Abstract
Background:
Growing epidemiological evidence suggests that organochlorine chemicals (OCCs), including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), may play a role in the pathogenesis of endometriosis.
Objectives:
We aimed to systematically review the experimental evidence (in vivo and in vitro) on the associations between exposure to OCCs and endometriosis-related end points.
Methods:
A systematic review protocol was developed following the National Toxicology Program /Office of Health Assessment and Translation (NTP/OHAT) framework and managed within a web-based interface. In vivo studies designed to evaluate the impact of OCCs on the onset or progression of endometriosis and proliferation of induced endometriotic lesions were eligible. Eligible in vitro studies included single-cell and co-culture models to evaluate the proliferation, migration, and/or invasion of endometrial cells. We applied the search strings to PubMed, Web of Science, and Scopus®. A final search was performed on 24 June 2020. Assessment of risk of bias and the level of evidence and integration of preevaluated epidemiological evidence was conducted using NTP/OHAT framework
Results:
Out of 812 total studies, 39 met the predetermined eligibility criteria (15 in vivo, 23 in vitro, and 1 both). Most studies () tested TCDD and other dioxin-like chemicals. In vivo evidence supported TCDD’s promotion of endometriosis onset and lesion growth. In vitro evidence supported TCDD’s promotion of cell migration and invasion, but there was insufficient evidence for cell proliferation. In vitro evidence further supported the roles of the aryl hydrocarbon receptor and matrix metalloproteinases in mediating steroidogenic disruption and inflammatory responses. Estrogen interactions were found across studies and end points.
Conclusion:
Based on the integration of a high level of animal evidence with a moderate level of epidemiological evidence, we concluded that TCDD was a known hazard for endometriosis in humans and the conclusion is supported by mechanistic in vitro evidence. Nonetheless, there is need for further research to fill in our gaps in understanding of the relationship between OCCs and their mixtures and endometriosis, beyond the prototypical TCDD. https://doi.org/10.1289/EHP8421
Introduction
Humans are exposed daily to complex mixtures of chemical pollutants, some of which may contribute to a disruption of endocrine functions and contribute to reproductive diseases. Chemicals with bioaccumulative properties are of particular concern, because they persist worldwide in the environment and ultimately may accumulate in human fatty tissues, despite the fact that many are now heavily regulated or banned (Pumarega et al. 2016; UNEP 2017). These chemicals include a large family of pollutants known as organochlorine chemicals (OCCs), characterized by their carbon–chlorine bonds, which comprise polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), polychlorinated biphenyls (PCBs), and organochlorine pesticides (OCPs) (Bernes 1998; Jones and De Voogt 1999). A number of OCCs such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) have been extensively reported to interact with the aryl hydrocarbon receptor (AhR) and/or estrogen receptors and disrupt the nervous, immune, and endocrine systems at various life stages (Gore et al. 2015; Lawrence and Vorderstrasse 2013; Barouki et al. 2012).
The female reproductive tract has been shown to be especially vulnerable to the presence of hormone-disrupting chemicals, potentially leading to early puberty, infertility, adverse pregnancy outcomes, fibroids, or endometriosis (Bruner-Tran et al. 2017; Gore et al. 2015; Ho et al. 2017; Hutz et al. 2006). Endometriosis is a hormone-dependent gynecological disease characterized by the presence and growth of ectopic endometrial tissues (Zondervan et al. 2018). The precise prevalence of endometriosis in the general population is largely unknown due to difficulties in diagnosis, underreporting, and unknown prevalence among asymptomatic individuals, but estimates of prevalence range widely from 5% to 45% of individuals who menstruate (Buck Louis et al. 2011; Rawson 1991). Many gaps also remain in fully understanding the etiology of endometriosis, but it is likely multifactorial, involving genetic and environmental factors (Sourial et al. 2014; van der Linden 1996).
In a previous systematic review and meta-analysis, we synthesized the associations between OCCs and endometriosis in human epidemiological studies and evaluated the quality of the body of evidence using the comprehensive National Toxicology Program/Office of Health Assessment and Translation (NTP/OHAT) framework (Cano-Sancho et al. 2019). The overall conclusion of this review supported the existence of positive associations between exposure to PCDDs, PCBs, and OCPs and endometriosis, which is consistent with subsequently published reviews (Freger and Foster 2020; Wen et al. 2019). However, the level of evidence was deemed moderate, with serious risk of bias: Major methodological limitations in epidemiological research of endometriosis included the lack of population-based study designs, the inherent constraints in classifying control populations, the heterogeneity of endometriosis subtypes, the use of different OCCs biomarkers, and narrow background exposure distributions.
Associations observed from epidemiological studies, however, provide an incomplete picture and fall short of being able to provide a biological explanation for the link between OCC exposure and endometriosis. Thus, from our point of view, experimental studies are necessary to advance our understanding of the underlying molecular mechanisms and provide the support of biological plausibility to the trends observed in human epidemiological studies. The nonhuman primate model of endometriosis is considered the most reliable because it mimics the pathophysiological conditions in women (Story and Kennedy 2004; Grümmer 2006). However, ethical considerations and high economic costs have favored the development of rodent models (Bruner-Tran et al. 2018). The process of surgically inducing lesions by implanting the animal’s autologous uterine horn was developed in rats (Vernon and Wilson 1985) and later adapted to mice (Cummings and Metcalf 1995a).
In addition, ex vivo and in vitro models, with primary cells and co-cultures from human biopsies, represent a straightforward way to gain insight into the molecular signaling pathways in endometrial cells (Fan 2020). To our knowledge, no studies to date are attempting to systematically gather and appraise the experimental evidence on OCC exposure and endometriosis, though some narrative reviews on the topic have been conducted (Birnbaum and Cummings 2002; Bruner-Tran and Osteen 2010; Guo et al. 2009; Rier and Foster 2003). In this context, the present work aimed to perform a systematic review applying the NTP/OHAT framework to a) systematically appraise experimental (in vivo and in vitro) studies reporting evidence on the associations between OCC exposure and endometriosis and b) to draw a conclusion based on the level of confidence of this body of evidence.
Methods
The present systematic review was conducted following the guidelines established in the NTP/OHAT handbook (NTP/OHAT 2015a), using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) principles. The protocol was registered in International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42018102618) on 17 July 17 2018, peer-reviewed, and published in January 2019 (Matta et al. 2019). Study selection, data extraction, data synthesis, and risk of bias (RoB) assessment were performed and managed using the Health Assessment Workspace Collaborative (HAWC; https://hawcproject.org/), an open-source, modular web-based content management system with a user interface (Shapiro et al. 2018). Numerical data from plots and graph images were extracted using WebPlotDigitizer (version 4.3; https://automeris.io/WebPlotDigitizer/). The RoB assessment was performed using the NTP/OHAT’s RoB rating tool to assess individual study quality (NTP/OHAT 2015b), and followed the guidance of the NTP protocol for the perfluorooctanesulfonic acid/perfluorooctanoic acid (PFOS/PFOA) monograph for the integration of in vivo and in vitro data (NTP 2016). Results are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al. 2009).
Eligibility Criteria
The literature search strategy was initially developed by using the list of all persistent organic pollutants (POPs) determined by the Stockholm Convention (UNEP 2017) to calibrate and refine the protocol during problem formulation. Accordingly, we developed a Populations, Exposures, Comparators, Outcomes (PECO) statement (Table 1) establishing the inclusion/exclusion criteria for our systematic review. In addition, we excluded any studies that did not contain original data, such as reviews, commentaries, editorials, or conference abstracts, as well as studies not available in English. Further details of problem formulation can be found in the previously published protocol (Matta et al. 2019).
Table 1.
Summary of Population, Exposure, Control, Outcome (PECO) statement for in vivo and in vitro studies.
| Inclusion | Exclusion | |
|---|---|---|
| P |
In vivo: Experimental animal models where endometriosis can either occur spontaneously or be induced In vitro: Human endometrial cells or tissues |
In vivo: Observational epidemiological studies In vitro: Cancer cells, nonuterine cells |
| E |
In vivo and in vitro: • Organochlorine chemicals (OCCs) |
In vivo and in vitro: • Pharmaceuticals, non-OCCs |
| C |
In vivo and in vitro: • Has reference or control group |
In vivo and in vitro: • Lacks reference or control group |
| O |
In vivo: • Onset or aggravation of endometriosis • Proliferation or growth of induced endometriotic lesions • Presence-of “endometriosis-like” phenotypes with human reference standard In vitro: Proliferation, migration, and/or invasion of endometrial cells |
In vivo and in vitro: • Outcomes unrelated to endometriosis |
Literature Search and Study Selection
The search string was developed to identify all relevant published evidence in experimental studies (in vivo, ex vivo, or in vitro) with primary data on the associations between controlled exposures to OCCs and endometriosis and endometriosis-related effects by a) reviewing MeSH terms and literature tags used by previously identified human epidemiology studies on endometriosis for relevant and appropriate terms; b) adapting existing chemical lists (UNEP 2017; Wassenaar et al. 2017); and c) extracting several potential endometriosis-related mechanistic outcomes (Liu and Zhao 2016). The complete search string can be found in Table S1. It comprised the exposure and outcome elements of the PECO statement nested through the Boolean operators “AND/OR.” A comprehensive list of 33 persistent organic pollutants identified in the Stockholm Convention with suspected endocrine-disrupting potential can be found in Table S2 (UNEP 2017). Because the scope of our review focused on OCCs, brominated congeners (i.e., decabromodiphenyl ether, hexabromobiphenyl, hexabromocyclododecane, hexabromodiphenyl ether, heptabromodiphenyl ether, tetrabromodiphenyl ether, and pentabromodiphenyl ether) and PFOS, its salts, and perfluorooctane sulfonyl fluoride were not included in the search terms. In addition to this list of chemicals, a set of OCC-related terminology was used to capture any other studies with relevant exposure. Synonyms of each chemical name were retrieved using the PubChem and ChemSpider database on 27 June 2018. An initial search was performed on 9 January 2019 in the databases PubMed/Medline (https://www.ncbi.nlm.nih.gov/pubmed), Scopus (https://www.scopus.com), and Web of Science (WoS; https://webofknowledge.com), with syntax customized for each database. A subsequent manual search of the bibliographic references of relevant articles and existing reviews was performed. A final search update was performed on 24 June 2020. No filter was applied to limit the date or language of publication during the search. The identified studies were exported to EndNote in RIS format to pool records for manual duplicate removal. Studies underwent a two-phase screening process by two independent researchers (K.M. and G.C.S.), based on the predefined inclusion and exclusion criteria of the PECO statement (Table 1). Phase I Screening was based on title and abstracts, and Phase II Screening was based on full text for the studies not excluded in Phase I. Any discrepancies were resolved by consensus or with the opinion of a third researcher (J.P.A.).
Data Extraction
Eligible studies underwent data extraction within the HAWC module according to a predefined standardized data extraction process (Table S3), explained below in detail, with illustrative examples from Foster et al. 1997 in the supplemental materials (Figures S1–S5). Results are stored and available for download in Excel format (https://hawcproject.org/assessment/812/).
First, studies were identified by citation (short and full), and type(s) of study data were indicated (i.e., “Animal bioassay,” hereafter referred to as in vivo and “in vitro”). Studies which contained both in vivo and in vitro data were indicated as such. In this initial stage of study preparation, general study data (i.e., conflicts of interest, funding source, author correspondence details) were extracted (Figure S1).
Studies with in vivo data comprised at least one animal “experiment” (Figure S2). Data extracted within each experiment included study name, study type (acute, short term, subchronic, chronic, mechanistic, reproductive, developmental, etc.), chemical identifiers (name, CAS, source), chemical purity, vehicle used, and details of animal husbandry and diet and compliance with any guidelines for methods (Figure S3). Each experiment consisted of at least one “animal group.” Data extracted within each animal group included animal species, strain, and sex, source of animals (laboratory or breeding details), life stage exposed and life stage assessed, observation duration, siblings (if animal groups were related), and any additional description of the animal group (Figure S4). A dosing regimen was also extracted for each animal group, including route of exposure, exposure duration, number of dose groups, control information (positive and/or negative controls), and any other information about dosing methodology (e.g., dose units, dose groups, details of dosing regimen). Dose–response information for end points was also extracted if such data were provided. Each animal group could have multiple end points, characterized by relevant biological system (i.e., reproductive), organ/tissue (i.e., endometrium), effect/effect subtype, diagnostic method, observation time, data set type (e.g., continuous, dichotomous, percent difference), variance type (standard error or standard deviation if reported or relevant), response units, data location in the literature, and other notes on the end point methodology and results (e.g., response direction that would be considered adverse, points of departure, monotonicity, statistical tests, trend tests, etc.) (Figure S4). A dose–response visualization was automatically generated for end points with dose–response data (Figure S5).
A similar data extraction process was performed for in vitro studies. First, cell type data was extracted, including species, strain, sex, and tissue of origin (typically female humans), as well as culture type (i.e., primary culture, immortalized cell line, transfected cell line, etc.) and source of cell cultures. Such data were extracted for each relevant cell type used in each study if more than one cell population were used. Chemical exposure data were then extracted, including chemical name, CAS number, source, purity (and purity confirmation details if available), and dilution storage notes. Again, such data were extracted for each unique chemical exposure for each study. Following this initial data extraction, an “experiment” for each cell type was created. If there were multiple cell types in an in vitro study, a separate experiment was created for each one. In each in vitro experiment, data extracted included dosing information (dosing regimen, duration, units), serum information (percent serum, serum type, and/or description), and control information (positive, negative, and/or vehicle controls). As with in vivo experiments, in vitro experiments with multiple outcomes were characterized by chemical exposure, assay type, outcome effect, data location in the literature, data set type (e.g., continuous, dichotomous, percent difference), variance type (standard error or standard deviation if reported or relevant), response units, observation time, points of departure, monotonicity, statistical tests, trend tests, and any other notes on the end point of the results. Dose–response data for end points were also extracted when such data were provided. Dose–response visualizations were similarly automatically generated for such end points. Corresponding authors were contacted by email for data unavailable in the published articles and for clarification of methods and risk of bias questions, and authors were provided 2 wk for response.
Synthesis of Results
End points were grouped into primary or secondary end points, as previously explained in the published protocol (Matta et al. 2019). In brief, four primary end points were measured (two in vivo and two in vitro). For in vivo studies, primary end points aimed to be the corollary to endometriosis in humans thus included a) the spontaneous onset of endometriosis and b) the growth or proliferation of induced endometriotic lesions. For in vitro studies, primary end points included c) cell migration/invasion and d) cell viability/proliferation. Secondary end points included gene expression or protein levels within the signaling pathways regulating the primary end points, markers of disrupted steroidogenic pathways, inflammatory biomarkers, such as cytokines [i.e., interleukins (IL)], or markers of extracellular matrix remodeling [i.e., matrix metalloproteinases (MPPs)]. End point results were summarized in data pivot figures displaying the significance and direction of the effects across exposure doses and studies. The heterogeneity of included studies precluded a quantitative meta-analysis.
Risk of Bias Assessment
The NTP/OHAT RoB Rating Tool was specifically adapted to the research question in the HAWC interface (NTP/OHAT 2015b) and tailored to in vitro studies as previously reported (NTP 2016). Briefly, the RoB tool consists of a set of questions tailored to each experimental stream of evidence, addressing six main bias domains listed below (i.e., selection bias, performance bias, attrition bias, detection bias, selective reporting bias, and other) (Table 2).
Table 2.
Risk of bias analysis: domains of bias and questions.
| Domain of bias | Risk of bias question |
|---|---|
| Selection bias | 1. Was administered dose or exposure level adequately randomized? |
| Selection bias | 2. Was allocation to study groups adequately concealed? |
| Performance bias | 3. Were experimental conditions identical across study groups?* |
| Performance bias | 4. Were the research personnel blinded to the study group during the study? |
| Attrition bias | 5. Were outcome data incomplete due to attrition or exclusion from analysis? |
| Detection bias | 6. Can we be confident in the exposure characterization?* |
| Detection bias | 7. Can we be confident in the outcome assessment?* |
| Selective reporting bias | 8. Were all measured outcomes reported? |
| Other | 9. Were there any other potential threats to internal validity? |
Note: Key elements considered for the tiered classification are marked by an asterisk (*).
Questions received one of five possible ratings: “Definitely Low Risk of Bias,” “Probably Low Risk of Bias,” “Probably High Risk of Bias,” “Definitely High Risk of Bias,” or “Not Reported,” based on prespecified criteria (Supplemental Materials, Section 3). The rating was determined by two independent assessors (K.M. and G.C.S.) and then finalized by discussion and consensus, with consultation by an additional member of the review team or technical advisors as needed. In the event that additional information was needed to make a rating determination, authors were contacted with questions specific to the RoB question and provided 2 wk to respond. Each RoB rating was justified based on the established criteria and the study text, and is stored and available in HAWC. Based on these ratings, individual studies were ranked on a three-tier scale of bias allowing the classification of specific bodies of evidence in “not serious,” “serious,” or “very serious” RoB to support decision-making for confidence rating (NTP/OHAT 2015a). More details of the three-tier scale can be found in the next section or in the previously published protocol.
Rating the Level of Evidence
Following the NTP/OHAT framework, we analyzed different domains affecting the confidence level for each primary end point–related body of evidence. Each stream of data was considered a body of evidence, and an assessment was performed for each primary end point to determine a confidence rating reflecting the confidence with which the study findings accurately reflect a true association between exposure to OCCs and the primary end points. The process is summarized in Table S4 and the NTP/OHAT systematic review handbook, based on GRADE guidelines (NTP/OHAT 2015a).
Briefly, each body of evidence was given an initial confidence rating, which was subsequently downgraded or upgraded according to factors that decrease or increase confidence in the results (NTP/OHAT 2015a). The initial confidence rating of “high” was determined by the presence of all four main features determined by the study design for both in vivo and in vitro evidence (Table S5). This high initial confidence rating was then either downgraded or upgraded, depending on the presence or absence of certain cross-studies flaws (i.e., risk of bias, inconsistency, indirectness, imprecision, publication bias) or strengths (i.e., consistency, large magnitude of effect, dose response), respectively.
Factors decreasing confidence.
RoB: As previously mentioned, the NTP/OHAT’s RoB-tiered approach considers three key elements of higher relevance to establish the classification criteria for each individual study [marked in Table 2 by an asterisk (*)]. Studies were subsequently categorized into three possible tiers depending on their responses to these key elements: Tier 1: Study must be rated as “Definitely Low” or “Probably Low” RoB for key elements AND have most other applicable items answered “Definitely Low” or “Probably Low”; Tier 2: Study meets criteria for neither Tiers 1 nor 3; Tier 3: Study must be rated as “Definitely High” or “Probably High” RoB for key elements AND have most other applicable items answered “Definitely High” or “Probably High.” “Not Reported” was counted as “Probably High.” Downgrading for RoB reflects the entire body of studies; therefore, the decision to downgrade was applied conservatively and reserved for cases with substantial RoB across most of the studies composing the body of evidence.
Unexplained inconsistency: Studies were considered for downgrading when there was inconsistency in results that were not explained by study design features, such as differences in cell model/animal species, timing or route of exposure, or health outcome assessment.
- Indirectness/applicability: The following points were used to assess the directness in the present study:
- Differences in population (applicability) and relevance of the animal model to outcome of concern.
- In vivo studies: Studies conducted in mammalian model systems were assumed relevant for humans (i.e., not downgraded) unless compelling evidence to the contrary was identified during the course of the evaluation (e.g., a biological system not present in humans).
- In vitro studies: Cell models were evaluated on the basis of the biological relevance in humans (human primary cell cultures or human immortalized cell lines).
- Differences in outcome measures or directness of the end points to the primary health outcome(s). For example, onset of endometriosis would be a direct end point, whereas development of “endometriosis-like” phenotypes is less direct.
-
Dose levels and route of administration in in vivo studies: External dose comparisons were used to reach confidence rating conclusions, because internal dosimetry in animal models can vary based on route of administration, species, age, diet, and other cofactors. The most commonly used routes of administration (i.e., oral, dermal, inhalation, subcutaneous injections) were considered direct for the purposes of establishing confidence ratings.The applicability of specific health outcomes or biological processes in animal models is outlined in the PECO inclusion/exclusion criteria, with the most accepted relevant/interpretable outcomes considered “primary,” and less direct measures, biomarkers of effect, or upstream measures of health outcome considered “secondary.”
Imprecision: Imprecision is typically assessed with confidence intervals for meta-analyses, but because a meta-analysis was not performed due to the heterogeneity of outcome measurements, the overall effects of the studies were considered for imprecision. Studies with high variability of effect estimates were at risk of imprecision bias.
Publication bias: Studies were considered for downgrading for publication bias when the study was uniformly small, especially when sponsored by industries, nongovernment organizations, or authors with conflicts of interest. Abstracts or other types of gray literature that do not appear as full-length articles within a reasonable time frame (3–4 y) may be another indication of publication bias; thus such literature has been excluded.
Factors increasing confidence.
Magnitude of effect: A large magnitude of effect was considered to upgrade the confidence.
Dose–response: Confidence was upgraded for dose–response if there was sufficient evidence of monotonic dose–response/gradient.
- Consistency: Consistency across animal studies, dissimilar populations, and study types were potential reasons to upgrade the confidence:
- Across animal studies: consistent results reported in multiple experimental animal models or species. Finding the same direction of change in the same outcome in more than two species would constitute sufficient evidence that a causal relationship has been established, by standards set by the International Agency for Research on Cancer. Though the health effect studied here is not cancer, the same principle can apply.
- Across dissimilar populations: consistent results reported across populations that differ in factors such as time, location, and/or exposure.
- Across study types: consistent results reported from studies with different design features (e.g., between chronic and multigenerational animal studies).
Rating the Level of Evidence
Subsequently, the confidence rating was translated to the level of evidence considering the presence or absence of the health effect and the direction or nature of the effect (Table S6). Level of evidence was established for each of four primary outcomes separately.
Evidence Integration and Hazard Identification
Based on the NTP/OHAT Hazard Identification Scheme (Figure S6) reported by Rooney et al. (2014), the level-of-evidence conclusion for human data can be considered together with nonhuman animal data to reach a hazard identification conclusion. At the first stage of integration, the level of evidence (i.e., “high,” “moderate,” “low”) from human and nonhuman animal studies is considered in order to establish a preliminary classification (i.e., “known,” “suspected,” “presumed,” “not classifiable”). In a second step, the impact of in vitro data can be used to bolster or downgrade this preliminary classification. We thus considered the epidemiological evidence meta-analyzed and evaluated in our previously published systematic review following the same guiding framework (Cano-Sancho et al. 2019) with the results of this review to reach a hazard identification conclusion.
The epidemiological evidence was grouped by main OCC families with enough studies to conduct a pooled analysis, including PCDD, PCDFs, PCBs, or OCPs. The body of evidence for PCDD/PCDFs consisted of 10 epidemiological studies, mainly reporting associations between TCDD, total dioxin toxic equivalents (TEQ), or TEQ for the group PCDD/PCDFs. The pooled effect size resulted in an odds ratio (95% confidence interval) of 1.65 (1.14, 2.39) with substantial heterogeneity (). The sources of heterogeneity were identified by stratified analysis, including the biological matrices or analytical methods, preventing the downgrading due to unexplained inconsistency. The confidence was rated as moderate and translated into a “moderate” level of evidence of health effect.
Results
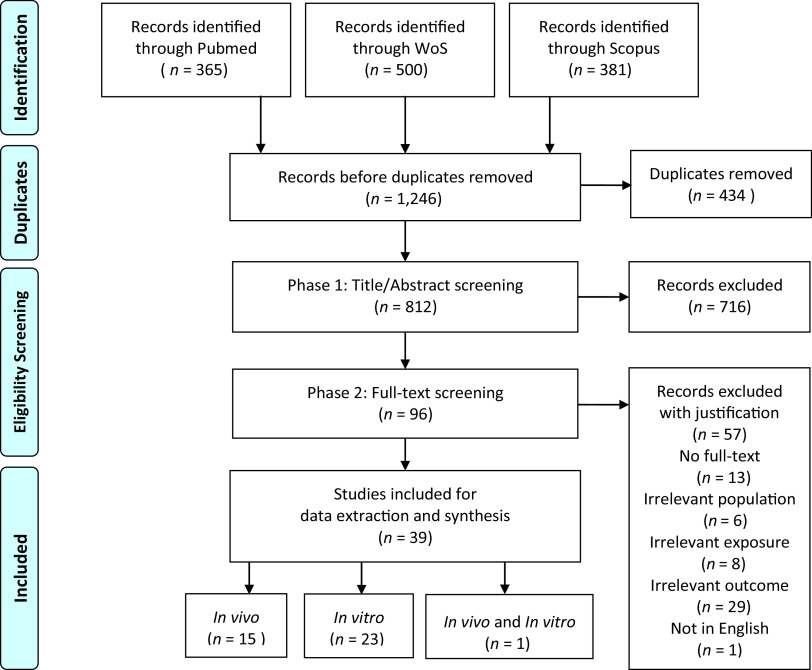
Through the search and selection process, we identified a total of 812 unique articles, of which 716 were excluded through Phase I screening based on title and abstract (Figure 1). The full texts of 96 articles were then examined during Phase II review. A total of 39 of these articles met the eligibility criteria (Table 1), 16 of which contained in vivo data and 24 in vitro data, among which one reported both data streams. The included articles are summarized in Tables 3 and 4.
Figure 1.
PRISMA flowchart displaying the results from the literature search and screening.
Table 3.
Summary table of selected in vivo studies.
| Reference | Species | Chemical | Study type | Size | Dosing duration/frequency | Route | Doses | Units | Reported end point | Consolidated end point |
|---|---|---|---|---|---|---|---|---|---|---|
| Arnold et al. 1996 | Rhesus monkey | Aroclor 1254 | Chr | 16 | 10 y | cap | 0/5/20/40/80 | g/kg body weight | Incidence | Onset |
| Rier et al. 1993 | Rhesus monkey | TCDD | Chr | 8 (24) | 4 y | diet | 0/5/25 | ppt | Incidence | Onset |
| Yang et al. 2000 | Cynomolgus monkey | TCDD | Chr | 5-6 | 1 y (1 capsule 5 d/wk) | cap | 0/1/5/25 | ng/kg/d | Diameter | Lesion growth |
| Bruner-Tran et al. 1999 | Nude mouse | TCDD | ST | 10 | Single dose | subq | 0/1 | nM | Amount | Onset |
| Nayyar et al. 2007 | C57BL/6 mouse | TCDD | Dev | 5-6 | 6 dose combinations Utero/prepubertal/pubertal |
gav | 0/10 | Like phenotypes | Onset | |
| Cummings et al. 1999 | B6C3F1 mouse | TCDD | Dev | 3 (12) | 2 doses (prenatal postnatal) | gav | 0-0/0-3/3-0/3-3/3-10 | ug/kg | Diameter | Lesion growth |
| SD rat | TCDD | Dev | 3 (12) | 2 doses (prenatal postnatal) | gav | 0-0/0-3/3-0/3-3/3-10 | mg/kg | Diameter | Lesion growth | |
| Cummings et al. 1996 | B6C3F1 mouse | TCDD | SChr | 8 (32) | 96 d (1 dose/3 wks) | gav | 0/3/10 | ug/kg | Diameter | Lesion growth |
| SD rat | TCDD | SChr | 8 (32) | 0, 3, 6, 9, 12 wk | gav | 0/3/10 | Diameter | Lesion growth | ||
| Foster et al. 1997 | B6C3F1 mouse | TCDD | SChr | 8, 5 | 30 d (daily dose) | subq | 0/100 | ng/kg/d | Diameter | Lesion growth |
| 4-CDE | SChr | 9, 6 | 30 d (daily dose) | subq | 0/150 | mg/kg/d | Diameter | Lesion growth | ||
| Huang et al. 2017 | B6C3F1 mouse | PCB 153 | ST | 12 | Single dose (10 or 20 d) | gav | 0/5/50 | mg/kg | Amount/Weight | Lesion growth |
| PCB 126 | ST | 12 | Single dose (10 or 20 d) | gav | 0/0.03/0.3 | mg/kg | Amount/Weight | Lesion growth | ||
| Johnson et al. 1997 | B6C3F1 mouse | TCDD | Chr | 10 | 15 wk (5 doses/3 wk) | gav | 0/1/3/10 | body weight | Diameter/Weight | Lesion growth |
| PCB 153 | Chr | 10 | 15 wk (5 doses/3 wk) | gav | 0/3/30 | mg/kg body weight | Diameter/Weight | Lesion growth | ||
| PCB 126 | Chr | 10 | 15 wk (5 doses/3 wk) | gav | 0/100/300/1000 | body weight | Diameter/Weight | Lesion growth | ||
| 1,3,6,8-TCDD | Chr | 10 | 15 wk (5 doses/3 wk) | gav | 0/2/20 | mg/kg body weight | Diameter/Weight | Lesion growth | ||
| 4-PeCDF | Chr | 10 | 15 wk (5 doses/3 wk) | gav | 0/10/30/100 | body weight | Diameter/Weight | Lesion growth | ||
| Khan et al. 2018 | C57BL/6 mouse | TCDD | SChr | 10 | 9 wk (3 doses/3 wk) | gav | 0/3 | body weight | Diameter | Lesion growth |
| Kitajima et al. 2004 | B6C3F1 mouse | TCDD | ST | 3 | Single dose (4 wk after induction) | subq | 0/10 | body weight | Diameter/gene expression | Lesion growth |
| Yang and Foster 1997 | B6C3F1 mouse | TCDD | ST | 5 | 28 d (daily dose) | subq | 0/10/50/100 | ng/kg/d | Diameter/Adhesion | Lesion growth |
| Yang et al. 1997 | B6C3F1 mouse | 4-CDE | ST | 5 (25) | 28 d (daily dose) | subq | 0/10/75/150 | mg/kg/d | Diameter | Lesion growth |
| Chiappini et al. 2019 | SD rat | HCB | SChr | 7 | 30 d (3 doses/wk) | gav | 0/1/10/100 | mg/kg body weight | Volume /Diameter | Lesion growth |
| Cummings and Metcalf 1995b | SD rat | MCX | ST | 10 | 21 d (daily dose) | gav | 0/250 | mg/kg/d | Diameter | Lesion growth |
Note: 4-CDE, 4-chlorodiphenyl ether; Chr, chronic; Dev, developmental; cap, oral capsule; gav, oral gavage; ST, short term; SD, Sprague Dawley; SChr, subchronic; subq, subcutaneous injection. Size listed is animals per dose group (total in parentheses when provided). Separate doses are separated by slashes (/), whereas multiple doses are separated by a dash (–).
Table 4.
Summary table of selected in vitro studies.
| Reference | Tissue/cell type | Chemical | Duration | Dose range | Unit | Assay | Reported end point | Consolidated end point | |
|---|---|---|---|---|---|---|---|---|---|
| Bofinger et al. 2001 | EExCU (endo and no endo) | 9-11 | TCDD | 24 h | 0.001–10 | nM | Northern blot (mRNA) EROD assay |
CYP1A1/B1 mRNA EROD activity |
Steroidogenesis(s) |
| Bredhult et al. 2007 | EEnC | 6 | op′-DDT | 24 h | 1–100 | ICC PCNA, BrdU Assay | Viability/proliferation | Viability/proliferation | |
| EEnC | 6 | TCDD | 24 h | 0.1–100 | nM | ICC PCNA, BrdU Assay | Viability/proliferation | Viability/proliferation | |
| EEnC | 6 | PCB 77, 126 | 24 h | 0.1–10 | ICC PCNA, BrdU Assay | Viability/proliferation | Viability/proliferation | ||
| Bredhult et al. 2008 | EEnC | 10 | op′-DDT | 24 h | 50 | BrdU Assay/qRT-PCR | Proliferation | Proliferation | |
| Chang et al. 2017 | ESC/monocyte* | 6 | TCDD | 48 h | 0.1–5 | nM | Flow cytometry | IL-10 | Inflammation(s) |
| Chiappini et al. 2016 | HUF, ESC (EU (endo and no endo)/EN), T-HESC | 3 | HCB | 24 h | 0.005–5 | MTT/WB | Viability MMP (, ) | Viability, inflammation%m steroidogenesis(s), migration/invasion | |
| Holloway et al. 2008 | ESC Granulosa Cells | 10/5 | Atrazine | 24 h | 0.001–100 | TWE | Aromatase activity | Steroidogenesis(s) | |
| Holloway et al. 2005 | ESC | 9 | p,p′-DDE | 24 h | 1–10,000 | ng/mL | TWE | Aromatase activity | Steroidogenesis(s) |
| Hu et al. 2018 | EU ESC (endo and no endo) | 9 | PCB 104 | 12 h–72 h | 2–10 | rtPCR/ELISA/CCK8/Crystal Violet | Gene expression/protein levels/proliferation/migration | Viability/proliferation, migration, inflammation(s) | |
| Huang et al. 2017 | ESCs (EU/EN) | 3 | PCB 126, 153 | 48 h | 0.3–60 | MTT/ELISA/WB | Cell viability IL-6, IL-8 s | Viability, inflammation | |
| Igarashi et al. 2005 | ESC-EEC* | 3 | TCDD (E) | 48–72 h | 0.1–20 | nM | RT-PCR/ WB | PR-B/PR-A ratio MMP (, ) | Steroidogenesis(s), inflammation |
| Kalinina et al. 2018 | Normal ESC | 5 | DDT | 24 h | 1–10 | RT-PCR | microRNA-190a/b | Migration/invasion | |
| Li et al. 2011 | 4 ESC (Normal, CD82-, EC, EU) | 6 | TCDD (E) | 48 h | 10 | nM | MTWA/ELISA | Invasion, Chemokine CCR2 | Invasion, inflammation(s) |
| Pitt et al. 2001 | EExCU | 11-13 | TCDD (EP) | 24–72 h | 0.001–10 | nM | qRT-PCR | AhR mRNA | Steroidogenesis(s) |
| Resuehr et al. 2012 | ESC | 5 | TCDD | 48 h | 10 | nM | qRT-PCR | CB1-R, PR-B | Inflammation(s)/steroidogenesis(s) |
| Shi et al. 2006 | ESC (EU/EN) ESC-PMC * | 3 | TCDD (E) | 48 h | 1 | nM | ELISA/IMS DNA | IL-8 /CXCR1 | Inflammation(s) |
| Shi et al. 2007 | ESC (EU/EN) | 3 | TCDD (E) | 48 h | 0.01–10 | nM | Flow cytometry | CCR8 | Inflammation(s) |
| Van den Brand et al. 2019 | ESC-EEC* | 3 | TCDD (EP) | 48 h | 1–500 | pM | TWE | AhR/CYP1A1 | Steroidogenesis(s) |
| Wang 2015 | ESC-U937 macrophage * | 3 | TCDD (E) | 48 h | 0.01–10 | nM | ELISA/flow cytometry | IL-10, IL-12/CD86 | Inflammation(s) |
| Wang et al. 2010b | ESC(EC/EU), ESC-U937* ESC-HMPC-U937 * | 3 | TCDD (E) | 48 h | 1 | nM | Western blot, Flow cytometry, ICC | TECK/CCR9 MMP (, ) | Migration/invasion, inflammation(s) |
| Wang et al. 2010a | ESC (EC/EU)-U937* ESC-HMPC-U937 * |
3 | TCDD (E) | 48 h | 1 | nM | Chemotaxis cell migration assay WB | Macrophage migration Chemokine CCR1 Expression |
Migration, inflammation(s) |
| Willing et al. 2011 | hTERT-EEC | 3 | TCDD (E) | 24 h | 1–10 | nM | LRGA/WB/proteomics Alamar Blue |
AhR /CYP1A1, migration Proliferation, ARNT |
Steroidogenesis(s), migration, proliferation |
| hTERT-EEC | 3 | PCB 126, 153 | 24 h | 100 | ng/mL | LRGA/WB/proteomics Alamar blue | AhR CYP1A1, migration Cell proliferation, ARNT |
Steroidogenesis(s), migration, proliferation | |
| Yang 1999 | ESC-SV40T | 3 | TCDD | 24 h | 0.1–100 | nM | qRT-PCR | AhR /IL-1B /PAI-2 mRNA | Steroidogenesis(s), inflammation(s) migration/invasion |
| Yu et al. 2008 | ESCs (EC/EU)-ESC/HMPC-U937* | 3 | TCDD (E) | 48 h | 1 | nM | Matrigel-based transwell ELISA, western blot | Cell invasion, secretion, RANTES, MMPs | Invasion, inflammation(s) |
| Zhao et al. 2002 | ESCs (normal/EN) | 3 | TCDD | 24 h | 10 | nM | PCR, ELISA, luciferase reporter gene | RANTES (mRNA, Protein secretion, gene expression) | Inflammation(s) |
Note: “Endo and no endo” is used to describe tissue/cell cultures drawn from women with and without endometriosis. Co-cultures are marked by an asterisk (*); refers to number of replicates or explants per dose group or cell type. Cultures concomitantly treated with estrogen and/or progesterone as well as TCDD are marked by E and/or P. Dose ranges exclude control group (0). Secondary outcomes are identified with “(s)” in table. DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; E, estrogen; EC, ectopic; EEnCs, human endometrial endothelial cells; ELISA, enzyme-linked immunosorbent assay; EExCU), endometrial explant culture; EN, endometriotic; EROD, 7-ethoxyresorufin O-deethylase; EECs, endometrial epithelial cell; ESCs, endometrial stromal cells; ESC-SV40T, ESCs immortalized with temperature-sensitive SV40 T antigen; EU, eutopic; hTERT-EEC, human telomerase immortalized reverse transcriptase endometrial epithelial cell; HUF, human uterine fibroblast; ICC, immunocytochemistry; IMS, immunostaining; LRGA, luciferase reporter gene assay; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; MTWA, Matrigel-based transwell western assay; P, progesterone; PCB, polychlorinatedbiphenyl; PCR, polymerase chain reaction; PMC, peritoneal mesothelial cell; qRT-PCR, quantitative real-time polymerase chain reaction; TDCC, 2,3,7,8-Tetrachlorodibenzo-p-dioxin; T-HESC, telomerase-immortalized human endometrial stromal cells; TWE, tritiated water-release; WB, Western-blot.
Study Characteristics
Main characteristics from in vivo studies are summarized in Table 3. Four animal models were identified, including nonhuman primates (), nude mice (), autologous mice (), and autologous rats (). Two studies tested both rat and mouse models simultaneously (Cummings et al. 1996, 1999). For primates and mouse models where spontaneous endometriosis or endometriosis-like phenotype could occur, the consolidated “endometriosis onset” end point was generated, grouping both incidence of phenotypes and attachment of injected endometrial tissue. For models in which endometriosis was surgically induced by sewing autologous endometrium to peritoneal tissues or vessels, lesion growth was the primary end point. Presence of endometriosis and size of endometriotic lesions were confirmed histologically, through laparoscopy or necropsy for all studies. A total of 14 OCCs were assessed across all studies (Table S7), but the majority of studies () assessed TCDD, the prototypical ligand of the AhR; other chemicals assessed by multiple studies were PCB 126 (), PCB 153 (), and DDT isomers (). PCBs 77 and 104; 1,3,6,8-TCDD; 4-PeCDF; and pesticides hexachlorobenzene (HCB), atrazine (ATR), and methoxychlor (MXC) were only assessed by a single study. Studies were either chronic (, ), subchronic (30–90 d, ), short term (, ), or developmental () (Table 3). We did include one review as an exception, because it presented primary data generated by the authors (Bruner-Tran et al. 1999).
Main characteristics from in vitro studies are summarized in Table 4. A variety of cell types have been assessed (Table S8), often with multiple cell types in a single study. Cell models included endometrial stromal cells (ESCs) (), ESC co-cultures (), endometrial endothelial cells (EEnCs) (), and endometrial explants (), as well as uterine fibroblasts (HUF), granulosa cells, and healthy noncancer endometrial cells. Three immortalized cell lines were included: ESCs immortalized with temperature-sensitive SV40 T antigen (ESC-SV40T), human telomerase reverse transcriptase (hTERT) EECs, and T-HESC, a uterine fibroblast immortalized with hTERT. All other cell models were primary cultures from human biopsies. Primary in vitro end points included cell migration/invasion as well as cell viability/proliferation.
Associations between Exposure to OCCs and Primary and Secondary Outcomes
OCCs and endometriosis onset.
Four in vivo studies measured the development of spontaneous endometriosis or endometriosis-like phenotypes, including two primate studies (Arnold et al. 1996; Rier et al. 1993) and two rodent studies (Bruner-Tran et al. 1999; Nayyar et al. 2007). One study with rhesus monkeys assessed exposure to Aroclor 1254 and the other studies to TCDD, with different animal models, exposure regimens, and outcome definition. The study on Aroclor 1254 did not find a significant relationship between incidence of endometriosis and dose treatment, regardless of concentrations (up to body weight per day) or follow-up duration. The high incidence in controls (37%) precluded the role of PCB exposure in increasing either incidence of endometriosis or the severity of the lesions [25% (16/64)] of treated monkeys (Arnold et al. 1996). The three studies on TCDD exhibited positive associations at different doses and using different models. Rier et al. (1993) noted a statistically significant dose-dependent increase in incidence and severity of endometriosis in a chronic study (4 y). Within the control group, 2 out of 6 (33%) monkeys developed endometriosis, whereas at the 5 and 25 ppt dose groups, incidence was 5 out of 7 (71%) and 6 out of 7 (86%), respectively (Rier et al. 1993). In a mouse model, the incidence of endometriotic-like lesions was measured 10 d after the mice were injected intraperitoneally with proliferative-phase endometrial tissues treated for 24 h with TCDD () combined with progesterone (P) or estradiol (E2) (Bruner-Tran et al. 1999). The combined treatment () led to 42 total lesions, whereas mice with the E2 treatment alone () or combined with P developed 20 total lesions or 0, respectively. In a developmental study, Nayyar et al. (2007) examined the presence of uterine phenotypes in mice, noting similarities in the phenotypes exhibited in the TCDD-treated animals to those found in humans with endometriosis. C57BL/6 mice were exposed to TCDD/kg body weight at different combinations of critical developmental life stages (six treatment groups, singly or in combination of: in utero/lactation, and/or prepubertal, and/or pubertal). Authors reported the similarities through immunohistochemical imaging but did not provide statistical analyses.
OCCs and endometriotic lesion growth.
A total of 12 in vivo studies measured the change in endometriotic lesion size in diameter, volume, and/or weight following the exposure to TCDD (Figure 2A) and other OCCs (Figure 2B). Most studies were performed on rodent models ( mice, rats), and one on cynomolgus monkeys. TCDD was analyzed in 8 out of 12 studies, with variable results, appearing mostly positively associated at the highest doses and null or negatively associated with lesion diameter and/or weight at the lowest doses (Figure 2A). The three studies which report negative associations had provided animals with a high dose pretreatment of E2 () or estrone (E1) () (Foster et al. 1997; Kitajima et al. 2004; Yang and Foster 1997). E2 did significantly increase epithelial height, stromal thickness, and proliferative activity of the endometriotic lesions, whereas coadministered TCDD reduced these effects (Kitajima et al. 2004), supporting the antiestrogenic effects of TCDD. Other dioxin-like chemicals were also tested, including 4-PeCDF, which significantly increased lesion weight at the highest dose ( body weight) (Johnson et al. 1997) and PCB 126 (dioxin toxic equivalency factor of 0.1), which led to increased lesion diameter and weight in two studies (Huang et al. 2017; Johnson et al. 1997). Two noncoplanar chemicals were also analyzed (1,3,6,8-TCDD and PCB 153), neither of which significantly affected lesion size (Johnson et al. 1997). Proestrogenic 4-chlorodiphenyl ether (4-CDE) was found to increase lesion diameter at all tested doses but only significantly at the highest dose (); this increase was not as much in comparison with that of the E2-treated positive control (Foster et al. 1997; Yang et al. 1997). MXC (, for 21 d by oral gavage) significantly increased lesion diameter in comparison with the vehicle control (Cummings and Metcalf 1995b). Lesion diameter increased dose dependently in rats treated with HCB through oral gavage (1, 10, and ), 3 times a week for 30 d (Chiappini et al. 2019). Overall, results support the hypothesis that exposure to TCDD and dioxin-like OCCs contribute to an increase in the growth of endometriotic lesions at the highest concentrations in comparison with negative or vehicle-treated controls, whereas exposure to noncoplanar PCBs and other OCCs did not have such a consistent effect, suggesting the involvement of the AhR signaling pathway.
Figure 2.
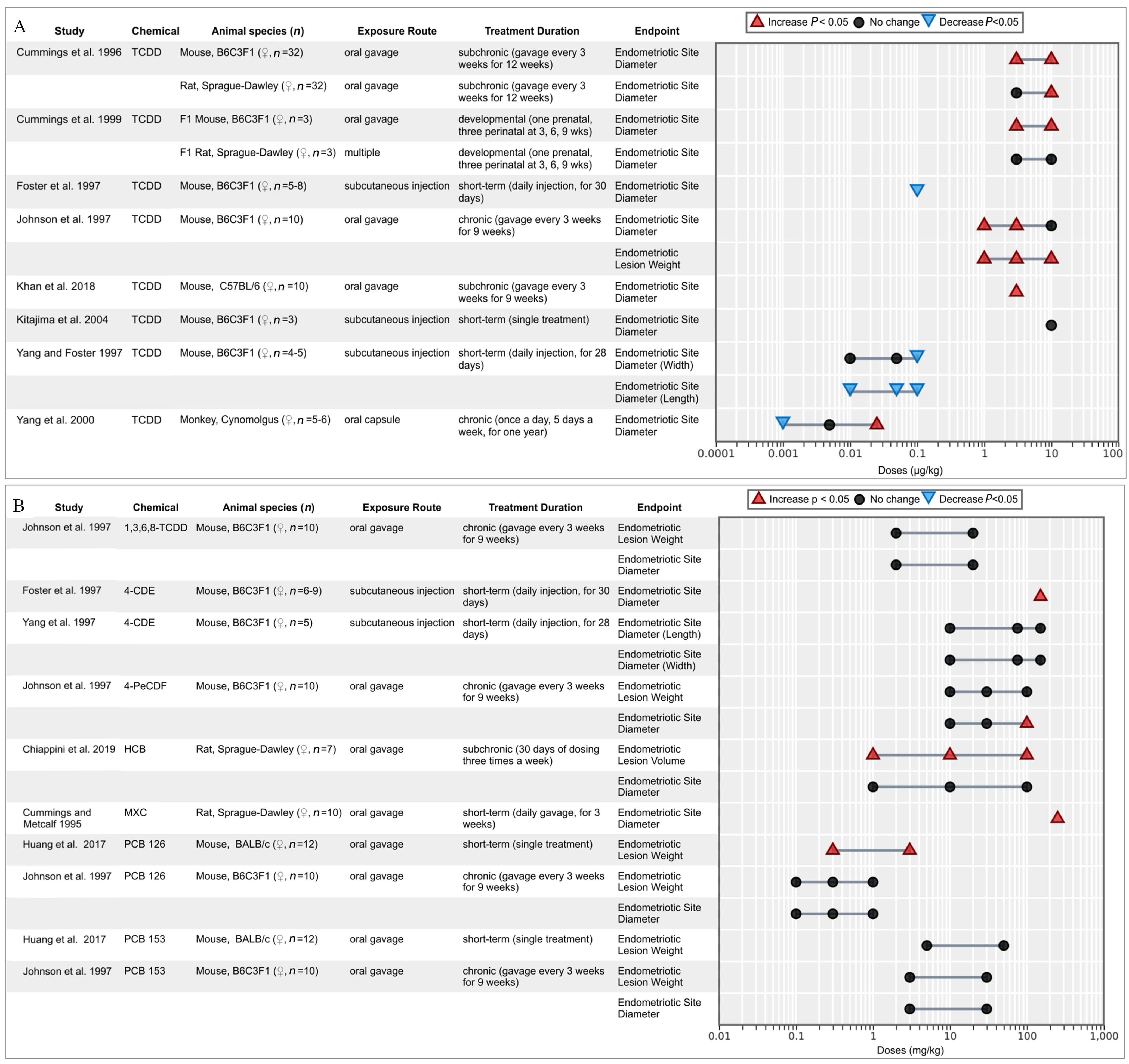
Associations between endometriotic lesion growth (measured in diameter, volume, and weight) reported from in vivo studies for TCDD (2A) and other organochlorine chemicals (2B). Lesion growth was measured in diameter, volume, and weight.
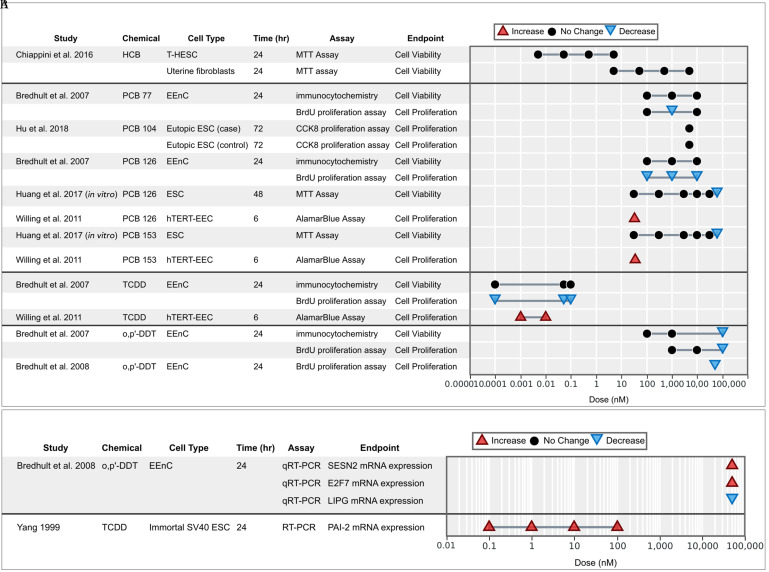
OCCs and endometrial cell viability/proliferation.
Cell viability was measured for six different chemical assays from three in vitro studies, and proliferation was measured for six chemicals in eight studies using five cellular models (EEnC, ESC, hTERT-EEC, HUF, and T-HESC). The overall evidence on viability and proliferation was inconclusive (Figure 3A,B). One study reported a significant increase of proliferation of endometrial cells dosed with PCB 126, PCB 153, and TCDD (Willing et al. 2011), whereas the rest reported mostly null results or significant decreases of viability or proliferation, especially at high doses. This discrepancy may likely be due to a trait in the immortalized hTERT-EEC cell line, which is often used for cancer studies; authors observed that TCDD has no antiestrogenic effects in hTERT-EEC, becayse it failed to inhibit estrogen-induced ER-alpha down-regulation in the presence of estrogen (Willing et al. 2011).
Figure 3.
Associations between exposure to organochlorine chemicals and (A) cell viability/proliferation and (B) molecular markers supporting viability/proliferation from in vitro studies. Endometrial stromal cells (ESCs) marked with case or control indicate that the cells were derived from women with endometriosis (case) or without (control).
OCCs and in vitro cell migration/invasion.
Six studies measured changes in cell migration and invasion for endometrial cells and co-cultures dosed with TCDD or PCBs (Figure 4A,B). Molecular markers supporting cell migration/invasion are reported in Figure 4B. Results across models and doses consistently showed increase in cell motility. In two studies, TCDD alone was not found to increase invasiveness, but the combination of TCDD with E2 did lead to a significant increase (Wang et al. 2010a; Yu et al. 2008). Three studies observed that the combination of E2 with TCDD had a synergistic effect on increased cell invasion (Li et al. 2011; Wang et al. 2010b; Yu et al. 2008). Additionally, ESCs in a co-culture with HPMC-U937 cells tended to have higher motility than ESCs cultured alone (Wang et al. 2010a; Yu et al. 2008). Overall, OCC exposures (especially in the presence of estrogens) was found to contribute to increased migration and invasion of endometrial cells.
Figure 4.
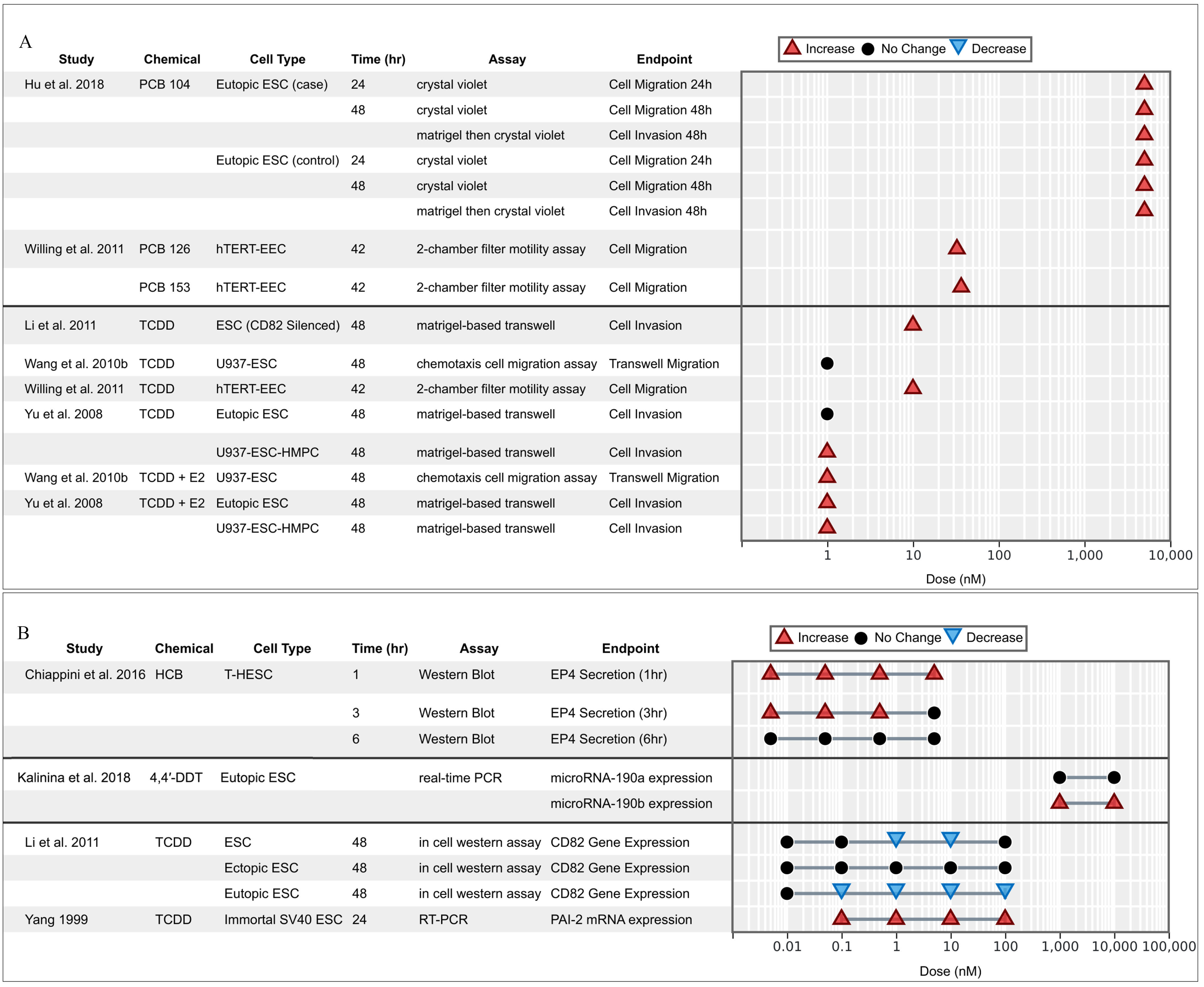
Associations between exposure to organochlorine chemicals and (A) migration/invasion and (B) molecular markers supporting migration/invasion reported from in vitro studies. Endometrial stromal cells (ESCs) marked with case or control indicate that the cells were derived from women with endometriosis (case) or without (control).
OCCs and extracellular matrix remodeling.
MMPs are a group of enzymes involved in the restructuring of endometrial tissue during the proliferative phase and are responsible for regulation of cell cycle and endometrial tissue remodeling (Nagase et al. 2006). Elevated MMP activities have been associated with extracellular matrix degradation and angiogenesis (Nanda et al. 2020) and play a critical role in the proliferation and invasion of endometriotic cells (Samartzis et al. 2019; Weigel et al. 2012). Six studies reported associations between OCC exposure and MMP activity and expression (Figure 5), exhibiting consistent positive associations for TCDD, PCBs, and HCB with varying degrees of significance. The exposure of eutopic ESCs to PCBs 104, 126, and 153 showed significant increases in MMP-2, MMP-3, MMP-9, and MMP-10 activity and expression. HCB increased MMP-2 and MMP-9 activities in HUF, T-HESC, and ESCs. MMP-9 levels were consistently elevated in all models, whereas MMP-2 was significantly elevated only in ESCs (Chiappini et al. 2016). One study reported a synergistic interaction when ESCs were dosed with a combination of TCDD and E2, significantly increasing MMP-2 and MMP-9 activities, whereas TCDD alone had no significant effect (Yu et al. 2008).
Figure 5.
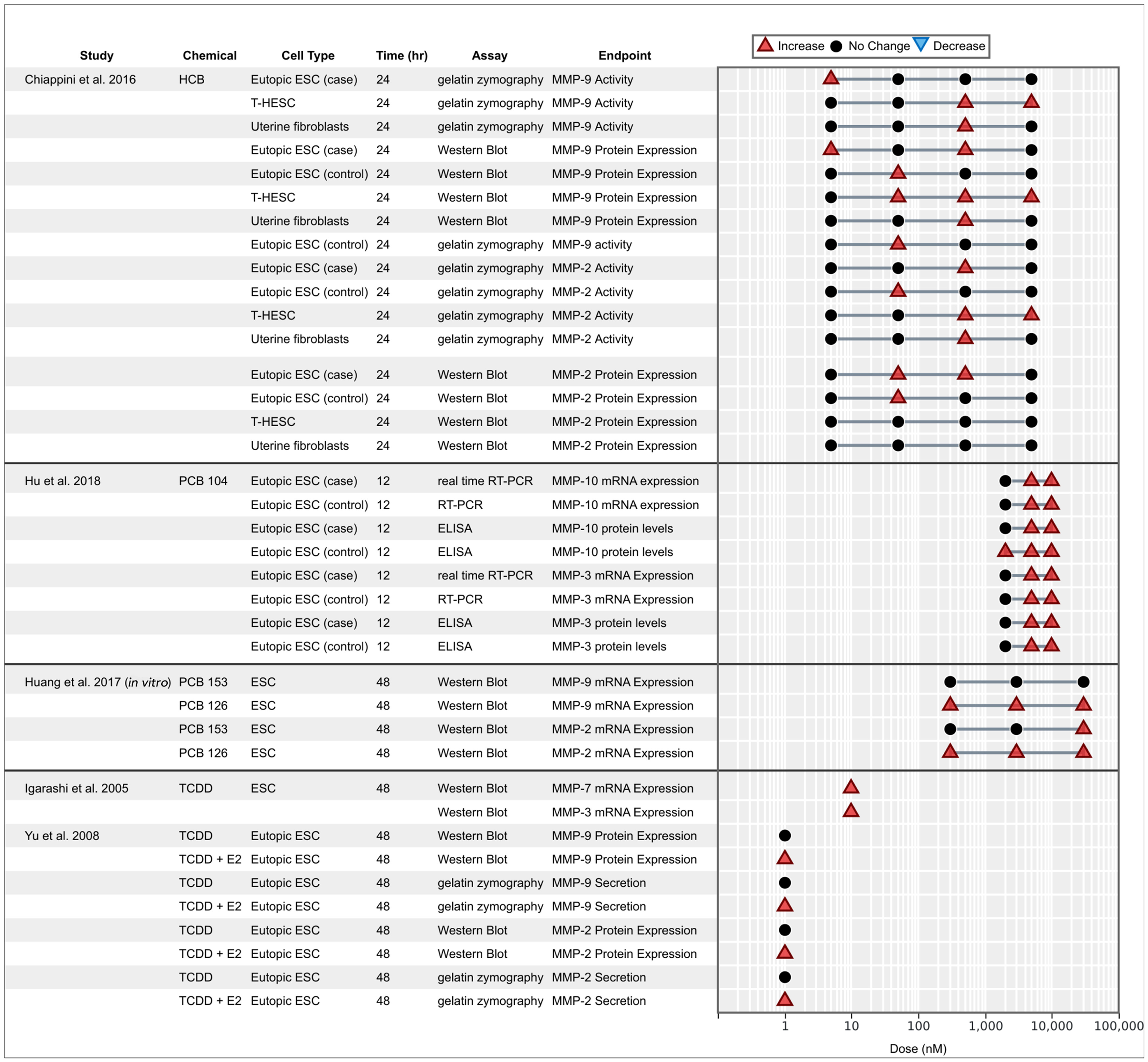
Associations between exposure to organochlorine chemicals and expression and secretion of matrix metalloproteinases reported from in vitro studies. Endometrial stromal cells (ESCs) marked with case or control indicate that the cells were derived from women with endometriosis (case) or without (control).
OCCs and markers of inflammation.
Markers of inflammation and immune dysfunction, including cytokine activity, were extensively evaluated in in vitro studies, representing the most diverse and substantiated body of indirect evidence of associations with endometriosis (Figure 6). The evaluation included studies mostly focused on the effects of TCDD (alone or combined with E2); few studies tested the effects of other OCCs on inflammatory cytokines, including o,p′-DDT, HCB, PCB 126, and PCB 153 (Bredhult et al. 2008; Chiappini et al. 2016; Huang et al. 2017). The results globally support a perturbation of immune function and inflammation of endometrial cells and co-cultures with macrophages, for the different OCCs.
Figure 6.
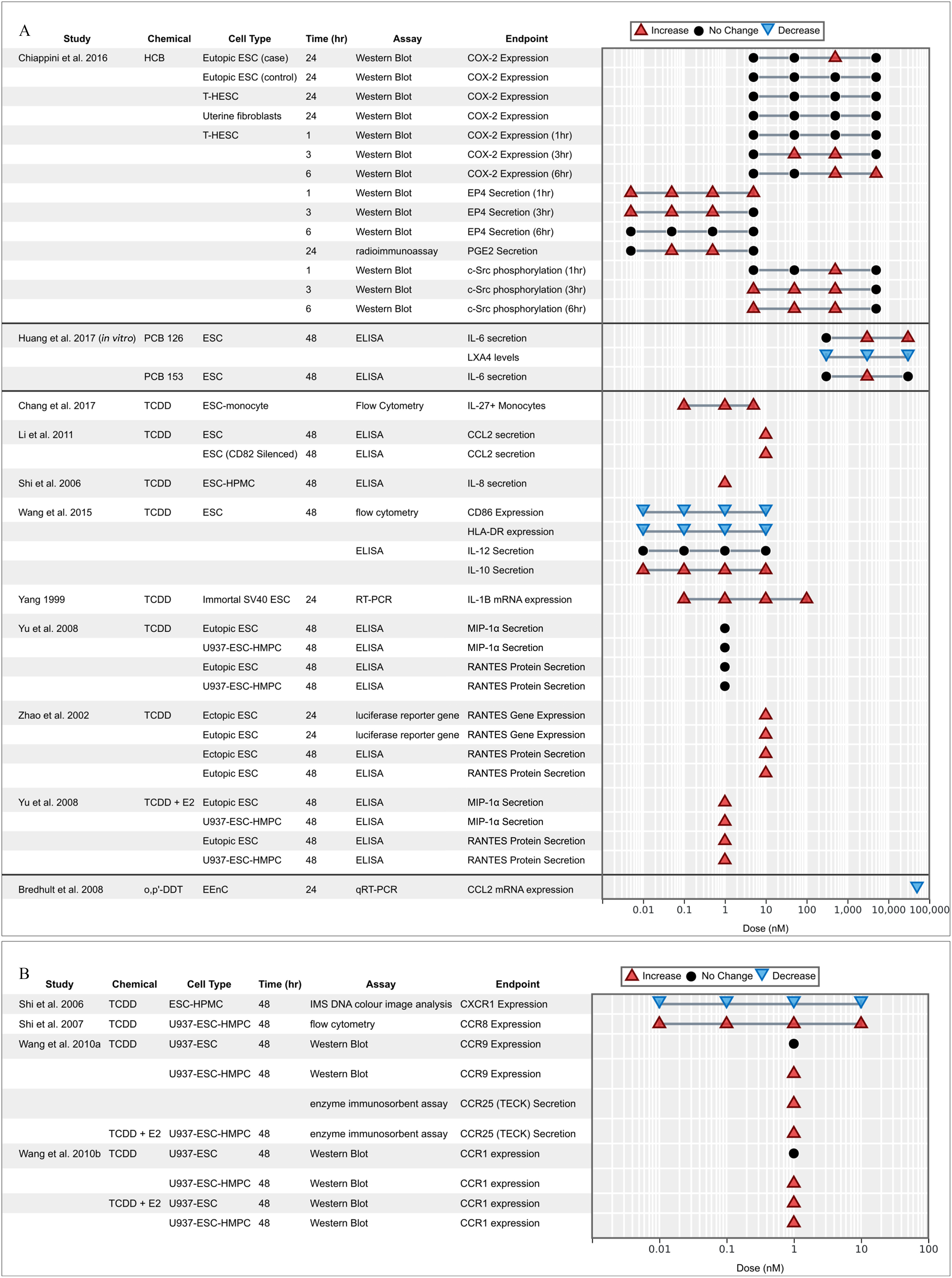
Associations between exposure to organochlorine chemicals and (A) chemokine expression and (B) their receptors reported from in vitro studies. Endometrial stromal cells (ESCs) marked with case or control indicate that the cells were derived from women with endometriosis (case) or without (control).
Prostaglandin E2 (PGE2) is an important mediator of inflammatory responses and is derived from arachidonic acid metabolized by cyclooxygenase-2 (COX-2) enzymes (Herr 2012; Park et al. 2006). In T-HESCs, HCB stimulated the c-Src kinase-induced COX-2 expression and activity, subsequently increasing the production and secretion of PGE2 and the expression of its mediator G-protein coupled receptor EP4 (Chiappini et al. 2016). Elevated COX-2 and PGE2 expression promoted the proliferation and invasion of ESCs (Takenaka et al. 2010). PGE2 was found to bind to EP4 and trigger c-Src kinase phosphorylation, MMP activation, and the migration and invasion of human immortalized endometriotic epithelial and stromal cells (Lee et al. 2011). Huang et al. (2017) examined the effects of nondioxin-like PCB 153 and dioxin-like PCB 126 on ESCs and found that exposure to PCB 126 decreased anti-inflammatory lipid mediator lipoxin A4 (LXA4). Secretion of IL-6, a proinflammatory interleukin, was also found to increase in ESCs in response to both PCBs 126 and 153 (Huang et al. 2017).
Protein secretion of proinflammatory chemokine RANTES (CCL5) was found to significantly increase in ESCs and U937-ESC-HMPC co-cultures in response to the combination of TCDD and E2, but not TCDD alone (Yu et al. 2008). Both RANTES gene expression and protein secretion increased in both ectopic and eutopic ESCs exposed to TCDD (Zhao et al. 2002). CCR8 expression increased in a bell shaped distribution with increasing dose of TCDD, especially in combination with E2, for eutopic and ectopic ESCs, and especially when co-cultured with U937 and HMPC cells (Shi et al. 2007). CCR9 expression increased in response to TCDD in U937-ESC-HMPC co-culture but not U937-ESC co-culture without HMPC, suggesting cell cross talk plays a role (Wang et al. 2010b). The CCL2 chemokine secretion and CCR2 protein levels (receptor expression) was significantly elevated in normal healthy ESCs following TCDD exposure—an increase that was even stronger in CD82 silenced cells (Li et al. 2011). In turn, CCR1 protein translation significantly increased in ESC co-cultures exposed to TCDD and E2 combined, as well as U937-ESC-HMPC triple co-culture treated with TCDD alone, but not U937-ESC double co-culture treated with TCDD alone, again supporting cross talk (Wang et al. 2010b). Secretion of IL-8 and expression of its receptor CXCR1 both increased in ESCs exposed to TCDD (Shi et al. 2006). Combined TCDD and E2 treatment inhibited CD86 expression in a dose-dependent manner in ESCs co-cultured with a macrophage cell line, which contributed to an increase in anti-inflammatory cytokine IL-10 (and not IL-12) production, providing evidence for the role of M2 macrophage activation in promoting endometriosis (Wang et al. 2015). IL-27, a member of IL-12 family, was significantly up-regulated in the ESC-monocyte co-culture after exposure to TCDD, further supporting cross talk between macrophages and ESCs (Chang et al. 2017). It is suggested that secretion of IL-27 by macrophages and ESCs induces IL-10 production in Th17 cells; this suggestion is also supported by in vivo evidence from the same study in which the IL treatments ( of IL-27, IL-10, and IL-17A) were tested on a nude C57BL/6 endometriosis mouse model, which increased the number and weight of endometriotic lesions (Chang et al. 2017). It is also suggested that the balance between M1 and M2 macrophages may contribute to angiogenesis and implantation of endometrial cells in pelvic endometriosis (Wang et al. 2013). The secretion of macrophage inflammatory protein was found to increase significantly after 48 h of exposure to TCDD (Yu et al. 2008). One study hypothesized that increased invasiveness could be due to increased secretion of the chemokine TECK (CCL25), which plays a key role in compartmentalization of the mucosal immune system through recruitment of localized immune cells (Wang et al. 2010b). In this regard, TECK was up-regulated in a ESC–HPMC–U937 co-culture exposed to 48 h of E2 () with TCDD (). This increase was observed in only the three-cell co-culture and not in ESCs tested alone, nor in either of the other cell types tested alone, suggesting cellular cross talks. Both the introduction of ESCs and the combination of E2 and TCDD increased TECK secretion in the endometriosis-associated cells and promoted the invasiveness of ESCs by increasing expression of MMP-2 and MMP-9 (Wang et al. 2010b).
OCCs and P resistance.
The disruption of P response is understood to be strongly linked to endometrial diseases (Fu et al. 2017) because the P receptor (PR) serves a protective role in modulating the impacts of estrogens and xenoestrogenic compounds (Yilmaz and Bulun 2019). Especially important is the relative expression of its isoforms PRA and PRB; PRB is often found to be suppressed or completely absent in tissues affected by endometrial diseases (Arnett-Mansfield et al. 2001; Brayman et al. 2006; Shao 2013). Overall, results consistently showed the suppression of the PRB in response to OCCs (Figure 7A). The PRB/PRA ratio was found to be significantly decreased in ESCs exposed to TCDD in all tested doses (Igarashi et al. 2005). TCDD also significantly inhibited overall PRB expression (Resuehr et al. 2012).
Figure 7.
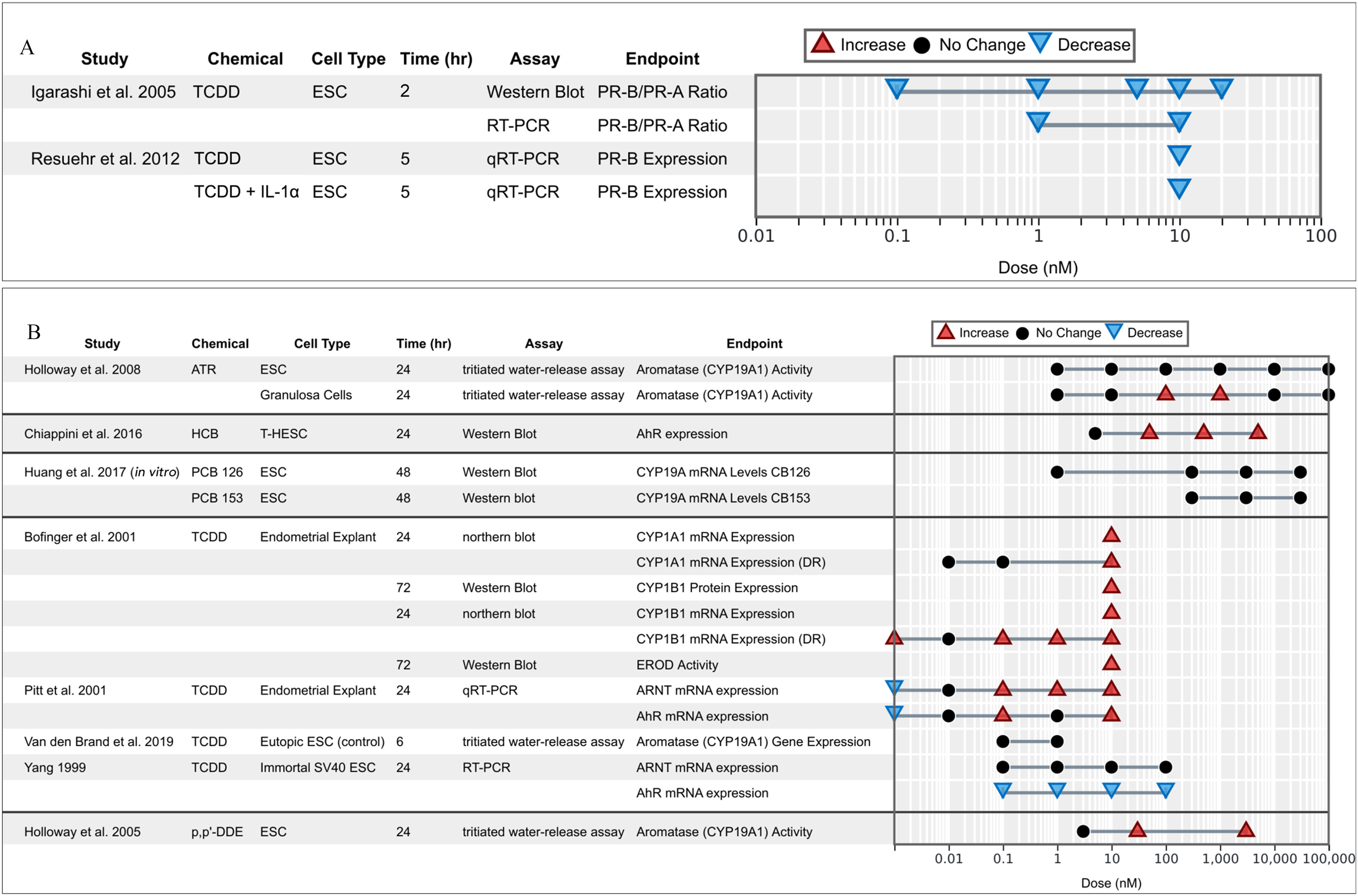
Associations between exposure to organochlorine chemicals and (A) the progesterone receptor and (B) AhR/ARNT and aromatase (CYO19A1) related activities and expression reported from in vitro studies. Endometrial stromal cells (ESCs) marked with case or control indicate that the cells were derived from women with endometriosis (case) or without (control).
Endometrial CB1-R immunoreactivity is dysregulated in women with endometriosis (Resuehr et al. 2012). P was found to induce endometrial cannabinoid receptor type 1 (CB1-R) mRNA expression in control ESCs (treated with E2; P) (Resuehr et al. 2012). Steroid-induced expression of this gene was inhibited by cotreatment with TCDD with or without . TCDD exposure significantly blocked P induction of CB1-R mRNA (cannabinoid type 1 receptor) expression in control ESCs; the addition of to TCDD had a synergistic effect in blocking this induction () (Resuehr et al. 2012).
OCCs and aryl hydrocarbon receptor/aromatase/steroidogenesis axis.
The AhR is a transcription factor that regulates gene expression and is involved in metabolism of xenobiotics. As an orphan nuclear receptor, its endogenous ligands are not known, and though some candidates have been suggested, the AhR remains most known perhaps for its role in binding with TCDD and other dioxin-like compounds. On activation, the AhR translocates into the cell nucleus and forms a dimer with the AhR nuclear translocator (ARNT), which leads to changes in gene transcription (Denison and Nagy 2003). We identified seven studies that measured AhR and AhR-related activities that may be relevant to endometriosis (Figure 7B). In endometrial explants, TCDD was found to increase both AhR and ARNT mRNA expressions (Pitt et al. 2001), but the relationship between TCDD and ARNT expression was mostly linked to covariates such as donor age and phase of the uterine cycle (Pitt et al. 2001). Yang (1999) reported similar results in ESC-SV40Ts: TCDD contributed to a significant increase in AhR mRNA expression with no impact on ARNT expression (Yang 1999).
Aromatase (CYP19A1), also known as estrogen synthase, is an enzyme responsible for a key final step in the biosynthesis of estrogens and is often regulated by the AhR (Lephart 1996). Aromatase is not normally expressed in healthy ESCs; elevated expression is thus associated with endometriotic diseases (Noble et al. 1996). This association is interesting in regard to the concomitant effect of TCDD and E2 on the pathogenesis of endometriosis (see above). Aromatase activity was found to increase significantly in granulosa cells at 1 and (), but remained unchanged in ESCs after atrazine exposure (Holloway et al. 2008). DDE increased aromatase activity in ESCs () at both 50 and (Holloway et al. 2005). Huang et al. (2017) did not observe a change in CYP19A1 mRNA levels in ESCs exposed to either PCB 126 or PCB 153, but found up-regulation of dehydrogenase 7 (HSD17B7) after exposing the cells to the dioxin-like PCB126. These effects were apparently mediated by the AhR because the specific inhibitor 3′,4′-dimethoxyflavone (DMF) counterbalanced the effects. CYP1A1 and CYP1B1 are involved in endocrine modulation through the metabolic activation of polycyclic aromatic hydrocarbons and by metabolizing E2 to the 2- and 4-hydroxylated derivatives, respectively (Coumoul et al. 2001; Hayes et al. 1996; Safe 1995). TCDD exposure was found to significantly increase CYP1A1 expression in endometrial explants (Bofinger et al. 2001) and hTERT-EECs (Willing et al. 2011). EROD, a biomarker of CYP1A1 induction (Whyte et al. 2000), was found to increase significantly () in explants after exposure to TCDD () and subsequent exposure for 48 h to hormones ( E2, P) (Bofinger et al. 2001). CYP1B1 was also found to increase in response to TCDD (Bofinger et al. 2001; Willing et al. 2011).
RoB Assessment of Individual Studies
The internal validity was assessed using the RoB tool developed by NTP/OHAT (NTP/OHAT 2015b) for each study that reported a primary outcome (16 in vivo studies and 9 in vitro studies) (Figure 8; percentage breakdown in Figure S7). Further details can be found online in HAWC for in vivo studies (https://hawcproject.org/study/assessment/812/rob-invivo/) and in vitro studies (https://hawcproject.org/study/assessment/812/rob-vitro/).
Figure 8.
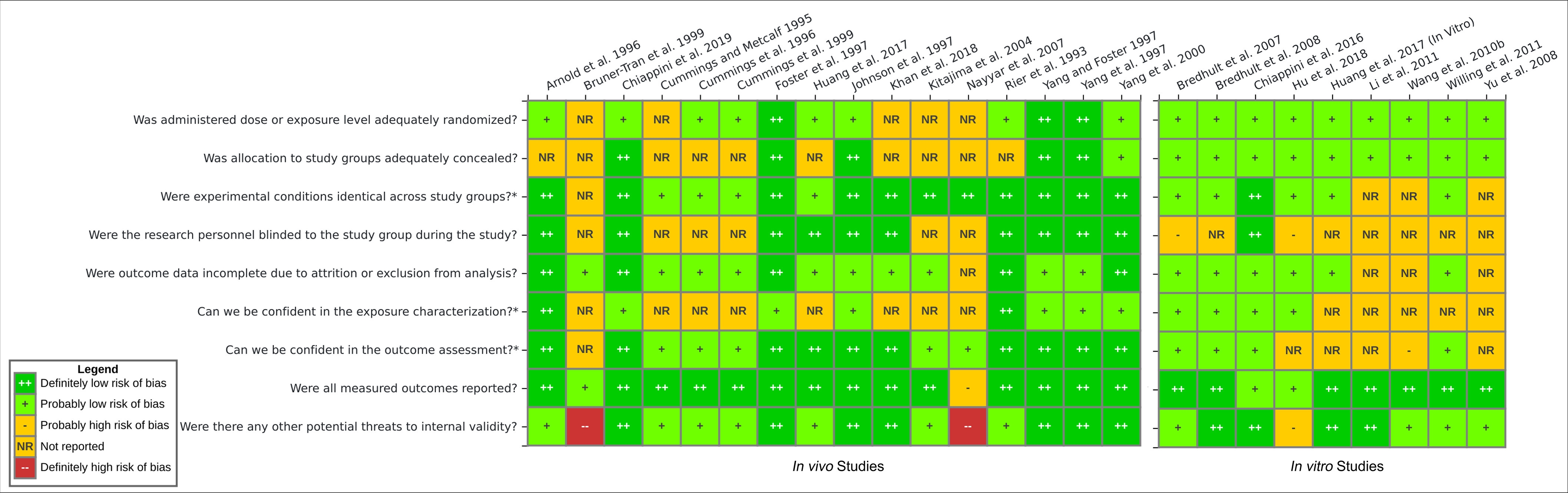
Risk of bias heatmap for the in vivo and in vitro studies that reported primary end points. Ratings were determined by two independent evaluators following the criteria adapted from OHAT RoB tool (NTP/OHAT 2015b). Additional details and justifications for each risk of bias rating are available online in HAWC interactive figures for in vivo studies (https://hawcproject.org/study/assessment/812/rob-invivo/) and in vitro studies (https://hawcproject.org/study/assessment/812/rob-vitro/). Note: HAWC, Health Assessment Workspace Collaborative; OHAT RoB, Office of Health Assessment and Translation Risk of Bias.
Most studies (80%, ) reported randomization in exposure allocation, whereas the remainder did not report the methods used for study group allocation. Although no studies reported methods of randomization or blinding of allocation, author correspondence confirmed allocation concealment for 15 studies (60%). A total of 24 studies (84%) explicitly reported identical experimental conditions and treatment vehicles across study groups, and the same proportion reported probably low or definitely low risk of attrition bias. Two studies explicitly documented the animals that died during the experiment and accounted for the deaths (Rier et al. 1993; Arnold et al. 1996). Research personnel were confirmed to be blinded to the study groups in fewer than half of the studies (44%, ). Twelve studies (48%) provided the source and purity of the chemical exposures, although only the studies on rhesus monkeys performed an independent test for impurities and contaminants studies (Arnold et al. 1996; Rier et al. 1993). Nearly all (94%, ) in vivo studies reported reliable outcome assessment. The gold standard for assessment of the presence of endometriosis or the growth of lesions is through laparoscopy and necropsy with measurement of lesions with calipers (Story and Kennedy 2004). Further confirmation performed through a histological examination made the difference between probably low and definitely low RoB. Almost all studies reported the measured outcomes expressed in the “Introduction” and “Methods” sections, with the exception of one review article that did not have a “Methods” section (Bruner-Tran et al. 1999) and another study that partially reported results (Nayyar et al. 2007). For other potential threats to internal validity, we considered whether or not the statistical analyses performed were appropriate, including a test for homogeneity of variances, the assumption necessary to reliably perform parametric tests.
Confidence Rating for the Body of Evidence
Additionally, a confidence rating was determined for each primary outcome and its association with TCDD exposure (Figures S8–S11) because TCDD was the most reported exposure in both in vivo and in vitro studies. Three key RoB criteria were used to categorize each primary outcome into three confidence tiers, which served as the basis of the level of evidence assessment (below). Among the in vivo studies reporting associations between TCDD and onset, one was classified in Tier 1 (Rier et al. 1993) (responses mostly “definitely low” and “probably low” for RoB) and two studies in Tier 3 (Bruner-Tran et al. 1999; Nayyar et al. 2007) (responses for RoB mostly “not reported” or “probably high” with some “definitely high”) (Figure S8). Because 2 out of 3 of the studies ranked in Tier 3, suggesting “serious risk” of bias for this end point, confidence rating was downgraded for onset. The body of evidence on associations between TCDD and in vivo lesion growth were rated Tier 1 and Tier 2. The RoB for this end point was thus deemed “not serious,” and the confidence rating was not downgraded (Figure S9). For in vitro migration/invasion, TCDD studies were rated either Tier 2 or Tier 3, mainly penalized by the underreporting of chemical standard details, blinding, or incomplete data, suggesting “serious risk” of bias (Figure S10). Confidence rating was thus downgraded for migration/invasion. Last, for in vitro viability/proliferation, TCDD studies were rated Tier 1 and Tier 2 (Figure S11). The RoB was deemed “not serious,” and thus confidence rating was not downgraded for viability/proliferation.
In addition to the RoB analysis, we considered several other categories to downgrade or upgrade confidence for each of the four primary outcomes following TCDD exposure. The level of evidence assessment is summarized in Table 5. To assess indirectness, we considered first relevance of the models to human health. Among animal studies, nonhuman primate models are considered the most relevant due to their ability to menstruate and spontaneously develop endometriosis. Rodent models were thus less direct, because endometriosis does not spontaneously occur in rodents. Nevertheless, the development and contribution of rodent models toward the understanding of endometrial phenotypes, the peritoneal microenvironment, and the immune system has been extensively documented (Bruner-Tran et al. 2002, 2018; Vernon and Wilson 1985). For in vitro models, only noncancer human endometrial cell models or explants were included in this review, and thus all were considered relevant for humans. Additionally, tested doses in animal and in vitro studies were plotted to compare their relevance to human exposure levels. Dose levels of in vitro studies fell within the levels detected in humans in previous epidemiological studies (Figure S12). On the other hand, most in vivo animal studies used doses that were a few orders of magnitude higher than the highest human internal exposures. Previously published literature has found that animals and humans respond at similar body burdens, especially for dioxin-related effects like induction of CYP19A (Devito et al. 1995). However, differences between internal and external dosing and species variability in metabolism and toxicokinetics fall within the uncertainty range commonly acknowledged in risk characterization. Following the guidance of the NTP/OHAT handbook, there is no downgrading for dose levels used in experimental animal studies for confidence rating determination (NTP/OHAT 2015a). Considering these elements, no end points were downgraded for indirectness.
Table 5.
Evidence profile table for the associations between TCDD and the primary outcomes related to endometriosis.
| Body of evidence | Initial rate of confidence | Downgrading factors | Upgrading factors | Final rate of confidence | Health effectd | Level of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of biasa | Unexplained inconsistencyb | Indirectness | Imprecision | Publication bias | Large magnitude | Dose–response | Consistencyc | |||||
| Onset (in vivo) | High | Downgrade | Upgrade | High | Effect | High | ||||||
| Lesion growth (in vivo) | High | High | Effect | High | ||||||||
| Migration/invasion (in vitro) | High | Downgrade | Upgrade | High | Effect | High | ||||||
| Viability/proliferation (in vitro) | High | Downgrade | Moderate | No effect | Inadequate | |||||||
aRating downgraded for risk of bias for onset and migration/invasion as the most studies ranked Tier 2 and Tier 3 due to underreporting on key elements (Figures S8–S11).
bRating downgraded for unexplained inconsistency for viability/proliferation because the two studies that reported on TCDD and proliferation found opposing directions of effect, which could not be explained by study design (Figure 3).
cRating upgraded for consistency for onset and migration/invasion because of consistent direction of effect across multiple study designs and animal/cell models (Figure 4).
dEvidence of health effect in onset, lesion growth, and migration/invasion. Evidence of no health effect in viability/proliferation. Based on this, determinations for the level of evidence were made (Table S6).
The consistency of results on in vivo onset and in vitro migration/invasion across study designs, models and doses merited an upgrade in confidence in those bodies of evidence where highly heterogeneity was expected. There were also inconsistencies for in vivo lesion size, but we found that these could be explained by differences in experimental design and animal models. Despite the often significant increases in lesion size, especially for murine models, in a monotonic dose–response manner (Cummings and Metcalf 1995b; Cummings et al. 1996; Johnson et al. 1997; Khan et al. 2018), the results were not consistent nor significant enough across all studies and models to merit an increase in confidence for dose response or magnitude of effect. Conversely, the divergence of results on viability/proliferation in vitro using similar doses and assays merited a downgrade in confidence due to unexplained inconsistency.
Considering the direction of health effects, we translated the confidence ratings into a level of evidence for health effect NTP/OHAT framework (Table S6) and displayed it in the Evidence Profile Table 5. For in vivo end points, there was high confidence in the body of evidence for health effect, translating to a high level of evidence linking TCDD exposure and increased endometriosis onset and lesion growth. For in vitro end points, there was high confidence for health effect on cell migration, translating to a high evidence linking TCDD exposure and increased endometrial cell migration/invasion. However, for endometrial cell viability or proliferation, there was moderate confidence in the body of evidence for no health effect, which translated to an inadequate level of evidence of no effect of TCDD on cell viability/proliferation (Table 5; Table S6). The evidence gathered from the rest of the OCCs further suggest a potential joint effect on cell migration, mainly by boosting the AhR/CYP1A1 pathway, promoting inflammation of the microenvironment or extracellular matrix-remodeling (Figure 9).
Figure 9.
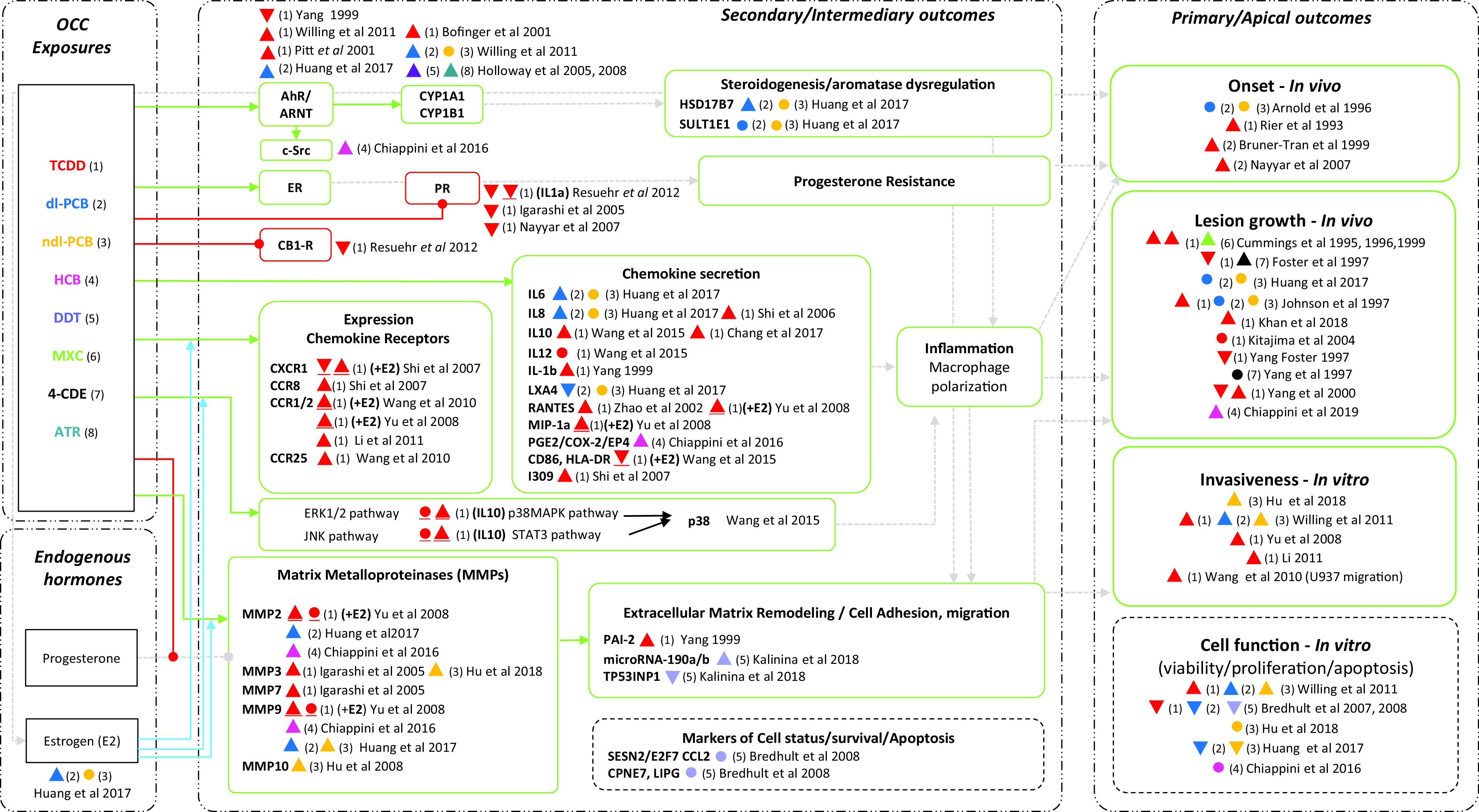
Network evidence plot summarizing the associations between exposure to organochlorine chemicals (OCCs) and the different end points related to endometriosis. Triangles and circles summarize the overall effect direction of the tested end point relative to the control. The triangle indicates significant increase, upside-down triangle significant decrease and the circle no significant effect. The colored number key in parenthesis identifies the specific OCC indicated in the “Exposure box,” also identified by the number in parenthesis [e.g., “red triangle (1)” indicates significant increase for TCDD]. In case of coexposure (i.e., with E2), the symbol appears underlined. Connecting lines ending with arrows indicate the overall evidence suggests up-regulation/increase, whereas connecting lines ending with circles indicate down-regulation/inhibition. Solid blue arrows indicate the presence of interactions, and dotted gray arrows stand for inferred associations between end points on the basis of current knowledge. The contour of end point boxes relates to the overall direction of effect toward positive associations (e.g., continuous line) or no conclusive effect (broken line). Note: AhR, aryl hydrocarbon receptor; ARNT, aryl hydrocarbon receptor nuclear translocator; ATR, atrazine; CB1-R, cannabinoid receptor type 1; CCR, chemokine receptor; COX, cyclooxygenase; CYP1A1, cytochrome P450; ER, estrogen receptor; HCB, hexachlorobenzene; IL, interleukin; LX, lipoxin; MXC, methoxychlor; MIP, macrophage inflammatory protein; MMP, matrix metalloproteinase; PCB, polychlorinated biphenyl; PGE, prostaglandin E; PR, progesterone receptor.
Epidemiological Evidence Integration and Hazard Identification
We previously established a moderate level of evidence for the associations between TCDD and dioxin-toxic equivalents and endometriosis in human epidemiological studies (), using the same evidence-based framework (Cano-Sancho et al. 2019). This was integrated with the high level of evidence established in the present systematic review for TCDD on related endometriosis outcomes (onset and lesion growth) in animal studies for a preliminary classification of “Presumed” hazard according the NTP/OHAT scheme (Figure S6). We then considered the different in vitro/mechanistic studies supporting the biological plausibility of such associations and consider some potential upgrading or downgrading. In this regard, although there was insufficient evidence of TCDD’s effects on cell viability/proliferation in in vitro studies, the strong support from the combined high level of evidence found for TCDD on cell migration/invasion in in vitro studies and the consistent effects from the supportive secondary outcomes justified the upgrading of the hazard identification of TCDD as a “Known” hazard for endometriosis in humans.
It is important to consider that this component-based approach may not reflect the realistic effect of TCDD on endometriosis, considering that TCDD is commonly found in combination with a number of other co-occurring OCCs in humans. Hence, taking a more realistic mixture-based approach, this hazard identification conclusion is even further supported by the potential additive and synergistic effects of those co-occurring OCCs (e.g., PCBs, HCB), with analog dioxin-like and estrogenic activities. Nevertheless, the goal of this classification is not intended for regulatory purposes but rather to provide a robust evaluation of the state of the science that currently exists for TCDD and dioxin-like chemicals on endometriosis outcomes. For the rest of the OCCs, we cannot establish conclusions in a component-based manner because the level of animal and in vitro evidence was considered inadequate.
Discussion
To the best of our knowledge, this is the first study to systematically gather and synthesize the experimental evidence linking OCC exposure to endometriosis and endometriosis-related outcomes. Most evidence is associated with TCDD exposure, which appears to consistently contribute to outcomes directly related to endometriosis, including in vivo lesion growth or in vitro invasiveness. Inflammation and extracellular matrix remodeling appear to be critical intermediary end points, which are disrupted by other OCCs, such as PCB126 or HCB, as well. The level of evidence was determined to be high for the associations of TCDD exposure on in vivo onset and lesion growth and in vitro cell migration/invasion. The level of evidence was deemed inadequate to conclude that TCDD has no effect on in vitro proliferation.
In this review, we also curated an assembling of evidence to facilitate a more robust dissertation on the potential mechanisms of action underlying the development of the disease linked to the exposure to OCCs. Inspired by the adverse outcome pathway (AOP) concept, we have organized the evidence in primary (i.e., adverse outcomes) and secondary end points (i.e., key events) that are initiated by various exposure and endogenous hormones (i.e., stressors), allowing the networking representation of intermediary and interrelated associations (Carvaillo et al. 2019). This framework also aligns with the “Key characteristics” approach proposed for carcinogens, endocrine disruptors, or reproductive toxicants (Guyton et al. 2018; La Merrill et al. 2020; Luderer et al. 2019). The ability of chemicals to alter some or all of the primary and secondary end points may help shed some light on the simultaneous effect of chemicals in realistic mixtures as commonly reported in epidemiological studies. In the synthetic proposed framework, we assume the oversimplification of the complex physiology of endometriosis, which has at least three phenotypically distinct subtypes (deep infiltrating, ovarian, and peritoneal/superficial) in humans, and only one of which (peritoneal) has been studied in these experimental models. Nonetheless, moving forward, we can see the potential of expanding the linear frameworks traditionally applied in hazard identification toward a network architecture. At the same time, the systematically gathered evidence in the present work may urge systems toxicologists to complete the much-needed AOP, initiated by stressors like environmental chemical exposures that potentially lead to endometriosis.
Despite the heterogeneity of the examined literature corpus, the reviewed data considered together suggest that TCDD and other dioxin-like chemicals are of particular concern in the pathogenesis and development of endometriosis. The role of AhR, commonly found at higher concentrations in endometriotic tissue than in healthy eutopic tissue, appears of major relevance to mediate the effects of dioxins and dioxin-like compounds such as PCB126 or HCB. In the case of HCB, two pathways were proposed involving a) the binding of HCB with the AhR-c-SRc complex triggering the c-Src phosphorylation and subsequent stimulation of growth factors receptors and activation of MMPs, and b) the translocation of HCB-AhR complex to the nucleus leading COX-2, VEGF and expression, subsequent synthesis, and stimulation of EP4 signaling, culminating with the activation of c-Src and MMPs (Chiappini et al. 2016, 2019). Dioxin-like PCBs were found to increase HSD17B7 expression through both decreasing HSD17B7 promoter methylation and by decreasing LXA4, which subsequently also increases inflammation (Huang et al. 2017). HSD17B7 is also involved in estrogen biosynthesis, and there is some evidence of increased expression of in endometriotic lesions (Delvoux et al. 2014) as well as in endometrial tumors and carcinomas (Lax 2017). The concomitant effect of OCCs on the activation of aromatase and steroidogenesis with the effects on P resistance may suggest the presence of a positive feedback loop promoting the activation of MMPs and proinflammatory microenvironment, facilitating the adhesion and migration of cells, translating to lesion growth in the animal models. P and retinoic acid, which is degraded by CYP1 induced by TCDD-activated AhR, are found to be natural inhibitors of endometrial MMP expression (Bruner-Tran et al. 1999) and may mitigate development of endometrial lesions. TCDD was found to inhibit P’s suppression of MMP expression, thus promoting endometrial lesion proliferation (Bruner-Tran et al. 1999). TCDD was also found to block P-mediated induction of , a necessary paracrine signal to inhibit MMP expression (Bruner-Tran et al. 1999). P- and TCDD-treated mice were thus unable to inhibit MMP suppression and the development of endometriotic lesions. Expression of P-induced was also found to be significantly decreased in the uteri of mice maximally exposed to TCDD (Nayyar et al. 2007). In vitro studies in this review have identified important cross talk between macrophages and ESC co-cultures exposed to TCDD and E2, providing evidence for the role of M2 macrophage activation by chemokines in promoting endometriosis; exposure to TCDD, especially in combination with E2—the synthesis of which is linked to aromatase—contributes to increased invasion in ESCs co-cultured with HMPCs, U937 cells, and/or macrophages, more than in ESCs cultured alone (Chang et al. 2017; Li et al. 2011; Shi et al. 2007; Wang et al. 2010b, 2015). One implication of macrophages not explored by the studies in this review are the neuroimmune interactions between macrophage-induced stimulation of inflammatory cytokines and peripheral nerves (Liang et al. 2018). These neuroinflammatory macrophage–nerve interactions have been strongly tied to endometriosis-related pain (Forster et al. 2019; Wu et al. 2017).
Sources of Heterogeneity
Experimental models.
As previously mentioned, a number of factors posed a challenge to evidence synthesis as sources of heterogeneity between studies. First, there were considerable differences in the various models used to study endometriosis. Within animals, three species of animal models were identified: monkey, mouse, and rat. Differences between rat and mouse models varied sometimes from magnitude of effect to even direction of effect (Cummings et al. 1996), suggesting there are species differences in the mechanisms mediating TCDD’s actions in promoting endometriosis. Indeed, even within a single species, there was potential for model variation. The majority of mouse studies included in this review were performed with induction of endometriotic lesions through the autologous implantation of the uterine horn method developed by Vernon and Wilson (1985) in rats and later adapted to mice by Cummings and Metcalf (1995a). However, one study used a nude mouse model (Bruner-Tran et al. 1999), a method also described by Bruner-Tran et al. (2002). Unlike the induced lesion model, the immunocompromised nude mouse model allows the establishment of endometriosis-like growth of human endometrium and endometriotic tissues in mice introduced to human tissues and OCC exposures. Because the endometriotic lesions or phenotypes develop “spontaneously” in these models, “onset” of endometriosis can be measured. No studies have been published systematically comparing these two models. There is also no such comparison between primate and rodent models, but it can be assumed that there may be differences in how these animals respond to OCCs. One study compared a human in vitro endometrial model with a rat in vitro endometrial model (van den Brand et al. 2019). Results showed clear species differences in AhR, PR, and estrogen receptor (ER)- in response to hormones, warranting more research into the applicability of certain animal models for endometriosis research. Nonetheless, models in which the spontaneous development of endometriosis or endometriosis-like phenotypes can occur are still a valuable source of information (Arnold et al. 1996; Bruner-Tran et al. 2002; Nayyar et al. 2007; Rier et al. 1993).
In in vitro studies, there was an even greater variety of cell populations used. The majority of studies used ESCs, including ESCs from both ectopic and eutopic endometriotic tissues, as well as eutopic ESCs from women with and without endometriosis. Differences in gene expression linked to infiltration and metastasis were found in eutopic vs. ectopic ESCs (Li et al. 2011) and in MMP expressions in the eutopic ESCs of control women vs. from women with endometriosis (Hu 2018). These examples highlight subtle but important differences in the variations of even the same cell type models. As expected, we also observed differences between cell models. Aromatase (CYP1A1) is not typically expressed in normal healthy ESCs and was thus not found to be affected in ESCs but was elevated in ectopic ESCs and granulosa cells (Holloway et al. 2005, 2008; Huang et al. 2017). Chemokine CCL2 increased in ESCs (Li et al. 2011) but decreased in EECs (Bredhult et al. 2008). It is hypothesized that this is because EECs express ER-beta (Critchley et al. 2001; Krikun et al. 2005) and are thus likely not to detect effects mediated by ER alpha. The roles each cell type play thus are likely to influence the results observed. Three studies used unique immortalized cell lines (Chiappini et al. 2016; Willing et al. 2011; Yang 1999), which sometimes displayed different characteristics than their nonimmortalized counterparts. For example, proliferative activity was found to significantly decrease in primary cultures of EECs (Bredhult et al. 2007, 2008) but had the opposite effect in hTERT-EECs (Willing et al. 2011); it is hypothesized that this effect difference is because TCDD has no antiestrogenic effects in hTERT-EECs. Last, several studies used cell co-cultures in different combinations (often combinations of ESCs, macrophage U937 cells involved in monocyte differentiation, and HMPCs that synthesize IL-6). Invasiveness increased in the three cell U937-ESC-HMPC co-cultures treated with TCDD but not in ESCs tested alone (and in neither of the other cell types tested alone) (Wang et al. 2010b). Several cytokine receptors (CCR1, CCR8, and CCR9) experienced similar effects (Shi et al. 2007; Wang et al. 2010b). This finding is evidence for cross talk between different cell types and highlights the importance of taking into consideration the complexity of these underlying cellular interactions in the real human system.
Exposures.
The articles included in this review measured 14 unique OCCs, but most studies () measured exposure to TCDD. Some used TCDD alone, and others tested in combination with hormones like E2 or P. Often, the addition of E2 created a positive synergistic effect (Li et al. 2011; Wang et al. 2010b; Yu et al. 2008). Other studies compared dioxin-like chemicals with nondioxin-like chemicals (Huang et al. 2017; Johnson et al. 1997). Although results varied in magnitude, they generally supported increases in onset and lesion growth in response to TCDD but less consistent for the other families. One of the most prominent nonhuman primate studies was conducted with the technical mixture Aroclor 1254 (Arnold et al. 1996), the exact composition and related toxicological effects of which have been shown to vary between lots and thus complicate the generalization of effects for individual congeners (Kodavanti et al. 2001). The specific Aroclor 1254 composition of that study was detailed in a previous report showing major concentrations for PCB77 (), PCB126 (), and PCB169 (), and because PCDDs are not detected at (Mes et al. 1989), that could help to explain the divergent results with Rier et al. (1993).
For animal studies, other elements associated with dosing regimens were also significant sources of heterogeneity, including life stage assessed, overall study duration, and route of exposure. Most studies included regimens that were chronic (multiple years for the monkey studies and several months for rodent studies); two rodent studies used developmental regimens, and other studies included subchronic or acute findings with a single dose. These multitudinous variations make it difficult to assess total final internal doses in the animals, unless serum concentrations of the exposures were measured. In the follow-up study after the original chronic TCDD treatment on rhesus monkeys (Rier et al. 1993), authors correlated serum levels of TCDD with serum levels of multiple unintended PCBs and other polyhalogenated aromatic hydrocarbons accumulated through diet which were also associated with the presence of endometriosis (Rier et al. 2001). The findings from this follow-up also suggest the presence of synergistic mechanisms of organochlorines through the alteration of normal dynamics of accumulation of OCCs, which may be extended to other non-OCCs with metabolic disruption potential.
In many studies the animals were pretreated with E1 or E2 to encourage lesion survival. TCDD has known antiestrogenic effects, so in two studies, TCDD treatment actually significantly decreased lesion size in animals that had been pretreated with a high dose of estrogenic compounds [i.e., E1 () or E2 (), respectively] (Foster et al. 1997; Yang and Foster 1997). Another study, which tested E2 treatment alone and in combination with TCDD, as well as TCDD alone without E2, found results that corroborate TCDD’s antiestrogenic effects (Kitajima et al. 2004). Interestingly, one group studied the effects of two different vehicle controls (Khan et al. 2018). Though TCDD treatment in both corn oil and DMSO both significantly increased lesion size in comparison with their vehicle controls, authors found that increased lesion size more than , suggesting that the vehicles selected may have an impact on the magnitude of effects observed.
Research Gaps
One limitation discussed above is the heterogeneity across studies included. In particular, there is a dearth of studies on other OCCs besides TCDD. Only 3 out of the 14 OCCs were investigated in more than two studies. Another three OCCs were investigated in two studies and the rest only in a single study. The current paradigm still focuses on singular exposure–outcome pairings, which fails to take into consideration the reality of the complex exposure mixtures to which humans are actually exposed. The strong correlation profiles of OCCs in epidemiological studies highlights that exposures to individual organochlorines are extremely unlikely (Cano-Sancho et al. 2018). We thus encourage future studies to explore mixed exposures or assessments of families of chemicals based on structural or biological similarities. For instance, there is evidence supporting the role of the estrogenic plasticizer bisphenol A (BPA) on the spontaneous development or progression of endometriosis lesions in rodents (Jones et al. 2018; Signorile et al. 2010). Thus, it may be speculated that synergistic interactions between pollutants like TCDD and nonpersistent estrogen-mimicking chemicals like BPA may occur as observed with E2.
The RoB assessment revealed a general lack of reporting of some key study features, including methods of randomization and blinding of research personnel to study group allocation, treatment throughout the study, and outcome assessment. A challenge in all controlled in vitro and in vivo studies is determining final total exposure or internal doses. Only one study verified dose content in the feed using gas chromatography and mass spectrometry over the 4-y treatment period and later performed a follow-up study that measured internal exposure levels in serum (Rier et al. 2001). Physiologically based toxicokinetic (PBTK) modeling would be needed to fill this lack of necessary experimental in vivo and in vitro data to calculate internal doses or dose relevance to humans more accurately. Although we acknowledge these lengths are often not feasible, we encourage researchers to be as thorough as possible in their study design, verification, and reporting methods. The RoB assessment of in vitro studies is a nascent field, and no tool currently exists specifically tailored to this capacity. We employed the RoB Tool set forth by NTP/OHAT, which was adapted for human and animal studies (NTP/OHAT 2015b; Rochester and Bolden 2015; Rooney et al. 2014) and in vitro studies during the evaluation of immunotoxicity of perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) (NTP 2016). However, we encourage future reviews to continue to explore different methods of assessing internal validity for in vitro studies.
Conclusion
The literature corpus included in the present review support the contribution of TCDD and other dioxin-like chemicals to the progression of endometriosis. Specifically, there was a high level of evidence of TCDD’s effects on endometriosis onset and lesion growth in vivo and on cell migration/invasion in vitro. Inflammation and extracellular matrix remodeling appeared to be the most likely mechanisms boosting adhesion, migration, and invasion of endometrial cells by OCCs. This conclusion, however, should be understood in the context of the limited number of available studies, considerable heterogeneity in the included studies, and the limitations in data reporting and evidence appraisal. These findings are consistent with an analogous systematic review and meta-analysis performed on the same family of chemicals in epidemiological studies (Cano-Sancho et al. 2019). Additionally, we provide an extended AOP-inspired framework to integrate multiple end points and intermediary endometriosis-related outcomes, which may support the understanding of the simultaneous effects of multiple chemical exposures. For instance, other endocrine-disrupting chemicals, such as bisphenol A, phthalates, or perfluoroalkyl acids may indirectly contribute to the global effects of OCCs on endometriosis progression, targeting some of the characterized intermediary outcomes (La Merrill et al. 2020). Overall, we deemed the level of evidence for the associations between OCC exposure and endometriosis outcomes to be high, with a particular focus on TCDD and dioxin-like chemicals. The integration with a moderate level of epidemiological evidence brought us to conclude that TCDD was a known hazard for endometriosis in humans, strongly supported by mechanistic in vitro evidence, according the NTP/OHAT framework. In any case, more experimental research is needed to fill the gaps in understanding the relationships between OCCs and endometriosis.
Supplementary Material
Acknowledgments
This SR was partially supported by a doctoral grant from French Ministry of Higher Education, Research and Innovation and the Région Pays de la Loire (No. 2018-05894, Etoiles Montantes 2019). This work was also supported by Institut national de la santé et de la recherche médicale (Inserm) et Université de Paris.
References
- Arnett-Mansfield RL, deFazio A, Wain GV, Jaworski RC, Byth K, Mote PA, et al. 2001. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res 61:4576–4582, PMID: 11389093. [PubMed] [Google Scholar]
- Arnold DL, Nera EA, Stapley R, Tolnai G, Claman P, Hayward S, et al. 1996. Prevalence of endometriosis in rhesus (Macaca mulatta) monkeys ingesting PCB (Aroclor 1254): review and evaluation. Fundam Appl Toxicol 31(1):42–55, PMID: 8998952, 10.1006/faat.1996.0074. [DOI] [PubMed] [Google Scholar]
- Barouki R, Aggerbeck M, Aggerbeck L, Coumoul X. 2012. The aryl hydrocarbon receptor system. Drug Metabol Drug Interact 27(1):3–8, PMID: 22718620, 10.1515/dmdi-2011-0035. [DOI] [PubMed] [Google Scholar]
- Bernes C. 1998. Persistent Organic Pollutants. Stockholm, Sweden: Swedish Environmental Protection Agency, 9–21. [Google Scholar]
- Birnbaum LS, Cummings AM. 2002. Dioxins and endometriosis: a plausible hypothesis. Environ Health Perspect 110(1):15–21, PMID: 11781160, 10.1289/ehp.0211015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofinger DP, Feng L, Chi LH, Love J, Stephen FD, Sutter TR, et al. 2001. Effect of TCDD exposure on CYP1A1 and CYP1B1 expression in explant cultures of human endometrium. Toxicol Sci 62(2):299–314, PMID: 11452143, 10.1093/toxsci/62.2.299. [DOI] [PubMed] [Google Scholar]
- Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD. 2006. Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol 20(10):2278–2291, PMID: 16740655, 10.1210/me.2005-0343. [DOI] [PubMed] [Google Scholar]
- Bredhult C, Bäcklin BM, Olovsson M. 2007. Effects of some endocrine disruptors on the proliferation and viability of human endometrial endothelial cells in vitro. Reprod Toxicol 23(4):550–559, PMID: 17493787, 10.1016/j.reprotox.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bredhult C, Sahlin L, Olovsson M. 2008. Gene expression analysis of human endometrial endothelial cells exposed to op′-DDT. Mol Hum Reprod 14(2):97–106, PMID: 18204070, 10.1093/molehr/gam091. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Gnecco J, Ding T, Glore DR, Pensabene V, Osteen KG. 2017. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: translating lessons from murine models. Reprod Toxicol 68:59–71, PMID: 27423904, 10.1016/j.reprotox.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Mokshagundam S, Herington JL, Ding T, Osteen KG. 2018. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev 14(2):173–188, PMID: 29861705, 10.2174/1573404813666170921162041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Osteen KG. 2010. Dioxin-like PCBs and endometriosis. Syst Biol Reprod Med 56(2):132–146, PMID: 20377312, 10.3109/19396360903381023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Rier SE, Eisenberg E, Osteen KG. 1999. The potential role of environmental toxins in the pathophysiology of endometriosis. Gynecol Obstet Invest 48(suppl 1):45–52, PMID: 10559664, 10.1159/000052868. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Webster-Clair D, Osteen KG. 2002. Experimental endometriosis - the nude mouse as a xenographic host. In: Endometriosis: Emerging Research and Intervention Strategies, vol. 955. Yoshinaga K, Parrott EC, eds. New York, NY: New York Acad Sciences, 328–342. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. 2011. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril 96(2):360–365, PMID: 21719000, 10.1016/j.fertnstert.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Sancho G, Labrune L, Ploteau S, Marchand P, Le Bizec B, Antignac JP. 2018. The challenging use and interpretation of circulating biomarkers of exposure to persistent organic pollutants in environmental health: comparison of lipid adjustment approaches in a case study related to endometriosis. Chemosphere 200:388–396, PMID: 29499519, 10.1016/j.chemosphere.2018.02.120. [DOI] [PubMed] [Google Scholar]
- Cano-Sancho G, Ploteau S, Matta K, Adoamnei E, Louis GB, Mendiola J, et al. 2019. Human epidemiological evidence about the associations between exposure to organochlorine chemicals and endometriosis: systematic review and meta-analysis. Environ Int 123:209–223, PMID: 30530163, 10.1016/j.envint.2018.11.065. [DOI] [PubMed] [Google Scholar]
- Carvaillo JC, Barouki R, Coumoul X, Audouze K. 2019. Linking bisphenol S to adverse outcome pathways using a combined text mining and systems biology approach. Environ Health Perspect 127(4):47005, PMID: 30994381, 10.1289/EHP4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H, et al. 2017. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORγt/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis 8(3):e2666, PMID: 28300844, 10.1038/cddis.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini F, Bastón JI, Vaccarezza A, Singla JJ, Pontillo C, Miret N, et al. 2016. Enhanced cyclooxygenase-2 expression levels and metalloproteinase 2 and 9 activation by hexachlorobenzene in human endometrial stromal cells. Biochem Pharmacol 109:91–104, PMID: 27038655, 10.1016/j.bcp.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Chiappini F, Sánchez M, Miret N, Cocca C, Zotta E, Ceballos L, et al. 2019. Exposure to environmental concentrations of hexachlorobenzene induces alterations associated with endometriosis progression in a rat model. Food Chem Toxicol 123:151–161, PMID: 30393115, 10.1016/j.fct.2018.10.056. [DOI] [PubMed] [Google Scholar]
- Coumoul X, Diry M, Robillot C, Barouki R. 2001. Differential regulation of cytochrome P450 1A1 and 1B1 by a combination of dioxin and pesticides in the breast tumor cell line MCF-7. Cancer Res 61(10):3942–3948, PMID: 11358810. [PubMed] [Google Scholar]
- Critchley HO, Brenner RM, Henderson TA, Williams K, Nayak NR, Slayden OD, et al. 2001. Estrogen receptor beta, but not estrogen receptor alpha, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab 86(3):1370–1378, PMID: 11238534, 10.1210/jcem.86.3.7317. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Hedge JM, Birnbaum LS. 1999. Effect of prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol Sci 52(1):45–49, PMID: 10568697, 10.1093/toxsci/52.1.45. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Metcalf JL. 1995a. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol 9(3):233–238, PMID: 7579907, 10.1016/0890-6238(95)00004-T. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Metcalf JL. 1995b. Effects of estrogen, progesterone, and methoxychlor on surgically induced endometriosis in rats. Fundam Appl Toxicol 27(2):287–290, PMID: 8529825, 10.1006/faat.1995.1135. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Metcalf JL, Birnbaum L. 1996. Promotion of endometriosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats and mice: time-dose dependence and species comparison. Toxicol Appl Pharmacol 138(1):131–139, PMID: 8658502, 10.1006/taap.1996.0106. [DOI] [PubMed] [Google Scholar]
- Delvoux B, D’Hooghe T, Kyama C, Koskimies P, Hermans RJJ, Dunselman GA, et al. 2014. Inhibition of type 1 17β-hydroxysteroid dehydrogenase impairs the synthesis of 17β-estradiol in endometriosis lesions. J Clin Endocrinol Metab 99(1):276–284, PMID: 24187399, 10.1210/jc.2013-2503. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. 2003. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43:309–334, PMID: 12540743, 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- DeVito MJ, Birnbaum LS, Farland WH, Gasiewicz TA. 1995. Comparisons of estimated human body burdens of dioxinlike chemicals and TCDD body burdens in experimentally exposed animals. Environ Health Perspect 103(9):820–831, PMID: 7498094, 10.1289/ehp.95103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H. 2020. In-vitro models of human endometriosis. Exp Ther Med 19(3):1617–1625, PMID: 32104212, 10.3892/etm.2019.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Sarginson A, Velichkova A, Hogg C, Dorning A, Horne AW, et al. 2019. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J 33(10):11210–11222, PMID: 31291762, 10.1096/fj.201900797R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WG, Ruka MP, Gareau P, Foster RA, Janzen EG, Yang JZ. 1997. Morphologic characteristics of endometriosis in the mouse model: application to toxicology. Can J Physiol Pharmacol 75(10–11):1188–1196, PMID: 9431442, 10.1139/y97-153. [DOI] [PubMed] [Google Scholar]
- Freger SM, Foster WG. 2020. The link between environmental toxicant exposure and endometriosis re-examined. In: Endometriosis. Marsh C, ed. London, UK: IntechOpen Ltd. 10.5772/intechopen.91002. [DOI] [Google Scholar]
- Fu J, Song H, Zhou M, Zhu H, Wang Y, Chen H, et al. 2017. Progesterone receptor modulators for endometriosis. Cochrane Database Syst Rev 7(7):Cd009881, PMID: 28742263, 10.1002/14651858.CD009881.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. 2015. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev 36(6):E1–E150, PMID: 26544531, 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grümmer R. 2006. Animal models in endometriosis research. Hum Reprod Update 12(5):641–649, PMID: 16775193, 10.1093/humupd/dml026. [DOI] [PubMed] [Google Scholar]
- Guo SW, Simsa P, Kyama CM, Mihalyi A, Fulop V, Othman EER, et al. 2009. Reassessing the evidence for the link between dioxin and endometriosis: from molecular biology to clinical epidemiology. Mol Hum Reprod 15(10):609–624, PMID: 19744969, 10.1093/molehr/gap075. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Rusyn I, Chiu WA, Corpet DE, van den Berg M, Ross MK, et al. 2018. Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis 39(4):614–622, PMID: 29562322, 10.1093/carcin/bgy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 1996. 17 Beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA 93(18):9776–9781, PMID: 8790407, 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr DR. 2012. Chapter two - potential use of g protein-coupled receptor-blocking monoclonal antibodies as therapeutic agents for cancers. In: International Review of Cell and Molecular Biology, vol. 297. Jeon KW, ed. San Diego, CA: Academic Press, 45–81. [DOI] [PubMed] [Google Scholar]
- Ho SM, Cheong A, Adgent MA, Veevers J, Suen AA, Tam NNC, et al. 2017. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod Toxicol 68:85–104, PMID: 27421580, 10.1016/j.reprotox.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway AC, Anger DA, Crankshaw DJ, Wu M, Foster WG. 2008. Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J Appl Toxicol 28(3):260–270, PMID: 17685393, 10.1002/jat.1275. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Stys KA, Foster WG. 2005. DDE-induced changes in aromatase activity in endometrial stromal cells in culture. Endocrine 27(1):45–50, PMID: 16077170, 10.1385/ENDO:27:1:045. [DOI] [PubMed] [Google Scholar]
- Hu T, Yao M, Fu X, Chen C, Wu R. 2018. Polychlorinated biphenyl 104 promotes migration of endometrial stromal cells in endometriosis. Toxicol Lett 290:19–28, PMID: 29535049, 10.1016/j.toxlet.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Huang Q, Chen Y, Chen Q, Zhang H, Lin Y, Zhu M, et al. 2017. Dioxin-like rather than non-dioxin-like PCBs promote the development of endometriosis through stimulation of endocrine–inflammation interactions. Arch Toxicol 91(4):1915–1924, PMID: 27663891, 10.1007/s00204-016-1854-0. [DOI] [PubMed] [Google Scholar]
- Hutz RJ, Carvan MJ, Baldridge MG, Conley LK, Heiden TK. 2006. Environmental toxicants and effects on female reproductive function. Tren Reprod Bio 2:1–11, PMID: 18516253, 10.1901/jaba.2006.2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, et al. 2005. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril 84(1):67–74, PMID: 16009159, 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Cummings AM, Birnbaum LS. 1997. Promotion of endometriosis in mice by polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls. Environ Health Perspect 105(7):750–755, PMID: 9294722, 10.1289/ehp.97105750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KC, De Voogt P. 1999. Persistent organic pollutants (POPs): state of the science. Environ Pollut 100(1–3):209–221, PMID: 15093119, 10.1016/S0269-7491(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Jones RL, Lang SA, Kendziorski JA, Greene AD, Burns KA. 2018. Use of a mouse model of experimentally induced endometriosis to evaluate and compare the effects of bisphenol A and bisphenol AF exposure. Environ Health Perspect 126(12):127004, PMID: 30675821, 10.1289/EHP3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina TS, Kononchuk VV, Ovchinnikov VY, Chanyshev MD, Gulyaeva LF. 2018. Expression of the miR-190 family is increased under DDT exposure in vivo and in vitro. Mol Biol Rep 45(6):1937–1945, PMID: 30421125, 10.1007/s11033-018-4343-0. [DOI] [PubMed] [Google Scholar]
- Khan Z, Zheng Y, Jones TL, Delaney AA, Correa LF, Shenoy CC, et al. 2018. Epigenetic therapy: novel translational implications for arrest of environmental dioxin-induced disease in females. Endocrinology 159(1):477–489, PMID: 29165700, 10.1210/en.2017-00860. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Khan KN, Fujishita A, Masuzaki H, Ishimaru T. 2004. Histomorphometric alteration and cell-type specific modulation of arylhydrocarbon receptor and estrogen receptor expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin and 17 beta-estradiol in mouse experimental model of endometriosis. Reprod Toxicol 18(6):793–801, PMID: 15279877, 10.1016/j.reprotox.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Kannan N, Yamashita N, Derr-Yellin EC, Ward TR, Burgin DE, et al. 2001. Differential effects of two lots of Aroclor 1254: congener-specific analysis and neurochemical end points. Environ Health Perspect 109(11):1153–1161, PMID: 11713001, 10.1289/ehp.011091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Schatz F, Taylor R, Critchley HO, Rogers PA, Huang J, et al. 2005. Endometrial endothelial cell steroid receptor expression and steroid effects on gene expression. J Clin Endocrinol Metab 90(3):1812–1818, PMID: 15613410, 10.1210/jc.2004-1814. [DOI] [PubMed] [Google Scholar]
- La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, et al. 2020. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol 16(1):45–57, PMID: 31719706, 10.1038/s41574-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax SF. 2017. Pathology of endometrial carcinoma. Adv Exp Med Biol 943:75–96, PMID: 27910065, 10.1007/978-3-319-43139-0_3. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Vorderstrasse BA. 2013. New insights into the aryl hydrocarbon receptor as a modulator of host responses to infection. Semin Immunopathol 35(6):615–626, PMID: 23963494, 10.1007/s00281-013-0395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Banu SK, Subbarao T, Starzinski-Powitz A, Arosh JA. 2011. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol Cell Endocrinol 332(1–2):306–313, PMID: 21111772, 10.1016/j.mce.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Lephart ED. 1996. A review of brain aromatase cytochrome P450. Brain Res Brain Res Rev 22(1):1–26, PMID: 8871783, 10.1016/0165-0173(96)00002-1. [DOI] [PubMed] [Google Scholar]
- Li MQ, Hou XF, Lv SJ, Meng YH, Wang XQ, Tang CL, et al. 2011. CD82 gene suppression in endometrial stromal cells leads to increase of the cell invasiveness in the endometriotic milieu. J Mol Endocrinol 47(2):195–208, PMID: 21685244, 10.1530/JME-10-0165. [DOI] [PubMed] [Google Scholar]
- Liang Y, Xie H, Wu J, Liu D, Yao S. 2018. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod Biol Endocrinol 16(1):122, PMID: 30518376, 10.1186/s12958-018-0441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Zhao M. 2016. A PubMed-wide study of endometriosis. Genomics 108(3–4):151–157, PMID: 27746014, 10.1016/j.ygeno.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Luderer U, Eskenazi B, Hauser R, Korach KS, McHale CM, Moran F, et al. 2019. Proposed key characteristics of female reproductive toxicants as an approach for organizing and evaluating mechanistic data in hazard assessment. Environ Health Perspect 127(7):075001, PMID: 31322437, 10.1289/EHP4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta K, Ploteau S, Coumoul X, Koual M, Le Bizec B, Antignac JP, et al. 2019. Associations between exposure to organochlorine chemicals and endometriosis in experimental studies: a systematic review protocol. Environ Int 124:400–407, PMID: 30682595, 10.1016/j.envint.2018.12.063. [DOI] [PubMed] [Google Scholar]
- Mes J, Arnold DL, Bryce F, Davies DJ, Karpinski K. 1989. The effect of long-term feeding of aroclor 1254 to female rhesus monkeys on their polychlorinated biphenyl tissue levels. Arch Environ Contam Toxicol 18(6):858–865, PMID: 2515810, 10.1007/BF01160301. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269, PMID: 19622511, 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. 2006. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69(3):562–573, PMID: 16405877, 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nanda A, K T, Banerjee P, Dutta M, Wangdi T, Sharma P, et al. 2020. Cytokines, angiogenesis, and extracellular matrix degradation are augmented by oxidative stress in endometriosis. Ann Lab Med 40(5):390–397, PMID: 32311852, 10.3343/alm.2020.40.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayyar T, Bruner-Tran KL, Piestrzeniewicz-Ulanska D, Osteen KG. 2007. Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod Toxicol 23(3):326–336, PMID: 17056225, 10.1016/j.reprotox.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LS, Simpson ER, Johns A, Bulun SE. 1996. Aromatase expression in endometriosis. J Clin Endocrinol Metab 81(1):174–179, PMID: 8550748, 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program). 2016. Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS). Research Triangle Park, NC: National Toxicology Program. [Google Scholar]
- NTP/OHAT (NTP/Office of Health Assessment and Translation). 2015a. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. http://ntp.niehs.nih.gov/go/38673 [accessed 15 May 2018].
- NTP/OHAT. 2015b. OHAT Risk of Bias Rating Tool for Human and Animal Studies. https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf [accessed 15 May 2018].
- Park JY, Pillinger MH, Abramson SB. 2006. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119(3):229–240, PMID: 16540375, 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Pitt JA, Feng L, Abbott BD, Schmid J, Batt RE, Costich TG, et al. 2001. Expression of AhR and ARNT mRNA in cultured human endometrial explants exposed to TCDD. Toxicol Sci 62(2):289–298, PMID: 11452142, 10.1093/toxsci/62.2.289. [DOI] [PubMed] [Google Scholar]
- Pumarega J, Gasull M, Lee D-H, López T, Porta M. 2016. Number of persistent organic pollutants detected at high concentrations in blood samples of the United States population. PLoS One 11(8):e0160432, PMID: 27508420, 10.1371/journal.pone.0160432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson JM. 1991. Prevalence of endometriosis in asymptomatic women. J Reprod Med 36(7):513–515, PMID: 1834839. [PubMed] [Google Scholar]
- Resuehr D, Glore DR, Taylor HS, Bruner-Tran KL, Osteen KG. 2012. Progesterone-dependent regulation of endometrial cannabinoid receptor type 1 (CB1-R) expression is disrupted in women with endometriosis and in isolated stromal cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Fertil Steril 98(4):948–956.e941, PMID: 22789143, 10.1016/j.fertnstert.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rier S, Foster WG. 2003. Environmental dioxins and endometriosis. Semin Reprod Med 21(2):145–154, PMID: 12917784, 10.1055/s-2003-41321. [DOI] [PubMed] [Google Scholar]
- Rier SE, Martin DC, Bowman RE, Dmowski WP, Becker JL. 1993. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol 21(4):433–441, PMID: 8253297, 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- Rier SE, Turner WE, Martin DC, Morris R, Lucier GW, Clark GC. 2001. Serum levels of TCDD and dioxin-like chemicals in rhesus monkeys chronically exposed to dioxin: correlation of increased serum PCB levels with endometriosis. Toxicol Sci 59(1):147–159, PMID: 11134554, 10.1093/toxsci/59.1.147. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL. 2015. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123(7):643–650, PMID: 25775505, 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122(7):711–718, PMID: 24755067, 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe SH. 1995. Modulation of gene expression and endocrine response pathways by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacol Ther 67(2):247–281, PMID: 7494865, 10.1016/0163-7258(95)00017-B. [DOI] [PubMed] [Google Scholar]
- Samartzis EP, Fink D, Stucki M, Imesch P. 2019. Doxycycline reduces MMP-2 activity and inhibits invasion of 12Z epithelial endometriotic cells as well as MMP-2 and -9 activity in primary endometriotic stromal cells in vitro. Reprod Biol Endocrinol 17(1):38, PMID: 30981279, 10.1186/s12958-019-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao R. 2013. Progesterone receptor isoforms A and B: new insights into the mechanism of progesterone resistance for the treatment of endometrial carcinoma. Ecancer 7:381, PMID: 24386010, 10.3332/ecancer.2013.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AJ, Antoni S, Guyton KZ, Lunn RM, Loomis D, Rusyn I, et al. 2018. Software tools to facilitate systematic review used for cancer hazard identification. Environ Health Perspect 126(10):104501, PMID: 30392397, 10.1289/EHP4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YL, Luo XZ, Zhu XY, Hua KQ, Zhu Y, Li DJ. 2006. Effects of combined 17 beta-estradiol with TCDD on secretion of chemokine IL-8 and expression of its receptor CXCR1 in endometriotic focus-associated cells in co-culture. Hum Reprod 21(4):870–879, PMID: 16517565, 10.1093/humrep/dei414. [DOI] [PubMed] [Google Scholar]
- Shi YL, Luo XZ, Zhu XY, Li DJ. 2007. Combination of 17 beta-estradiol with the environmental pollutant TCDD is involved in pathogenesis of endometriosis via up-regulating the chemokine I-309-CCR8. Fertil Steril 88(2):317–325, PMID: 17693327, 10.1016/j.fertnstert.2006.11.129. [DOI] [PubMed] [Google Scholar]
- Signorile PG, Spugnini EP, Mita L, Mellone P, D’Avino A, Bianco M, et al. 2010. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol 168(3):318–325, PMID: 20350546, 10.1016/j.ygcen.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Sourial S, Tempest N, Hapangama DK. 2014. Theories on the pathogenesis of endometriosis. Int J Reprod Med 2014:179515, PMID: 25763392, 10.1155/2014/179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story L, Kennedy S. 2004. Animal studies in endometriosis: a review. ILAR J 45(2):132–138, PMID: 15111732, 10.1093/ilar.45.2.132. [DOI] [PubMed] [Google Scholar]
- Takenaka Y, Taniguchi F, Miyakoda H, Takai E, Terakawa N, Harada T. 2010. Lipopolysaccharide promoted proliferation and invasion of endometriotic stromal cells via induction of cyclooxygenase-2 expression. Fertil Steril 93(1):325–327, PMID: 19647233, 10.1016/j.fertnstert.2009.06.042. [DOI] [PubMed] [Google Scholar]
- UNEP (United Nations Environmental Programme). 2017. Global Monitoring Plan for Persistent Organic Pollutants Under the Stockholm Convention Article 16 on Effectiveness Evaluation. Second Global Monitoring Report. UNEP/POPS/COP.8/INF/38. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.8-INF-38.English.pdf [accessed 10 October 2018].
- van den Brand AD, Rubinstein E, de Jong PC, van den Berg M, van Duursen MBM. 2019. Primary endometrial 3D co-cultures: a comparison between human and rat endometrium. J Steroid Biochem Mol Biol 194:105458, PMID: 31465845, 10.1016/j.jsbmb.2019.105458. [DOI] [PubMed] [Google Scholar]
- van der Linden PJ. 1996. Theories on the pathogenesis of endometriosis. Hum Reprod 11(suppl 3):53–65, PMID: 9147102, 10.1093/humrep/11.suppl_3.53. [DOI] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA. 1985. Studies on the surgical induction of endometriosis in the rat. Fertil Steril 44(5):684–694, PMID: 4054348. [PubMed] [Google Scholar]
- Wang Y, Chen H, Wang NL, Guo HY, Fu YL, Xue SG, et al. 2015. Combined 17β-estradiol with TCDD promotes M2 polarization of macrophages in the endometriotic milieu with aid of the interaction between endometrial stromal cells and macrophages. PLoS One 10(5):e0125559, PMID: 25950905, 10.1371/journal.pone.0125559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fu Y, Xue S, Ai A, Chen H, Lyu Q, et al. 2013. The M2 polarization of macrophage induced by fractalkine in the endometriotic milieu enhances invasiveness of endometrial stromal cells. Int J Clin Exp Pathol 7(1):194–203, PMID: 24427339. [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y, Wang L, et al. 2010a. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol 45(5):291–299, PMID: 20732991, 10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu J, Luo X, Wang X, Li M, Wang L, et al. 2010b. Abnormal regulation of chemokine TECK and its receptor CCR9 in the endometriotic milieu is involved in pathogenesis of endometriosis by way of enhancing invasiveness of endometrial stromal cells. Cell Mol Immunol 7(1):51–60, PMID: 20081876, 10.1038/cmi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar PNH, Trasande L, Legler J. 2017. Systematic review and meta-analysis of early-life exposure to bisphenol a and obesity-related outcomes in rodents. Environ Health Perspect 125(10):106001, PMID: 28982642, 10.1289/EHP1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel MT, Krämer J, Schem C, Wenners A, Alkatout I, Jonat W, et al. 2012. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol 160(1):74–78, PMID: 22056701, 10.1016/j.ejogrb.2011.09.040. [DOI] [PubMed] [Google Scholar]
- Wen X, Xiong Y, Qu X, Jin L, Zhou C, Zhang M, et al. 2019. The risk of endometriosis after exposure to endocrine-disrupting chemicals: a meta-analysis of 30 epidemiology studies. Gynecol Endocrinol 35(8):645–650, PMID: 30907174, 10.1080/09513590.2019.1590546. [DOI] [PubMed] [Google Scholar]
- Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE. 2000. Ethoxyresorufin-o-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit Rev Toxicol 30(4):347–570, PMID: 10955715, 10.1080/10408440091159239. [DOI] [PubMed] [Google Scholar]
- Willing C, Peich M, Danescu A, Kehlen A, Fowler PA, Hombach-Klonisch S. 2011. Estrogen-independent actions of environmentally relevant AhR-agonists in human endometrial epithelial cells. Mol Hum Reprod 17(2):115–126, PMID: 20876610, 10.1093/molehr/gaq081. [DOI] [PubMed] [Google Scholar]
- Wu J, Xie H, Yao S, Liang Y. 2017. Macrophage and nerve interaction in endometriosis. J Neuroinflammation 14(1):53, PMID: 28288663, 10.1186/s12974-017-0828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH. 1999. Expression of dioxin-responsive genes in human endometrial cells in culture. Biochem Biophys Res Commun 257(2):259–263, PMID: 10198199, 10.1006/bbrc.1999.0451. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Agarwal SK, Foster WG. 2000. Subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin modulates the pathophysiology of endometriosis in the cynomolgus monkey. Toxicol Sci 56(2):374–381, PMID: 10910996, 10.1093/toxsci/56.2.374. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Foster WG. 1997. Continuous exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibits the growth of surgically induced endometriosis in the ovariectomized mouse treated with high dose estradiol. Toxicol Ind Health 13(1):15–25, PMID: 9098947, 10.1177/074823379701300102. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Yagminas A, Foster WG. 1997. Stimulating effects of 4-chlorodiphenyl ether on surgically induced endometriosis in the mouse. Reprod Toxicol 11(1):69–75, PMID: 9138635, 10.1016/S0890-6238(96)00198-0. [DOI] [PubMed] [Google Scholar]
- Yilmaz BD, Bulun SE. 2019. Endometriosis and nuclear receptors. Hum Reprod Update 25(4):473–485, PMID: 30809650, 10.1093/humupd/dmz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wang Y, Zhou WH, Wang L, He YY, Li DJ. 2008. Combination of estrogen and dioxin is involved in the pathogenesis of endometriosis by promoting chemokine secretion and invasion of endometrial stromal cells. Hum Reprod 23(7):1614–1626, PMID: 18456672, 10.1093/humrep/den125. [DOI] [PubMed] [Google Scholar]
- Zhao D, Pritts EA, Chao VA, Savouret JF, Taylor RN. 2002. Dioxin stimulates RANTES expression in an in-vitro model of endometriosis. Mol Hum Reprod 8(9):849–854, PMID: 12200463, 10.1093/molehr/8.9.849. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. 2018. Endometriosis. Nat Rev Dis Primers 4(1):9, PMID: 30026507, 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.