Abstract
Background
The novel coronavirus SARS-CoV2 that causes COVID-19 has resulted in the death of more than 2.5 million people, but no cure exists. Although passive immunization with COVID-19 convalescent plasma (CCP) provides a safe and viable therapeutic option, the selection of optimal units for therapy in a timely fashion remains a barrier.
Study design and methods
Since virus neutralization is a necessary characteristic of plasma that can benefit recipients, the neutralizing titers of plasma samples were measured using a retroviral-pseudotype assay. Binding antibody titers to the spike (S) protein were also determined by a clinically available serological assay (Ortho-Vitros total IG), and an in-house ELISA. The results of these assays were compared to a measurement of antibodies directed to the receptor binding domain (RBD) of the SARS-CoV2 S protein (Promega Lumit Dx).
Results
All measures of antibodies were highly variable, but correlated, to different degrees, with each other. However, the anti-RBD antibodies correlated with viral neutralizing titers to a greater extent than the other antibody assays.
Discussion
Our observations support the use of an anti-RBD assay such as the Lumit Dx assay, as an optimal predictor of the neutralization capability of CCP.
Introduction
Since its emergence in late 2019 the novel coronavirus SARS-CoV2 has crippled healthcare systems and taken the lives of at least 2.5M. With no consistently effective therapeutic interventions, the use of COVID-19 Convalescent Plasma (CCP) remains a viable treatment option [1–3]. Passive immunization relies on neutralizing antibodies that can prevent infection of respiratory epithelial cells by the virus. Although verification of virus neutralization is the gold standard for determining the therapeutic value of any single unit of CCP [4], this is not practical for the scale of the pandemic. Alternatively, antibody measurements by commercial serological assay systems currently available correlate modestly with the neutralization activity of CCP [5–7]. In general, and supported by data from our lab [8], the presence of anti-spike (S) protein antibodies is more predictive of virus neutralization than antibodies directed to other viral antigens. Among these, antibodies targeting the S protein receptor binding domain (RBD) in theory offer the best chance of viral neutralization by preventing interaction of the S protein with the ACE2 receptor on epithelial cells [9]. Here, a novel single step immunoassay, Lumit Dx SARS-CoV-2 (Promega), was used to measure RBD specific immunoglobulins in 111 convalescent plasma donors. Neutralizing titers for these samples were also determined using a retroviral pseudotype assay [10]. These antibody measures were then compared with total anti-S Ig measured with a commercial serological assay and anti-S IgG measured with in-house ELISAs.
Materials and methods
Samples and patient data
CCP donor samples were obtained from the American Red Cross with no tracible patient identifiers from donations between 4/10/202 to 12/7/2020. Plasma samples from CCP recipients were obtained as deidentified samples collected between 4/12/2020 and 12/7/2020 by the University of Wisconsin Cancer Centers biobank under a sperate IRB approved protocol for banking of COVID-19 related clinical samples. This study was reviewed and approved by the IRBs of the American Red Cross (protocol # 2020–018) and the Health Sciences IRB at the University of Wisconsin (IRB# 2020–0914) with waiver from informed consent granted.
ELISA
A modified ELISA, based on protocols published by Robbiani et al. and Routhu et al. [5, 11], were used to evaluate antibody binding to SARS-CoV-2 spike protein extracellular domain. 96-well plates were coated with 1μg/mL SARS-CoV-2 spike protein (S1+S2 ECD, Sino Biologicals, Cat. No. 40589-V08B1) in PBS and stored at 4°C overnight. Plates were washed twice with wash buffer (PBS with 0.05% Tween-20) and incubated with blocking buffer (PBS, 5% nonfat milk, 1% FBS, and 0.05% Tween-20). Plates were washed and serum/plasma samples were added at a 1:200 starting dilution followed by 7 threefold serial dilutions. Rhesus Anti-SARS CoV Spike monoclonal antibody (NHP Reagent Resource, Clone CR3022), anti-Dengue monoclonal antibody (Clone DEN3), and 6 negative control plasma samples were added to each plate for validation. After 1h at 37°C, plates were washed and incubated with anti-human IgG secondary antibody conjugated to horseradish peroxidase (HRP) (Jackson Immunoresearch, Cat.no 109-036-088, Lot: 147168) in blocking buffer (1:5000 dilution) at 37°C for 1 h. TMB substrate was added (ThermoFisher), and absorbance read at 405 nm with a plate reader (Victor X4, Perkin Elmer). To evaluate IgA and IgM binding levels, the same plates were washed and incubated with HRP inhibitor (0.02% sodium azide in blocking buffer) for 30min. Complete loss of absorbance at 405 nm with TMB under these conditions was confirmed during assay development. Plates were washed before incubating with anti-human IgA (Jakcson Immunoresearch, Cat.no 109-036-011, Lot: 148461) or IgM conjugated to HRP (1:5000 dilution) (Jackson Immunoresearch, Cat.no 109-035-129, Lot: 149274) and absorbance data collected. Area under the curve (AUC) was calculated (Graphpad Prism) as a measure of the anti-S antibody titers.
Lumit Dx immunoassay
The Promega Lumit Dx SARS-CoV-2 Immunoassay was used according to the manufacturer’s protocol for detection of antibody binding to the RBD of SARS-CoV-2 spike protein. The assay utilizes luminescence to determine antibody binding to SARS-CoV-2 RBD and was measured using a Victor X4 plate reader (Perkin Elmer).
Neutralization assay
The neutralization assay was described previously [8, 10]. 293T cells were obtained from ATCC and transduced with a retroviral vector that package a bicistronic vector encoding the ACE2 receptor and puromycin acetyl transferase. The resultant 293T-ACE2 cells were selected and maintained with D10 media (DMEM plus 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B, and 2mM L-glutamine) supplemented 0.5 mg/ml Puromycin. Expression of ACE2 was confirmed by flow cytometry using anti-ACE2 rabbit polyclonal antibody (Proteintech, Cat.no. 21115-1-AP). 293T-ACE2 cells were seeded in 96 well plates. The following day, SARS-CoV-2 convalescent serum or plasma was diluted in D10 media and mixed with retroviral particles that package a firefly luciferase reporter and pseudotyped with the SARS-CoV-2 spike protein. 1h later, the mixture was incubated with 293T-ACE2 cells for 48h. The cell lysates were then measured for luciferase activity with the Perkin Elmer Britelite system and the Victor X4 plate reader. Relative infectivity was determined by normalizing serum or plasma treated cells to those of no serum or plasma controls after subtracting signal from uninfected cells. The dilution at which the sample reduced infectivity to half the maximum was determined and reported as IC50 values.
Results
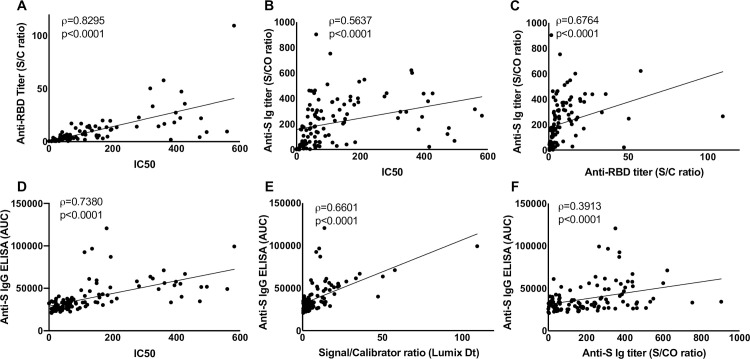
Serum or plasma samples from 111 CCP donors provided by the American Red Cross (ARC) were assayed for anti-SARS-CoV-2 S protein antibodies by the assays described. Regardless of the assay, the measures of anti-spike antibodies were highly variable. Neutralization across the samples also varied with a wide range of half-maximal neutralization titers, IC50 (range <8 to 581.4). Whereas neutralizing titers predict the efficacy of the CCP in a transfusion recipient, the assays are impractical for the scale of the pandemic. In order to evaluate the ability of commercially available test kits to predict neutralizing capability, the total Ig to the S protein (Ortho-Vitros) and to the RBD (Lumit Dx, Promega) were measured. Whereas a majority of CCP units had low titers of anti-RBD antibodies, a few samples had higher anti-RBD titers. This trend was largely similar to the neutralizing titers of these samples. In agreement with the idea that anti-RBD antibodies are usually neutralizing [9], these assays were highly correlated (ρ of 0.8295) (Fig 1A). Although the total anti S Ig as measured with the Ortho-Vitros assay also correlated reasonably with the IC50 values, the correlation coefficient was much lower (ρ = 0.5637) (Fig 1B). The Ortho-Vitros assay correlated with the anti-RBD antibody titers, as expected (ρ = 0.6764) (Fig 1C). These comparisons portray the value of the anti-RBD assay over other serological assays in identifying a CCP unit of higher neutralizing titer.
Fig 1. Correlative analysis of anti-SARS-CoV-2 immunoassays.
Assays were performed to detect anti spike- Immunoglobulin (Ig) using the Ortho-Vitros assay (represented by signal to cut-off ratios (S/CO)), or anti-spike IgG with ELISA (represented by area under the curve, AUC), anti-RBD Ig using the Lumit Dx assay (represented by sample signal to calibrator ratios (S/C)) and 50% neutralizing titers with a retroviral-pseudotype assay (represented by IC50). Analysis of correlation were performed using Spearman’s correlation test for (A) anti-RBD titer, S/C vs IC50, (B) total anti-S Ig titer, S/CO vs IC50 and(C) anti-RBD titer, S/C vs total anti-S Ig titer, S/CO. These assay results were also qualified by comparing IC50 (D), anti-RBD titer, S/C (E) and total anti-S Ig titer, S/CO (F) against an in-house anti-spike protein IgG ELISA. Spearman’s correlation coefficient, ρ, and p values are indicated for each correlation.
Anti-S IgG, IgA and IgM were also measured with an ELISA developed in-house in the 111 CCP samples. The IC50 values did not correlate well with anti-S IgA (ρ = 0.2838) and anti-S IgM titers (ρ = 0.2377). Similarly, the anti-RBD titers also did not correlate well with anti-S IgA (ρ = 0.3198) and anti-S IgM titers (ρ = 0.1708). However, anti-S IgG strongly correlated with the IC50 titer (ρ = 0.7380) (Fig 1D) and with the anti-RBD titer (ρ = 0.6601) (Fig 1E). These correlations suggest that a majority of the neutralizing antibodies are likely to be of the IgG isotype. Perhaps the most surprising comparison showed that although, the anti-S IgG (Fig 1F), IgA and IgM titers individually correlated with the total Ig titers from the Ortho-Vitros assay, these correlations were not as strong as those with the anti-RBD titers. This suggests that the Ortho-Vitros assay, which is designed to be qualitative, may not be amenable to accurate quantitation.
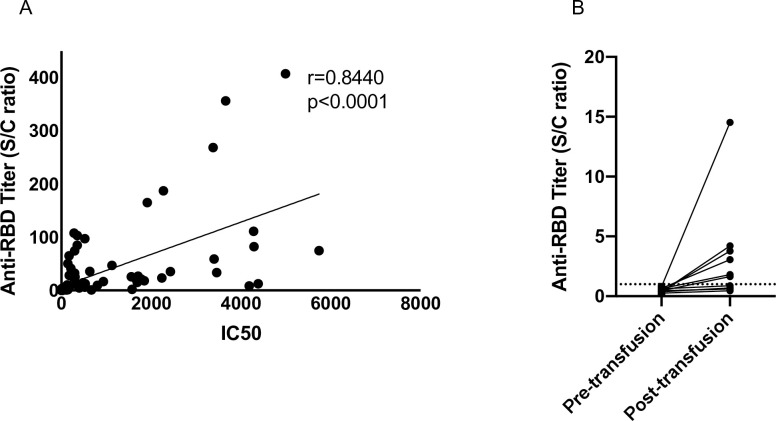
Taken together, these results confirm the idea that measuring anti-RBD antibodies is predictive of neutralizing titers to a greater degree than other assays. The therapeutic benefit of CCP may also be immediately evaluated by sampling transfusion recipients and measuring antibody titers before and after transfusion. The anti-RBD antibody titers in CCP recipients sampled longitudinally also confirmed the high degree of correlation between the two assays (ρ = 0.8440) (Fig 2A). Furthermore, by analyzing the anti-RBD titers in plasma recipients, transmissible antibody titers with the transfusion of CCP in at least 6 patients were observed (Fig 2B).
Fig 2. Neutralizing titer and anti-RBD antibody titers in CCP recipients, pre- and post- transfusion.
(A) Plasma or serum samples from CCP recipients before or after transfusion were assayed for neutralizing ability and for anti-RBD Ig titers using the Lumit Dx assay. Correlation analysis was performed using Spearman’s test and the correlation coefficient, ρ, and p values are reported. (B) Plasma or serum samples from antibody naïve CCP recipients were analyzed for anti-RBD antibodies using the Lumit Dx assay system. Dashed line indicates the criteria to determine seropositivity of CCP units.
Discussion
The antibody responses to SARS-CoV2 infection are highly variable in COVID-19 patients and several clinical characteristics have been associated with strong anti-SARS-CoV2 antibody production [12]. Although these characteristics can be used to increase chances of selecting a CCP donor with the potential to produce a robust antibody response, the identification of a CCP with therapeutic value remains cumbersome. CCP with higher antibody titers that can neutralize the virus is essential to the success of plasma transfusion [4]. Whereas analysis of neutralization is not practical in a clinical setting, several commercial assay platforms are currently available to test anti-SARS-CoV2 antibody titers. Of these, measures of anti-spike antibodies correlate best with the neutralizing titer [5, 7]. In this study, using measures of anti-spike antibodies from three different platforms, and neutralizing capability of CCP, wide range of antibody responses in COVID-19 patients was confirmed.
Since virus neutralization is a necessary attribute for therapy [4], commercial assays were evaluated to test if they can predict the neutralizing ability of CCP. Several studies have documented the correlation of neutralization titers with different measures of antibodies in COVID-19 patients and with anti-RBD antibodies in particular [5–7, 13, 14]. In studies where neutralizing titers of antibodies were not known, or were predicted with sub-optimal assay platforms, the effect of high antibody titer CCP has likely been underestimated [15, 16]. In this study, the Lumit Dx assay system was evaluated as a surrogate system for detecting anti-RBD antibodies and to predict CCP units with higher neutralization titers.
The presence of neutralizing antibodies can be detected in patients as early as one week after the onset of symptoms [17]. In approximately 1% of patients, where the neutralizing titer is extremely high within 7 days post onset of symptoms, neutralizing antibodies are detectable at high levels at least 2 months later [18]. On the other hand, in patients with lower titers early on, neutralizing activity diminishes soon after [18]. These trends are largely in line with antibody measurements against the S or the nucleocapsid protein of SARS-CoV2 [7, 14, 19, 20]. The duration of the neutralization response is an important consideration when evaluating plasma for transfusion therapy [21]. Transfusion of CCP with lower antiviral activity could result in the generation of neutralization escape variants, and also affect the likelihood of re-infection in plasma recipients. In several studies, antibody levels required to theoretically expect a positive intervention were not met [4]. In a previous study, we have reported that up to 50% of locally collected CCP units may have the neutralizing activity necessary [8] and this study extends these observations.
Other systems to measure anti-RBD are also available commercially [14]. The importance of these assay platforms lies in the global scale of the pandemic and the necessity to quickly evaluate and deploy convalescent plasma for treatment. While the efficacy of the different vaccines against newer and more contagious strains of SARS-CoV2 are evaluated, it is necessary to maximize the use of available treatment modes. To effectively make decisions to manage the scale of the pandemic, an assay platform that is quick, and deployable with limited training is crucial. Of note, the Lumit Dx assay can provide a measure of anti-RBD antibodies, and neutralizing potential, in 45 minutes and can be scaled for a high throughput format of the assay. This assay can also be automated with a liquid-handling robot to further increase throughput.
In conclusion, with the analysis of over 200 clinical samples, we demonstrate the utility of the Lumit Dx assay in predicting the neutralizing ability of CCP. The convenience of the Lumit Dx assay system makes the measurement of anti-RBD antibodies amenable to a high throughput setup with a rapid turnaround time. Despite the assay-design being qualitative, the dynamic range of the output extends over several decades and thus can be used to extrapolate a quantitative readout of anti-RBD antibody titers.
Supporting information
(XLSX)
Acknowledgments
The authors would like to thank Drs. Susan Stramer and Erin Goodhue of the American Red Cross for their assistance with providing donor samples for this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by Research and Development funds provided to Joseph Connor, MD by the University of Wisconsin Department of Pathology and Laboratory Medicine. Parts of this study were supported by the University of Wisconsin Carbone Cancer Center (UWCCC) through the use of its shared services (BioBank). These shared services are supported in part by NIH/NCI P30 CA014520- UW Comprehensive Cancer Center Support.
References
- 1.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(5):460–70. doi: 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. New England Journal of Medicine. 2021;384(7):610–8. doi: 10.1056/NEJMoa2033700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu STH, Lin H-M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study. Nature Medicine. 2020;26(11):1708–13. doi: 10.1038/s41591-020-1088-9 [DOI] [PubMed] [Google Scholar]
- 4.Katz LM. (A Little) Clarity on Convalescent Plasma for Covid-19. New England Journal of Medicine. 2021;384(7):666–8. doi: 10.1056/NEJMe2035678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–42. doi: 10.1038/s41586-020-2456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar E, Kuchipudi SV, Christensen PA, Eagar TN, Yi X, Zhao P, et al. Relationship between Anti-Spike Protein Antibody Titers and SARS-CoV-2 In Vitro Virus Neutralization in Convalescent Plasma. bioRxiv. 2020:2020.06.08.138990. doi: 10.1101/2020.06.08.138990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C, et al. Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents. medRxiv: the preprint server for health sciences. 2020:2020.08.05.20169128. doi: 10.1101/2020.08.05.20169128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janaka SK HW, Mou H, Farzan M, Stramer S, Goodhue E, Weiss J, et al. Donor Anti-Spike Immunity is Related to Recipient Recovery and Can Predict the Efficacy of Convalescent Plasma Units. Manuscript under review. 2021. doi: 10.1101/2021.02.25.21252463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zost SJ, Gilchuk P, Chen RE, Case JB, Reidy JX, Trivette A, et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nature Medicine. 2020;26(9):1422–7. doi: 10.1038/s41591-020-0998-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Jackson CB, Mou H, Ojha A, Peng H, Quinlan BD, et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nature Communications. 2020;11(1):6013. doi: 10.1038/s41467-020-19808-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Routhu NK, Gangadhara S, Cheedarla N, Shiferaw A, Rahman SA, Sahoo A, et al. Modified Vaccinia Ankara Based SARS-CoV-2 Vaccine Expressing Full-Length Spike Induces Strong Neutralizing Antibody Response. bioRxiv. 2020:2020.06.27.175166. doi: 10.1101/2020.06.27.175166 [DOI] [Google Scholar]
- 12.Wu F, Liu M, Wang A, Lu L, Wang Q, Gu C, et al. Evaluating the Association of Clinical Characteristics With Neutralizing Antibody Levels in Patients Who Have Recovered From Mild COVID-19 in Shanghai, China. JAMA Internal Medicine. 2020;180(10):1356–62. doi: 10.1001/jamainternmed.2020.4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidner L, Gänsdorfer S, Unterweger S, Weseslindtner L, Drexler C, Farcet M, et al. Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. Journal of Clinical Virology. 2020;129:104540. 10.1016/j.jcv.2020.104540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papenburg J, Cheng MP, Corsini R, Caya C, Mendoza E, Manguiat K, et al. Evaluation of a Commercial Culture-free Neutralization Antibody Detection Kit for Severe Acute Respiratory Syndrome-Related Coronavirus-2 and Comparison with an Anti-RBD ELISA Assay. medRxiv. 2021:2021.01.23.21250325. doi: 10.1101/2021.01.23.21250325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv. 2020:2020.08.12.20169359. doi: 10.1101/2020.08.12.20169359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K, Long QX, Deng HJ, Hu J, Gao QZ, Zhang GJ, et al. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/08/04. doi: 10.1093/cid/ciaa1143 ; PubMed Central PMCID: PMC7454328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature Microbiology. 2020;5(12):1598–607. doi: 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein S, Pekosz A, Park H-S, Ursin R, Shapiro J, Benner S, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. medRxiv: the preprint server for health sciences. 2020:2020.06.26.20139063. doi: 10.1101/2020.06.26.20139063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko JH, Joo EJ, Park SJ, Baek JY, Kim WD, Jee J, et al. Neutralizing Antibody Production in Asymptomatic and Mild COVID-19 Patients, in Comparison with Pneumonic COVID-19 Patients. Journal of clinical medicine. 2020;9(7). Epub 2020/07/28. doi: 10.3390/jcm9072268 ; PubMed Central PMCID: PMC7408950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Tong X, Chen H, He R, Lv Q, Yang R, et al. Characteristics and serological patterns of COVID-19 convalescent plasma donors: optimal donors and timing of donation. Transfusion. 2020;60(8):1765–72. Epub 2020/07/07. doi: 10.1111/trf.15918 ; PubMed Central PMCID: PMC7361741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.