Abstract
OBJECTIVES:
We assessed the prognostic value of the preoperative magnetization transfer ratio (MTR) and morphometrics of the spinal cord in patients with degenerative cervical myelopathy (DCM) in a longitudinal cohort study.
METHODS:
Thirteen subjects with DCM underwent 3T magnetization transfer imaging. The MTR was calculated for the spinal cord regions and specific white matter tracts. Morphometric measures were extracted. Clinical (modified Japanese Orthopaedics Association [mJOA] and Nurick scale scores) and health-related quality of life scores were assessed before and after cervical decompression surgery. The association between the magnetic resonance imaging (MRI) metrics and postoperative recovery was assessed (Spearman’s correlation). Receiver operating characteristics were used to assess the accuracy of MRI metrics in identifying ≥50% recovery in function.
RESULTS:
Preoperative anterior cord MTRs were associated with recovery in mJOA scores (ρ = 0.608; P = 0.036; area under the curve [AUC], 0.66). Preoperative lateral cord MTR correlated with the neck disability index (ρ = 0.699; P = 0.011) and pain interference scale (ρ = 0.732; P = 0.007). Preoperative rubrospinal tract MTR was associated with mJOA score recovery (ρ = 0.573; P = 0.041; AUC, 0.86). Preoperative corticospinal tract and reticulospinal MTRs were related to recovery in pain interference scores (ρ = 0.591; P = 0.033; and ρ = 0.583; P = 0.035, respectively). Eccentricity of the cord was associated with Nurick scores (ρ = 0.606; P = 0.028) and mJOA scores (ρ = 0.651; P = 0.025; AUC, 0.92).
CONCLUSIONS:
Preoperative MTR and eccentricity measurements of the spinal cord have prognostic value in assessing the response to surgery and recovery in patients with DCM. Advanced MRI and atlas-based postprocessing techniques can inform interventions and advance the healthcare received by patients with DCM.
Keywords: Degenerative spondylotic myelopathy, Magnetic resonance imaging, Prognostic utility, Spinal cord, Surgical outcomes
INTRODUCTION
Degenerative cervical myelopathy (DCM) is the most common cause of chronic spinal cord injury.1,2 Common impairments include reductions of proprioception, motor weakness, and gait imbalances.3,4 In patients presenting with moderate to severe symptoms, early decompression surgery has been shown to be beneficial.5–7 However, decisions regarding the care of patients with mild to moderate symptoms are complex and challenging, being largely dependent on radiological characteristics, the clinical presentation, practitioner experience, and patient preference.8 Studies comparing the efficacy of surgical versus conservative management have demonstrated clinical equipoise regarding the functional outcomes.9–12 Furthermore, patients treated surgically have shown variable and limited neurological functional recovery,13,14 with 4 of 10 patients reporting <50% improvement in 1 study.15 This variability in outcome has underscored the need to refine surgical decision-making.
Previous studies have assessed the utility of clinical, imaging, and neurophysiological features in predicting the surgical outcomes for patients with DCM. Advanced age,16 symptom severity, and a longer symptom duration have been the most common preoperative factors associated with negative outcomes.6 Magnetic resonance imaging (MRI)-based factors, including T1-weighted hypointensity, T2-weighted hyperintensity, and morphological parameters, might have a role in predicting outcomes.17–19 Although these factors can cumulatively serve as guidelines to inform postoperative functional improvement, accurate prognostic markers are lacking. Advances in MRI acquisition and postprocessing software have enabled quantification of demyelination and volumetric changes in regions of the spinal cord.20,21 Patients with DCM revealed significant myelin loss22 and decreased white and gray matter volumes compared with healthy counterparts.23,24 Additionally, these MRI parameters have been associated with clinical disability.22–24 Although these reports associated the radiographic findings with symptoms, they did not show how these markers are associated with the response to surgical intervention.
In the present study, we used magnetization transfer (MT) imaging to assess whether the preoperative MRI metrics are associated with the postoperative outcomes in patients with DCM. We hypothesized that the MRI markers used to quantify demyelination of the spinal cord regions and white matter tracts will demonstrate prognostic value for functional recovery at 6 months after decompression surgery.
METHODS
Participants
A total of 22 subjects (13 patients and 9 healthy controls) were recruited. All participants with DCM were recruited from a single academic spine practice of 5 board-certified neurosurgeons. Participants were included if they had reported the characteristic signs of cervical myelopathy, including upper extremity weakness, sensory loss, a lack of hand and leg coordination, and gait instability, combined with spinal imaging indicating cervical spinal cord compression. These participants have also been studied uniquely and separately.25 Those with major neurodegenerative diseases such as Alzheimer disease or multiple sclerosis (MS), spinal tumors or trauma, tremor, diabetes, systemic rheumatologic disease, peripheral or vascular neuropathy, and/or a history of spinal surgery were excluded. The healthy controls were also screened for current spinal conditions, neck pain, or other neurological deficits. All the participants provided written informed consent. The institutional review board approved the present study.
Clinical and Health-Related Quality of Life Scores
All subjects completed the modified Japanese Orthopaedic Association (mJOA) and the Nurick scale, neck and arm numerical rating scale (NRS), neck disability index (NDI), pain interference scale (Pain-6a), and the 36-item short-form health survey (SF-36), including the physical (SF-36P) and mental component scales (health and well-being survey). The mJOA questionnaire is a widely used scale used to assess upper and lower extremity sensorimotor function and sphincter dysfunction.26 The Nurick scale is used to assess ambulatory status.27 The NRS is used to quantify pain and discomfort on a scale of 0–10 (0, no discomfort/pain; 10, extreme discomfort/pain).28 The SF-36 is a questionnaire widely used to assess limitations in physical activity and bodily pain due to health problems.29 Participants also completed these questionnaires at their 6-month follow-up examination. The recovery rate was defined as (preoperative minus postoperative)/(normal minus preoperative) × 100 and was calculated for each variable.30
MRI Assessment
All imaging data were collected using a 3.0-Tesla Siemens Prisma MRI scanner (Siemens, Erlangen, Germany) equipped with a 64-channel head/neck coil. The participants were placed supine on the scanner bed, and a localizer scan was obtained to identify the location of the intervertebral discs of the cervical spine. Six transverse scans were acquired at each intervertebral disc using a multi-echo T2*-weighted gradient-echo sequence (repetition time, 300 ms; echo time, 18 ms; flip angle, 30°; field of view, 180 × 180; matrix size, 384 × 384; inplane resolution, 0.47 × 0.47 mm2; slice thickness, 6 mm; and number of averages, 2) with (MTC1) and without (MTC0) an MT pulse.31
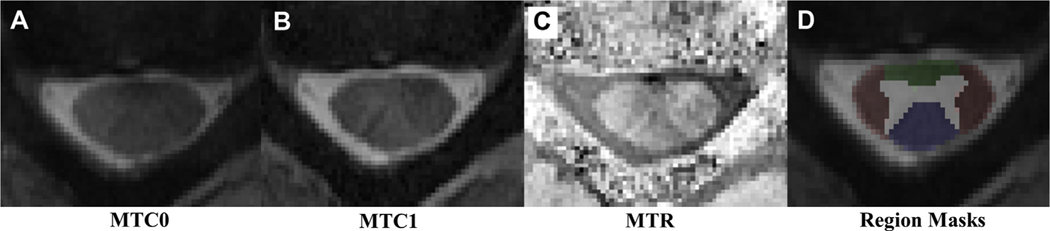
Image processing and analysis were performed using the Spinal Cord Toolbox, version 3.0 (open source software).21 For each slice, the spinal cord was first manually segmented. The MTC1 image was registered with the corresponding MTC0 image. The gray and white matter were segmented from the spinal cord using the MTC0 image and a multiatlas method, which registers a template white and gray matter segmentation to the image. Next, the PAM50 template was registered to the MTC0 image at each slice using the subject’s native space as the registration destination and the spinal cord mask to initialize the registration.32 MT images were then generated using the coregistered images and the following equation: MTR = 100 × (MTC0 – MTC1)/MTC0). MT ratio (MTR) metrics were extracted from the anterior, left and right lateral, and posterior white matter regions of the spinal cord (Figure 1) using the maximum a posteriori approach.33 The MTRs for the ventral corticospinal tract, ventral reticulospinal tract, medial reticulospinal tract, lateral corticospinal tract, rubrospinal tract, lateral reticulospinal tract, and ventrolateral reticulospinal tract were extracted.
Figure 1.
Illustration of magnetization transfer (MT) imaging: (A) without the MT pulse (MTC0), (B) with the MT pulse (MTC1), (C) MT Ratio (MTR), (D) region masks over the spinal cord, anterior cord (green), posterior cord (blue), and lateral cord (red).
The shape descriptor parameters of eccentricity of the spinal cord were extracted. A board-certified radiologist, not associated with the patient’s clinical care, assessed the compression grades at each level of the cervical spine. The region of compression was defined as 3 levels: the level of maximum compression and 1 level above and below that level. The mean eccentricity across all levels and at the region of compression was calculated. This method is consistent with previous work that assessed demyelination (changes in MTR values) in patients with DCM.22
Statistical Analysis
We performed 2-sample t tests to assess the significant differences between the subject groups. The mean values ±standard deviation are reported. Spearman’s correlations were computed to assess the associations between the radiographic parameters (MTRs) and postoperative recovery rate in patients. Receiver operating characteristic curves were conducted to assess the accuracy of the MTR metrics and morphometric parameters in identifying participants with ≥50% of recovery in symptoms after surgery. All statistical analyses were performed using SPSS software, version 24 (IBM Corp., Armonk, New York, USA). Statistical significance was set at P < 0.05.
RESULTS
Participant Characteristics (Demographic, Radiographic, and Clinical and Health-Related Quality of Life Scores)
The patient cohort included 13 subjects (4 women and 9 men) and the control group included 9 subjects (6 women and 3 men). The mean age of the patient and control group was 55.9 ± 14.0 years and 53.2 ± 7.2 years, respectively (P = 0.602). The patients had lower mJOA scores than those of the control group (14.5 ± 2.0; range, 12–17; and 18.0 ± 0, respectively; P < 0.001). The patients had higher Nurick scores than those of the controls (1.85 ± 0.90; range, 1–4; and 0 ± 0, respectively; P < 0.001). The patients also had greater neck discomfort (4.1 ± 2.3 vs. 0.44 ± 0.73; P < 0.001) and arm discomfort (3.54 ± 2.84 vs. 0.22 ± 0.44; P = 0.003). The NDI (13.54 ± 7.53 vs. 2.11 ± 2.75; P < 0.001) and Pain-6a scores (57.68 ± 8.10 vs. 43.14 ± 6.13; P < 0.001) were also higher and the SF-36P scores were lower (38.91 ± 14.96 vs. 56.25 ± 6.17; P = 0.004) compared with those of the controls. Seven patients had maximum compression at C5-C6, 3 patients at C6-C7, 2 patients at C3-C4, and 1 patient at C4-C5.
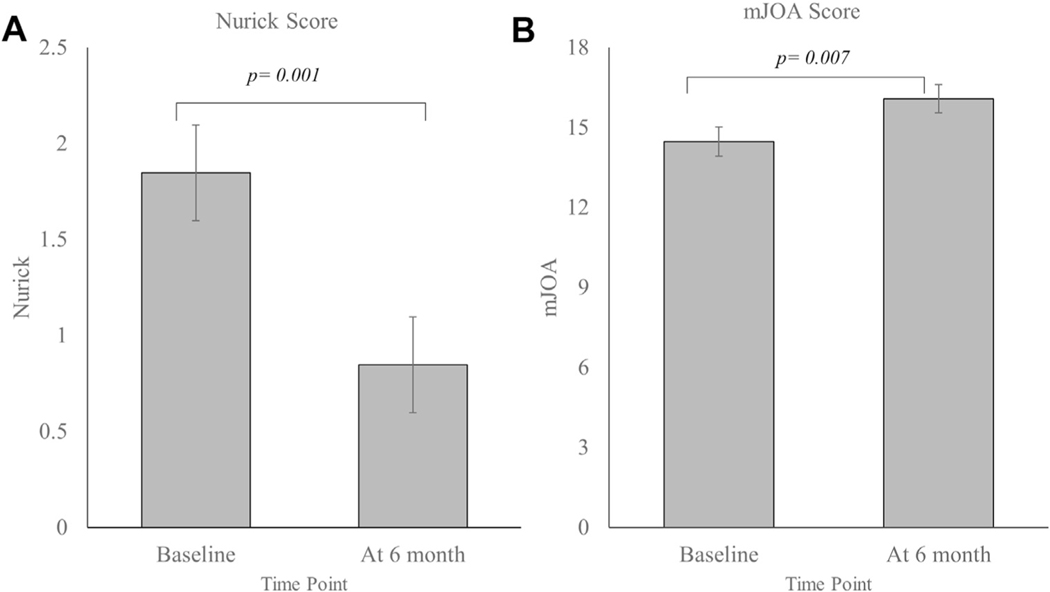
The patients had experienced significant improvement in their function at 6 months after decompression surgery. The Nurick score had decreased (0.85 ± 0.89 vs. 1.85 ± 0.89; P = 0.001) and the mJOA score had increased (16.10 ± 1.89 vs. 14.50 ± 1.98; P = 0.007) after surgery (Figure 2). The patients reported less neck discomfort (1.46 ± 1.71 vs. 4.10 ± 2.25; P = 0.07), less arm discomfort (2.15 ± 2.73 vs. 3.54 ± 2.85; P = 0.220), lower NDI scores (6.77 ± 5.76 vs. 13.54 ± 7.53; P = 0.002), lower Pain-6a scores (50.56 ± 7.18 vs. 57.68 ± 8.10; P = 0.010), and higher SF-36P scores (50.12 ± 8.74 vs. 38.9 ± 14.96; P = 0.027).
Figure 2.
Functional recovery in patients with degenerative cervical myelopathy at 6 months after surgery: (A) Nurick score and (B) modified Japanese Orthopaedic Association (mJOA) score. Data reported as mean ± standard error.
Associations with Spinal Cord Regions
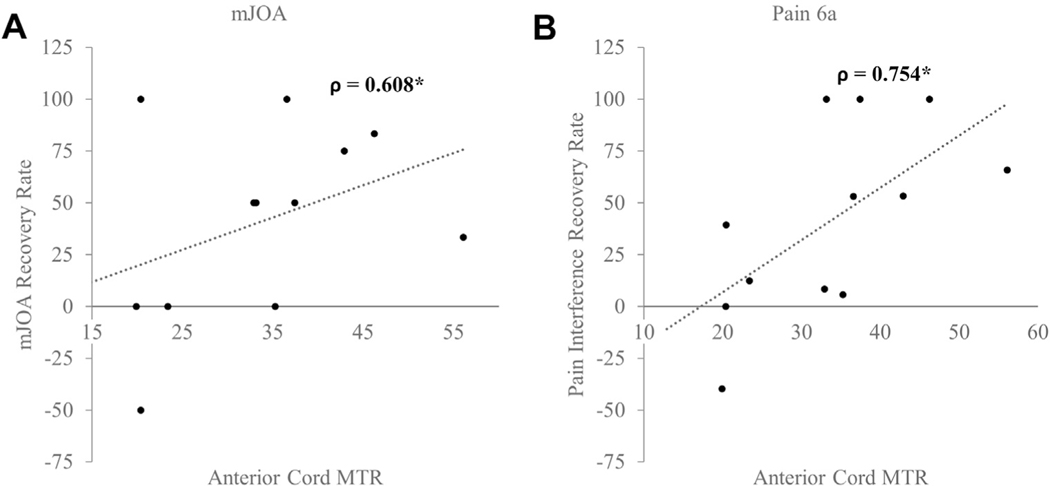
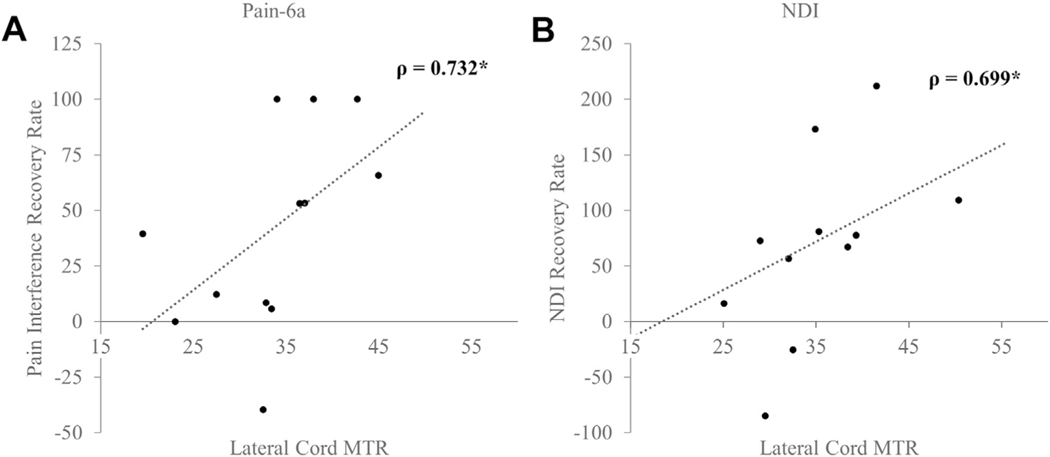
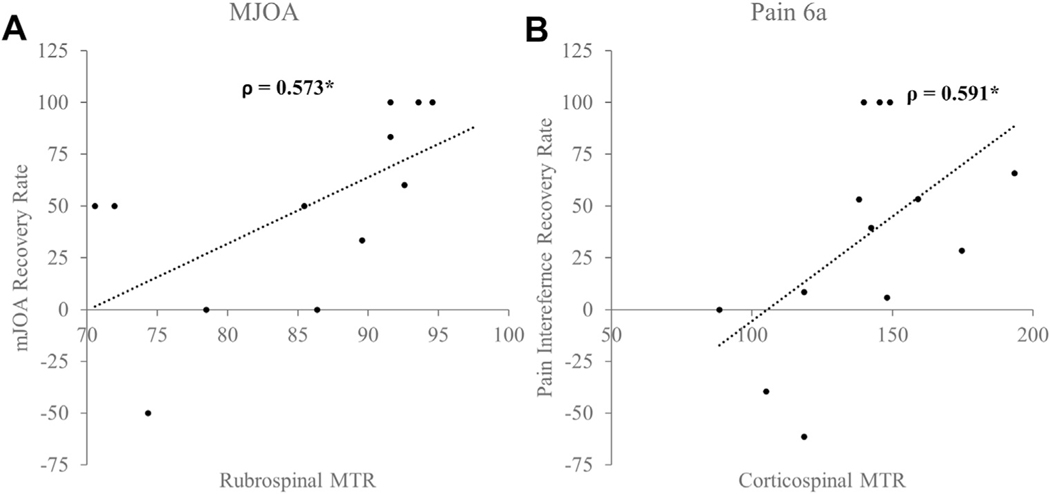
Several spinal cord regions had significant associations with the rate of recovery as assessed by the clinical and health-related quality of life scores. A positive improvement in the mJOA score was associated with the anterior cord MTR (ρ = 0.608; P = 0.036; Figure 3A). A higher anterior cord MTR also correlated positively with changes in the reported Pain-6a scores (ρ = 0.754; P = 0.005) and NDI (ρ = 0.545; P = 0.067; Figure 3B). The anterior cord MTR was also accurate in distinguishing patients with DCM who had experienced ≥50% recovery in their mJOA score (area under the receiver operating characteristic curve [AUC], 0.66), Pain-6a score (AUC, 0.97), and NDI (AUC, 0.84). The lateral cord regions were also associated with functional recovery. The right lateral cord was associated with the neck NRS (ρ = 0.582; P = 0.047), the right and left lateral cord ascending pathways were related to the NDI (ρ = 0.699; P = 0.011) and Pain-6a (ρ = 0.732; P = 0.007; Figure 4). Similarly, the lateral cord MTR was highly accurate in classifying 50% recovery in the NDI (AUC, 0.90), neck NRS score (AUC, 0.78), and Pain-6a score (AUC, 0.80).
Figure 3.
Association between anterior cord magnetization transfer ratio (MTR) and functional recovery rates: (A) modified Japanese Orthopaedic Association (mJOA) score and (B) pain interference scale (Pain-6a) score. Spearman’s ρ is reported.
Figure 4.
Association between lateral cord magnetization transfer ratio (MTR) and functional recovery rates: (A) pain interference scale (Pain-6a) score and (B) neck disability index (NDI). Spearman’s ρ is reported.
Associations with White Matter Tracts
Secondarily, we analyzed the specific MTRs for the white matter tracts in relation to the functional recovery rates. Several white matter tracts (i.e., corticospinal, spinocerebellar, and rubrospinal tracts) showed significant associations with the postoperative changes in the clinical and health-related quality of life scores. An improvement in the mJOA scores was associated with the preoperative mean rubrospinal MTR (ρ = 0.573; P = 0.041; Figure 5A) across all levels and at the region of compression (ρ = 0.554; P = 0.050) and the spinocerebellar tract MTR (across the entire cervical spinal cord, ρ = 0.662; P = 0.014; across the compression region, ρ = 0.576; P = 0.039). The MTRs for the rubrospinal and spinocerebellar tracts were 86% and 88% accurate in correctly identifying patients with ≥50% recovery in their mJOA scores after surgery. Similarly, recovery in the Nurick score was significantly associated with the spinocerebellar tract MTR (ρ = 0.626; P = 0.022). The spinocerebellar and rubrospinal tract MTRs were both highly likely to correctly bifurcate participants with and without <50% recovery in their postoperative Nurick scores (AUC, 0.85 and 0.80, respectively). An improvement in neck discomfort (i.e., arm NRS score) was significantly related to the spinocerebellar tract MTR (ρ = 0.565; P = 0.044; AUC for ≥50% recovery, 0.69). Likewise, a postoperative change in the Pain-6a score correlated with a mean corticospinal and reticulospinal tract MTR (ρ = 0.591; P = 0.033; and ρ = 0.583; P = 0.035; Figure 5B). The corresponding AUC for ≥50% recovery in the Pain-6a score was 0.76 and 0.73.
Figure 5.
Association between white matter tract magnetization transfer ratio (MTR) and functional recovery rates: (A) rubrospinal MTR related to modified Japanese Orthopaedic Association (mJOA) score and (B) corticospinal MTR related to pain interference scale (Pain-6a) score. Spearman’s ρ is reported.
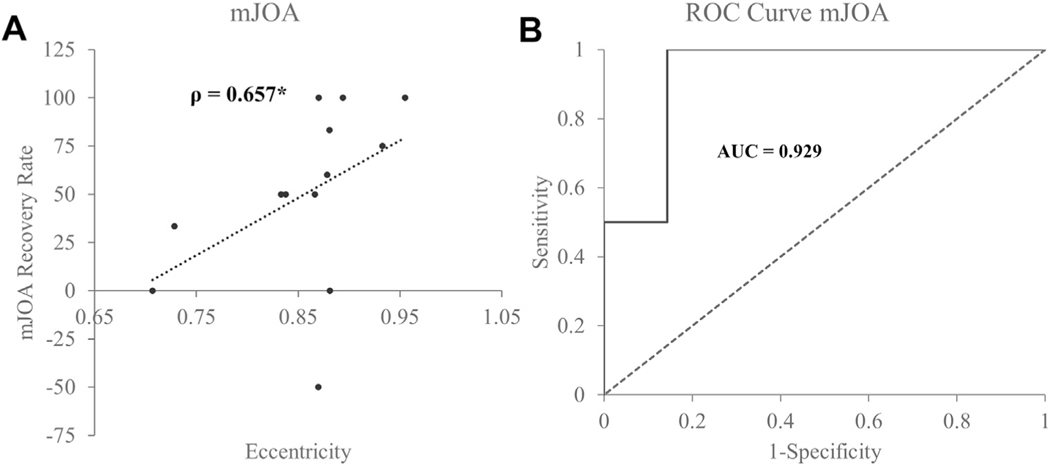
Associations with Morphological Parameters
Preoperative eccentricity (shape distortion) of the spinal cord was associated with a postoperative recovery in the Nurick score (ρ = 0.606; P = 0.028) and mJOA score (ρ = 0.651; P = 0.025; Figure 6A). Eccentricity of the spinal cord was 92% accurate in identifying ≥50% recovery in the postoperative mJOA scores (Figure 6B).
Figure 6.
Association between cord eccentricity and functional recovery rate. (A) Eccentricity related to modified Japanese Orthopaedic Association (mJOA) score. Spearman’s ρ is reported. (B) Receiver operating characteristic (ROC) curve denoting accuracy of eccentricity in identifying ≥50% mJOA recovery. Area under the curve is reported.
DISCUSSION
In the present study, we found that patients with a higher preoperative MTR experienced greater improvement in clinical myelopathy and quality of life and might represent a cohort of patients who would benefit from early surgical intervention. The MTR provides a measure of myelin integrity,34 and its use has been validated in demyelinating pathologies such as MS and Alzheimer disease.35,36 Evidence is emerging for its use in other common conditions such as whiplash injury.37,38 In patients with MS, decreases in MTR are related to clinical disability39,40 and occur before the appearance of lesions on conventional MRI scans. As such, the MTR can be useful in predicting the age of such lesions.41–43 Numerous studies have reported the clinical use of MTR as a well-accepted diagnostic and therapeutic marker in MS; however, literature on the utility of MTR for spinal diseases such as DCM is sparse.
Regional MTRs are associated with the functions of the spinal cord. In patients with chronic spinal cord injury, the ventrolateral region MTR explained motor disability, and posterior cord MTR was related to sensory disability measured using the American Spinal Injury Association scale.34 Similarly, Zackowski et al.44 showed that in patients with MS, column-specific spinal MT imaging is related to sensorimotor deficits and functional measures of walking and balance. Patients with DCM present with increased reflexes, decreases in proprioception, imbalance, and gait difficulties, suggesting the potential for some pathophysiological overlap. Previous work has demonstrated that patients with DCM have lower anterior cord MTRs, which were associated with greater disability.22 In the present study, we found that the preoperative anterior cord MTR is significantly related to the recovery in symptoms of myelopathy after intervention. The lateral cord MTR was associated with recovery in arm and neck disability and pain. These findings suggest that intervening before the occurrence of any significant demyelination might be crucial for the resolution of symptoms and functional recovery. Additionally, regional MTR analysis might be useful in understanding the variance in specific sensorimotor deficits in patients with equivalent clinical scores and their response to surgery.
Our findings are consistent with the current literature. Hopkins et al.23 showed that white matter tract volumes are significantly lower in patients with DCM. The corticospinal tracts contribute to skilled motor control, and patients exhibit sprouting and reorganization of corticospinal tract axons after spinal cord injury, likely to facilitate functional recovery.45 The reticulospinal tract is a major descending pathway essential for postural control and can assist in motor activity after corticospinal lesions. Baker and Perez46 have shown that reticulospinal tract neurons contribute to gross hand function in patients after spinal cord injury. We found that greater preoperative myelin loss or inflammation of the descending pathways is detrimental to improvement in physical function, pain and discomfort, ambulation, and the reduction in reflexes after surgery. It can be hypothesized that patients with DCM use similar mechanisms to regain function after surgery; thus, preserved preoperative tract neurons promote reorganization in these tracts. However, further electrophysiological investigation is needed to understand the underlying roles of intact neural tracts and axonal myelination in the mechanism of recovery in patients with DCM. Additionally, studies on the function and organization of the rubrospinal tract after spinal cord injury in humans are scarce. The findings from animal studies in primates have suggested that rubrospinal tracts play a role in the control of motor function in upper extremities in humans.47 Several animal studies have reported regeneration of rubrospinal tracts and their contribution to skilled hand movements and locomotion.48–50 A reduced rubrospinal tract volume has been demonstrated in patients with DCM.23 We also observed that lower preoperative rubrospinal damage is related to greater improvement in function after surgery. The exact function of this tract in humans and its role in DCM symptoms and recovery are under investigated and require further research.
Although factors such as T2-weighted signal intensity, combined T1-and T2-weighted signal changes, and maximum canal compromise are linked to poor outcomes, it has been recommended that surgeons consider these collectively when estimating the risks of poor neurological recovery.51–53 In the present study, we have demonstrated that the morphological parameter of eccentricity is related to postoperative functional recovery. Thus, patients with greater structural distortion of the spinal cord are likely to benefit more from decompression surgery. The results have shown that although MRI is widely used in diagnostics in DCM, it might also prognostic utility.
The findings have primarily demonstrated that advanced MRI techniques and atlas-based spinal cord mapping can provide important prognostic markers that could inform surgical decision-making and enhance treatment efficacy for patients with DCM. MTR analysis can assist clinicians in (1) identifying which patients are more likely to benefit the most from surgery, (2) informing decisions about the treatment of patients with asymptomatic stenosis, and (3) counseling patients accordingly. The prescription of operative versus nonoperative treatment, especially in cases of mild to moderate DCM is often challenging. Our results suggest that the timing the surgery before any significant demyelination or inflammation in spinal white matter can potentially maximize clinical outcomes and recovery. Based on these findings, an early intervention to halt spinal cord neuronal destruction is recommended to combat the progressive nature of the disease.
One of the limitations of the present study was that partial volume averaging at the boundaries during image acquisition and probability mapping could have been present, which might have affected the extracted volumes, especially of the small tracts. Second, continued improvements in function might occur after 6 months. Therefore, analysis of longitudinal data from 1- and 2-year follow-up evaluations could provide for a more comprehensive predictive model. Third, the small sample size in the present study limited further analysis. Although the present study only used linear correlation, the next steps could focus on development of multivariate regression models and machine learning algorithms to accurately predict surgical outcomes. Finally, although their associations are varied, studies have shown that the baseline clinical, demographic, spinal alignment, and conduction characteristics correlate with postoperative recovery.54–58 The inclusion of these metrics with MRI-derived metrics could generate better and more reliable prognostic models.
CONCLUSIONS
Higher preoperative spinal cord MTRs demonstrated a preliminary prognostic value of better postoperative outcomes for patients. Advanced MRI and evolving atlas-based techniques can be clinically useful, inform interventions, and complement the healthcare received by patients with various levels of DCM.
ACKNOWLEDGMENTS
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest statement: Zachary Adam Smith received funding from the National Institute on Neurological Disorders and Stroke (grant K23NS104211). Kenneth A. Weber II received funding from National Institute of Neurological Disorders and Stroke (grants K23NS104211 and L30NS108301). Sean Mackey received funding from National Institute of Drug Abuse (grant K24DA029262). The sponsors played no role in the study design, data collection, decision to publish, or preparation of the report
Abbreviations and Acronyms
- AUC
Area under the receiver operating characteristic curve
- DCM
Degenerative cervical myelopathy
- mJOA
Modified Japanese Orthopaedic Association scale
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- MT
Magnetization transfer
- MTR
Magnetization transfer ratio
- NDI
Neck disability index
- NRS
Numerical rating scale
- Pain-6a
Pain interference scale
- SF-36
36-Item short-form health survey
- SF-36P
36-Item short-form health survey physical component score
REFERENCES
- 1.Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19:409–421. [DOI] [PubMed] [Google Scholar]
- 2.Karadimas SK, Gatzounis G, Fehlings MG. Pathobiology of cervical spondylotic myelopathy. Eur Spine J. 2015;24(suppl 2):132–138. [DOI] [PubMed] [Google Scholar]
- 3.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976). 2015;40:E675–E693. [DOI] [PubMed] [Google Scholar]
- 4.Bakhsheshian J, Mehta VA, Liu JC. Current diagnosis and management of cervical spondylotic myelopathy. Glob Spine J. 2017;7:572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acharya S, Srivastava A, Virmani S, Tandon R. Resolution of physical signs and recovery in severe cervical spondylotic myelopathy after cervical laminoplasty. Spine (Phila Pa 1976). 2010;35: E1083–E10837. [DOI] [PubMed] [Google Scholar]
- 6.Cheung WY, Arvinte D, Wong YW, Luk KD, Cheung KM. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy—a prospective study. Int Orthop. 2008;32:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9:152–157. [DOI] [PubMed] [Google Scholar]
- 8.Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Glob Spine J. 2017;7(suppl):70s–83s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee J, Tetreault LA, Chapman JR, et al. Nonoperative versus operative management for the treatment degenerative cervical myelopathy: an updated systematic review. Glob Spine J. 2017; 7(suppl):35s–41s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadanka Z, Bednarik J, Novotny O, Urbanek I, Dusek L. Cervical spondylotic myelopathy: conservative versus surgical treatment after 10 years. Eur Spine J. 2011;20:1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadanka Z, Mares M, Bednanik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine (Phila Pa 1976). 2002;27: 2201–2205. [DOI] [PubMed] [Google Scholar]
- 12.Kong LD, Meng LC, Wang LF, Shen Y, Wang P, Shang ZK. Evaluation of conservative treatment and timing of surgical intervention for mild forms of cervical spondylotic myelopathy. Exp Ther Med. 2013;6:852–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiban E, Meyer B. Treatment considerations of cervical spondylotic myelopathy. Neurol Clin Pract. 2014;4:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbraith JG, Butler JS, Dolan AM, O’Byrne JM. Operative outcomes for cervical myelopathy and radiculopathy. Adv Orthop. 2012;2012:919153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JT, Wang LF, Wang S, Li J, Shen Y. Risk factors for poor outcome of surgery for cervical spondylotic myelopathy. Spinal Cord. 2016;54: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 16.Zhang RJ, Shen CL, Zhang JX, et al. Clinical features and surgical outcomes of cervical spondylotic myelopathy in patients of different ages: a retrospective study. Spinal Cord. 2018;56:7–13. [DOI] [PubMed] [Google Scholar]
- 17.Kim B, Yoon DH, Shin HC, et al. Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament. Spine J. 2015;15: 875–884. [DOI] [PubMed] [Google Scholar]
- 18.Liu FJ, Sun YP, Shen Y, Ding WY, Wang LF. Prognostic value of magnetic resonance imaging combined with electromyography in the surgical management of cervical spondylotic myelopathy. Exp Ther Med. 2013;5:1214–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nouri A, Tetreault L, Zamorano JJ, et al. Role of magnetic resonance imaging in predicting surgical outcome in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2015;40:171–178. [DOI] [PubMed] [Google Scholar]
- 20.Grossman RI, Gomori JM, Ramer KN, Lexa FJ, Schnall MD. Magnetization transfer: theory and clinical applications in neuroradiology. Radiographics. 1994;14:279–290. [DOI] [PubMed] [Google Scholar]
- 21.De Leener B, Levy S, Dupont SM, et al. SCT: spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017; 145(Pt A):24–43. [DOI] [PubMed] [Google Scholar]
- 22.Cloney MB, Smith ZA, Weber KA II, Parrish TB. Quantitative magnetization transfer MRI measurements of the anterior spinal cord region are associated with clinical outcomes in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2018; 43:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopkins BS, Weber KA II, Cloney MB, Paliwal M, Parrish TB, Smith ZA. Tract-specific volume loss on 3T MRI in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2018;43: E1204–E1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith ZA, Weber KA II, Paliwal M, et al. MRI atlas-based volumetric mapping of the cervical cord gray matter in cervical canal stenosis. World Neurosurg. 2020;134:e497–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith ZA, Barry AJ, Paliwal M, Hopkins BS, Cantrell D, Dhaher Y. Assessing hand dysfunction in cervical spondylotic myelopathy. PLoS One. 2019;14:e0223009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata K, Yoshimura N, Hashizume H, et al. The prevalence of cervical myelopathy among subjects with narrow cervical spinal canal in a population-based magnetic resonance imaging study: the Wakayama Spine Study. Spine J. 2014;14:2811–2817. [DOI] [PubMed] [Google Scholar]
- 27.Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100. [DOI] [PubMed] [Google Scholar]
- 28.Walton DM, Elliott JM, Salim S, Al-Nasri I. A reconceptualization of the pain numeric rating scale: anchors and clinically important differences. J Hand Ther. 2018;31:179–183. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30: 473–483. [PubMed] [Google Scholar]
- 30.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976). 1981;6: 354–364. [DOI] [PubMed] [Google Scholar]
- 31.Held P, Dorenbeck U, Seitz J, Frund R, Albrich H. MRI of the abnormal cervical spinal cord using 2D spoiled gradient echo multiecho sequence (MEDIC) with magnetization transfer saturation pulse: a T2* weighted feasibility study. J Neuroradiol. 2003;30:83–90. [PubMed] [Google Scholar]
- 32.De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J. PAM50: unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2018; 165:170–179. [DOI] [PubMed] [Google Scholar]
- 33.Levy S, Benhamou M, Naaman C, Rainville P, Callot V, Cohen-Adad J. White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage. 2015;119:262–271. [DOI] [PubMed] [Google Scholar]
- 34.Cohen-Adad J, El Mendili MM, Lehericy S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55: 1024–1033. [DOI] [PubMed] [Google Scholar]
- 35.Vavasour IM, Laule C, Li DK, Traboulsee AL, MacKay AL. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J Magn Reson Imaging. 2011;33:713–718. [DOI] [PubMed] [Google Scholar]
- 36.Kabani NJ, Sled JG, Shuper A, Chertkow H. Regional magnetization transfer ratio changes in mild cognitive impairment. Magn Reson Med. 2002; 47:143–148. [DOI] [PubMed] [Google Scholar]
- 37.Elliott JM, Dewald JP, Hornby TG, Walton DM, Parrish TB. Mechanisms underlying chronic whiplash: contributions from an incomplete spinal cord injury? Pain Med. 2014;15:1938–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AC, Parrish TB, Hoggarth MA, et al. Potential associations between chronic whiplash and incomplete spinal cord injury. Spinal Cord Ser Cases. 2015;1:15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkhof F. MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS). Mult Scler. 1999;5:283–286. [DOI] [PubMed] [Google Scholar]
- 40.Gass A, Barker GJ, Kidd D, et al. Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Ann Neurol. 1994; 36:62–67. [DOI] [PubMed] [Google Scholar]
- 41.Pike GB, De Stefano N, Narayanan S, et al. Multiple sclerosis: magnetization transfer MR imaging of white matter before lesion appearance on T2-weighted images. Radiology. 2000;215:824–830. [DOI] [PubMed] [Google Scholar]
- 42.Filippi M, Rocca MA, Martino G, Horsfield MA, Comi G. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol. 1998;43:809–814. [DOI] [PubMed] [Google Scholar]
- 43.Tomiak MM, Rosenblum JD, Prager JM, Metz CE. Magnetization transfer: a potential method to determine the age of multiple sclerosis lesions. AJNR Am J Neuroradiol. 1994;15:1569–1574. [PMC free article] [PubMed] [Google Scholar]
- 44.Zackowski KM, Smith SA, Reich DS, et al. Sensorimotor dysfunction in multiple sclerosis and column-specific magnetization transferimaging abnormalities in the spinal cord. Brain. 2009;132(Pt 5):1200–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol. 2012;590: 3647–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker SN, Perez MA. Reticulospinal contributions to gross hand function after human spinal cord injury. J Neurosci. 2017;37:9778–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang HS, Kwon HG, Hong JH, Hong CP, Jang SH. The rubrospinal tract in the human brain: diffusion tensor imaging study. Neurosci Lett. 2011;504: 45–48. [DOI] [PubMed] [Google Scholar]
- 48.Williams PT, Martin JH. Motor cortex activity organizes the developing rubrospinal system. J Neurosci. 2015;35:13363–13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris R, Whishaw IQ. A proposal for a rat model of spinal cord injury featuring the rubrospinal tract and its contributions to locomotion and skilled hand movement. Front Neurosci. 2016;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon BK, Liu J, Messerer C, et al. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99: 3246–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C, Das SK, Yang DJ, Yang HF. Application of magnetic resonance imaging in cervical spondylotic myelopathy. World J Radiol. 2014;6:826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tetreault LA, Dettori JR, Wilson JR, et al. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(suppl 1):S89–S110. [DOI] [PubMed] [Google Scholar]
- 53.Fehlings MG, Tetreault LA, Wilson JR, Skelly AC. Cervical spondylotic myelopathy: current state of the art and future directions. Spine (Phila Pa 1976). 2013;38(suppl 1):S1–S8. [DOI] [PubMed] [Google Scholar]
- 54.Deftereos SN, Kechagias E, Ioakeimidou C, Georgonikou D. Transcranial magnetic stimulation but not MRI predicts long-term clinical status in cervical spondylosis: a case series. Spinal Cord. 2015;53(suppl 1):S16–S18. [DOI] [PubMed] [Google Scholar]
- 55.Oshima Y, Seichi A, Takeshita K, et al. Natural course and prognostic factors in patients with mild cervical spondylotic myelopathy with increased signal intensity on T2-weighted magnetic resonance imaging. Spine (Phila Pa 1976). 2012;37:1909–1913. [DOI] [PubMed] [Google Scholar]
- 56.Tetreault L, Palubiski LM, Kryshtalskyj M, et al. Significant predictors of outcome following surgery for the treatment of degenerative cervical myelopathy: a systematic review of the literature. Neurosurg Clin North Am. 2018;29:115–127.e35. [DOI] [PubMed] [Google Scholar]
- 57.Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24(suppl 2):236–251. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Tian Y, Wang C, et al. Prognostic value of intraoperative MEP signal improvement during surgical treatment of cervical compressive myelopathy. Eur Spine J. 2016;25:1875–1880. [DOI] [PubMed] [Google Scholar]