologous (and perhaps also allogeneic) cell-based DMRT in kidney disease.
Conclusions and Future Directions
As we have reviewed here, extensive preclinical research has greatly increased our understanding of the in vivo distribution, longevity, and mechanisms of action of MSCs and other potential regenerative therapies in the setting of kidney diseases. These insights have extended the rationale for regenerative therapies beyond the initial concept of engraftment and differentiation into functional tissues to include modulatory effects of, typically, short-lived cells, vesicles, or biomaterials that ameliorate disease processes through complex, paracrine interactions with host cells and tissues which promote inherent mechanisms of repair and regeneration. Well-conducted small- and large-animal model studies have been essential to determining the optimal parameters for clinical application of MSCs and other DMRT to kidney diseases—including the route of delivery and localization after administration; the key responder compartments and cell responses within the kidney; and the source, timing of release, and activity of the most important soluble mediators. In keeping with the literature reviewed in this article, we believe that these critical parameters must be defined for each specific disease and therapy. Conversely, lack of concordance between animal-model research and human clinical application of regenerative therapies continues to be a major challenge to the field. For the four distinct renal-disease areas we have focused on, translation of DMRT into clinical nephrology practice remains at an early stage. Indeed, of the translational strategies we review here, only MSC therapy for RVD and T-reg therapy in KTx could be said to have shown preliminary (early-phase) clinical trial evidence of superior efficacy compared with conventional pharmacotherapy and interventional procedures. Nonetheless, research in this area has generated a wealth of novel scientific insight and a growing number of informative clinical trial experiences with MSC- and regulatory immune cell–based investigational medicinal products. Reassuringly, the safety profiles for such cell therapies in patients with kidney diseases and KTx enrolled into clinical trials has, thus far, proven to be very good. For some of these clinical applications, preliminary signals of in vivo disease modulation have also emerged (26,33,84,86,88,100,101,104), whereas others have lacked evidence of efficacy (58).
In considering how future clinical effect could be maximized for cell-based DMRT that have undergone early-phase clinical trials in patients with kidney disease, a number of critical missing elements should be highlighted: (1) development of disease-specific assays to quantify potency of, and patient response to, DMRT to account for complexities, such as interindividual heterogeneity and changes in cell functionality, that occur during ex vivo expansion; (2) definition of optimal dosing, distribution, and frequency of administration of DMRT for specific clinical targets; (3) consensus on the influence of cryopreservation, which may negatively affect the consistency of DMRT therapeutic effects (6,7,113); (4) increased understanding of relative clinical efficacy of autologous and allogeneic cell therapies for specific patient groups; (5) innovations in manufacturing procedures that will eventually allow for cost-effective delivery of DMRT to large numbers of patients. As is clear from Figure 1, other DMRT for which preclinical evidence bases and technological developments are building await definitive clinical translation (13,36,114). Increasingly, crossdiscipline research and innovation has brought the potential for combinatorial advanced therapies to the forefront of translational initiatives in regenerative medicine. Incorporation of gene editing and biomaterials science holds future promise for stabilizing and enhancing the key mechanisms of action of cellular therapies identified from preclinical studies or patient profiling in early-phase clinical trials. Similarly, clever use of combined pharmacotherapy and DMRT is likely to be critical for optimizing and broadening the clinical applications of regenerative medicine. For example, patient conditioning through the coadministration of antisenescence (senolytic) agents to enhance MSC survival and anti-inflammatory/immune regulatory responses (31,111).
Viewed through the lens of four distinct areas of clinical nephrology practice, we conclude that the potential for our patients to substantially benefit from DMRT within the next decade is high and will be driven by a spirit of “joined-up thinking” among basic scientists, biomedical engineers, technology innovators, clinical trialists, clinicians, and funding and regulatory bodies.
Disclosures
M. Griffin reports receiving honoraria from American Society of Nephrology, Hebei Medical University (China), and National Institutes of Health; being an associate editor for JASN, being on the editorial boards for Frontiers in Antigen Presenting Cell Biology, Frontiers in Renal Pharmacology, Kidney International, and Transplantation; being a section editor for Mayo Clinic Proceedings; and receiving research funding from Randox Laboratories for research not related to this article. S. Herrmann reports having patents and inventions with Pfizer, but these are not related to this research. All remaining authors have nothing to disclose.
Funding
L. Hickson is supported by Regenerative Medicine Minnesota grant RMM 091718, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants DK109134 and DK123492, and NIDDK Diabetic Complications Consortium grants DK076169 and DK115255 (RRID:SCR_001415, www.diacomp.org). S. Herrmann is supported by NIDDK grant DK118120, and by a Mary Kathryn and Michael B. Panitch Career Development Award. M. Griffin is supported by European Commission grants 634086 (Horizon 2020 Collaborative Health Project NEPHSTROM) and 602470 (FP7 Collaborative Health Project VISICORT), Science Foundation Ireland grants 09/SRC-B1794 (REMEDI Strategic Research Cluster) and 13/RC/2073 (CÚRAM Research Centre), and the European Regional Development Fund.
Author Contributions
M. Griffin was responsible for funding acquisition; and M. Griffin, S. Herrmann, L. Hickson, and B. McNicholas conceptualized the article, wrote the original draft, and reviewed and edited the manuscript.
References
- 1.Haseltine WA: The emergence of regenerative medicine: A new field and a new society. J Regen Med 2: 17–23, 2004 [Google Scholar]
- 2.Mao AS, Mooney DJ: Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci U S A 112: 14452–14459, 2015. 10.1073/pnas.1508520112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oxburgh L, Carroll TJ, Cleaver O, Gossett DR, Hoshizaki DK, Hubbell JA, Humphreys BD, Jain S, Jensen J, Kaplan DL, Kesselman C, Ketchum CJ, Little MH, McMahon AP, Shankland SJ, Spence JR, Valerius MT, Wertheim JA, Wessely O, Zheng Y, Drummond IA: (Re)Building a kidney. J Am Soc Nephrol 28: 1370–1378, 2017. 10.1681/ASN.2016101077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little MH, Hale LJ, Howden SE, Kumar SV: Generating kidney from stem cells. Annu Rev Physiol 81: 335–357, 2019. 10.1146/annurev-physiol-020518-114331 [DOI] [PubMed] [Google Scholar]
- 5.Nishinakamura R: Human kidney organoids: Progress and remaining challenges. Nat Rev Nephrol 15: 613–624, 2019. 10.1038/s41581-019-0176-x [DOI] [PubMed] [Google Scholar]
- 6.Galipeau J, Sensébé L: Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell 22: 824–833, 2018. 10.1016/j.stem.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squillaro T, Peluso G, Galderisi U: Clinical trials with mesenchymal stem cells: An update. Cell Transplant 25: 829–848, 2016. 10.3727/096368915X689622 [DOI] [PubMed] [Google Scholar]
- 8.Zwang NA, Leventhal JR: Cell therapy in kidney transplantation: Focus on regulatory T cells. J Am Soc Nephrol 28: 1960–1972, 2017. 10.1681/ASN.2016111206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood KJ, Bushell A, Hester J: Regulatory immune cells in transplantation. Nat Rev Immunol 12: 417–430, 2012. 10.1038/nri3227 [DOI] [PubMed] [Google Scholar]
- 10.Arcolino FO, Zia S, Held K, Papadimitriou E, Theunis K, Bussolati B, Raaijmakers A, Allegaert K, Voet T, Deprest J, Vriens J, Toelen J, van den Heuvel L, Levtchenko E: Urine of preterm neonates as a novel source of kidney progenitor cells. J Am Soc Nephrol 27: 2762–2770, 2016. 10.1681/ASN.2015060664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leuning DG, Reinders ME, Li J, Peired AJ, Lievers E, de Boer HC, Fibbe WE, Romagnani P, van Kooten C, Little MH, Engelse MA, Rabelink TJ: Clinical-grade isolated human kidney perivascular stromal cells as an organotypic cell source for kidney regenerative medicine. Stem Cells Transl Med 6: 405–418, 2017. 10.5966/sctm.2016-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rani S, Ryan AE, Griffin MD, Ritter T: Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol Ther 23: 812–823, 2015. 10.1038/mt.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McFetridge ML, Del Borgo MP, Aguilar MI, Ricardo SD: The use of hydrogels for cell-based treatment of chronic kidney disease. Clin Sci (Lond) 132: 1977–1994, 2018. 10.1042/CS20180434 [DOI] [PubMed] [Google Scholar]
- 14.US Renal Data System , US Renal Data System 2016 annual data report 2016. Available at: https://www.ajkd.org/article/S0272-6386(16)30703-X/fulltext. Accessed February 12, 2020
- 15.Long DA, Norman JT, Fine LG: Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol 8: 244–250, 2012. 10.1038/nrneph.2011.219 [DOI] [PubMed] [Google Scholar]
- 16.Herrmann SM, Textor SC: Renovascular hypertension. Endocrinol Metab Clin North Am 48: 765–778, 2019. 10.1016/j.ecl.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eirin A, Textor SC, Lerman LO: Emerging paradigms in chronic kidney ischemia. Hypertension 72: 1023–1030, 2018. 10.1161/HYPERTENSIONAHA.118.11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB Sr, Dworkin LD; CORAL Investigators: Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014. 10.1056/NEJMoa1310753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, Textor SC: Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv 6: 428–435, 2013. 10.1161/CIRCINTERVENTIONS.113.000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eirin A, Textor SC, Lerman LO: Novel therapeutic strategies for renovascular disease. Curr Opin Nephrol Hypertens 28: 383–389, 2019. 10.1097/MNH.0000000000000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA: Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 22: 1–9, 2013. 10.1097/MNH.0b013e32835b36c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch WJ, Baumgärtl H, Lübbers D, Wilcox CS: Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int 59: 230–237, 2001. 10.1046/j.1523-1755.2001.00483.x [DOI] [PubMed] [Google Scholar]
- 23.Udani S, Lazich I, Bakris GL: Epidemiology of hypertensive kidney disease. Nat Rev Nephrol 7: 11–21, 2011. 10.1038/nrneph.2010.154 [DOI] [PubMed] [Google Scholar]
- 24.Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO: Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 30: 1030–1041, 2012. 10.1002/stem.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira-Sales EB, Maquigussa E, Semedo P, Pereira LG, Ferreira VM, Câmara NO, Bergamaschi CT, Campos RR, Boim MA: Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PLoS One 8: e78464, 2013. 10.1371/journal.pone.0078464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF, McKusick MA, Misra S, Bjarnason H, Armstrong AS, Gastineau DA, Lerman LO, Textor SC: Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. J Am Soc Nephrol 28: 2777–2785, 2017. 10.1681/ASN.2017020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ, Lerman LO: Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant 27: 1080–1095, 2018. 10.1177/0963689718780942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO: Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 92: 114–124, 2017. 10.1016/j.kint.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grange C, Skovronova R, Marabese F, Bussolati B: Stem cell-derived extracellular vesicles and kidney regeneration. Cells 8: 1240, 2019. 10.3390/cells8101240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG: Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19: 1769–1779, 2011. 10.1038/mt.2011.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM: Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front Immunol 9: 2837, 2018. 10.3389/fimmu.2018.02837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saad A, Zhu XY, Herrmann S, Hickson L, Tang H, Dietz AB, van Wijnen AJ, Lerman L, Textor S: Adipose-derived mesenchymal stem cells from patients with atherosclerotic renovascular disease have increased DNA damage and reduced angiogenesis that can be modified by hypoxia. Stem Cell Res Ther 7: 128, 2016. 10.1186/s13287-016-0389-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abumoawad A, Saad A, Ferguson CM, Eirin A, Herrmann SM, Hickson LJ, Goksu BB, Bendel E, Misra S, Glockner J, Dietz AB, Lerman LO, Textor SC: In a phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int 97: 793–804, 2020. 10.1016/j.kint.2019.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eirin A, Zhu XY, Ebrahimi B, Krier JD, Riester SM, van Wijnen AJ, Lerman A, Lerman LO: Intrarenal delivery of mesenchymal stem cells and endothelial progenitor cells attenuates hypertensive cardiomyopathy in experimental renovascular hypertension. Cell Transplant 24: 2041–2053, 2015. 10.3727/096368914X685582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinsey GR, Sharma R, Okusa MD: Regulatory T cells in AKI. J Am Soc Nephrol 24: 1720–1726, 2013. 10.1681/ASN.2013050502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcheque J, Bussolati B, Csete M, Perin L: Concise reviews: Stem cells and kidney regeneration: An update. Stem Cells Transl Med 8: 82–92, 2019. 10.1002/sctm.18-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A: Acute kidney injury in sepsis. Intensive Care Med 43: 816–828, 2017. 10.1007/s00134-017-4755-7 [DOI] [PubMed] [Google Scholar]
- 38.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC: The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801–810, 2016. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Federspiel CK, Itenov TS, Mehta K, Hsu RK, Bestle MH, Liu KD: Duration of acute kidney injury in critically ill patients. Ann Intensive Care 8: 30, 2018. 10.1186/s13613-018-0374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devaney J, Horie S, Masterson C, Elliman S, Barry F, O’Brien T, Curley GF, O’Toole D, Laffey JG: Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 70: 625–635, 2015. 10.1136/thoraxjnl-2015-206813 [DOI] [PubMed] [Google Scholar]
- 41.Luo CJ, Zhang FJ, Zhang L, Geng YQ, Li QG, Hong Q, Fu B, Zhu F, Cui SY, Feng Z, Sun XF, Chen XM: Mesenchymal stem cells ameliorate sepsis-associated acute kidney injury in mice. Shock 41: 123–129, 2014. 10.1097/SHK.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 42.Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, Yarmush ML: Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant 19: 823–830, 2010. 10.3727/096368910X508942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laroye C, Lemarié J, Boufenzer A, Labroca P, Cunat L, Alauzet C, Groubatch F, Cailac C, Jolly L, Bensoussan D, Reppel L, Gibot S: Clinical-grade mesenchymal stem cells derived from umbilical cord improve septic shock in pigs. Intensive Care Med Exp 6: 24, 2018. 10.1186/s40635-018-0194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ: Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182: 1047–1057, 2010. 10.1164/rccm.201001-0010OC [DOI] [PubMed] [Google Scholar]
- 45.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E: Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production [published correction appears in Nat Med 15: 462, 2009]. Nat Med 15: 42–49, 2009. 10.1038/nm.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA: Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302: L1003–L1013, 2012. 10.1152/ajplung.00180.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss ARR, Dahlke MH: Immunomodulation by mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol 10: 1191, 2019. 10.3389/fimmu.2019.01191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F: Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med 9: eaam7828, 2017. 10.1126/scitranslmed.aam7828 [DOI] [PubMed] [Google Scholar]
- 49.Fazekas B, Griffin MD: Mesenchymal stromal cell-based therapies for acute kidney injury: Progress in the last decade. Kidney Int 97: 1130–1140, 2020. 10.1016/j.kint.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 50.Rabani R, Volchuk A, Jerkic M, Ormesher L, Garces-Ramirez L, Canton J, Masterson C, Gagnon S, Tatham KC, Marshall J, Grinstein S, Laffey JG, Szaszi K, Curley GF: Mesenchymal stem cells enhance NOX2-dependent reactive oxygen species production and bacterial killing in macrophages during sepsis. Eur Respir J 51: 1702021, 2018. 10.1183/13993003.02021-2017 [DOI] [PubMed] [Google Scholar]
- 51.Rocheteau P, Chatre L, Briand D, Mebarki M, Jouvion G, Bardon J, Crochemore C, Serrani P, Lecci PP, Latil M, Matot B, Carlier PG, Latronico N, Huchet C, Lafoux A, Sharshar T, Ricchetti M, Chrétien F: Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun 6: 10145, 2015. 10.1038/ncomms10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsoyi K, Hall SRR, Dalli J, Colas RA, Ghanta S, Ith B, Coronata A, Fredenburgh LE, Baron RM, Choi AMK, Serhan CN, Liu X, Perrella MA: Carbon monoxide improves efficacy of mesenchymal stromal cells during sepsis by production of specialized proresolving lipid mediators. Crit Care Med 44: e1236–e1245, 2016. 10.1097/CCM.0000000000001999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva JD, Lopes-Pacheco M, de Castro LL, Kitoko JZ, Trivelin SA, Amorim NR, Capelozzi VL, Morales MM, Gutfilen B, de Souza SAL, Weiss DJ, Diaz BL, Rocco PRM: Eicosapentaenoic acid potentiates the therapeutic effects of adipose tissue-derived mesenchymal stromal cells on lung and distal organ injury in experimental sepsis. Stem Cell Res Ther 10: 264, 2019. 10.1186/s13287-019-1365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos ACW, de Jonge-Muller ESM, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW: Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 29: 1549–1558, 2011. 10.1002/stem.698 [DOI] [PubMed] [Google Scholar]
- 55.Park J, Kim S, Lim H, Liu A, Hu S, Lee J, Zhuo H, Hao Q, Matthay MA, Lee J-W: Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax 74: 43–50, 2019. 10.1136/thoraxjnl-2018-211576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McIntyre LA, Stewart DJ, Mei SHJ, Courtman D, Watpool I, Granton J, Marshall J, Dos Santos C, Walley KR, Winston BW, Schlosser K, Fergusson DA; Canadian Critical Care Trials Group; Canadian Critical Care Translational Biology Group: Cellular immunotherapy for septic shock. A phase I clinical trial. Am J Respir Crit Care Med 197: 337–347, 2018. 10.1164/rccm.201705-1006OC [DOI] [PubMed] [Google Scholar]
- 57.Schlosser K, Wang JP, Dos Santos C, Walley KR, Marshall J, Fergusson DA, Winston BW, Granton J, Watpool I, Stewart DJ, McIntyre LA, Mei SHJ; Canadian Critical Care Trials Group and the Canadian Critical Care Translational Biology Group: Effects of mesenchymal stem cell treatment on systemic cytokine levels in a phase 1 dose escalation safety trial of septic shock patients. Crit Care Med 47: 918–925, 2019. 10.1097/CCM.0000000000003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, Lellouche F, Fox-Robichaud A, Atta MG, Melby S, Mehta RL, Wald R, Verma S, Mazer CD; ACT-AKI investigators: Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol 29: 260–267, 2018. 10.1681/ASN.2016101150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sung P-H, Chang C-L, Tsai T-H, Chang L-T, Leu S, Chen Y-L, Yang C-C, Chua S, Yeh K-H, Chai H-T, Chang H-W, Chen H-H, Yip H-K: Apoptotic adipose-derived mesenchymal stem cell therapy protects against lung and kidney injury in sepsis syndrome caused by cecal ligation puncture in rats. Stem Cell Res Ther 4: 155, 2013. 10.1186/scrt385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.NCD Risk Factor Collaboration (NCD-RisC): Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4·4 million participants [published correction appears in Lancet 389: e2, 2017 10.1016/S0140-6736(16)32060-8]. Lancet 387: 1513–1530, 2016. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai C-Y, Floyd T, Al-Aly Z: Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94: 567–581, 2018. 10.1016/j.kint.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 62.Reidy K, Kang HM, Hostetter T, Susztak K: Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014. 10.1172/JCI72271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiley CD: Role of senescent renal cells in pathophysiology of diabetic kidney disease. Curr Diab Rep 20: 33, 2020. 10.1007/s11892-020-01314-y [DOI] [PubMed] [Google Scholar]
- 64.Griffin TP, Martin WP, Islam N, O’Brien T, Griffin MD: The promise of mesenchymal stem cell therapy for diabetic kidney disease. Curr Diab Rep 16: 42, 2016. 10.1007/s11892-016-0734-6 [DOI] [PubMed] [Google Scholar]
- 65.Fadini GP, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A: Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care 36: 943–949, 2013. 10.2337/dc12-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heerspink HJL, Parving H-H, Andress DL, Bakris G, Correa-Rotter R, Hou F-F, Kitzman DW, Kohan D, Makino H, McMurray JJV, Melnick JZ, Miller MG, Pergola PE, Perkovic V, Tobe S, Yi T, Wigderson M, de Zeeuw D; SONAR Committees and Investigators: Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial [published correction appears in Lancet 393: 1936, 2019 10.1016/S0140-6736(19)30939-0]. Lancet 393: 1937–1947, 2019. 10.1016/S0140-6736(19)30772-X [DOI] [PubMed] [Google Scholar]
- 67.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu P-L, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 68.Torres Crigna A, Daniele C, Gamez C, Medina Balbuena S, Pastene DO, Nardozi D, Brenna C, Yard B, Gretz N, Bieback K: Stem/stromal cells for treatment of kidney injuries with focus on preclinical models. Front Med (Lausanne) 5: 179, 2018. 10.3389/fmed.2018.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.An X, Liao G, Chen Y, Luo A, Liu J, Yuan Y, Li L, Yang L, Wang H, Liu F, Yang G, Yi S, Li Y, Cheng J, Lu Y: Intervention for early diabetic nephropathy by mesenchymal stem cells in a preclinical nonhuman primate model. Stem Cell Res Ther 10: 363, 2019. 10.1186/s13287-019-1401-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lv S-S, Liu G, Wang J-P, Wang W-W, Cheng J, Sun A-L, Liu H-Y, Nie H-B, Su M-R, Guan G-J: Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol 17: 275–282, 2013. 10.1016/j.intimp.2013.05.031 [DOI] [PubMed] [Google Scholar]
- 71.Yang J, Dai C, Liu Y: A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol 16: 68–78, 2005. 10.1681/ASN.2003090795 [DOI] [PubMed] [Google Scholar]
- 72.Cheung TS, Dazzi F: Mesenchymal-myeloid interaction in the regulation of immunity. Semin Immunol 35: 59–68, 2018. 10.1016/j.smim.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 73.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y: Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14: 493–507, 2018. 10.1038/s41581-018-0023-5 [DOI] [PubMed] [Google Scholar]
- 74.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Saijo Y, Tsuchida H, Ishioka S, Nishikawa A, Saito T, Fujimiya M: Umbilical cord extracts improve diabetic abnormalities in bone marrow-derived mesenchymal stem cells and increase their therapeutic effects on diabetic nephropathy. Sci Rep 7: 8484, 2017. 10.1038/s41598-017-08921-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G, Brizzi MF: Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep 9: 4468, 2019. 10.1038/s41598-019-41100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luk F, de Witte SF, Korevaar SS, Roemeling-van Rhijn M, Franquesa M, Strini T, van den Engel S, Gargesha M, Roy D, Dor FJ, Horwitz EM, de Bruin RW, Betjes MG, Baan CC, Hoogduijn MJ: Inactivated mesenchymal stem cells maintain immunomodulatory capacity. Stem Cells Dev 25: 1342–1354, 2016. 10.1089/scd.2016.0068 [DOI] [PubMed] [Google Scholar]
- 77.Negi N, Griffin MD: Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance. Stem Cells 38: 596–605, 2020. 10.1002/stem.3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stenvinkel P, Wadström J, Bertram T, Detwiler R, Gerber D, Brismar TB, Blomberg P, Lundgren T: Implantation of autologous selected renal cells in diabetic chronic kidney disease stages 3 and 4—clinical experience of a “first in human” study. Kidney Int Rep 1: 105–113, 2016. 10.1016/j.ekir.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruce AT, Ilagan RM, Guthrie KI, Rivera E, Choudhury S, Sangha N, Spencer T, Bertram TA, Jain D, Kelley RW, Basu J: Selected renal cells modulate disease progression in rodent models of chronic kidney disease via NF-κB and TGF-β1 pathways. Regen Med 10: 815–839, 2015. 10.2217/rme.15.43 [DOI] [PubMed] [Google Scholar]
- 80.Chen S, Shi J, Zhang M, Chen Y, Wang X, Zhang L, Tian Z, Yan Y, Li Q, Zhong W, Xing M, Zhang L, Zhang L: Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep 5: 18104, 2015. 10.1038/srep18104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Packham DK, Fraser IR, Kerr PG, Segal KR: Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: A randomized, placebo-controlled, dose escalation study. EBioMedicine 12: 263–269, 2016. 10.1016/j.ebiom.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stegall MD, Gaston RS, Cosio FG, Matas A: Through a glass darkly: Seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol 26: 20–29, 2015. 10.1681/ASN.2014040378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Podestà MA, Remuzzi G, Casiraghi F: Mesenchymal stromal cells for transplant tolerance. Front Immunol 10: 1287, 2019. 10.3389/fimmu.2019.01287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C: Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 307: 1169–1177, 2012. 10.1001/jama.2012.316 [DOI] [PubMed] [Google Scholar]
- 85.Dreyer GJ, Groeneweg KE, Heidt S, Roelen DL, van Pel M, Roelofs H, Huurman VAL, Bajema IM, Moes DJAR, Fibbe WE, Claas FHJ, van Kooten C, Rabelink RJ, de Fijter JW, Reinders MEJ: Human leukocyte antigen selected allogeneic mesenchymal stromal cell therapy in renal transplantation: The Neptune study, a phase I single-center study. Am J Transplant 20: 2905–2915, 2020. 10.1111/ajt.15910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erpicum P, Weekers L, Detry O, Bonvoisin C, Delbouille M-H, Grégoire C, Baudoux E, Briquet A, Lechanteur C, Maggipinto G, Somja J, Pottel H, Baron F, Jouret F, Beguin Y: Infusion of third-party mesenchymal stromal cells after kidney transplantation: A phase I-II, open-label, clinical study. Kidney Int 95: 693–707, 2019. 10.1016/j.kint.2018.08.046 [DOI] [PubMed] [Google Scholar]
- 87.Mudrabettu C, Kumar V, Rakha A, Yadav AK, Ramachandran R, Kanwar DB, Nada R, Minz M, Sakhuja V, Marwaha N, Jha V: Safety and efficacy of autologous mesenchymal stromal cells transplantation in patients undergoing living donor kidney transplantation: A pilot study. Nephrology (Carlton) 20: 25–33, 2015. 10.1111/nep.12338 [DOI] [PubMed] [Google Scholar]
- 88.Perico N, Casiraghi F, Todeschini M, Cortinovis M, Gotti E, Portalupi V, Mister M, Gaspari F, Villa A, Fiori S, Introna M, Longhi E, Remuzzi G: Long-term clinical and immunological profile of kidney transplant patients given mesenchymal stromal cell immunotherapy. Front Immunol 9: 1359, 2018. 10.3389/fimmu.2018.01359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reinders MEJ, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FHJ, van Miert PPMC, Roelen DL, van Kooten C, Fibbe WE, Rabelink TJ: Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: Results of a phase I study. Stem Cells Transl Med 2: 107–111, 2013. 10.5966/sctm.2012-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W, Li X, Chen Z, Ma J, Liao D, Li G, Fang J, Pan G, Xiang AP: Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: A clinical pilot study [published correction appears in Transplantation 97: e37, 2014]. Transplantation 95: 161–168, 2013. 10.1097/TP.0b013e3182754c53 [DOI] [PubMed] [Google Scholar]
- 91.Perico N, Casiraghi F, Gotti E, Introna M, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Noris M, Remuzzi G: Mesenchymal stromal cells and kidney transplantation: Pretransplant infusion protects from graft dysfunction while fostering immunoregulation. Transpl Int 26: 867–878, 2013. 10.1111/tri.12132 [DOI] [PubMed] [Google Scholar]
- 92.Pool M, Eertman T, Sierra Parraga J, ’T Hart N, Roemeling-Van Rhijn M, Eijken M, Jespersen B, Reinders M, Hoogduijn M, Ploeg R, Leuvenink H, Moers C: Infusing mesenchymal stromal cells into porcine kidneys during normothermic machine perfusion: Intact MSCs can be traced and localised to glomeruli. Int J Mol Sci 20: 3607, 2019. 10.3390/ijms20143607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brasile L, Henry N, Orlando G, Stubenitsky B: Potentiating renal regeneration using mesenchymal stem cells. Transplantation 103: 307–313, 2019. 10.1097/TP.0000000000002455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lohmann S, Eijken M, Møldrup U, Møller BK, Hunter J, Moers C, Leuvenink H, Ploeg RJ, Clahsen-van Groningen MC, Hoogduijn M, Baan CC, Keller AK, Jespersen B: Ex vivo administration of mesenchymal stromal cells in kidney grafts against ischemia reperfusion injury - effective delivery without kidney function improvement posttransplant [published online ahead of print September 18, 2020]. Transplantation 10.1097/TP.0000000000003429 [DOI] [PubMed] [Google Scholar]
- 95.Cheung TS, Galleu A, von Bonin M, Bornhäuser M, Dazzi F: Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: Implications for the monitoring of mesenchymal stromal cell activity. Haematologica 104: e438–e441, 2019. 10.3324/haematol.2018.214767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G: Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol 10: 43, 2019. 10.3389/fimmu.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morath C, Schmitt A, Zeier M, Schmitt M, Sandra-Petrescu F, Opelz G, Terness P, Schaier M, Kleist C: Cell therapy for immunosuppression after kidney transplantation. Langenbecks Arch Surg 400: 541–550, 2015. 10.1007/s00423-015-1313-z [DOI] [PubMed] [Google Scholar]
- 98.Mathew JM, H-Voss J, LeFever A, Konieczna I, Stratton C, He J, Huang X, Gallon L, Skaro A, Ansari MJ, Leventhal JR: A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep 8: 7428, 2018. 10.1038/s41598-018-25574-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chandran S, Tang Q, Sarwal M, Laszik ZG, Putnam AL, Lee K, Leung J, Nguyen V, Sigdel T, Tavares EC, Yang JYC, Hellerstein M, Fitch M, Bluestone JA, Vincenti F: Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant 17: 2945–2954, 2017. 10.1111/ajt.14415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, Tang Q, Guinan EC, Battaglia M, Burlingham WJ, Roberts ISD, Streitz M, Josien R, Böger CA, Scottà C, Markmann JF, Hester JL, Juerchott K, Braudeau C, James B, Contreras-Ruiz L, van der Net JB, Bergler T, Caldara R, Petchey W, Edinger M, Dupas N, Kapinsky M, Mutzbauer I, Otto NM, Öllinger R, Hernandez-Fuentes MP, Issa F, Ahrens N, Meyenberg C, Karitzky S, Kunzendorf U, Knechtle SJ, Grinyó J, Morris PJ, Brent L, Bushell A, Turka LA, Bluestone JA, Lechler RI, Schlitt HJ, Cuturi MC, Schlickeiser S, Friend PJ, Miloud T, Scheffold A, Secchi A, Crisalli K, Kang SM, Hilton R, Banas B, Blancho G, Volk HD, Lombardi G, Wood KJ, Geissler EK: Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 395: 1627–1639, 2020. 10.1016/S0140-6736(20)30167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roemhild A, Otto NM, Moll G, Abou-El-Enein M, Kaiser D, Bold G, Schachtner T, Choi M, Oellinger R, Landwehr-Kenzel S, Juerchott K, Sawitzki B, Giesler C, Sefrin A, Beier C, Wagner DL, Schlickeiser S, Streitz M, Schmueck-Henneresse M, Amini L, Stervbo U, Babel N, Volk H-D, Reinke P: Regulatory T cells for minimising immune suppression in kidney transplantation: Phase I/IIa clinical trial. BMJ 371: m3734, 2020. 10.1136/bmj.m3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ochando J, Ordikhani F, Jordan S, Boros P, Thomson AW: Tolerogenic dendritic cells in organ transplantation. Transpl Int 33: 113–127, 2020. 10.1111/tri.13504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, Oberg HH, Pascher A, Lützen U, Janssen U, Broichhausen C, Renders L, Thaiss F, Scheuermann E, Henze E, Volk H-D, Chatenoud L, Lechler RI, Wood KJ, Kabelitz D, Schlitt HJ, Geissler EK, Fändrich F: Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol 187: 2072–2078, 2011. 10.4049/jimmunol.1100762 [DOI] [PubMed] [Google Scholar]
- 104.Morath C, Schmitt A, Kleist C, Daniel V, Opelz G, Süsal C, Ibrahim E, Kälble F, Speer C, Nusshag C, Pego da Silva L, Sommerer C, Wang L, Ni M, Hückelhoven-Krauss A, Czock D, Merle U, Mehrabi A, Sander A, Hackbusch M, Eckert C, Waldherr R, Schnitzler P, Müller-Tidow C, Hoheisel JD, Mustafa SA, Alhamdani MSS, Bauer AS, Reiser J, Zeier M, Schmitt M, Schaier M, Terness P: Phase I trial of donor-derived modified immune cell infusion in kidney transplantation. J Clin Invest 130: 2364–2376, 2020. 10.1172/JCI133595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hickson LJ, Eirin A, Lerman LO: Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int 89: 767–778, 2016. 10.1016/j.kint.2015.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kornicka K, Houston J, Marycz K: Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem Cell Rev Rep 14: 337–345, 2018. 10.1007/s12015-018-9809-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reinders MEJ, Roemeling-van Rhijn M, Khairoun M, Lievers E, de Vries DK, Schaapherder AFM, Wong SWS, Zwaginga JJ, Duijs JM, van Zonneveld AJ, Hoogduijn MJ, Fibbe WE, de Fijter JW, van Kooten C, Rabelink TJ, Roelofs H: Bone marrow-derived mesenchymal stromal cells from patients with end-stage renal disease are suitable for autologous therapy. Cytotherapy 15: 663–672, 2013. 10.1016/j.jcyt.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 108.Bian X, Griffin TP, Zhu X, Islam MN, Conley SM, Eirin A, Tang H, O’Shea PM, Palmer AK, McCoy RG, Herrmann SM, Mehta RA, Woollard JR, Rule AD, Kirkland JL, Tchkonia T, Textor SC, Griffin MD, Lerman LO, Hickson LJ: Senescence marker activin A is increased in human diabetic kidney disease: Association with kidney function and potential implications for therapy. BMJ Open Diabetes Res Care 7: e000720, 2019. 10.1136/bmjdrc-2019-000720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, Prata LG, van Dijk TH, Verkade E, Casaclang-Verzosa G, Johnson KO, Cubro H, Doornebal EJ, Ogrodnik M, Jurk D, Jensen MD, Chini EN, Miller JD, Matveyenko A, Stout MB, Schafer MJ, White TA, Hickson LJ, Demaria M, Garovic V, Grande J, Arriaga EA, Kuipers F, von Zglinicki T, LeBrasseur NK, Campisi J, Tchkonia T, Kirkland JL: Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18: e12950, 2019. 10.1111/acel.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I: A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15: 1082–1087, 2009. 10.1038/nm.2014 [DOI] [PubMed] [Google Scholar]
- 111.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL: Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease [published correction appears in EBioMedicine 52: 102595, 2020 10.1016/j.ebiom.2019.12.004]. EBioMedicine 47: 446–456, 2019. 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao L, Hu C, Zhang P, Jiang H, Chen J: Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J Cell Mol Med 24: 25–33, 2020. 10.1111/jcmm.14769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang Q, Vincenti F: Transplant trials with Tregs: Perils and promises. J Clin Invest 127: 2505–2512, 2017. 10.1172/JCI90598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nargesi AA, Lerman LO, Eirin A: Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther 17: 29–42, 2017. 10.2174/1566523217666170412110724 [DOI] [PMC free article] [PubMed] [Google Scholar]