Abstract
Objectives:
The aim of this study was to determine if lysyl oxidase-like 1 (LOXL1) and Fibulin-5 (Fib-5), two crucial proteins in the elastin metabolism pathway, are detectable in the vaginal secretions of women with and without pelvic organ prolapse (POP). We then sought to quantify levels of these proteins in relation to prolapse.
Methods:
Vaginal secretions were obtained from 48 subjects (13 (27.1%) without and 35 (72.9%) with POP-Q Stage 2–4 prolapse). Eleven (22.9%) subjects were premenopausal and 37 (77.1%) were postmenopausal. Presence of LOXL-1 and Fibulin-5 within specimens were first identified via western blotting. Enzyme-Linked Immunosorbent Assays specific for LOXL1 and Fibulin-5 were conducted to quantify total protein secretion.
Results:
LOXL1 was detected in 45/48 (93.8%) and Fibulin-5 was seen in 24/48 (50%) of subjects. LOXL1 values were lower in women without prolapse (13.3 ng/100 mg median, 24.4 IQR) vs. those with prolapse (26.4 ng/100 mg, 102.2 IQR). On multivariate analysis controlling for age, women with prolapse had a 544% (p=0.0042 higher LOXL1 protein level compared to those without. There was no significant differences in LOXL1 or Fibulin-5 protein detection with relation to menopausal status in bivariate analysis.
Conclusions:
This is the first published report of non-invasively measuring urogenital LOXL1 and Fibulin-5. In vaginal secretions, LOXL1 protein is higher in subjects with POP than those without.
Keywords: Lysyl oxidase-like 1, LOXL-1, Fibulin-5, elastin, Pelvic Organ Prolapse
Introduction:
Pelvic Organ Prolapse (POP) is a common condition that negatively impacts the quality of life of millions of women.1 Although the exact cause is unknown, it is believed that POP is a multifactorial disorder associated with vaginal childbirth, aging, and weakening of the pelvic support structures.2 While injury to the pelvic floor is presumed responsible for sequalae years or even decades later resulting in anatomic defects, the two are not perfectly correlated, with some women developing POP while others do not. For example, women suffering from Ehlers-Danlos Syndrome, a connective tissue disorder, are predisposed to spontaneous POP in the absence of other risk factors.3 This discrepancy has led to an investigation of genetic factors that may be implicated in the pathogenesis of POP. One such widely studied pathway involves metabolism of elastin and collagen within the pelvic floor.
Fibulin-5 and Lysyl oxidase-like 1 (LOXL1) play an essential role in the synthesis and assembly of elastic fibers in the uterosacral ligaments from women with advanced pelvic organ prolapse compared to controls.4 LOXL1 is thought to play a role in the cross linking of tropoelastin monomers to create elastin polymers, while Fibulin-5 facilitates the transfer of elastin aggregates to microfibrils, the microfibrils acting as scaffolding of elastic fibers.5
Many recent studies have reported the changes in gene expression and downstream protein expression of Fibulin-5 and LOXL-1 associated with POP. The most widely used methods include gene expression from tissue removed during surgical resection or as a small biopsy.4,6–8 To date, it has never been reported if LOXL-1 and Fibulin-5 proteins are expressed in vaginal secretions and if these levels are above the minimum threshold of detection in commercially available assays. Our aim was to conduct a feasibility study to determine whether LOXL-1 and Fibulin-5 can be detected in vaginal fluids and to measure the level of these proteins in women with and without pelvic organ prolapse.
Materials & Methods:
Subject Selection:
The study protocol was approved by the University of California Irvine Institutional Review Board. Subjects were screened and enrolled through either the Female Pelvic Medicine & Reconstructive Surgery or General Gynecology clinics into two groups: those with prolapse and those without. Prolapse was defined as a Pelvic Organ Prolapse Quantification stage of II or greater. All women older than age 18 were eligible for enrollment. Current pregnancy, history of connective tissue disease, and current use of steroid therapy were excluded. Demographic data along with a targeted history were obtained at the time of enrollment.
Sample Collection:
Initial study design involved sample collection via a tampon placed vaginally for at least six hours however this was technically difficult for some subjects with advanced prolapse as the tampon would not remain in place. Collection was then modified to using a large tip cotton swab (Scopettes, BirchWood Laboratories). In a systematic fashion, the apex, anterior, and posterior vaginal walls were swabbed for 5 seconds each to collect a sufficient specimen. The sample was then kept on ice and stored in a −80⁰C freezer until the time of processing.
The samples were then thawed at room temperature and lysate buffer with protease inhibitor (100:1) was added. The liquid was strained from the tampons or swabs which were then discarded, and the solution was centrifuged at 1000 rpm for 15 minutes. Samples were then sonicated and re-centrifuged twice to collect the cellular lysate. A bicinchoninic acid (BCA) assay was performed to determine total protein concentration of the specimen which was then adjusted with phosphate buffered saline (PBS) to a standardized 1 ug/uL across all specimens.
Western Blot Analysis
Western blotting was performed initially to determine proof of concept and confirm the presence of protein from both tampon and swab samples prior to initiation of enzyme linked immunosorbent analysis (ELISA). 30 μg of total protein were loaded and separated on mini-SDS-PAGE gels (10%). After electrophoresis, samples were electroblotted onto a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA) at room temperature. The resultant membranes were blocked with Bovine Serum Albumin (BSA) 5% solution and subsequently incubated with primary LOXL1 Antibody (SC-166632, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Fibulin-5 Antibody (BS-0810R, Santa Cruz Biotechnology, Santa Cruz, CA, USA), both in a 1:200 dilution with 5% BSA overnight at 4⁰C. The membranes were then incubated with a 1:3000 dilution of goat anti-mouse IgG secondary antibody (32430 Thermo Fischer Scientific, Waltham, Massachusetts, USA) for LOXL-1, and a 1:1000 dilution of goat anti-rabbit IgG secondary antibody (32460 Thermo Fischer Scientific, Waltham, Massachusetts, USA) for Fibulin-5 for 1 hour at room temperature. Immunoreactive bands were then seen with the ECL detection system (Amersham Life Science, Buckinghamshire, UK).
Quantification of these results via a densometry analysis with ImageJ of the resultant western blotting bands was considered; however, given the potential for considerable cross staining via the region of the interest and the potential subjectivity of this method, the decision was made to use ELISAs.
ELISA Analysis
Vaginal secretion specimens were collected, and protein concentration was determined via BCA assay and diluted to 1 μg/μL as above. LOXL-1 and Fibulin-5 protein levels were determined ELISA testing (LOXL-1 LS-F7825 & Fibulin-5 LS-F21810, Lifespan Biosciences, Seattle, WA, USA). Each specimen was prepared for the ELISA by generating a standard curve based on optical density.
Threshold for detection on the base of the ELISA used are 0.156 ng/mL for LOXL-1 and 3.125 ng/mL for Fibulin-5. For the purposes of this study, any value below this minimal threshold for each protein was considered undetectable. 100 μL of protein from each specimen was assayed in duplicate for the ELISA. In order to account for the potential differences in vaginal secretion volume (LOXL-1/Fibulin-5 protein) from tampon versus vaginal swab specimens results were standardized as a ratio of nanogram (ng) / 100 mg protein for LOXL-1 and picogram (pg)/100 mg protein for Fibulin-5.
Statistical Analysis:
Fisher’s exact test and two sample t test were used to compare distribution of characteristics variables between patients with or without prolapse. Descriptive statistics of LOXL-1 and Fibulin-5 by patient’s demographic, clinical characteristics and prolapse status were calculated and presented in median and interquartile range. Non-parametric Wilcoxon two sample tests were used to examine the difference between characteristics categories. Multivariate linear regression models were fitted for the two proteins. Due to non-normal distribution of outcomes, protein levels were transformed using natural logarithm log (X+1), in which X is protein level. Estimates from regression model were transformed back for interpretation. All statistical analysis analyses were performed on SAS 9.4 (SAS Institute Cary, NC.). Statistical significance was set at P<0.05, using 2-tailed tests.
Results:
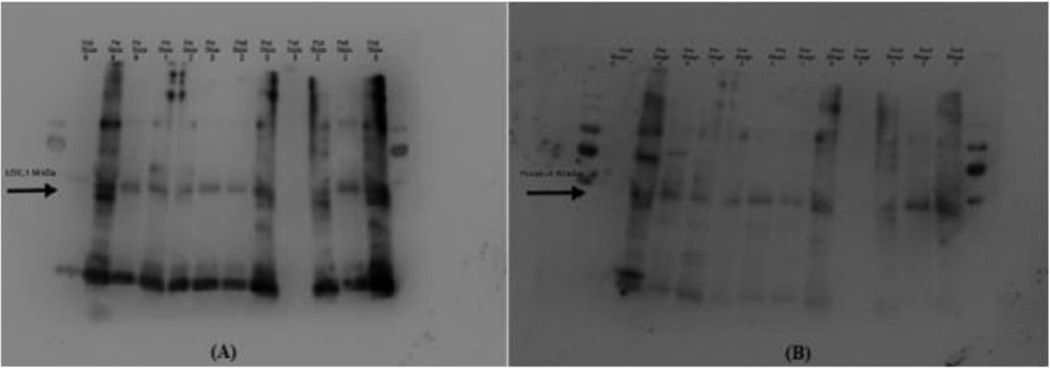
Subject demographics can be seen in Table 1. Forty-eight subjects were included in the analysis with 11 (22.9%) premenopausal and 37 (77.1%) post-menopausal subjects. There were 35 (72.9%) women with prolapse (POP-Q Stages II-IV) and 13 (27.1%) without prolapse. Aside from older age and menopausal status in the prolapse cohorts, there were no significant differences between the two groups. Initial proof of concept that LOXL-1 and Fibulin-5 are detectable in the vaginal secretions of both pre and post-menopausal women with various stages of prolapse is shown on a Western Blot in Figure 1.
Table 1.
Distribution of patient’s characteristics by prolapse status
| Total (n=48) | No prolapse (n=13, 27.1%) | Prolapse (n=35, 72.9%) | p value a | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| History of estrogen use | 1.0000 | ||||||
| No | 33 | 68.8 | 9 | 69.2 | 24 | 68.6 | |
| Yes | 15 | 31.3 | 4 | 30.8 | 11 | 31.4 | |
| Gravidity | 0.7234 | ||||||
| 0 or 1 | 15 | 34.9 | 5 | 41.7 | 10 | 32.3 | |
| 2 or more | 28 | 65.1 | 7 | 58.3 | 21 | 67.7 | |
| Parity | 0.7366 | ||||||
| 0 or 1 | 16 | 37.2 | 5 | 41.7 | 11 | 35.5 | |
| 2 or more | 27 | 62.8 | 7 | 58.3 | 20 | 64.5 | |
| History of vaginal delivery | 0.1704 | ||||||
| No | 8 | 20.0 | 4 | 33.3 | 4 | 14.3 | |
| Yes | 32 | 80.0 | 8 | 66.7 | 24 | 85.7 | |
| Race | 0.1160 | ||||||
| Asian | 11 | 22.9 | 5 | 38.5 | 6 | 17.1 | |
| Hispanic | 4 | 8.3 | 2 | 15.4 | 2 | 5.7 | |
| White | 33 | 68.8 | 6 | 46.2 | 27 | 77.1 | |
| Pelvic surgery | 0.5147 | ||||||
| No | 28 | 59.6 | 9 | 69.2 | 19 | 55.9 | |
| Yes | 19 | 40.4 | 4 | 30.8 | 15 | 44.1 | |
| Hysterectomy | 0.1362 | ||||||
| No | 35 | 74.5 | 12 | 92.3 | 23 | 67.6 | |
| Yes | 12 | 25.5 | 1 | 7.7 | 11 | 32.4 | |
| Menopausal status | 0.0044 | ||||||
| Pre | 11 | 22.9 | 7 | 53.8 | 4 | 11.4 | |
| Post | 37 | 77.1 | 6 | 46.2 | 31 | 88.6 | |
| Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Age | 63.0±16.3 | 50.5±14.8 | 67.6±14.5 | <0.0001 | |||
SD, standard deviation
P value from Fisher exact test or two sample t test
Figure 1:
Western Blot Analysis of a sample of 12 subjects with various stages of prolapse. Arrows illustrate the location of the protein of interest LOXL-1 (Panel A) and Fibulin-5 (Panel B). Pre=premenopausal, Post=postmenopausal. Numbers indicate POP-Q Stage of prolapse.
On ELISA analysis 45/48 (93.8%) subjects had detectable LOXL-1 in vaginal secretions compared to 24/48 (50%) for Fibulin-5. Table 2 illustrates the bivariate and multivariate analysis for LOXL-1 and Fibulin-5 values. LOXL1 values were higher in women with prolapse (26.4 ng/100 mg protein, 102.2 IQR) compared to those without (13.3 ng/100 mg protein, 24.4 IQR) (p = 0.01). On multivariate analysis factoring age as an independent variable, women in the prolapse group had a 544% (p<0.004) increase in LOXL1 protein compared to those without. There were no significant differences in LOXL1 or Fibulin-5 protein detection with relation to menopausal status.
Table 2.
Bivariate and multivariate analysis for LOXL-1 and Fibulin-5 values
| LOXL-1 (ng/100mg protein) |
FIB-5 (pg/100mg protein) |
|||||
|---|---|---|---|---|---|---|
| Median | IQR | p value a | Median | IQR | p value a | |
| All sample | 24.0 | 86.3 | 87.2 | 1613.7 | ||
| History of estrogen use | 0.2717 | 0.7230 | ||||
| No | 26.0 | 173.2 | 174.5 | 1497.4 | ||
| Yes | 22.4 | 39.2 | 0.0 | 2547.9 | ||
| Gravidity | 0.8892 | 0.3593 | ||||
| 0 or 1 | 24.7 | 106.7 | 440.1 | 2858.3 | ||
| 2 or more | 24.0 | 69.4 | 170.0 | 1613.7 | ||
| Parity | 0.7740 | 0.2348 | ||||
| 0 or 1 | 25.5 | 98.9 | 665.3 | 2813.1 | ||
| 2 or more | 23.4 | 89.0 | 0.0 | 1497.4 | ||
| History of vaginal delivery | 0.1586 | 0.2561 | ||||
| No | 12.9 | 61.9 | 1008.8 | 3138.4 | ||
| Yes | 30.3 | 96.7 | 170.0 | 2109.7 | ||
| Race | 0.0922 | 0.8918 | ||||
| Asian | 17.7 | 32.4 | 362.8 | 2547.9 | ||
| Hispanic | 673.3 | 1638.0 | 170.0 | 2564.8 | ||
| White | 23.4 | 86.9 | 0.0 | 1189.8 | ||
| Pelvic surgery | 0.4454 | 0.8634 | ||||
| No | 25.3 | 93.7 | 321.1 | 1613.7 | ||
| Yes | 23.2 | 91.1 | 0.0 | 2609.0 | ||
| Hysterectomy | 0.9323 | 0.4949 | ||||
| No | 23.4 | 110.2 | 340.0 | 2489.5 | ||
| Yes | 25.6 | 59.1 | 0.0 | 814.9 | ||
| Menopausal status | 0.8644 | 0.6115 | ||||
| Pre | 24.7 | 99.1 | 340.0 | 2756.7 | ||
| Post | 23.4 | 85.6 | 0.0 | 1189.8 | ||
| Prolapse | 0.0140 | 0.3323 | ||||
| No | 13.3 | 24.4 | 362.8 | 2609.0 | ||
| Yes | 26.4 | 102.2 | 0.0 | 1497.4 | ||
| Multivariate linear regression result b | ||||||
| Prolapse | ||||||
| No | referent | referent | ||||
| Yes | 544% higher | 0.0042 | 50% lower | 0.6160 | ||
IQR, interquartile range
p value from non-parametric Wilcoxon two-sample test or Kruskal-Wallis test
Multivariate linear regression model use log (X+1) as outcome (X=protein value); independent variables included age and prolapse status
Discussion:
The pelvic floor is supported by bones, muscles, and ligaments. The connective tissue of these ligaments contain elastic fibers which consist of single units of tropoelastin joined together via polymerization by lysyl oxidase.9 In addition, a scaffolding structure consisting of microfibrils made of fibrillin is required for the creation of elastin.10 LOXL1 is suspected to play a role in elastogenesis because POP is present in lysyl oxidase knockout mice by 1–2 days postpartum. Interestingly, the elastic fibers were normal prior to pregnancy suggesting LOXL1’s role in elastin remodeling as opposed to initial assembly.11 Nulliparous Fibulin-5 knockout mice, on the other hand, displayed evidence of POP as early as 3 months of age with 92% affected by 6 months.12
With this background potentially implicating these two proteins, further studies aimed at characterizing the role of LOXL1 and Fibulin-5 in the development of POP in humans (Table 3). These experiments focused on determination of mRNA and protein expression in a biopsied specimen from either the uterosacral ligament, cardinal ligament or vaginal epithelium.
Table 3:
LOX1 & Fib-5 Expression Across All Biopsy Sites
| Author | Bx Location | Sample Size | Menopausal Status | Method | LOXL1 | Fib 5 |
|---|---|---|---|---|---|---|
| Klutke [13] | Uterosacral | 31 POP/29 Control | Mixed | Real time PCR | ↓ mRNA 5x in POP | ↑ mRNA 3x in POP |
| Joo Jung [4] | Uterosacral | 30 POP/30 Control | Postmen | Real time PCR Western Blot | ↑ mRNA in POP ↑ protein in POP |
↓ mRNA in POP ↓ protein in POP |
| Zhao [7] | Uterosacral | 30 POP/ 30 control | Postmen | Immunohistochem | ↓ protein in POP | ↓ protein in POP |
| Zhou [6] | Cardinal Ligament | 53 POP/25 control | Mixed 48 Postmen/5 Premeno | Immunohistochem | ↓ protein in POP | ↓ protein in POP |
| Takacs [17] | Vaginal | 12 POP/ 10 control | Mixed | Realtime PCR & Immunohistochem | n/a | ↓ mRNA in POP ↓ protein in POP |
| Alarab [15] | Vaginal | 15 POP/ 11 control | Premen Prolif phase | Real time PCR, Immunoblotting, Immunohistochem | ↓ mRNA ↓ protein in POP |
n/a |
| Kow [8] | Vaginal | 10 premen POP/ 10 postmen POP/ 10 premen control | Mixed | Real time PCR ELISA | ↓ mRNA in premen POP ↑ mRNA in postmen POP No difference in protein |
n/a |
Assessment of mRNA expression of the uterosacral ligaments using polymerase chain reaction (PCR) in a mixed cohort of pre and postmenopausal women showed decreased LOXL1 mRNA expression in subjects with POP.13 Another group looked at uterosacral protein expression of elastin, Fibulin-5, and LOXL1 in a cohort of postmenopausal subjects with POP using immunohistochemistry and showed decreased protein expression relative to controls. They also confirmed that both proteins were expressed near the site of elastin, but that in POP subjects LOXL1 and Fibulin-5 appeared grossly abnormal and disorganized.7 Other groups have noted decreased Fibulin-5 protein in the uterosacral ligaments of subjects with POP.4,16 Evaluation of biopsy samples from the cardinal ligaments of pre and postmenopausal women has shown that both LOXL1 and Fibulin-5 are decreased in women with advancing prolapse stage.6
Evaluation of LOXL1 and Fibulin-5 in the vagina has also been an area of interest as some women develop POP in the anterior or posterior vaginal walls. Additionally, the collagen content of vaginal tissue is homologous to the uterosacral ligaments and thus can be considered a proxy.14 Amongst premenopausal women in the proliferative phase of the menstrual cycle with POP, LOXL1 mRNA and protein expression is significantly decreased in vaginal tissue.15 Another analysis of LOXL1 in vaginal biopsy specimens showed decreased mRNA in premenopausal POP and increased mRNA in postmenopausal POP subjects compared to premenopausal controls. While there was not a statistically significant decrease in LOXL1 protein in subjects with POP relative to controls, there was an overall downward trend suggesting the potential role of LOXL1 in POP.8 Additionally the study was not powered to detect differences in protein concentration so further exploration is warranted. Furthermore, decreased Fibulin-5 mRNA and protein expression is also seen in women with anterior compartment vaginal prolapse.16
In contrast to the trends described above, only one study showed an increase in LOXL1 protein in the uterosacral ligaments of postmenopausal women with POP compared to controls4 and one study showed an increase in Fibulin-5 mRNA of women with POP.13 In all cases the studies point to a difference in elastin synthesis with regard to POP development.
While POP can be easily determined with a physical exam, variations in proteins implicated in the pathogenesis of prolapse could ultimately function as predictors for risk of developing prolapse or serve as therapeutic targets in the future. The aim of this study was to determine if LOXL1 and Fibulin-5 could be detected in vaginal secretions with the ultimate goal of developing a minimally invasive (not requiring a biopsy or tissue sample) protocol that could facilitate the measurement of these proteins easily and on a large scale.
Initial pilot testing for this project involved a comparison of collection of secretions via a vaginal tampon versus a large cotton swab with no significant difference in results. The decision was made to swab all three compartments (anterior, posterior, apical vaginal wall) to get a sufficient sample. Successful expression of LOXL1 and Fibulin-5 was first demonstrated via western blotting (Figure 1). While banding can be seen in the area of interest suggestive of detection of protein, there was considerable cross staining near the region of interest (56 kDa for LOXL1 and 50 kDa for Fibulin-5). As a result, qualitative evaluation of protein via a banding densometry analysis would have likely yielded inaccurate results.
In order to accurately characterize protein expression, ELISA was selected as a processing medium. Quantification via ELISA involves the development of a standard curve with known concentrations of protein, which then allows calculation of unknown samples. In the event where the concentration of unknown could not be determined, it was assumed to be zero. LOXL1 was found to be much more detectable than Fibulin-5 (93.8% versus 50%) in vaginal secretions. As it has been reported that Fibulin-5 is expressed in the vaginal epithelium17,18, one possible explanation is that the molecule is not as easily extruded through the vaginal epithelium as LOXL1 or was more susceptible to protein degradation during processing. Another possibility is that the loss of Fibulin-5 is directly implicated in the pathogenesis of prolapse.4 While the decreased Fibulin-5 in subjects with POP noted in our study did not reach statistical significance, this trend is supported in the literature.4,6,7,17
LOXL1 was highly detectable and there was a statistically significant increase in protein secreted in women with prolapse. While this increase in protein is supported in the literature4, others have shown either a decrease in LOXL1 protein6,7,15 or no significant difference.8 Prior to comparing the results of this study to the current literature however, a future study must seek to validate vaginal secretion findings against biopsy specimens to determine if there is a positive correlation and if the former can be used as a proxy. Additionally, much larger sample sizes are needed to confirm that our results are not due to statistical (Type I or Type II) error. These two key factors notwithstanding possible explanations for this heterogeneity include variable biopsy sites (uterosacral, cardinal, vaginal), the hormonal status of subjects, ethnicity/genetics, or other factors such as parity, smoking, or previous pelvic surgery.
On multivariate analysis with age as a variable, women with prolapse had a 544% (p<0.004) increase in LOXL1 relative to those without. It has been postulated that the increase in LOXL1 protein expression seen in our results could potentially be attributed to a compensatory mechanism in women with POP;4 however, a larger sample size would be needed to explore this further.
The main strength of this study is the development and implementation of a novel method for noninvasive detection of LOXL1 and Fibulin-5 as this was intended as a small pilot study and our chief aim was to determine proof of concept. This study has multiple limitations. The initial design involved collection of vaginal secretions via a tampon placed vaginally for 6 hours, however this presented both technical and logistical challenges. Some subjects reported that a tampon would not remain in place, which could potentially be attributed to their degree of prolapse or other anatomical factors. Additionally, the process of specimen collection and transfer on ice back to study investigators was not feasible for many subjects. In order to address these challenges specimen collection via vaginal swab was attempted and protein detection was confirmed with Western Blotting. In order to account for the larger quantity of vaginal secretions (aggregate protein) collected from the 6-hour tampon compared to the swab method, the final results were reported as a ratio of amount of LOXL1 or Fibulin-5 / total concentration of protein. While this change in protocol is a limitation, the results reported have been standardized by method, and the vaginal swab collection method has been shown to be feasible. This pilot project enrolled a small sample size with unevenly distributed cohorts of those with and without prolapse. Prolapse was defined at stage II on the POP-Q exam as this can be considered clinically significant given descent to the level of the hymen.
This manuscript determined proof of concept via Western Blotting technique and further showed that LOXL1/Fibulin-5 protein concentration could be quantified using ELISA. Future studies could utilize quantitative real-time polymerase chain reaction (qRT-PCR) which would facilitate further understanding of the expression of these proteins in real time. A future study could potentially evaluate the levels of protein secreted in four cohorts of women: premenopausal with and without POP, and post-menopausal women with and without POP. Our cohort also had a small number of women with advanced stage prolapse, and a future study with an even distribution based on stage of prolapse would be beneficial.
Acknowledgments
The authors acknowledge Thomas Lechuga PhD for his contribution on this project.
Funding: This pilot study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant (UL1TR001414).
A portion of this work was presented in at American Urogynecologic Society (AUGS) 38th Annual Scientific Meeting in Rhode Island 2017.
Footnotes
Conflict of Interest for all authors: None
References:
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6 [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Zhao Y, Pawlyk B, Damaser MS, Li T. Failure of Elastic Fiber Homeostasis Leads to Pelvic Floor Disorders. Am J Pathol. 2006;168:519–528. doi: 10.2353/ajpath.2006.050399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carley ME, Schaffer J. Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers-Danlos syndrome. 2000. doi: 10.1067/mob.2000.105410 [DOI] [PubMed] [Google Scholar]
- 4.Jung HJ, Jeon J, Yim GW, Kim SK, Choi JR, Bai SW. Changes in expression of fibulin-5 and lysyl oxidase-like 1 associated with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2009;145:117–122. doi: 10.1016/j.ejogrb.2009.03.026 [DOI] [PubMed] [Google Scholar]
- 5.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res Part C - Embryo Today Rev. 2007;81(4):229–240. doi: 10.1002/bdrc.20111 [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Ling O, Bo L. Expression and significance of lysyl oxidase-like 1 and fibulin-5 in the cardinal ligament tissue of patients with pelvic floor dysfunction. J Biomed Res. 2013;27(1):23–28. doi: 10.7555/JBR.27.20110142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao BH, Zhou JH. Decreased expression of elastin, fibulin-5 and lysyl oxidase-like 1 in the uterosacral ligaments of postmenopausal women with pelvic organ prolapse. J Obstet Gynaecol Res. 2012;38(6):925–931. doi: 10.1111/j.1447-0756.2011.01814.x [DOI] [PubMed] [Google Scholar]
- 8.Kow N, Ridgeway B, Kuang M, Butler RS, Damaser MS. Vaginal Expression of LOXL1 in Premenopausal and Postmenopausal Women with Pelvic Organ Prolapse. Female Pelvic Med Reconstr …. 2016. doi: 10.1097/SPV [DOI] [PubMed] [Google Scholar]
- 9.Kagan HM, Li W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88(4):660–672. doi: 10.1002/jcb.10413 [DOI] [PubMed] [Google Scholar]
- 10.Kielty C, Sherratt M, Shuttleworth C. Elastic Fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1016/j.ijid.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase–like 1 protein. Nat Genet. 2004;36(2):178–182. doi: 10.1038/ng1297 [DOI] [PubMed] [Google Scholar]
- 12.Drewes PG, Yanagisawa H, Starcher B, et al. Pelvic Organ Prolapse in Fibulin-5 Knockout Mice. Am J Pathol. 2007;170(2):578–589. doi: 10.2353/ajpath.2007.060662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klutke J, Ji Q, Campeau J, et al. Decreased endopelvic fascia elastin content in uterine prolapse. Acta Obstet Gynecol Scand. 2008;87(1):111–115. doi: 10.1080/00016340701819247 [DOI] [PubMed] [Google Scholar]
- 14.Phillips CH, Anthony F, Benyon C, Monga AK. Collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. BJOG An Int J Obstet Gynaecol. 2006;113(1):39–46. doi: 10.1111/j.1471-0528.2005.00773.x [DOI] [PubMed] [Google Scholar]
- 15.Alarab M, Bortolini MAT, Drutz H, Lye S, Shynlova O. LOX family enzymes expression in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Int Urogynecol J. 2010;21(11):1397–1404. doi: 10.1007/s00192-010-1199-9 [DOI] [PubMed] [Google Scholar]
- 16.Takacs P, Nassiri M, Candiotti K, Yang J, Yavagal S, Medina CA. Differential expression of fibulins in the uterosacral ligaments of women with uterine prolapse. Arch Gynecol Obstet. 2010;282(4):389–394. doi: 10.1007/s00404-009-1262-2 [DOI] [PubMed] [Google Scholar]
- 17.Takacs P, Gualtieri M, Nassiri M, Candiotti K, Fornoni A, Medina CA. Fibulin-5 Expression is decreased in women with anterior vaginal wall prolapse: A pilot study. Int Urogynecol J. 2009;20(8):985–990. doi: 10.1007/s00192-009-0876-z [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Kira Y, Hamuro A, Takase A, Tachibana D, Koyama M. Differential gene expression of extracellular-matrix-related proteins in the vaginal apical compartment of women with pelvic organ prolapse. doi: 10.1007/s00192-018-3637-z [DOI] [PubMed] [Google Scholar]