Abstract
Objective
To evaluate temporal correlations between CSF and neuroimaging (PET and MRI) measures of amyloid, tau, and neurodegeneration in relation to Alzheimer disease (AD) progression.
Methods
A total of 371 cognitively unimpaired and impaired participants enrolled in longitudinal studies of AD had both CSF (β-amyloid [Aβ]42, phosphorylated tau181, total tau, and neurofilament light chain) and neuroimaging (Pittsburgh compound B [PiB] PET, flortaucipir PET, and structural MRI) measures. The pairwise time interval between CSF and neuroimaging measures was binned into 2-year periods. Spearman correlations identified the time bin when CSF and neuroimaging measures most strongly correlated. CSF and neuroimaging measures were then binarized as biomarker-positive or biomarker-negative using Gaussian mixture modeling. Cohen kappa coefficient identified the time bin when CSF measures best agreed with corresponding neuroimaging measures when determining amyloid, tau, and neurodegeneration biomarker positivity.
Results
CSF Aβ42 and PiB PET showed maximal correlation when collected within 6 years of each other (R ≈ −0.5). CSF phosphorylated tau181 and flortaucipir PET showed maximal correlation when CSF was collected 4 to 8 years prior to PET (R ≈ 0.4). CSF neurofilament light chain and cortical thickness showed low correlation, regardless of time interval (Ravg ≈ −0.3). Similarly, CSF total tau and cortical thickness had low correlation, regardless of time interval (Ravg < −0.2).
Conclusions
CSF Aβ42 and PiB PET best agree when acquired in close temporal proximity, whereas CSF phosphorylated tau precedes flortaucipir PET by 4 to 8 years. CSF and neuroimaging measures of neurodegeneration have low correspondence and are not interchangeable at any time interval.
Alzheimer disease (AD) often involves β-amyloid (Aβ) and tau protein accumulation over a relatively long prodromal phase before the emergence of clinical symptoms.1,2 Recent guidelines define Alzheimer pathologic change as biomarker evidence of amyloid (either PET amyloid or CSF Aβ42 or Aβ42/Aβ40 ratio), tau (either CSF phosphorylated tau181 [p-tau181] or PET tau),3 and neurodegeneration (either structural MRI or CSF total tau [t-tau] or neurofilament light chain [NfL]). This amyloid-tau-neurodegeneration (AT[N]) framework suggests CSF and neuroimaging measures within each biomarker group are generally interchangeable. However, the extent to which they are interchangeable when there is a time interval between the 2 assessments has not been well investigated.
Currently, the interval between CSF and neuroimaging acquisition has been included as a possible study confound4-15 or has been limited to a predefined time window (1–4 years). A consensus does not exist for each AT(N) biomarker. If the correlation between CSF and neuroimaging measures is similar for time periods outside the 1–4 years window, many studies could be unnecessarily limiting their data. Identifying the precise time intervals that still yield comparable CSF and neuroimaging measures, which are invasive and carry risk of radiation, respectively, may also allow research studies to strategically reduce participant burden and participant dropout. Finally, exploring the temporal relationship between CSF and neuroimaging measures can provide us with a better understanding of disease biology. In this work, we identify the time interval that maximizes the correlation between CSF and neuroimaging measures for the AT(N) criteria.
Methods
Participants
A total of 371 participants enrolled in longitudinal studies at the Washington University in St. Louis Knight Alzheimer Disease Research Center were evaluated. Recruitment was performed using previously described methods.16 All participants were assessed annually to determine the presence or absence of cognitive impairment, operationalized as either “unimpaired” (Clinical Dementia Rating [CDR] 0) or “impaired” (CDR >0).17 For this analysis, the CDR status was reported from the assessment closest to the participant's PET scan. Participants were included who had either normal cognition (CDR 0) or who were cognitively impaired (CDR > 0) with an AD phenotype (encompassing both mild cognitive impairment due to AD and AD dementia), diagnosed in accordance with a uniform protocol and standard criteria.18 Exclusion criteria included an unwillingness or inability to complete MRI or PET imaging, a known mutation that leads to AD, or medical conditions that could interfere with longitudinal evaluations. DNA samples were also genotyped to determine each participant's APOE ε4 genotype. Genotyping was done using either an Illumina 610 or Omniexpress chip as previously described.19 Participants were included for analysis if they completed 1 structural MRI, 1 Pittsburgh compound B (PiB) PET scan, and had at least 1 CSF analysis. A subset of participants (n = 200) completed a flortaucipir PET scan, as this method was recently introduced.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Washington University in St. Louis Institutional Review Board, and each participant provided signed, informed consent.
Structural MRI
MRI were obtained on 3T Siemens scanners. T1-weighted scans were segmented with FreeSurfer5.3.20 Previous work has identified the temporal (inferior, middle, and superior) cortex, parietal (inferior and superior) cortex, entorhinal cortex, precuneus, and hippocampus as areas most affected by AD.21,22 Cortical thickness measurements from these regions were converted to Z scores separately for the left and right hemispheres relative to the entire cohort, and then averaged to obtain a normalized AD cortical thickness for each participant. Because no predefined MRI cut point exists, participants were considered neurodegeneration-positive if the AD cortical thickness was below average (z < 0).
PET Imaging
PET amyloid imaging was conducted with [11C]-PiB as the tracer using previously described methods.23 Participants received a single IV bolus of PiB infused over 20 seconds and a 3D dynamic PET scan was acquired. Quantitative PET analysis for regional target-to-reference intensity ratio, standardized uptake value ratio (SUVR), utilized the 30–60 minutes postinjection time window.23 The cerebellum cortex served as the reference region.
The PET Unified Pipeline (github.com/ysu001/PUP) was used for processing. Images were smoothed to achieve a common spatial resolution of 8 mm.24 Interframe motion correction for dynamic PET images was performed with standard registration techniques.25 Corresponding structural images were obtained from MRI scans.
PET registration to the MRI was performed using a vector-gradient algorithm in a symmetric fashion.26 Regional values were obtained from the FreeSurfer5.3 segmentation mentioned above. Regional values were partial volume corrected using a regional spread function–based approach.27 Global amyloid burden was calculated by obtaining the arithmetic mean of regional SUVRs from the lateral orbitofrontal, medial orbitofrontal, rostral middle frontal, superior frontal, superior temporal, middle temporal, and precuneus cortices. Participants were considered amyloid-positive if the global amyloid burden SUVR was greater than 1.42.23
PET tau imaging was performed with [18F]-flortaucipir (FTP) and processed using the same pipeline with data from the 80–100 minutes postinjection window converted to SUVRs.23 A summary measure of overall PET tau burden, herein referred to as tauopathy, was defined as the arithmetic mean of regional SUVRs from the amygdala, entorhinal cortex, inferior temporal cortex, and lateral occipital cortex. Participants were considered tau-positive if the FTP PET summary SUVR was greater than 1.22.23
CSF Acquisition
CSF was collected as previously described.28 Participants underwent a lumbar puncture (LP) following overnight fasting. Approximately 25 mL of CSF was collected in a 50 mL polypropylene tube via gravity drip using an atraumatic Sprotte 22-gauge spinal needle. The sample was gently mixed to disrupt potential gradient effects and then centrifuged at low speed to pellet any debris. The CSF was aliquoted into polypropylene tubes and stored at −80°C until assays were performed. CSF Aβ42, t-tau, and p-tau181 were measured with corresponding Elecsys immunoassays on a Roche cobas e601 analyzer.29 For each analyte, a single lot of reagents was used. NfL was measured with an immunoassay kit manufactured by Uman Diagnostics.
Statistical Analysis
For each biomarker in the AT(N) framework, the following time bins were defined by the number of years between CSF collection and neuroimaging acquisition (PiB PET scan for amyloid, FTP PET scan for tau, and MRI scan for neurodegeneration): CSF collected 10–13 years before the scan ([−13, −10], 8–10 years before the scan [−10, −8], 6–8 years before the scan [−8, −6], 4–6 years before the scan [−6, −4], 2–4 years before the scan [−4, −2], 0–2 years before the scan [−2, 0], 0–2 years after the scan [0, 2], or 2–4 years after the scan [2, 4]). Two-year bins were utilized due to the common practice of limiting studies to CSF and PET measures collected within 2 years of each other. If results indicated that the maximal correlation aligned within ±2 years of separation, single-year bins were applied in a further analysis. Longitudinal CSF samples were available for some participants. If a participant had more than 1 CSF sample taken within a 2-year time bin, samples were randomly dropped so that each participant only had 1 CSF sample per time bin, minimizing the introduction of a random effect due to individual variation.30 If there were fewer than 20 individuals in a time bin, that time bin was dropped due to insufficient sample size.
In order to compare participant demographics, we performed an analysis of variance with binned time between CSF and neuroimaging as the grouping variable, using the R package tableOne.31 We then calculated Spearman correlations between the CSF measure and PET or MRI scan for each time bin. We calculated the correlations between CSF Aβ42, p-tau181, t-tau, or NfL and overall cortical PiB PET burden, FTP PET burden, or cortical thickness. We also calculated the correlation between CSF p-tau181/Aβ42 ratio and PiB PET. We performed correlation calculations for all participants and for only PET amyloid-positive participants. Due to the limited number of individuals with pathologic changes due to AD, we were unable to calculate a correlation value for the (−13, −10) cohort. Because there were an unequal number of participants in each of the time bins, we then applied Fisher z transformation to compare values across groups.32 Finally, we performed a Tukey test between correlations across time bins.33
To calculate an appropriate CSF cut point for identifying biomarker positivity, we employed a model-based clustering approach. Using the R package mclust to fit a Gaussian mixture model to the CSF biomarker data, we first determined the optimal number of components (1–9) and their variance (equal or unconstrained) through model selection.34 We found that a 3-component model with unconstrained variances best fits the data when evaluated by the Bayesian Information Criterion. With the third component likely describing high outlier values, we focused on the first 2 components, believed to capture low and high CSF biomarker values. The CSF biomarker value inferred to have an equal conditional probability of being assigned to either the low or high component was considered the CSF biomarker positivity cut point. Using these calculated cut points, individuals were classified as either CSF biomarker-positive or CSF biomarker-negative. For each time bin, we calculated Cohen kappa coefficient (κ) to measure the agreement between CSF and neuroimaging biomarker positivity status.
Data Availability
All data are available upon request from the Knight Alzheimer Disease Research Center at Washington University in St. Louis through documented procedures.
Results
Overall, participants were well-matched across time bins with regards to sex, APOE ε4 status, years of education, and CDR status. Participants had on average 16 years of education and were approximately 40% male. Most participants were cognitively normal (CDR 0) and approximately a third were APOE ε4 positive. Amyloid measures were available for individuals who had undergone an LP up to 10 years prior to or 6 years following a PiB PET scan. Tau measures were available for individuals who had undergone an LP up to 13 years before to 2 years after an FTP PET scan. Similarly, neurodegenerative measures (both NfL and t-tau) were available for LPs obtained 13 years before to 2 years after MRI scan.
Amyloid
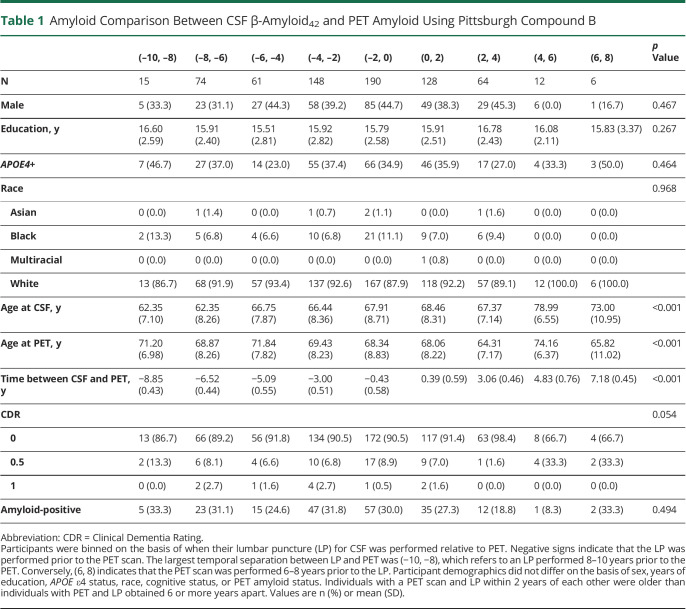
After excluding time bins with less than 20 samples, 665 data points were included in the analysis (table 1). The average age at LP ranged from 62 to 68 years and 64 to 71 years at PET imaging. The overall correlation between CSF Aβ42 and PiB PET was R = −0.488. Minimal correlation was observed when the LP occurred 2–4 years after PiB PET imaging (R = −0.324, 95% CI, −0.528, −0.0854), and maximal correlation was observed when the LP occurred 0–2 years before PiB PET imaging (R = −0.563, 95% CI, −0.653, −0.458) (figure 1, A and C). When the time bins were further refined to single years, higher correlation (not significant; p > 0.10) was observed when the LP occurred 1–2 years prior to the PiB PET scan (R = −0.622, 95% CI, −0.788, −0.374) compared to 0–1 year prior to the PiB PET scan (R = −0.552, 95% CI, −0.652, −0.433). Overall, there was no significant difference in correlation for the following time bins: ([−6, −4], [−4, −2], [−2, 0], [0, 2]). When this analysis was repeated for the AD pathologic change cohort (as categorized by PET amyloid positivity), the greatest correlation between CSF Aβ42 and PET occurred when the LP was performed 4–6 years prior to the PiB PET scan (R = −0.700, 95% CI, −0.892, −0.292) (figure 1, B and D). Trend-level evidence suggested that the correlation for (−6, −4) was significantly stronger than (−2, 0) (p = 0.09). The maximum agreement between CSF-defined and PET-defined amyloid positivity occurred when the LP was performed 2–4 years after the PiB PET scan (κ = 0.57). Except for the (−8, −6) time bin, the 2 measures generally agreed across all time bins (κ > 0.51). Finally, to test the specificity of the relationship between CSF Aβ42 and PiB PET, we calculated the correlation between PiB PET and CSF p-tau, t-tau, and NfL. The overall correlation was consistently lower (R < 0.46), and the maximum correlation was observed in the (−8, −6) time bin for all 3 measures.
Table 1.
Amyloid Comparison Between CSF β-Amyloid42 and PET Amyloid Using Pittsburgh Compound B
Figure 1. Correlation of Amyloid Biomarkers.
Biomarkers of amyloid showed reasonably good correlation for a wide range of time bins (A). For individuals classified as amyloid-positive with a PET scan using Pittsburgh compound B (PiB), the greatest inverse correlation between CSF β-amyloid (Aβ)42 and PET PiB was when the lumbar puncture (LP) was performed 4–8 years prior to the PET (B). The timeframes with the greatest correlation for all participants (C), as well as those for participants with Alzheimer disease pathologic change (D), are shown.
We also calculated the correlation between CSF p-tau/Aβ42 and PiB PET and measured an overall correlation of R = 0.63. The minimum correlation was measured when the LP occurred 2–4 years after PiB PET imaging (R = 0.51, 95% CI, 0.298, 0.669). Maximum correlation was observed when the LP occurred 2–4 years prior to the PiB PET scan (R = 0.71, 95% CI, 0.617, 0.780). When this analysis was repeated for individuals with AD pathologic change (as categorized by PET amyloid positivity), the maximum correlation between CSF p-tau/Aβ42 and PiB PET was measured when the LP occurred 6–8 years prior to PiB PET imaging (R = 0.86, 95% CI, 0.699, 0.940). Finally, we measured the agreement between amyloid positivity defined by CSF p-tau/Aβ42 and PiB PET-defined positivity. The greatest agreement was measured when the LP occurred 4–6 years prior to the PiB PET scan (κ = 0.86).
Tau
A total of 321 data points were included in this analysis (table 2). The age at LP ranged from 63 to 70 years across the time bins. The average age at FTP PET imaging was approximately 71 years and did not significantly differ across the time bins. The overall correlation between CSF p-tau181 and FTP PET was 0.369. Minimal correlation between these values was observed when the LP occurred 2–4 years before FTP PET (R = 0.240, 95% CI, 0.014, 0.443), and the maximal correlation was observed when the LP occurred 4–6 years before FTP PET imaging (R = 0.541, 95% CI, 0.312, 0.711) (figure 2, A and C). This correlation was significantly stronger than correlations between CSF p-tau181 and FTP PET scan for the 2–4 years and 0–2 year time bins (p = 0.01, p = 0.02, respectively). When this analysis was repeated for the AD pathologic change only cohort (as categorized by PET amyloid positivity), the greatest correlation between CSF p-tau181 and FTP PET scan occurred at 6–8 years between measurements (R = 0.603, 95% CI, 0.153, 0.846) (figure 2, B and D). This correlation was significantly stronger than correlations between CSF p-tau181 and FTP PET scan for the 4–6 and 0–2 year time bins (p = 0.04, p = 0.01, respectively). CSF p-tau181 was most predictive of PET tau positivity when the LP occurred 4–6 years before the corresponding FTP PET imaging (κ = 0.38).
Table 2.
Tau Comparison Between CSF p-Tau181 and PET Tau Using Flortaucipir
Figure 2. Correlation of Tau Biomarkers.
Biomarkers of tau show reasonably good correlation when the lumbar puncture (LP) was performed several years prior to the PET scan (A). For individuals classified as amyloid-positive using a PET amyloid (Pittsburgh compound B) scan, correlation between CSF (phosphorylated tau [p-tau]) and flortaucipir PET was highest when the LP was performed 4–8 years prior to the PET (B). The timeframes with greatest measurement correlation for all participants (C), as well as those for participants with documented Alzheimer disease pathology (D), are shown.
Neurodegeneration
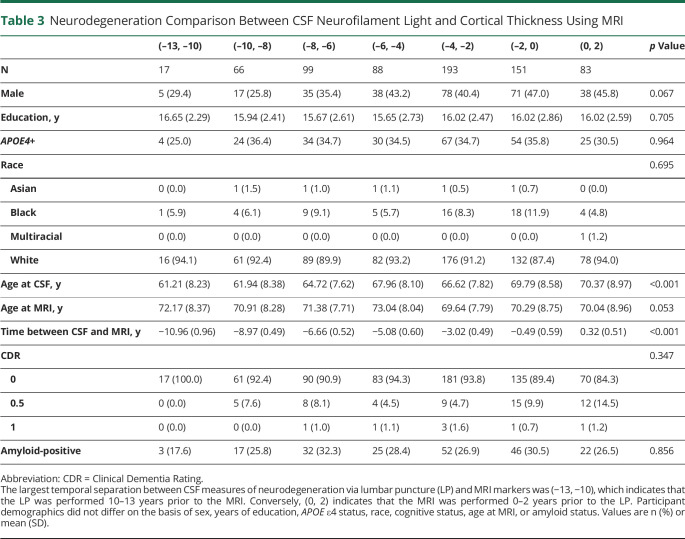
A total of 697 data points were included in this analysis (table 3). The age at LP ranged from 61 to 70 years across the time bins. The average age at MRI was approximately 71 years and did not significantly differ across time bins. The overall correlation between CSF NfL and AD cortical thickness was R = −0.306 (95% CI, −0.374, −0.236). Minimal correlation was observed when the LP occurred 10–13 years before imaging (R = 0.007, 95% CI, −0.526, 0.535), and maximal correlation was observed when the LP occurred 8–10 years after the MRI (R = −0.371, 95% CI, −0.562, −0.142) (figure 3, A and C). When this analysis was repeated for individuals with AD pathologic change (as categorized by PET amyloid positivity), the greatest correlation was observed when NfL was collected 2 years after the MRI (R = −0.318, 95% CI, −0.659, 0.132) (figures 3, B and D) but was not significantly different from any of the other time bins. In addition to relatively weak correlations between NfL and cortical thickness, CSF NfL was not predictive of below-average cortical thickness (z < 0). The largest κ observed was 0.19 in the (−8, −6) time window.
Table 3.
Neurodegeneration Comparison Between CSF Neurofilament Light and Cortical Thickness Using MRI
Figure 3. Correlation of Neurodegeneration Biomarkers.
Biomarkers of neurodegeneration (Alzheimer disease [AD] cortical thickness and CSF neurofilament light [NfL]) showed relatively poor correlation, regardless of the time bin (A). For individuals classified as amyloid-positive with a PET amyloid (Pittsburgh compound B) scan, correlation between cortical thickness and CSF NfL increased with age. The greatest correlation occurred when the lumbar puncture (LP) was performed 0–2 years after the structural MRI (B). For all participants, the greatest correlation between CSF NfL and structural MRI occurred when the LP was performed 2–4 years prior to the MRI (C). However, for participants with documented AD pathology, the greatest correlation was observed when the LP was performed 0–2 years after the MRI (D).
We also compared the relationships between CSF t-tau and AD cortical thickness. This analysis found even lower correlation between these measures, regardless of time frame (overall R = −0.185, 95% CI, −0.256, −0.113) (data not shown).
Discussion
In this demographically well-matched cohort, we observed reasonably good correlation between CSF Aβ42 and PET amyloid for individuals with AD pathologic change when the LP was performed from 6 years before to 2 years after PiB PET imaging. Similarly, a relatively high (κ > 0.51) for PET amyloid-positivity classification was identified throughout this time window. The greatest correlation between CSF p-tau181 and FTP PET occurred when the LP was performed 4–6 years prior to FTP PET imaging for all individuals, and 6–8 years prior to FTP PET imaging when the participants were limited to only individuals with AD pathologic change. CSF p-tau181 also showed maximal ability to classify tau-positive individuals when it was obtained 4–6 years prior to FTP PET. Markers of neurodegeneration, including NfL and AD cortical thickness, had a substantially weaker correlation with each other compared to amyloid or tau biomarkers, regardless of time bin, and had relatively poor performance in identifying individuals with below-average cortical thickness. A summary of these observations is provided in table 4.
Table 4.
Summary of the Temporal Correlation of CSF and Neuroimaging Markers
Our reported correlation (R ≈ −0.5) between CSF Aβ42 and PiB PET is somewhat weaker than previous published values, which were closer to R ≈ −0.75,7 but were based on participants with more advanced AD pathology. When we only considered individuals with AD pathologic change, we identified a similar level of correlation between measures. We are the first to have identified the optimal time window for these 2 amyloid biomarkers, with the highest level of correlation observed when CSF Aβ42 was collected 4–6 years prior to PiB PET acquisition. Our results are in agreement with hypothesized changes in CSF Aβ42 occurring prior to the formation of mature plaques that are measured by PET amyloid tracers.35,36 Prior work that has classified individuals as amyloid-positive or amyloid-negative on the basis of either CSF or PET has identified more individuals as CSF+/PET– for amyloid than CSF–/PET+ for amyloid, providing additional support to the hypothesis that CSF Aβ42 changes prior to PET amyloid.37
For the full cohort, including individuals with and without AD pathologic change, no substantial difference in correlation was observed between CSF and neuroimaging measures when data were collected as much as 6 years apart. Based on our results, it appears that many researchers may be unnecessarily limiting their cohorts when placing stringent criteria of 1- to 2-year time restrictions on the temporal separation between CSF and PET, specifically for individuals with AD pathologic changes. Given the findings from both the continuous and dichotomous analyses, particularly in the context of cognitively normal individuals in the early stages of preclinical AD, we suggest that researchers evaluating individuals for amyloid accumulation can include samples for which CSF was acquired from as many as 6 years before to 2 years after the PiB PET scan.
Previous efforts to classify individuals based on either of these amyloid biomarkers have been relatively successful. CSF Aβ42 and PET amyloid have essentially identical areas under the curve (AUCs) for distinguishing between cognitively normal and cognitively impaired individuals.38 CSF Aβ42 showed fairly good ability to predict amyloid positivity using PiB (AUC 0.85)28,29,39,40 when limiting data to results collected within a 1-year period. We observed high κs when CSF Aβ42 was used to classify PET amyloid positivity; however, we identified similar performance across the (−6, 2) year window, supporting our above recommendation.
The ratio of CSF p-tau/Aβ42, while combining a measure of amyloid and tau into a single term, has shown to be a strong predictor of PiB PET amyloid positivity (AUC 0.96).29 This was supported by our supplemental analysis showing improved agreement between PET-defined and CSF-defined amyloid positivity when using CSF p-tau/Aβ42 ratio vs Aβ42 only. We also observed comparable results up to 2 years earlier than Aβ42 alone and sustained correlation at 2 to 4 years and upwards of 6 years prior to PiB PET. The CSF biomarker ratio likely reflects the essential interplay between the 2 proteinopathies necessary for conversion to PiB PET amyloid positivity, hence the improved and sustained correlation. Given these results, future analyses using CSF Aβ42/Aβ40, a purely CSF amyloid marker with known high concordance with PiB PET, likely will also show improved correlations with PiB PET over Aβ42 alone.
In individuals with AD pathologic change, the greatest correlation between CSF p-tau181 and FTP PET was when these measures were separated by 6–8 years. PET tau has demonstrated strong correlation with cognitive performance and brain atrophy and has discriminatory power in classifying individuals with and without AD.23 Given its relatively later changes with regard to AD progression, it is notable that CSF p-tau181 changes occurred 4–8 years prior to FTP PET imaging. This suggests that rather than treating CSF and PET as equivalent measures of tau pathology, there may be mechanistic differences between the 2 measures that should be considered. A recent study showed FTP PET uptake corresponds with accumulation of tau in the form of threads and tangles.41 Another longitudinal study measured elevated CSF p-tau181 years prior to tau accumulation.42 Our results support these findings and suggest CSF p-tau181 may be considered a precursor to future mature tauopathy as measured by PET tau.3 The results of our analysis also have important implications for future efforts to compare CSF p-tau181 and PET tau. Prior studies have not reported the time elapsed between CSF p-tau181 and PET tau acquisition10,11,13-15 or applied a time window where CSF and PET measurements were obtained within 1.5 years of each other.15 We have demonstrated that the temporal separation between measurements is relevant for determining how closely correlated these biomarkers are related. Future work that includes both CSF tau and PET tau should report the time interval, recognizing that measures taken further apart may show stronger correlations.
When classifying individuals as tau-positive or tau-negative, we observed that positivity defined by CSF p-tau181 has the greatest agreement with PET-defined tau positivity when CSF was collected 4–6 years prior to FTP PET imaging. Across all time windows, the maximum κ achieved was only 0.38, which is lower than the predictive ability achieved for amyloid biomarkers. This may reflect relative differences in what CSF p-tau181 and FTP PET are measuring as compared to what CSF Aβ42 and PiB PET represent. However, it could also reflect the relative quality of the tau-positivity cutoff as compared to the amyloid-positivity cutoff. PET tau is a more recently developed measure, and efforts to dichotomize individuals into tau-positive and tau-negative classifications are in the early phase of development compared to PET amyloid.43
Overall, we observed relatively low correlation between CSF NfL and AD cortical thickness, regardless of the time bin. Given the differing underlying biology of these 2 markers, it is not surprising that correlations between axonal damage and structural atrophy were only loosely related. Although both of these markers have previously been found to discriminate between health and disease, weak agreement was reported.12 Similarly, our results did not show NfL to be predictive of below-average cortical thickness, regardless of time bin. Previous studies have shown a fairly strong correlation between NfL and age.44 Under the AT(N) framework, we would expect that this relatively healthy cohort of primarily cognitively normal individuals was not experiencing significant neurodegeneration as these changes occur late in AD progression. Perhaps interesting relationships between NfL and AD cortical thickness would be observed in a cohort with more advanced AD. Furthermore, previous studies have shown lower levels of NfL in individuals diagnosed with AD compared to neuroinflammatory disorders or other dementia disorders, such as frontotemporal dementia.44 Our cohort is likely experiencing changes due to aging rather than AD or AD-related diseases.
CSF t-tau, which is explicitly cited to be included in neurodegeneration criteria of the AT(N) framework,3 has even lower correlation with cortical thickness. It displays extremely high correlation with CSF p-tau (R = 0.987) (data not shown) and only moderate correlation with CSF NfL (R = 0.511) (data not shown). Based on the low correlation values between CSF t-tau and cortical thickness, as well as our inability to identify individuals with below-average cortical thickness (z < 0) using CSF t-tau (κ < 0.2), we conclude that CSF t-tau, while possibly measuring another aspect of neurodegeneration, does not measure the same process measured by either CSF NfL or cortical thickness.
Our study has a number of limitations. Many participants were recruited because they had a family history of AD. As a result, this cohort has a higher frequency of the APOE ε4 allele compared to the general population and are likely highly motivated to undergo LP and imaging studies, introducing possible biases. Future studies should apply similar methods to individuals more reflective of the general clinic population. This cohort was also limited to relatively healthy, cognitively normal individuals. Subsequent studies in a cohort with more individuals with symptomatic AD is necessary to ascertain the relationships between neurodegenerative markers in later disease stages. Because most participants only had 1 or 2 CSF samples and were included in only 1 or 2 time bins, this study is considered cross-sectional. Even though important demographic characteristics such as sex, race, or APOE ε4 status are not significantly different across time bins, participant differences may exist and could influence the results. The cross-sectional nature limits our ability to draw decisive conclusions regarding temporal relationships. A future longitudinal analysis with the same participants included in each time bin would be ideal to validate our findings. The relatively poor discriminative ability of CSF p-tau and, more acutely, CSF NfL, may be reflective of the lower correlation of CSF to imaging measures as compared to the CSF Aβ42/PET amyloid relationship; however, it could also be a reflection of the relative quality of existing cutoffs. PET tau is a relatively new measure, and few studies attempt to classify individuals as neurodegeneration-positive based on a quantitative structural MRI value. As future efforts identify more robust cutoffs for tau and neurodegeneration, repeated analysis of this nature may improve κs for classification. Regardless of the prospective future of improved cutoffs, the correlations associated with evaluation of each measure on a continuous scale suggest that amyloid measures are similar but not the same, tau measures are somewhat similar but certainly not equivalent, and the 3 neurodegenerative measures are not evaluating the same entity, but rather, similar and related measures. It may be inappropriate to treat 2 measures as equivalent when they are, in fact, reflective of different disease stages.
For the biomarkers evaluated in this article, CSF measures should not be treated as equivalent proxies for imaging measures. Instead, CSF Aβ42 and CSF p-tau181 should be thought of as early biomarkers, while imaging measures are late biomarkers of pathology. CSF NfL and AD cortical thickness may both be markers of neurodegenerative processes, but they are not markers of the same process, and thus should not be used interchangeably given their relatively poor correlation. Our recommendations for use are presented in table 4 and may influence subsequent clinical trials using these various biomarkers.
In preclinical AD cohorts, it is our recommendation that researchers dichotomizing individuals for amyloid accumulation expand their criteria for correspondence between acquisition of PET and CSF beyond what is commonly used, allowing for samples in which CSF was acquired as many as 6 years before to 2 years after PET collection. These recommendations on appropriate time frames extend to when CSF is used for binary classification of pathology positivity or negativity, which has important clinical relevance. These results suggest abnormal amyloid levels in one measure will likely be reflected in the other. These measures are likely evaluating similar features of amyloid. Therefore, only a single measure (either PET or CSF) is needed to evaluate amyloid, reducing patient burden and demand for scarce resources. In contrast, we suggest that CSF p-tau values should only be used as proxies for PET tau when CSF is collected 4–8 years prior to PET. Similarly, the 3 neurodegeneration markers CSF NfL, CSF t-tau, and AD cortical thickness do not correlate across the time windows examined. These results suggest biomarkers of tau and especially neurodegeneration may not be measuring the same entity. It may be difficult to use a single biomarker of tau or neurodegeneration to determine positivity for a patient. Beyond the clinical setting, this may have important implications for clinical trials that may use a particular tau or neurodegeneration biomarker to assess the efficacy of a therapeutic.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- AT[N]
amyloid-tau-neurodegeneration
- AUC
area under the curve
- CDR
Clinical Dementia Rating
- FTP
18F-flortaucipir
- LP
lumbar puncture
- NfL
neurofilament light
- p-tau181
phosphorylated tau181
- PiB
Pittsburgh compound B
- SUVR
standardized uptake value ratio
- t-tau
total tau
Appendix. Authors

Study Funding
Supported by NIH grants R01NR012907, R01NR012657, R01NR014449, P01AG00391, P01AG026276, and P01AG005681. Additional funding was provided from Barnes-Jewish Hospital, the Washington University Institute of Clinical and Translational Sciences Foundation (UL1 TR000448), the Hope Center for Neurologic Disorders, the Paula and Rodger O. Riney Fund, the Daniel J. Brennan MD Fund, and the Fred Simmons and Olga Mohan Fund.
Disclosure
Anna H. Boerwinkle, Julie K. Wisch, Charles D. Chen, Brian A. Gordon, Omar H. Butt, Suzanne E. Schindler, Courtney Sutphen, Shaney Flores, and Aylin Dincer report no disclosures. Tammie L.S. Benzinger has consulted on clinical trials with Biogen, Roche, Janssen, and Eli Lilly and receives research support from Eli Lilly and Avid Radiopharmaceuticals. Avid Radiopharmaceuticals provided the AV-1451 used in this study. Anne M. Fagan has received research funding from the National Institute on Aging of the NIH, Biogen, Centene, Fujirebio, and Roche Diagnostics; is a member of the scientific advisory boards for Roche Diagnostics, Genentech, and AbbVie; and consults for Araclon/Grifols, Diadem, DiamiR, and Otsuka. John C. Morris is funded by NIH grants P30 AG066444, P01AG003991, P01AG026276, U19 AG032438, and U19 AG024904. Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. Beau M. Ances reports no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Berg L, McKeel DW, Miller JP, Baty J, Morris JC. Neuropathological indexes of Alzheimer's disease in demented and nondemented persons aged 80 years and older. Arch Neurol. 1993;50(4):349-358. [DOI] [PubMed] [Google Scholar]
- 2.Mattsson N, Schöll M, Strandberg O, et al. 18 F‐AV‐1451 and CSF t‐tau and p‐tau as biomarkers in Alzheimer's disease. EMBO Mol Med. 2017;9(9):1212-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwan MD, Rinne JO, Hasselbalch SG, et al. Use of amyloid-PET to determine cutpoints for CSF markers. Neurology. 2016;86(1):50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolboom N, Van Der Flier WM, Yaqub M, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009;50(9):1464-1470. [DOI] [PubMed] [Google Scholar]
- 6.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80(19):1784-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strozyk D, Blennow K, White LR, Launer LJ. CSF Aß 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652-656. [DOI] [PubMed] [Google Scholar]
- 8.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29(10):1456-1465. [DOI] [PubMed] [Google Scholar]
- 9.Leuzy A, Carter SF, Chiotis K, Almkvist O, Wall A, Nordberg A. Concordance and diagnostic accuracy of [11C]PIB PET and cerebrospinal fluid biomarkers in a sample of patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2015;45(4):1077-1088. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Q, Liu M, Ha L, Zhou Y. Quantitative 18F-AV1451 brain tau PET imaging in cognitively normal older adults, mild cognitive impairment, and Alzheimer's disease patients. Front Neurol Front Media. 2019;10:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med. 2016;8(338). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allison SL, Koscik RL, Cary RP, et al. Comparison of different MRI-based morphometric estimates for defining neurodegeneration across the Alzheimer's disease continuum. Neuroimage Clin. 2019;23:101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon BA, Friedrichsen K, Brier M, et al. The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain. 2016;139(8):2249-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schöll M, Maass A, Mattsson N, et al. Biomarkers for tau pathology. Mol Cell Neurosci. 2019;97:18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Joie R, Bejanin A, Fagan AM, et al. Associations between [18F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurol. 2018;90(4):E282-E290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol Am Med Assoc. 2019;76(3):264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17(2):101-118. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC, Weintraub S, Chui HC, et al. The uniform data set (UDS): clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis Assoc Disord. 2006;20(4):210-216. [DOI] [PubMed] [Google Scholar]
- 19.Cruchaga C, Kauwe JSK, Harari O, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer's disease. Neuron. 2013;78(2):256-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11-22. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Benzinger TL, Hassenstab J, et al. Spatially distinct atrophy is linked to β-amyloid and tau in preclinical Alzheimer disease. Neurology. 2015;84(12):1254-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra S, Gordon BA, Su Y, et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. Neuroimage. 2017;161:171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su Y, Blazey TM, Snyder AZ, et al. Partial volume correction in quantitative amyloid imaging. Neuroimage. 2015;107:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajnal JV, Saeed N, Soar EJ, Oatridge A, Young IR, Bydder GM. A registration and interpolation procedure for subvoxel matching of serially acquired MR images. J Comput Assist Tomogr. 1995;19(2):289-296. [DOI] [PubMed] [Google Scholar]
- 26.Rowland DJ, Garbow JR, Laforest R, Snyder AZ. Registration of [18F]FDG microPET and small-animal MRI. Nucl Med Biol. 2005;32(6):567-572. [DOI] [PubMed] [Google Scholar]
- 27.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39(5):904-911. [PubMed] [Google Scholar]
- 28.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512-519. [DOI] [PubMed] [Google Scholar]
- 29.Schindler SE, Gray JD, Gordon BA, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimers Dement. 2018;14(11):1460-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison XA, Donaldson L, Correa-Cano ME, et al. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ. 2018;6:e4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida K, Bohn J. Package “Tableone.” R; 2019. [Google Scholar]
- 32.Myers L, Sirois MJ. Differences between spearman correlation coefficients. Encycl Stat Sci. 2006;12:1-2. [Google Scholar]
- 33.Revelle W. Psych: Procedures for Personality and Psychological Research; 2015. Available at: personality-project.org/r/psych-manual.pdf. [Google Scholar]
- 34.Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc. 2002;97(458):611-631. [Google Scholar]
- 35.Findeis MA. The role of amyloid beta peptide 42 in Alzheimer's disease. Pharmacol Ther. 2007;116(2):266-286. [DOI] [PubMed] [Google Scholar]
- 36.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131(6):1630-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med. 2009;1(8-9):371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattsson N, Insel PS, Landau S, et al. Diagnostic accuracy of CSF Ab42 and florbetapir PET for Alzheimer's disease. Ann Clin Transl Neurol. 2014;1(8):534-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vos SJB, Gordon BA, Su Y, et al. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewczuk P, Matzen A, Blennow K, et al. Cerebrospinal fluid aβ42/40 corresponds better than Aβ42 to amyloid PET in alzheimer's disease. J Alzheimers Dis. 2017;55(2):813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CD, Holden TR, Gordon BA, et al. Ante‐ and postmortem tau in autosomal dominant and late‐onset Alzheimer's disease. Ann Clin Transl Neurol. 2020;7(12):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barthélemy NR, Li Y, Joseph-Mathurin N, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer's disease. Nat Med. 2020;26(3):398-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James OG, Doraiswamy PM, Borges-Neto S. PET imaging of tau pathology in Alzheimer’s disease and tauopathies. Front Neurol. 2015;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 2019;76(9):1035-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request from the Knight Alzheimer Disease Research Center at Washington University in St. Louis through documented procedures.