Abstract
Heat stress accounts for substantial economic loss in the poultry industry by altering the health and performance of chickens. Alpha-lipoic acid (ALA) is a water and fat-soluble antioxidant which is readily absorbed from the intestine resulting in maximum bioavailability. Moreover, ALA acts as a coenzyme in glucose metabolism and helps generate other antioxidants. Considering these benefits, we hypothesized that dietary supplementation of ALA would help mitigate heat stress in poultry. A total of 72 Day-old broiler chicks were randomly assigned into three treatment groups: no heat stress (NHS), heat stress with basal diet (HS), and heat stress with alpha-lipoic acid (HS+ALA); each treatment group had 6 replicate pens with 4 birds in each pen (n = 24/group). The allocated birds were raised under standard husbandry practices for 3 weeks. After 21 d, birds in the HS and HS+ALA groups were exposed to heat stress (33°C for 8 hours during the day) for 3 weeks, while the NHS group was reared under normal conditions (22–24°C). The HS+ALA group received a basal finisher diet fortified with ALA (500 mg/kg) during the treatment period (22 to 42 d), while other birds were provided with the basal finisher diet. Weekly body weight and feed intake were recorded. The cecum digesta for volatile fatty acids (VFAs) analysis and 16S rRNA sequencing for the gut microbiota analysis; and the ileum tissue samples for histological and gene expression analyses were collected on d 42. Exposure to heat stress decreased (P<0.05) average daily gain (ADG) and final body weight (FBW) in the HS group compared to the NHS group, the supplementation of ALA improved (P<0.05) ADG and FBW in heat-stressed birds. Furthermore, birds in the HS+ALA group had increased (P<0.05) expression of HSP90, PRDX1, GPX3, SOD2, OCLN, and MUC2 genes and higher (P<0.05) concentrations of major VFAs (acetate, propionate, and butyrate). The dietary ALA supplementation also improved the villus height and villus height to crypt depth ratio in the HS+ALA group. The microbial diversity analysis revealed significant abundance (P<0.05) of beneficial bacteria Lactobacillus and Peptostreptococcaceae in the cecum of the ALA group. These results indicate that dietary ALA supplementation effectively mitigates the negative effects of heat stress in broilers by improving the expression of heat-shock, tight-junction, antioxidants, and immune-related genes in the intestine, improving villus structures, increasing concentration of major VFAs, and enriching the beneficial microbiota.
Introduction
Poultry meat is a good source of white meat containing a low amount of fat and high proteins. Considering this benefit of poultry meat, the consumers’ preference for poultry meat has rapidly increased in the past decade. This demand has been largely fulfilled by intensive selection and breeding of the chickens for rapid growth and heavier breast muscle [1]. However, these improved breeding are accompanied by one major problem, i.e., highly susceptible to higher environmental temperature due to higher metabolic rate, lack of sweat glands, and the presence of feathers [2]. Thus, with the rising global temperature, heat stress is a significant problem in the poultry industry resulting in massive economic loss in the tropical regions [3]. Heat stress exhibits negative effects on poultry health and production performance by altering different physiological, neuroendocrine, immunological, and behavior responses [2]. As a result, there has been a huge surge in the research regarding mitigating the heat stress in poultry. Researchers have recently tried and tested different compounds such as polyphenols and antioxidants to ameliorate the heat stress in poultry [2, 4–7].
Alpha-lipoic acid (ALA), also known as Universal antioxidant, is produced in small amounts inside the cell [8]. ALA is both water and fat-soluble antioxidants, are readily absorbed from the intestine, and can easily cross the blood-brain barrier resulting in optimum bioavailability. Besides this, both ALA and dihydrolipoic acid (DHLA)- the reduced form of ALA, can quench free radicals both in the liquid and aqueous domains [9]. Moreover, ALA and DHLA also possess metal-chelating activity, acts as a coenzyme in glucose metabolism, and generates other antioxidants such as ascorbate, vitamin E, and glutathione (GST) [8, 10]. Considering these benefits, ALA has recently gained huge attention in normal and heat stress conditions as a potential feed supplement in poultry.
Studies have shown that dietary fortification of ALA was able to improve the growth performance indices, immunological and biochemical characteristics, lipid metabolism, and oxidative stress in the poultry [9]. Likewise, the storability of poultry meat and meat product was also found to increase with the fortification of ALA in the feed [9]. However, there has not been any study focusing on ALA supplementation on gastrointestinal physiology and the growth performance in heat-stressed broiler birds. Thus, this study was carried out to unveil ALA supplementation’s mitigatory effects on the growth performance, immune parameters, gut microbiota, and gut health of heat-stressed broiler birds.
Material and methods
Birds and husbandry
The animal experimentation was carried out at the small animal facility of the University of Hawaii at Manoa, and the experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Hawaii (Approval no. 17–2605). A total of 72 day-old Cobb-500 unsexed chicks were sourced from a local hatchery (Asagi Hatchery Inc., Honolulu, HI). On d 1, birds were weighed individually, winged tagged, and placed equally and randomly to one of 3 treatments with 6 replicates of each treatment (n = 24 birds/treatment; 4 birds/pen). The treatment groups were: 1) No heat stress (NHS), 2) Heat stress with basal diet (HS), and 3) heat stress with alpha-lipoic acid (HS+ALA). For the first 21 d, all the birds were raised following the standard broiler rearing guidelines on the floor pen system with access to ad libitum feed and water. After 21 d, birds in the HS, and HS+ALA were exposed to the heat of 33–35°C (8 am to 6 pm) and 21–22°C (6 pm to 8 am) with 50% relative humidity for 3 weeks. Birds in the NHS group were reared at the normal room temperature (22°C-24°C) with 50% relative humidity. The lighting regime was 23 h light and 1 h dark periods. The size of the pen was 1 m x 0.71 m, and the stocking density was 1500 cm2/bird.
Diets
Birds were fed the corn-soybean meal-based mash diets in two phases, starter (1–21 d) and finisher (22–42 d), to meet the nutrients requirements of broilers [11]. The energy and protein requirements of the diet was met following the NRC (1994); however, Ca and P level was lower than the commercial requirements. All the birds were provided with the normal starter diet for the first 14 d; afterward, 500 mg/kg ALA was supplemented on the starter diet of the HS+ALA group from 14 to 21 d, while the other two groups (HS and NHS) were provided with the normal starter diet. From 22 to 42 d, NHS and HS birds were fed with the basal finisher diets, and 500 mg/kg ALA was supplemented in the finisher diet of the HS+ALA group. The dose rate of ALA was considered as 500 mg/kg feed based on a previous study [12] in the broiler birds. The diet formulation and their nutrient profiles are presented in Table 1.
Table 1. Ingredients and nutrient composition of the experimental diets.
| Starter | Finisher | |||
|---|---|---|---|---|
| Ingredients % | Basal | Basal+ALA | Basal | Basal+ ALA |
| Corn | 54.86 | 54.86 | 63.14 | 63.14 |
| SBM | 39.5 | 39.5 | 29.6 | 29.6 |
| Soybean oil | 2 | 2 | 4.5 | 4.5 |
| Limestone | 1.27 | 1.27 | 0.85 | 0.85 |
| Monocalcium phosphate | 0.75 | 0.75 | 0.5 | 0.5 |
| Lysine | 0.23 | 0.23 | 0.18 | 0.18 |
| Methionine | 0.14 | 0.14 | 0.12 | 0.12 |
| Threonine | 0.2 | 0.2 | 0.16 | 0.16 |
| NaCl | 0.43 | 0.43 | 0.35 | 0.35 |
| Sodium bicarbonate | 0.12 | 0.12 | 0.1 | 0.1 |
| Vitamin + Mineral mix* | 0.5 | 0.5 | 0.5 | 0.5 |
| ALA, mg/kg (top dressing) | 0 | 500 | 0 | 500 |
| Calculated nutrient content, % | ||||
| MEn, kcal/kg | 2909 | 2909 | 3203 | 3203 |
| CP | 22.09 | 22.09 | 18.07 | 18.07 |
| Ca | 0.75 | 0.75 | 0.52 | 0.52 |
| Total P | 0.57 | 0.57 | 0.47 | 0.47 |
| Available P | 0.3 | 0.3 | 0.23 | 0.23 |
| Lysine | 1.39 | 1.39 | 1.10 | 1.10 |
| Methionine | 0.48 | 0.48 | 0.41 | 0.41 |
| Cystine | 0.43 | 0.43 | 0.38 | 0.38 |
| Threonine | 1.03 | 1.03 | 0.85 | 0.85 |
| Tryptophan | 0.33 | 0.33 | 0.26 | 0.26 |
| Methionine + Cysteine | 0.91 | 0.91 | 0.8 | 0.8 |
| Arginine | 1.61 | 1.61 | 1.31 | 1.31 |
| Valine | 1.22 | 1.22 | 1.03 | 1.03 |
| Isoleucine | 0.93 | 0.93 | 0.76 | 0.76 |
| Leucine | 1.89 | 1.89 | 1.63 | 1.63 |
| dig Lys | 1.25 | 1.25 | 0.99 | 0.99 |
| dig Met | 0.45 | 0.45 | 0.39 | 0.39 |
| dig Thr | 0.85 | 0.85 | 0.69 | 0.69 |
| NDF | 9.13 | 9.13 | 8.78 | 8.78 |
| CF | 3.97 | 3.97 | 3.46 | 3.46 |
| Na | 0.22 | 0.22 | 0.18 | 0.18 |
| Cl | 0.30 | 0.30 | 0.25 | 0.25 |
| Choline (mg/kg) | 1419 | 1419 | 1200 | 1200 |
*Providing the following (per kg of diet): vitamin A (trans-retinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 3,000 IU; vitamin E (all-rac-tocopherol-acetate), 30 mg; vitamin B1, 2 mg; vitamin B2, 8 mg; vitamin B6, 4 mg; vitamin B12 (cyanocobalamin), 0.025 mg; vitamin K3 (bisulphatemenadione complex), 3mg; choline (choline chloride), 250 mg; nicotinic acid, 60 mg; pantothenic acid (D-calcium pantothenate), 15 mg; folic acid, 1.5 mg; betaíne anhydrous, 80 mg; D-biotin, 0.15 mg; zinc (ZnO), 80 mg; manganese (MnO), 70 mg; iron (FeCO3), 60 mg; copper (CuSO4·5H2O), 8 mg; iodine (KI), 2 mg; selenium (Na2SeO3), 0.2 mg.
Growth performance
The birds were individually weighed on d 1, 7, 14, 21, 28, 35, and 42. Feed provided to the individual pen was noted, and leftover feed in the pen was also weighed weekly. The feed intake per replicate pen was determined by subtracting the leftover feed over to the total feed consumed during the week. The mortality of the birds was monitored daily. The ADG, ADFI, and FCR were calculated after adjusting for mortality, if any.
Sample collection
At 42 d, two birds/pen (n = 12) from each treatment group were selected randomly and were euthanized by carbon dioxide asphyxiation. A small piece of the ileum (5 cm posterior to the ileocecal transition) was collected (n = 6/treatment; one from each pen), snap-frozen in liquid nitrogen, and stored at -80°C for subsequent gene expression study. The cecum was excised, snap-frozen, and placed at -80°C for microbiota (n = 6/treatment; one from each pen) and VFA (n = 12/treatment; two from each pen) analysis while, approximately 1 cm of the ileum sample (6 cm posterior to the ileocecal junction) was excised, washed with normal saline and preserved in 10% neutral buffered formalin (NBF, pH 7) for the ileum histomorphology analysis (n = 4/treatment).
Total RNA extraction
The 50–100 mg of frozen ileum tissues were used for the total RNA extraction. The total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. The RNA concentration was determined using NanoDrop one (Thermo Fisher Scientific, Madison, WI), while quality was accessed by running RNA samples on 2% agarose gel. Extracted RNA was stored at -80°C until further analysis.
Quantitative real-time PCR (qPCR) assay
The specific oligonucleotide primers used for the qPCR assay were designed using the NCBI Primer-Blast tool and are presented in Table 2. The gene expression analysis was carried out as previously described [13]. In short, for cDNA synthesis, 1 μg of total RNA (20 μL reaction of reverse transcriptase mixture) was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The newly synthesized cDNA (20 μL) was diluted (25X) with 480 μL of nuclease-free water and was stored at -20°C until further analysis. The qPCR was carried out by using StepOne Plus real-time PCR system (Applied Biosystems, Foster City, CA) where10 μL reaction mixture containing 3 μL of cDNA, 5 μL of PowerUp SYBR Green Master Mix (Applied Biosystems, Foster City, CA), and 1 μL each of forward and reverse primers specific to the gene target was used. The amplification conditions were 50°C for 2 minutes (hold), 95°C for 2 minutes (hold), followed by 40 repeat cycles of 95°C for 15 seconds (denaturation), 60°C for 15 seconds (annealing), and 72°C for 1 minute (extension). Based on the consistency of expression in the ileum tissue, β-actin was chosen for normalization. The target genes were analyzed in duplicates, and an average value was taken from the experimental replicate. The expression level of target genes was determined using the cycle threshold (Ct) values and changes in the gene expression were calculated by the 2-ΔΔCt method compared to the control group. The relative mRNA expression was normalized to the endogenous reference gene B-actin.
Table 2. Primers used to quantify the expression of the genes by qPCR.
| Gene | Accession no. | Primer Sequence | Amplicon (bp) |
|---|---|---|---|
| SOD1 | NM_205064.1 | F: CAACACAAATGGGTGTACCA | 119 |
| R: CTCCCTTTGCAGTCACATTG | |||
| SOD2 | NM_204211.1 | F: CCTTCGCAAACTTCAAGGAG | 160 |
| R: AGCAATGGAATGAGACCTGT | |||
| GPX1 | NM_001277853.2 | F: AATTCGGGCACCAGGAGAA | 101 |
| R: CTCGAACATGGTGAAGTTGG | |||
| GPX3 | NM_001163232.2 | F: GAGGGAGAAGGTGAAATGCT | 192 |
| R: CCCAGCTCATTTTGTAGTGC | |||
| TXN | NM_205453.1 | F: GGCAATCTGGCTGATTTTGA | 79 |
| R: ACCATGTGGCAGAGAAATCA | |||
| PRDX1 | NM_001271932.1 | F: GGTATTGCATACAGGGGTCT | 101 |
| R: AGGGTCTCATCAACAGAACG | |||
| NRF2 | NM_205117.1 | F: CCCTGCCCTTAGAGATTAGAC | 248 |
| R: CAAGTTCATGTCCTTTTCTCTGC | |||
| HSF1 | NM_001305256.1 | F: AAGGAGGTGCTCCCAAAGTA | 221 |
| R: TTCTTTATGCTGGACACGCTG | |||
| HSF3 | NM_001305041.1 | F: TTCAGCGATGTGTTTAACCCT | 244 |
| R: GGAGGTCTTTTGGATCCTCT | |||
| HSP90 | NM_001109785.1 | F: GATAACGGTGAACCTTTGGG | 120 |
| R: GGGTAGCCAATGAACTGAGA | |||
| HSP70 | NM_001006685.1 | F: TCTCATCAAGCGTAACACCAC | 104 |
| R: TCTCACCTTCATACACCTGGAC | |||
| OCLN | NM_205128 | F: CCGAGGACAGCCCTCAATAC | 82 |
| R: CTTTGGTAGTCTGGGCTCCG | |||
| CLDN1 | NM_001013611 | F: TACCCCAAAAATGCCCCCTC | 109 |
| R: GCGGCATTGTAGTGTCCTCT | |||
| MUC2 | NM_001318434 | F: GTGGTCTGTGTGGCAACTT | 71 |
| R: GTCTCTTGCAGCCCATTCCT | |||
| IL4 | NM_001030693 | F: TGTGCCCACGCTGTGCTTACA | 155 |
| R: CTTGTGGCAGTGCTGGCTCTCC |
*F = Forward; R = Reverse.
Ileum histomorphology
The 10% NBF fixed ileal tissues were shipped to the Histology core facility, John A. Burns School of Medicine, UH Manoa, where samples were dehydrated with the series of ethanol solutions (70%, 80%, 95%, and 100%) and embedded in paraffin blocks. The embedded ileum tissue was sectioned at 6 μm thickness and stained with hematoxylin and eosin staining. A total of 18 well oriented villus-crypts unit per sample was selected and observed under an 8× objective lens by using an Olympus microscope (U-TV0.63XC, Tokyo, Japan). Different intestinal morphological parameters such as villus height (VH)- distance from the tip of villus to the crypt, crypt depth (CD)- distance from villus base to the muscularis mucosa, and villus height to crypt depth ratio (VH/CD) [14] were measured along with the apparent villus surface area by using Infinite Analyze software (Lumenera Corporation, Ottawa, ON, Canada).
Volatile fatty acid
The VFA analysis was carried out as previously described [15]. The VFA profile was analyzed by using gas chromatography (Trace 1300, Thermo Scientific, Waltham, MA) equipped with a flame ionization detector (FID), AS 1310 series automatic liquid sampler, and a 30 m × 0.53 mm internal diameter column (Stabilwax-DA, Restek, Restek Corporation, Bellefonte, PA). Helium was supplied as a carrier gas at a flow rate of 14.5 mL/min, and run time was set for 15 min. The temperature of the injector port and detector port was set at 200°C and 240°C, respectively. Trimethyl acetate (TMA) was used as an internal standard. Data handling and processing were performed on ChromeleonTM 7.2 software (Thermo Scientific, Waltham, MA).
DNA extraction and 16S rRNA gene sequencing
Total genomic DNA was extracted from the cecal content using the QIAamp® DNA Stool Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instruction. The extracted bacterial DNA concentration was determined using NanoDrop one (Thermo Fisher Scientific, Madison, WI) and stored at -20°C until further analysis. The V3 and V4 hypervariable regions of the 16S rRNA gene were amplified as outlined in Illumina 16S Metagenomic Sequencing Library guideline (Illumina) with the following modifications: Platinum Taq DNA Polymerase High Fidelity (Invitrogen, Life Technologies Corporation, Grand Island, NY) was used to set up the PCR reaction, and Mag-Bind Total Pure NGS beads (Omega Bio-Tek) were used for PCR Clean-Ups, and 35 cycles were used in the PCR. Briefly, 16S rRNA sequencing involved the following steps: 1) 1st stage PCR: Amplicon PCR, 2) PCR Clean-Up, 3) 2nd Stage PCR: Index PCR, 4) 2nd PCR Clean-Up 5) Library quantification, normalization, and pooling, and 6) Library denaturing and MiSeq sample loading. The specific sequence of 16S rRNA gene used for amplified were forward primer (5′-CCTACGGGNGGCWGCAG-3′) and reverse primer (5′GACTACHVGGGTATCTAATCC- 3′) [16]. Finally, amplicons were normalized and pooled, and subsequently sequenced on the Illumina MiSeq sequencer at the University of Hawaii at Manoa in Genomics, Proteomics, and Bioinformatics core facility.
Sequencing processing
The Qiagen CLC Genomics Workbench 12.0.1 and the CLC Microbial Genomics module was used for microbial bioinformatics. The sequencing analysis procedures were followed as described in the OTU clustering step-by-step tutorial (Qiagen, Hilden, Germany). The sequencing files (ending with ‘fastq’) were imported in the CLC workbench; files were then paired, setting the minimum distance to 200 and maximum distance to 500. The reads were then trimmed, and the read with the low coverage was removed from the analysis. Thus, obtained reads were clustered as operational taxonomical units (OTUs), based on 97% sequence similarity against the Greengenes v13_8 97% database using the CLC Microbial Genomics module. Chimeras and OTUs with lower abundance (less than 10 reads) were removed. For alpha and beta diversity analysis, the phylogenetic tree was constructed using a maximum likelihood approach based on multiple sequence alignment (MSA) of the OTU sequences generated by MUSCLE in the workbench. The Simpson’s index and Shanon entropy were calculated to determine alpha diversity, while the unweighted UniFrac and weighted UniFrac distances were calculated to determine beta diversity. Permutational multivariate analysis of variance (PERMANOVA) analysis was carried out to measure the significance of beta diversity. Differences in microbiome among treatment groups were analyzed by one-way ANOVA to determine significant difference at different taxa level.
Statistical analysis
Growth performance, gene expression, ileum histomorphology, and significantly abundant microbiome were analyzed using the R-studio and presented as mean ± SEM. These data were analyzed by using one-way analysis of variance (ANOVA), and mean separation between treatment groups was done by the Tukey-Kramer test. Kruskal-Wallis pairwise test and pairwise PERMANOVA test were carried out for alpha and beta diversity to determine the potential difference between treatment groups using the CLC Microbial Genomics module. The spearman correlation analysis was conducted to analyze associations between differential abundance taxonomic groups and measured change parameters using statistical software JMP v14 (SAS Institute Inc., Cary, NC). Values with P<0.05 were regarded as statistically significant.
Results
Effects of alpha-lipoic acid in growth performance
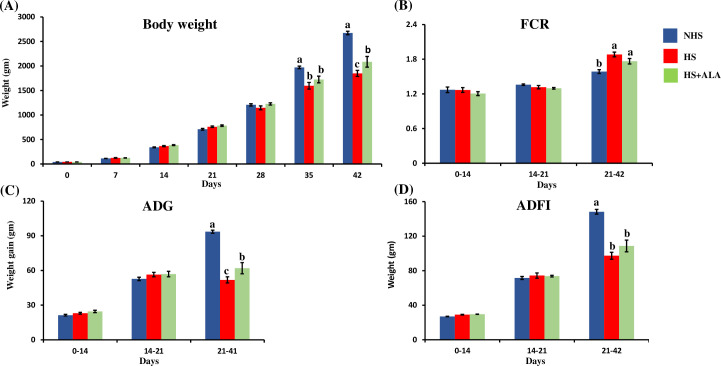
The growth performance of birds in different treatment groups is shown in Fig 1. Supplementation of the ALA significantly improved (P<0.05) the final bodyweight in the heat-stressed broiler birds as compared to the HS group. There was no significant difference (P>0.05) in body weight between the treatment groups until 28 days. However, on 35 d, body weight was significantly decreased (P<0.05) in the HS group compared to the NHS group; dietary ALA supplementation in the heat-stressed broiler birds could not significantly improve the body weight. Nevertheless, on d 42, dietary ALA supplementation significantly improved (P<0.05) the bodyweight in the heat-stressed broiler birds. During the heat stress period (21–42 d), the ADFI was significantly lowered (P<0.05) in both HS and HS+ALA groups as compared to the NHS group. The ADG, on the other hand, was significantly lowered (P<0.05) in the HS group as compared to the NHS group, and dietary supplementation of ALA significantly improved (P<0.05) ADG in heat-stressed broiler birds during the heat stress period (21–42 d). FCR was significantly high (P<0.05) in the HS group and the ALA group compared to the NHS group. Dietary ALA supplementation was not able to significantly improve (P>0.05) the FCR in the heat-stressed broiler birds. There was no mortality in the NHS group, while one bird died in the HS group and the ALA group.
Fig 1. Effects of ALA on the growth performance of heat-stressed broilers.
(A) Bodyweight, (B) FCR, (C) ADG, and (D) ADFI. Data presented as the mean± SEM (n = 24/treatment). The effect of treatment was statistically different at * P<0.05 for body weight, ADG, ADFI, and FCR. Different letters indicate the significant difference among the treatments.
Effects of ALA on the intestinal gene expression
Expression of heat shock protein-related genes
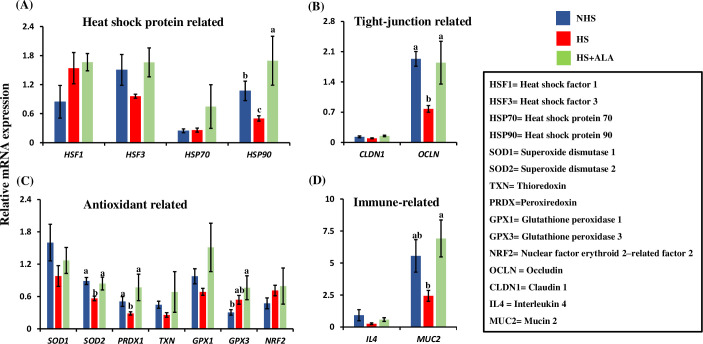
The mRNA expression of heat shock protein-related genes (HSF1, HSF3, HSP70, and HSP90) in the ileum of birds in different treatment groups is shown in Fig 2(A). The mRNA expression of HSF1, HSF3, and HSP70 remains unchanged across the treatment groups. The mRNA expression of HSP90 was significantly decreased (P<0.05) in the HS group as compared to the NHS group, while dietary ALA supplementation significantly increased (P<0.05) the mRNA expression of HSP90 in heat-stressed broiler birds.
Fig 2. Effects of ALA supplementation on the ileal gene expression of heat-stressed broilers.
(A) heat sock protein, (B) tight-junction related, (C) antioxidants related, and (D) immune-related genes. Data presented as the mean ±SEM (n = 6/treatment). Different letters indicate the significant difference among the treatments (* P<0.05).
Expression of tight-junction related genes
The mRNA expression of the tight-junction related genes (OCLN and CLDN1) in the ileum of birds in different treatment groups is shown in Fig 2(B). The heat stress significantly decreased (P<0.05) the mRNA expression of OCLN in the HS group as compared to the NHS group, while dietary ALA supplementation significantly increased (P<0.05) the expression of OCLN in heat-stressed broiler birds. The mRNA expression of CLDN1 remains unchanged between treatment groups.
Expression of antioxidant related genes
The mRNA expression of the antioxidant-related genes (SOD1, SOD2, PRDX1, TXN, GPX1, GPX3, and NRF2) in the ileum of birds in different treatment groups are shown in Fig 2(C). The mRNA expression of the SOD2 and PRDX1 was significantly decreased (P<0.05) in the HS group as compared to the NHS group, while ALA supplementation significantly increased (P<0.05) their expressions in heat-stressed birds. Dietary ALA supplementation in the heat-stressed birds significantly increased (P<0.05) the mRNA expression of GPX3 as compared to the NHS group. The mRNA expression of the SOD1, TXN, GPX1, and NRF2 remain unchanged between the treatment groups.
Expression of immune-related genes
The mRNA expression of the immune-related genes (IL4 and MUC2) between different treatment groups is shown in Fig 2(D). Dietary ALA supplementation in the heat-stressed broiler birds significantly increased (P<0.05) the expression of MUC2 in heat-stressed broiler birds, as compared to the heat-stressed birds provided with just basal diet (HS group). The mRNA expression of the IL4 remained unchanged between the treatment groups.
Ileum histomorphology
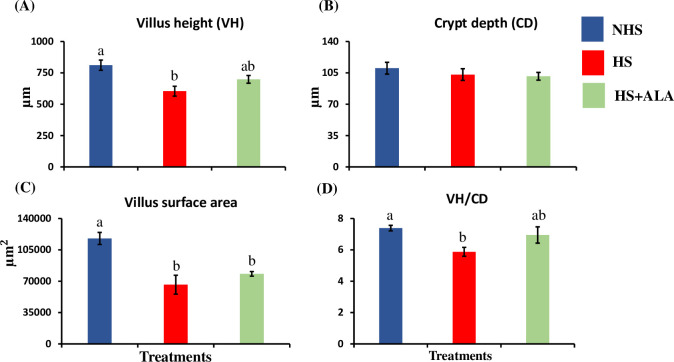
The ileum histomorphology of birds in different treatment groups is shown in Fig 3. Villus height and Villus height to crypt depth ratio were significantly lowered (P<0.05) in the HS group as compared to the NHS group, while ALA supplementation significantly improved (P>0.05) these parameters in heat-stressed broiler birds. The villus surface area was significantly decreased (P<0.05) in the heat-stressed birds compared to the NHS group. Dietary ALA supplementation did not improve (P>0.05) the surface area in heat-stressed broiler birds. The crypt depth remained unchanged across the treatment groups.
Fig 3. Effects of ALA supplementation on the ileum histomorphology of the heat-stressed broilers.
(A) Villus height (VH), (B) Crypt depth (CD), (C) Villus surface area, (D) Villus height (VH): Crypt depth (CD). Data presented as the mean ±SEM (n = 4/treatment). The effect of treatment was statistically different at * P<0.05. Different letters indicate the significant difference among the treatments.
Volatile fatty acids
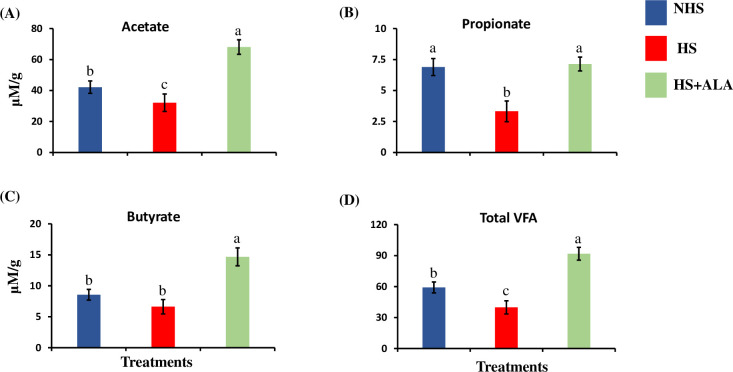
The major VFA in the cecal digesta of birds in different treatment groups is shown in Fig 4. The amount of propionate was significantly decreased (P<0.05) in the HS groups as compared to the NHS groups. Simultaneously, dietary ALA supplementation significantly increased (P<0.05) propionate concentration in the cecal digesta compared to the HS group. The amount of acetate was significantly decreased (P<0.05) in the HS group as compared to the NHS group, while dietary ALA supplementation in heat-stressed broiler birds significantly increased its amount as compared to the HS group. Dietary ALA supplementation in the heat-stressed broiler birds also significantly increased (P<0.05) the concentration of butyrate as compared to the other two groups. Overall, total VFA was significantly lower (P<0.05) in the HS group as compared to the NHS group, and dietary ALA supplementation significantly increased (P<0.05) the concentration of the total VFA.
Fig 4. Effects of ALA supplementation on the major volatile fatty acids in the cecal digesta of the heat-stressed broilers.
(A) Acetate, (B) Propionate, (C) Butyrate, and (D) Total VFA. Data presented as the mean ±SEM (n = 12/treatment). The effect of treatment was statistically different at * P<0.05. Different letters indicate the significant difference among the treatments.
Alpha and beta diversity of cecal microbiota
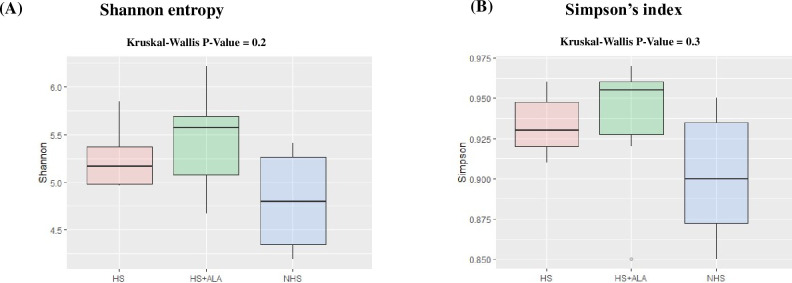
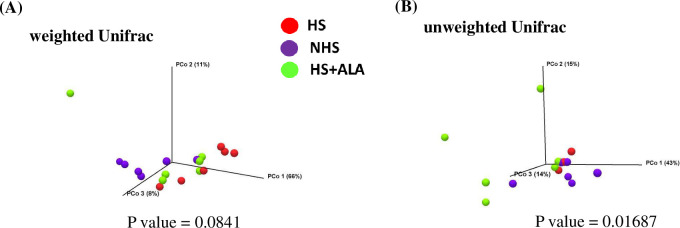
Shannon entropy was significantly higher (P<0.05) in the HS+ALA group as compared to the NHS group, while no differences (P>0.05) were observed in the Simpson index between these two groups (Fig 5). The unweighted UniFrac based PCoA revealed that bacterial composition in cecal content was significantly different (PERMANOVA analysis, P value = 0.01687) between the treatments, while no difference was observed in weighted UniFrac (Fig 6).
Fig 5. Box plot showing the effects of ALA supplementation on microbial alpha diversity in heat-stressed broilers.
(A) Shannon entropy, and (B) Simpson’s index.
Fig 6. Effects of ALA supplementation on microbial beta diversity in heat-stressed broilers.
(A) weighted UniFrac, and (B) unweighted UniFrac.
Cecal microbial composition
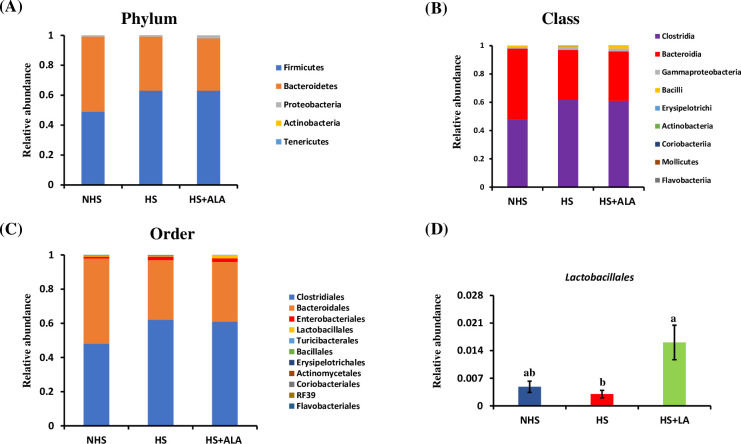
At the phylum level, HS and HS+ALA groups were dominated by Firmicutes (63%, 63%), followed by Bacteroidetes (36%, 35%), while the NHS group has an almost equal abundance of Firmicutes (49%) and Bacteroidetes (50%) (Fig 7(A)). At the class level, the taxon-based analysis revealed that Clostridia and Bacteroidia were dominant classes across different treatment groups. However, these dominant taxa were not significantly different (P>0.05) across treatment groups (Fig 7(B)). At the order level, Clostridales and Bacteroidales were dominant in different treatment groups (Fig 7(C)). These dominant orders were not statistically different between treatments. Dietary ALA supplementation, however, significantly increased (P<0.05) Lactobacillales in the heat-stressed birds (Fig 7(D)) as compared to the HS group.
Fig 7. Cecal microbial compositions.
(A) Relative abundance at Phylum level, (B) Relative abundance at Class level, (C) Relative abundance at the Order level, and (D) Significantly abundance of Lactobacillales.
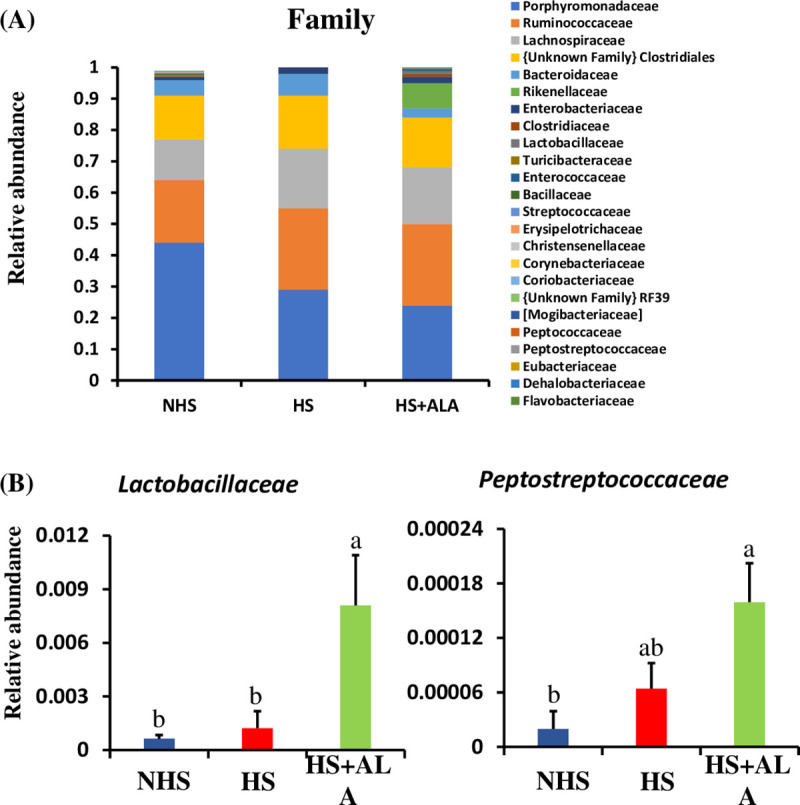
The Porphyromonadaceae, Ruminococcaceae, Lachnospiraceae, and unknown Family of Clostridiales were the dominant family found across the treatment groups. The families Lactobacillaceae and Peptostreptococcaceae were significantly enriched (P<0.05) in the HS+ALA group compared to other groups (Fig 8).
Fig 8. Cecal microbial composition at the family level. (A) Relative abundance of microbiota, and (B) Significantly abundance microbiota within the family level.
At the genus level, Parabacteroides was the most dominant genus across all groups- HS (29%), HS+LA (24%), and NHS (44%), followed by unknown family_Clostridales. The relative abundance of these dominant genera was not significantly different among the groups. Dietary ALA supplementation, however, significantly enriched (P<0.05) the Lactobacillus and unknown genus of Peptostreptococcaceae in the heat-stressed birds (Fig 9).
Fig 9. Cecal microbial composition at the genus level. (A) Relative average abundance of microbiota, and (B) significantly abundance microbiota at the genus level.
Correlation between the differential microbial species and measured parameters
The significant correlations observed after performing Spearman correlation analysis are shown in Table 3. The results showed that the expression of SOD2 and OCLN were positively (P<0.05) associated with order Lactobacillales. Acetate, butyrate, and total VFAs were positively correlated (P<0.05) with order Lactobacillales, family Lactobacillaceae, family Peptostreptococcaceae, genus Lactobacillus, and unknown_genus_Peptostreptococcaceae; While propionate was positively correlated (P<0.05) only with order Lactobacillales.
Table 3. Spearman correlation between the differential microbial species and changed measure parameters.
| Variables | Differential enriched microbes | Spearman ρ | Prob>|ρ |
|---|---|---|---|
| SOD2 | O_Lactobacillales | 0.5119 | 0.0299* |
| OCLN | O_Lactobacillales | 0.4891 | 0.0394* |
| Acetate | O_Lactobacillales | 0.5595 | 0.0158* |
| Acetate | F_Lactobacillaceae | 0.7004 | 0.0012* |
| Acetate | F_Peptostreptococcaceae | 0.5199 | 0.0270* |
| Acetate | G_Lactobacillus | 0.7004 | 0.0012* |
| Acetate | G_Unknown_Genus_Peptostreptococcaceae | 0.5199 | 0.0270* |
| Propionate | O_Lactobacillales | 0.4871 | 0.0404* |
| Butyrate | O_Lactobacillales | 0.5429 | 0.0199* |
| Butyrate | F_Lactobacillaceae | 0.6715 | 0.0023* |
| Butyrate | F_Peptostreptococcaceae | 0.4795 | 0.0441* |
| Butyrate | G_Lactobacillus | 0.6715 | 0.0023* |
| Butyrate | G_Unknown_Genus_Peptostreptococcaceae | 0.4795 | 0.0441* |
| Total VFA | O_Lactobacillales | 0.5905 | 0.0099* |
| Total VFA | F_Lactobacillaceae | 0.7128 | 0.0009* |
| Total VFA | F_Peptostreptococcaceae | 0.471 | 0.0485* |
| Total VFA | G_Lactobacillus | 0.7128 | 0.0009* |
| Total VFA | G_Unknown_Genus_Peptostreptococcaceae | 0.471 | 0.0485* |
Discussion
This study was carried out to evaluate the effectiveness of ALA (500 mg/kg of feed) to mitigate the negative effects of heat stress in poultry by observing growth performance along with different GI physiological parameters. Dietary supplementation of the ALA (500 mg/kg) significantly improved the final body weight, ADG, expressions of antioxidant, heat-shock, tight-junction, and immune-related genes, increased concentration of VFAs, and enriched beneficial gut microbiota in the heat-stressed broiler birds.
Heat stress negatively affects the production performance of broilers by decreased feed intake and growth [2, 17]. We also observed a decreased growth performance of broilers under heat stress. Several nutritional strategies are employed to mitigate the negative effects of heat stress in poultry. Among them, dietary supplementation of ALA as potential feed additives during heat stress has gained popularity in recent years and is found effective in mitigating the negative effects of heat stress in poultry [9]. In accord with those, we also observed significant improvement in the final body weight and ADG in heat-stressed broiler birds supplemented with ALA.
Further, we were interested in delineating the underlying GI physiological changes, leading to improved growth performance in heat-stressed birds supplemented with ALA. The intestine is an important organ that helps in nutrient digestion and absorption. High environmental temperature is found to have detrimental effects on the intestine [18]. Amongst different intestine parts, the ileum, a terminal part of the small intestine, is mainly susceptible to heat stress [19]. Different groups of genes (heat shock protein, antioxidant, tight junction, and immune-related genes) were then analyzed to detail the effects of ALA on heat-stressed broiler birds.
Heat shock proteins (HSPs)–chaperons–are the proteins that help properly fold the protein during the stress condition and possess cytoprotective action [20]. Different HSPs and their transcriptional factors were analyzed to determine the protective action of ALA during stress. Unlike previous studies [19, 21], in acute stress, where expression of HSP90 was higher in heat-stressed birds, expression HSP90 was lowered in this study. There have been varied expressions of HSFs and HSPs regarding tissue sample and the stress duration (i.e., acute vs. chronic stress) [22]. The spatiotemporal expression study of the HSPs and HSFs is therefore warranted. The lower expression of these genes in our study may be due to the exhaustion of the protective mechanism within the cells during chronic stress. Interestingly, dietary supplementation of ALA significantly increased the expression of HSP90.
Antioxidants are the molecules that scavenge the free radicals produced inside the cell. Heat stress elicits oxidative stress resulting in excess production of free radicals within the cell [23]. Therefore, different antioxidant-related genes were analyzed in this study to determine the effectiveness of ALA at the cellular level. The expressions of SOD2, PRDX1, and GPX3 were significantly improved in the heat-stressed broiler birds supplemented with ALA. SOD2 is mitochondrial manganese (Mn) containing enzymes that dismutase the superoxide radicals into hydrogen peroxide [24]. GPX3 reduces hydroperoxides and H2O2 by glutathione [25], while PRDX1 reduces H2O2 and hydroperoxides by using thioredoxin [26]. Like this study, previous studies have also demonstrated beneficial effects of ALA in improving total antioxidant capacity and antioxidant enzyme activity in oxidative stress conditions in broilers [27, 28].
The expression of tight junction protein and immune-related genes was then analyzed. The OCLN, a transmembrane protein, is one of the major tight junction protein that regulates paracellular permeability and also plays a role in barrier functions [29]. Heat stress impairs the tight-junction protein in the intestine by reducing the blood flow and ultimately generating hypoxia in the intestine [30]. In light of that, the OCLN was lowered in the heat-stressed broiler birds in this study, while ALA supplementation improved the expression of OCLN in heat-stressed broiler birds. Besides, MUC2 expression was also improved in ALA supplemented heat-stressed broiler birds. Mucin is essential in protecting the gut from pathogens, acidic environment, digestive enzymes, and nutrient digestion and absorption [31]. These results indicate that ALA supplementation improved the intestinal membrane integrity and protected the gut from pathogen and acidic environment, which might be one reason for improving the body weight and ADG in heat-stressed birds.
Heat stress impairs the intestinal morphology, decreases villus height, and villus height to crypt depth ratio [32–34]. In this study, heat stress altered the villus’ morphology in the ileum, which may be due to intestinal ischemia, leading to epithelial shedding [35]. Although not statistically significant, the dietary ALA supplementation improved villus height and villus height to crypt depth ratio in heat-stressed birds. The improvement observed in the villus structure was possibly due to the VFAs produced in the gut as VFAs are exerting trophic effects on the intestinal morphology [36]. This observation drove us to analyze the major VFAs produced in the gut of heat-stressed birds. All major VFAs (acetate, propionate, and butyrate) concentrations were significantly increased in heat-stressed broiler birds supplemented with ALA. The improvement in the VFAs in heat-stressed birds was possibly due to the change in the microbiota as VFA is the microbial fermentation product [37]. Thus, we directed our study towards analyzing the microbiota changes in heat-stressed birds supplemented with ALA.
Dietary ALA supplementation significantly improved the relative abundance of Lactobacillales (order), Lactobacillaceace (family), Peptostreptococcaceae (family), Lactobacillus, and unknown genus of Peptostreptococcaceae in the heat-stressed broiler birds.
Peptostreptococcaceae belong to the phylum Firmicutes and play a role in gut homeostasis [38]. They are also found to produce VFAs in the intestine [39]. Spearman’s correlation analysis in this study too revealed a positive association between Peptostreptococcaceae and acetate, butyrate, and total VFAs. Lactobacillus is the gram-positive bacteria that produce lactic acid, which reduces the pH and prevents the growth of pathogenic bacteria [40]. Thus, significant dominance of the Lactobacillus probably reduced the risk of pathogen amplification and invasion in heat-stressed birds supplemented with ALA. Besides, lactobacillus is found to have antioxidant capacity and remove the ROS to mitigate the damage induced by oxidative stress [41]. Moreover, Lactobacillus is also considered as a potent probiotic to improve gut health [42, 43]. Additionally, a positive association was observed between Lactobacillus and different gut health parameters such as expressions of SOD2 and OCLN; and concentration of acetate, propionate, butyrate, and total VFAs. Considering these results, improved body weight and ADG in heat-stressed broilers supplemented with the ALA can be mainly attributed to improved gut microbiota and VFAs. More specifically, Lactobacillus and Peptostreptococcaceae might have played a vital role in improving growth performance in heat-stressed birds.
The alteration of the microbiota on supplementation of ALA can be attributed to the difference in acquisition and use of the lipoic acid among the different microbial species. There is diversity in the lipoate metabolism among different microbial species. One such example is a difference in lipoate metabolism between Helicobacter pylori and Pseudomonas aeruginosa. Protein encoding the lipoate metabolism is absent in the H. pylori, while P. aeruginosa contains the genome that encodes both the lipoate synthesis and lipoate scavenging enzymes as well as the components of the five lipoate complexes [44].
Conclusion
The results of this study revealed that the dietary supplementation of ALA (500 mg/kg) significantly improved the final body weight and ADG; improved expressions of antioxidant, heat shock, immune-related, and tight-junction related genes in the ileum; increased concentration of VFAs and enriched the beneficial bacteria in the gut of heat-stressed broilers. Thus, ALA supplementation can be considered as one of the potential strategies to mitigate heat stress in broilers.
Supporting information
(XLSX)
Acknowledgments
We sincerely thank Socorro Tauyan for helping in animal experimentation, Dr. Amit Singh in sampling, and Dr. Mohammad Arif for providing the facilities for Microbiome Analysis.
Data Availability
Additional supplemental files having the minimal data set is submitted along with this manuscript.
Funding Statement
This work was supported by a Start-up grant from CTAHR University of Hawaii at Manoa, and USDA Multistate (2052R) to B.M.
References
- 1.Griffin H, Goddard C. Rapidly growing broiler (meat-type) chickens. Their origin and use for comparative studies of the regulation of growth. Int J Biochem. 1994;26: 19–28. doi: 10.1016/0020-711x(94)90190-2 [DOI] [PubMed] [Google Scholar]
- 2.Wasti S, Sah N, Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 2020;10. doi: 10.3390/ani10081266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St-Pierre NR, Cobanov B, Schnitkey G. Economic Losses from Heat Stress by US Livestock Industries. J Dairy Sci. 2003;86: E52–E77. doi: 10.3168/JDS.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- 4.Lin H, Jiao HC, Buyse J, Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult Sci J. 2006;62: 71–86. doi: 10.1079/WPS200585 [DOI] [Google Scholar]
- 5.Shimamoto S, Nakamura K, Tomonaga S, Furukawa S, Ohtsuka A, Ijiri D. Effects of Cyclic High Ambient Temperature and Dietary Supplementation of Orotic Acid, a Pyrimidine Precursor, on Plasma and Muscle Metabolites in Broiler Chickens. Metabolites. 2020;10: 189. doi: 10.3390/metabo10050189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh AA, Eltantawy MS, Gawish EM, Younis HH, Amber KA, El-Moneim A, et al. Impact of Dietary Organic Mineral Supplementation on Reproductive Performance, Egg Quality Characteristics, Lipid Oxidation, Ovarian Follicular Development, and Immune Response in Laying Hens Under High Ambient Temperature. Biol Trace Elem Res. 2019; 1–9. doi: 10.1007/s12011-019-01861-w [DOI] [PubMed] [Google Scholar]
- 7.Saleh AA, Kirrella AA, O Dawood MA, Ebeid TA, Ahmed Ali Saleh C. Effect of dietary inclusion of cumin seed oil on the performance, egg quality, immune response and ovarian development in laying hens under high ambient temperature. J Anim Physiol Anim Nutr. 2019;103: 1810–1817. doi: 10.1111/jpn.13206 [DOI] [PubMed] [Google Scholar]
- 8.Packer L, Witt EH, Tritschler HJ. ALPHA-LIPOIC ACID AS A BIOLOGICAL ANTIOXIDANT. Free Radic Biol Med. 1995;19: 227–250. doi: 10.1016/0891-5849(95)00017-r [DOI] [PubMed] [Google Scholar]
- 9.Sohaib M, Anjum FM, Nasir M, Saeed F, Arshad MS, Hussain S. Alpha-lipoic acid: An inimitable feed supplement for poultry nutrition. J Anim Physiol Anim Nutr (Berl). 2017;102: 33–40. doi: 10.1111/jpn.12693 [DOI] [PubMed] [Google Scholar]
- 10.Bilska A, Wodek L. Lipoic acid–the drug of the future? Pharamacological Reports. 2005;57: 570–577. [PubMed] [Google Scholar]
- 11.NRC. Nutrient Requirements of Poultry. Ninth Revi. Washington, DC: National Academies Press; 1994. [Google Scholar]
- 12.Guo ZY, Li JL, Zhang L, Jiang Y, Gao F, Zhou GH. Effects of alpha-lipoic acid supplementation in different stages on growth performance, antioxidant capacity and meat quality in broiler chickens. Br Poult Sci. 2014;55: 635–643. doi: 10.1080/00071668.2014.958057 [DOI] [PubMed] [Google Scholar]
- 13.Sah N, Kuehu DL, Khadka VS, Deng Y, Peplowska K, Jha R, et al. RNA sequencing-based analysis of the laying hen uterus revealed the novel genes and biological pathways involved in the eggshell biomineralization. Sci Rep. 2018;8: 16853. doi: 10.1038/s41598-018-35203-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma MK, Dinh T, Adhikari PA. Production performance, egg quality, and small intestine histomorphology of the laying hens supplemented with phytogenic feed additive. J Appl Poult Res. 2020. doi: 10.1016/j.japr.2019.12.001 [DOI] [Google Scholar]
- 15.Singh AK. In Ovo and Post-hatch Nutritional Programming to Improve Broiler Performance and Gut Health. University of Hawai’i at Manoa. 2019. [Google Scholar]
- 16.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic acids Res. 2013;41: e1–e1. doi: 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasti S, Sah N, Singh AK, Lee CN, Jha R, Mishra B. Dietary supplementation of dried plum: a novel strategy to mitigate heat stress in broiler chickens. J Anim Sci Biotechnol. 2021;12: 1–17. doi: 10.1186/s40104-020-00531-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87: E101–E108. doi: 10.2527/jas.2008-1339 [DOI] [PubMed] [Google Scholar]
- 19.Varasteh S, Braber S, Akbari P, Garssen J, Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10: e0138975. doi: 10.1371/journal.pone.0138975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegele H, Müller L, Buchner J. Hsp70 and Hsp90—a relay team for protein folding. In Reviews of physiology, biochemistry and pharmacology. Berlin, Heidelberg: Springer; 2004. pp. 1–44. [DOI] [PubMed] [Google Scholar]
- 21.Flees J, Rajaei-Sharifabadi H, Greene E, Beer L, Hargis BM, Ellestad L, et al. Effect of Morinda citrifolia (Noni)-enriched diet on Hepatic Heat Shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front Physiol. 2017;8: 919. doi: 10.3389/fphys.2017.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J, Tang L, Lu L, Zhang L, Xi L, Liu HC, et al. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PLoS One. 2014;9. doi: 10.1371/journal.pone.0102204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra B, Jha R. Oxidative stress in the poultry gut: Potential challenges and interventions. Frontiers in Veterinary Science. Frontiers Media S.A.; 2019. doi: 10.3389/fvets.2019.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surai PF. Antioxidant systems in poultry biology: superoxide dismutase. J Anim Res Nutr. 2016;1: 8. [Google Scholar]
- 25.Surai PF, Kochish II, Fisinin VI. Glutathione peroxidases in poultry biology: Part 1. Classification and mechanisms of action. Worlds Poult Sci J. 2018;74: 185–198. [Google Scholar]
- 26.Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424: 561. doi: 10.1038/nature01819 [DOI] [PubMed] [Google Scholar]
- 27.Li W, Wei F, Xu B, Sun Q, Deng W, Ma H, et al. Effect of stocking density and alpha-lipoic acid on the growth performance, physiological and oxidative stress and immune response of broilers. Asian-Australasian J Anim Sci. 2019;32: 1914–1922. doi: 10.5713/ajas.18.0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Senousey HK, Chen B, Wang JY, Atta AM, Mohamed FR, Nie QH. Effects of dietary Vitamin C, Vitamin E, and alpha-lipoic acid supplementation on the antioxidant defense system and immune-related gene expression in broilers exposed to oxidative stress by dexamethasone. Poult Sci. 2018;97: 30–38. doi: 10.3382/ps/pex298 [DOI] [PubMed] [Google Scholar]
- 29.Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13: 11. doi: 10.5217/ir.2015.13.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One. 2013;8: e70215. doi: 10.1371/journal.pone.0070215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montagne L, Piel C, Lalles JP. Effect of diet on mucin kinetics and composition: nutrition and health implications. Nutr Rev. 2004;62: 105–114. doi: 10.1111/j.1753-4887.2004.tb00031.x [DOI] [PubMed] [Google Scholar]
- 32.Burkholder KM, Thompson KL, Einstein ME, Applegate TJ, Patterson JA. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult Sci. 2008;87: 1734–1741. doi: 10.3382/ps.2008-00107 [DOI] [PubMed] [Google Scholar]
- 33.Sohail MU, Hume ME, Byrd JA, Nisbet DJ, Ijaz A, Sohail A, et al. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. 2012;91: 2235–2240. doi: 10.3382/ps.2012-02182 [DOI] [PubMed] [Google Scholar]
- 34.Yi D, Hou Y, Tan L, Liao M, Xie J, Wang L, et al. N-acetylcysteine improves the growth performance and intestinal function in the heat-stressed broilers. Anim Feed Sci Technol. 2016;220: 83–92. [Google Scholar]
- 35.Rivera LR, Thacker M, Pontell L, Cho HJ, Furness JB. Deleterious effects of intestinal ischemia/reperfusion injury in the mouse enteric nervous system are associated with protein nitrosylation. Cell Tissue Res. 2011;344: 111–123. doi: 10.1007/s00441-010-1126-x [DOI] [PubMed] [Google Scholar]
- 36.Wong JM, De Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40: 235–243. doi: 10.1097/00004836-200603000-00015 [DOI] [PubMed] [Google Scholar]
- 37.Jha R, Fouhse JM, Tiwari UP, Li L, Willing BP. Dietary fiber and intestinal health of monogastric animals. Front Vet Sci. 2019;6. doi: 10.3389/fvets.2019.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng Y, Yi M, Fan J, Bai Y, Ge Q, Yao G. Effects of acute intra-abdominal hypertension on multiple intestinal barrier functions in rats. Sci Rep. 2016;6: 22814. doi: 10.1038/srep22814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kisuse J, La-ongkham O, Nakphaichit M, Therdtatha P, Momoda R, Tanaka M, et al. Urban diets linked to gut microbiome and metabolome alterations in children: A comparative cross-sectional study in Thailand. Front Microbiol. 2018;9: 1345. doi: 10.3389/fmicb.2018.01345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He J, He Y, Pan DD, Cao J, Sun YY, Zeng X. Associations of gut microbiota with heat stress-induced changes of growth, fat deposition, intestinal morphology and antioxidant capacity in ducks. Front Microbiol. 2019;10: 903. doi: 10.3389/fmicb.2019.00903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin J, Zeng D, Wang H, Ni X, Yi D, Pan K, et al. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol. 2014;98: 6817–6829. doi: 10.1007/s00253-014-5752-1 [DOI] [PubMed] [Google Scholar]
- 42.Martín R, Chamignon C, Mhedbi-Hajri N, Chain F, Derrien M, Escribano-Vázquez U, et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep. 2019;9: 5398. doi: 10.1038/s41598-019-41738-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jha R, Das R, Oak S, Mishra P. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals. 2020;10: 1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spalding MD, Prigge ST. Lipoic Acid Metabolism in Microbial Pathogens. Microbiol Mol Biol Rev. 2010;74: 200–228. doi: 10.1128/MMBR.00008-10 [DOI] [PMC free article] [PubMed] [Google Scholar]