Abstract
A series of fifteen new 2-[3-(3-chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-4(2H)-yl]-N′-arylmethyleneacetohydrazides (5a–o) were synthesized and screened for their anti-HIV-1 and cytotoxicity activity. Out of fifteen pyrazolobenzothiazine-based hydrazones, thirteen were found to be active inhibitors of HIV with EC50 values <20 μM. Moreover, the cytotoxicity results showed that most of the compounds were toxic to PBM, CEM and Vero cell lines. This information could be used for structural modifications to acquire good candidates of HIV drugs.
Keywords: 1,2-Benzothiazine 1,1-dioxides; Pyrazolobenzothiazine; Anti-HIV-1; Cytotoxicity activity
Introduction
Benzothiazine family consists of many structural isomers having varied positions of S and N atoms in the ring. Interestingly, all the isomers have found their applications in various biological activities. The carboxamide derivatives of 1,2-benzothiazine 1,1-dioxides are frequently reported for their analgesic and anti-inflammatory potential (Gannarapu et al., 2014). The oxicam family of nonsteroidal anti-inflammatory drugs (NSAIDs) is in use for the treatment of various kinds of inflammatory disorders (Olkkola et al., 1994; Katzmann, 1990; Hadadzadeh et al., 2012; Balfour et al., 1996). Various other derivatives of benzothiazine family have been reported as antimicrobial (Abbas and Farghaly, 2010), antimalarial (Barazarte et al., 2008), anti-inflammatory (Gannarapu et al., 2014), anti-tumor/anti-cancer agents (Coughlin et al., 1995; Abbas and Farghaly, 2010; Zięba et al., 2013; Peng et al., 2011; Kamel et al., 2010; Coughlin et al., 1995), antiviral (Aslam et al., 2014a, b) and acetylcholinesterase inhibitors (Aslam et al., 2014c).
We had been involved in the syntheses of many new libraries of benzothiazine derivatives having anti-HIV activities (Ahmad et al., 2014; Aslam et al., 2014a, b). Human immunodeficiency virus (HIV) is a continuous threat for human being. Since its discovery in 1983, various ways have been devised to treat it. Just after 2 years of its discovery, zidovudine (AZT) was invented. A better understanding of viral life cycle led to develop better methods for its growth inhibition. HIV is now being controlled with highly active antiretroviral therapy (HAART). HAART consists of a combination of various antiviral drugs, each inhibiting a different stage of viral life cycle. By now, HAART has improved the quality of life of patients living with HIV and has controlled both morbidity and mortality rate (Clercq, 2007). Literature reveals a good number of heterocyclic compounds as nonnucleoside reverse transcriptase inhibitors (NNRTIs) (Fig. 1; Xu et al., 2014; Valuev-Elliston et al., 2012; Vadivelan et al., 2011; Reed and Daar, 2006; Meng et al., 2014; Kukhanova, 2012; Jochmans, 2008; He et al., 2005; El Safadi et al., 2007; Mehellou, 2010). There is still a need for the development of more effective drugs with better selectivity and less toxicity. The current study deals with the anti-HIV potential of a novel series of pyrazolobenzothiazine dioxides. Pyrazolobenzothiazine ring system has been constructed on 1,2-benzothiazine ring with the hope to get a synergistic effect (Scheme 1).
Fig. 1.
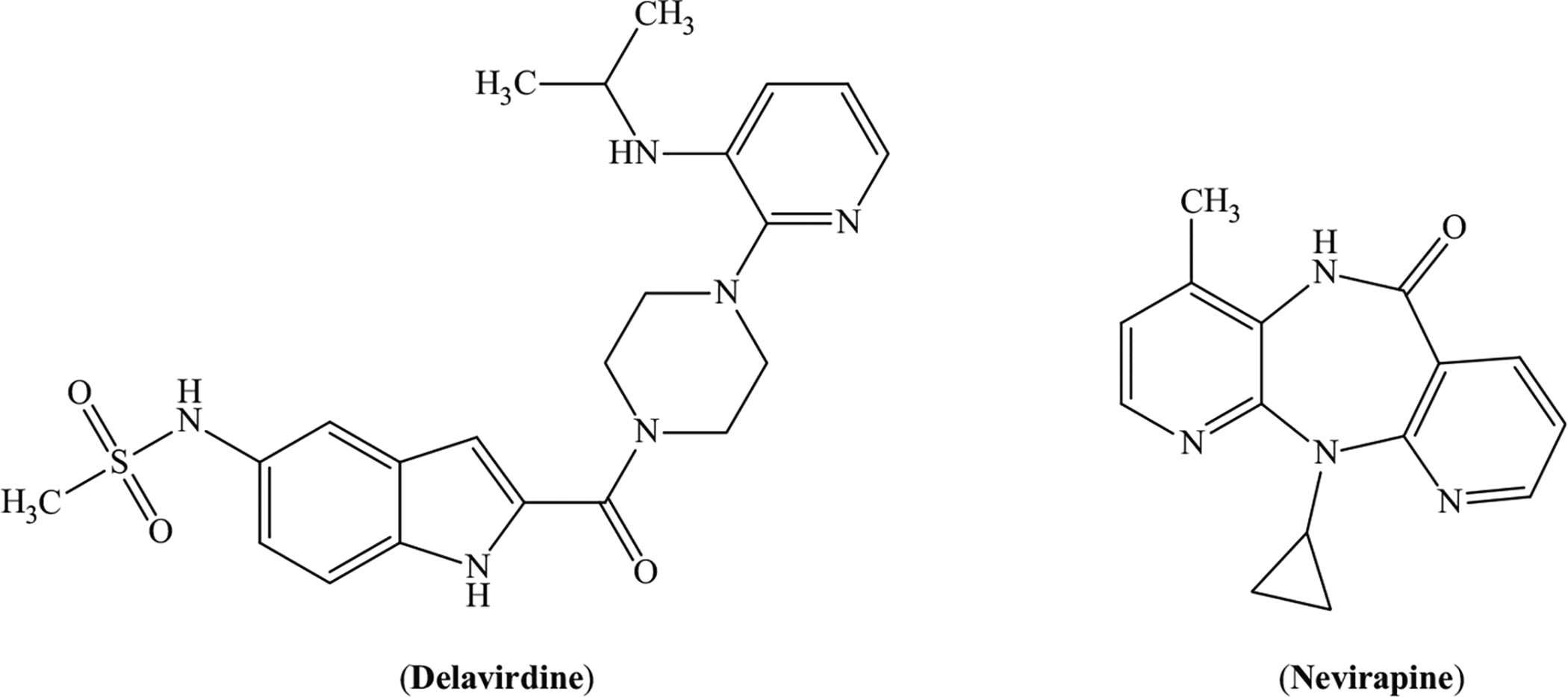
Structures of delavirdine and nevirapine, FDA-approved anti-HIV NNRTIs
Scheme 1.
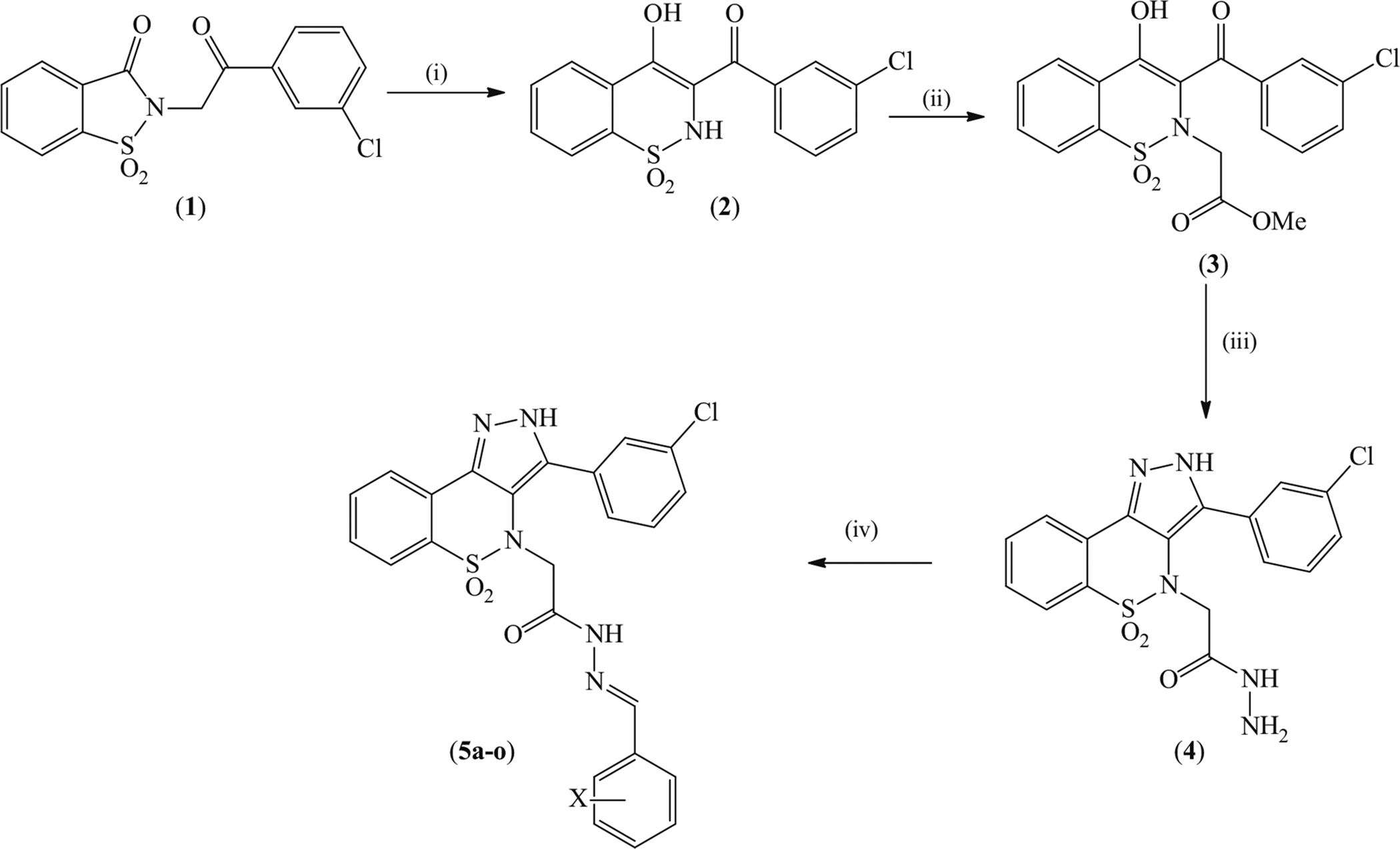
Synthesis of 2-[3-(3-chlorophenyl)-5,5-dioxidobenzo[e] pyrazolo[4,3-c][1,2]thiazin-4(2H)-yl]-N′-arylmethyleneacetohydrazides. Reagents and conditions: (i) NaOMe/MeOH, reflux, 40 min. (ii) ClCH2CO2Me/MeCN/K2CO3, reflux, 10 h. (iii) NH2NH2·H2O/EtOH, reflux, 4 h. (iv) ArCHO/cat. AcOH/EtOH, reflux, 4–5 h
Experimental work
All the chemicals were purchased from Alfa Aesar and were used without purification. 1H NMR spectra were recorded on a Bruker DPX-400 instrument at 400 MHz. Chemical shifts are reported in ppm referenced to the residual solvent signal. FT-IR spectra were recorded on a Thermo Nicolet IR 200 spectrometer. Melting points were recorded on a Gallenkamp melting point apparatus and are uncorrected. NMR spectra were recorded on Bruker DPX nuclear magnetic resonance spectrometer (400 MHz). Low-resolution electron impact mass spectra were recorded on MAT312 AND MASPEC system (msw/A091). X-ray crystallography of crystalline compound was carried out on Bruker–Nonius Kappa CCD diffractometer, while SHELXTL and SHELXL97 software was used for its data analysis. (3-Chlorophenyl)(4-hydroxy-1,1-dioxido-2H-benzo[e][1,2] thiazin-3-yl)methanone (2) was synthesized from saccharin by reacting with 3-chlorophenacyl bromide followed by Gabriel Colman rearrangement (Khalid et al., 2010a, b).
Methyl 2-[3-(3-chlorobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl]acetate (3)
(3-Chlorophenyl) (4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-3-yl)methanone (2) (6.0 g, 20 mmol) was dissolved in acetonitrile (40 mL), and anhydrous potassium carbonate (3.31 g, 24 mmol) was added in the solution. Methyl chloroacetate (2.60 g, 24 mmol) was added, and the reaction mixture was heated under reflux for 10 h. The completion of reaction was monitored by TLC. The solvent was removed under vacuum. The residues were dissolved in cold water and neutralized with diluted hydrochloric acid to get the pale yellow crystals of the product which were filtered and dried. Yield: 82 %; m.p. 143 °C; FT-IR (KBr) vmax/(cm−1) : 2961, 1754, 1328, 1179, 801, 748; 1H NMR: (DMSO-d6, 400 MHz) δ: 3.31 (s, 3H, O-CH3), 3.97 (s, 2H, N-CH2), 7.63 (t, 1H, J = 8.0 Hz, Ar–H), 7.74 (d, 1H, J = 8.0 Hz, Ar–H), 7.92–7.96 (m, 5H, Ar–H), 8.16 (t, 1H, J = 4.2 Hz, Ar–H), 14.65 (s, 1H, O–H); EI-MS: m/z: 409 (11 %, M + 2], 407 (28, M+), 330 (6), 284 (24), 244 (11), 139 (100).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl)]acetohydrazide (4)
A mixture of methyl 2-[3-(3-chlorobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl]acetate (3) (5.0 g, 13.4 mmol) and hydrazine monohydrate (4.9 mL, 99 mmol) in ethanol (50 mL) was refluxed for four hours. The unreacted hydrazine monohydrate and ethanol were removed under vacuum. The residue was treated with ice-cold water. The precipitates were filtered and recrystallized from ethanol. Light yellow; Yield: 73 %. m.p: 267 °C; FT-IR (KBr) vmax/(cm−1) : 3432, 3375, 1687, 1333, 1162, 783, 763; 721 cm−1; 1H NMR: (DMSO-d6, 400 MHz) δ: 4.06 (s, 2H(53%), CH2), 4.10 (s, 2H(47%), CH2), 4.48 (s(broad), 2H, NH2), 7.59–7.64 (m, 3H, Ar–H), 7.79–7.83 (m, 4H, Ar–H), 7.99 (d, 1H(53%), J = 7.2 Hz, Ar–H), 8.02 (d, 1H(47%), J = 7.6 Hz, Ar–H), 8.97(s, 1H, N–H), 13.87(s, 1H(53%), N–H), 14.23 (s, 1H(47%), N–H); EI-MS: m/z 405 (7 %, M + 2), 403 (21, M+), 331 (67), 309 (4), 269 (80), 217 (21), 138 (60), 104 (100).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-arylmethyleneacetohydrazides (5a–o)
Equimolar quantities of acetohydrazide 4 and benzaldehydes were refluxed in ethyl alcohol for 4–5 h. After completion of reaction, as indicated by TLC, the contents of the flask were concentrated on rotary evaporator. The precipitates were filtered, washed with excess ethanol and dried.
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-(4-fluorobenzylidene)acetohydrazide (5a)
Off white powder; m.p. 249 °C; FT-IR (KBr) vmax/(cm−1) : 3714, 3309, 1687, 1599, 1325,1153 cm−1; 1H NMR: (DMSO-d6, 400 MHz) δ: 4.21 (s, 2H(53%), N-CH2), 4.27 (s, 2H(47%), N-CH2), 7.18 (t, 2H, J = 8.4 Hz, Ar–H), 7.41 (s, 1H, Ar–H), 7.52–7.65 (m, 3H, Ar–H), 7.75–7.85 (m, 5H, CH, Ar–H), 7.88 (d, 1H(59%), J = 11.6 Hz, Ar–H), 8.0 (d, 1H(31%), J = 6.4 Hz, Ar–H), 11.25 (s, 1H(69%), N–H), 11.35 (s, 1H(31%), N–H), 13.88 (s, 1H(53%), N–H), 14.24 (s, 1H(47%), N–H); 13C NMR(DMSO-d6, 100 MHz) δ: 51.6 (CH2), 115.5 (Ar–C), 118.1 (Ar–C), 122.7 (Ar–C), 124.4 (Ar–C), 125.6 (Ar–C), 127.4 (Ar–C), 128.7 (Ar–C), 129.1 (Ar–C), 129.5 (Ar–C), 130.8 (Ar–C), 132.5 (Ar–C), 132.7 (Ar–C), 133.1 (Ar–C), 134.2 (Ar–C), 134.5 (Ar–C), 134.9 (Ar–C), 135.4 (Ar–C), 135.7 (Ar–C), 137.5 (Ar–C), 139.1 (Ar–C), 142.8 (Ar–C), 149.8 (=CH), 168.9 (C=O); EI-MS (m/z): 511 (1 %, M + 2), 509 (3, M+), 445 (1), 326 (11), 280 (100), 217 (22).
N′-(3-Chlorobenzylidene)-2-[3-(3-chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-4(2H)-yl]acetohydrazide (5b)
Off white powder; m.p. 233 °C; FT-IR (KBr) vmax/(cm−1) : 3707, 3305, 1693, 1609, 1326, 1158 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ: 4.19 (s, 2H(53%), N-CH2), 4.26 (s, 2H(47%), N-CH2), 7.03 (d, 2H, J = 6.8 Hz, Ar–H), 7.50–7.57 (m, 3H, Ar–H), 7.63 (s, 1H, Ar–H), 7.74 (s, 1H, C–H), 7.81–7.85 (m, 4H, Ar–H), 8.01–8.03 (m, 2H, Ar–H), 11.38 (s, 1H(69%), N–H), 11.48 (s, 1H, N–H), 13.90 (s, 1H(53%), N–H), 14.25 (s, 1H(47%), N–H); 13C NMR (DMSO-d6, 100 MHz) δ: 51.3 (CH2), 121.1 (Ar–C), 122.5 (Ar–C), 123.8 (Ar–C), 124.5 (Ar–C), 125.5 (Ar–C), 127.3 (Ar–C), 128.8 (Ar–C), 129.3 (Ar–C), 129.7 (Ar–C), 130.9 (Ar–C), 132.5 (Ar–C), 132.9 (Ar–C), 133.4 (Ar–C), 133.8 (Ar–C), 134.2 (Ar–C), 134.5 (Ar–C), 134.7 (Ar–C), 135.5 (Ar–C), 135.9 (Ar–C), 137.7 (Ar–C), 142.9 (Ar–C), 148.7 (=CH), 169.1 (C=O); EI-MS (m/z): 526 (1 %, M+), 491(3), 326 (3), 280 (100), 217 (9).
N′-(4-Chlorobenzylidene)-2-[3-(3-chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-4(2H)-yl]acetohydrazide (5c)
Off white powder; m.p: 267 °C; FT-IR (KBr) vmax/(cm−1) : 3747, 3315, 1685, 1609, 1328, 1155 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ: 4.22 (s, 2H(53%), N-CH2), 4.27(s, 2H(47%), N-CH2), 7.39–7.42 (m, 3H, Ar–H), 7.52–7.67 (m, 4H, Ar–H), 7.75–7.87 (m, 5H, CH, Ar–H), 7.99 (d, 1H(53%), J = 7.2, Ar–H), 8.06 (d, 1H(47%), J = 7.2 Hz, Ar–H), 11.32 (s, 1H(69%), N–H), 11.42 (s, 1H(31%), N–H), 13.89 (s, 1H(53%), N–H) 14.25 (s, 1H(47%), N–H); 13C NMR (DMSO-d6, 100 MHz) δ: 51.4 (CH2), 121.0 (Ar–C), 122.7 (Ar–C), 123.7 (Ar–C), 124.4 (Ar–C), 125.7 (Ar–C), 127.1 (Ar–C), 128.7 (Ar–C), 129.1 (Ar–C), 129.3 (Ar–C), 129.8 (Ar–C), 130.8 (Ar–C), 132.5 (Ar–C), 132.8 (Ar–C), 133.7 (Ar–C), 134.1 (Ar–C), 134.5 (Ar–C), 134.7 (Ar–C), 135.4 (Ar–C), 135.7 (Ar–C), 137.9 (Ar–C), 142.8 (Ar–C), 149.1 (=CH), 169.3 (C=O); EI-MS (m/z): 527 (2.4 %, M + 2), 525 (2.8, M+), 405 (3), 326 (12), 280 (100), 217(17).
N′-(3-Bromobenzylidene)-2-[3-(3-chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-4(2H)-yl]acetohydrazide (5d)
Off white crystalline; m.p. 239 °C; FT-IR (KBr) vmax/(cm−1) : 3741, 3303, 1694, 1609, 1326, 1158 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ: 4.19 (s, 2H(53%), N-CH2), 4.26 (s, 2H(47%), N-CH2), 7.24–7.32 (m, 2H, Ar–H), 7.59–7.62 (m, 3H, Ar–H), 7.69 (s, 1H, Ar–H),7.73 (s, 1H, CH), 7.82–7.89 (m, 4H, Ar–H), 8.01–8.04 (m, 2H, Ar–H), 11.38 (s, 1H(41%), NH), 11.48 (s, 1H(59%), NH), 13.89 (s, 1H(53%), NH), 14.24 (s, 1H(47%), NH); 13C NMR(DMSO-d6, 100 MHz) δ: 51.6 (CH2), 117.3 (Ar–C), 121.5 (Ar–C), 123.8 (Ar–C), 124.5 (Ar–C), 125.9 (Ar–C), 127.4 (Ar–C), 128.6 (Ar–C), 128.9 (Ar–C), 129.4 (Ar–C), 129.8 (Ar–C), 131.1 (Ar–C), 132.3 (Ar–C), 132.7 (Ar–C), 133.3 (Ar–C), 134.2 (Ar–C), 134.4 (Ar–C), 134.7 (Ar–C), 135.5 (Ar–C), 135.8 (Ar–C), 137.6 (Ar–C), 142.7 (Ar–C), 144.1 (=CH), 169.9 (C=O); EI-MS (m/z): 451 (21 %), 326 (1), 280 (100), 217 (15).
N′-(4-Bromobenzylidene)-2-(3-(3-chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-4(2H)-yl)acetohydrazide (5e)
Off white powder; m.p. 259 °C; FT-IR (KBr) vmax/(cm−1) : 3737, 3325, 1685, 1609, 1328, 1154 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ: 4.23 (s, 2H53%, N-CH2), 4.28 (s, 2H47%, N-CH2), 7.31 (t, 2H, J = 7.2 Hz, Ar–H), 7.51–7.59 (m, 3H, Ar–H), 7.71 (s, 1H, Ar–H),7.73 (s, 1H, CH), 7.82–7.86 (m, 5H, Ar–H), 8.03, 8.06 (d, 1H(31%), J1 = 7.2 Hz, Ar–H), 8.06 (d, 1HB(69%), J = 7.2 Hz, Ar–H), 11.32 (s, 1HA(53%), NH), 11.42 (s, 1HB(47%), NH), 13.90 (s, 1HA(53%), NH), 14.26 (s, 1HB(47%), NH); 13C NMR (DMSO-d6, 100 MHz) δ: 51.4 (CH2), 119.5 (Ar–C), 122.3 (Ar–C), 123.9 (Ar–C), 124.5 (Ar–C), 125.7 (Ar–C), 127.3 (Ar–C), 128.7 (Ar–C), 129.1 (Ar–C), 129.9 (Ar–C), 130.9 (Ar–C), 132.2 (Ar–C), 132.8 (Ar–C), 133.2 (Ar–C), 133.7 (Ar–C), 134.1 (Ar–C), 134.5 (Ar–C), 134.8 (Ar–C), 135.4 (Ar–C), 135.9 (Ar–C), 137.7 (Ar–C), 142.9 (Ar–C), 147.7 (=CH), 170.1 (C=O); EI-MS: m/z 568.1 (M+), 570.1(M++2).570 (2 %, M+), 451 (5), 326 (18), 280 (100), 217 (23).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-(2,4-dichlorobenzylidene)acetohydrazide (5f)
Light yellow powder; m.p. 251 °C; FT-IR (KBr) vmax/(cm−1) : 3648, 3313, 1686, 1601, 1324, 1157 cm−1. 1H NMR (DMSO-d6, 400 MHz) δ: 4.21 (s, 2H(57%), N-CH2), 4.27 (s, 2H(43%), N-CH2), 7.36–7.61 (m, 5H, Ar–H), 7.65 (s, 1H, CH), 7.74–7.88 (m, 5H, Ar–H), 8.00 (d, 1H(44%), J = 7.2 Hz, Ar–H), 8.07 (d, 1H(66%), J = 8.0 Hz, Ar–H), 8.09 (s, 1H, Ar–H), 11.47 (s, 1H(29%), NH), 11.62 (s, 1H(71%), NH), 13.88 (s, 1H(53%), NH), 14.25 (s, 1H(47%), NH). 13C NMR(DMSO-d6, 400 MHz) δ: 51.3, 122.8, 123.9, 124.7, 125.9, 127.1, 127.8, 128.1, 128.7, 129.0, 129.5, 129.8, 131.8, 132.5, 132.7, 134.1, 134.2, 134.5, 134.9, 135.3, 135.5, 137.7, 139.8, 142.8, 149.1, 170.7. EI-MS (m/z): 561 (2, M + 2), 559 (1, M+), 440 (5), 326 (16), 280 (100), 217 (22).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-(2,6-dichlorobenzylidene)acetohydrazide (5g)
Dirty yellow powder; m.p. 277 °C; FT-IR (KBr) vmax/(cm−1) : 3737, 3222, 1692, 1606, 1324, 1158 cm−1. 1H NMR (DMSO-d6, 400 MHz) δ: 4.18 (s, 2H(56%), N-CH2), 4.24 (s, 2H(44%), N-CH2), 7.27 (t, 2H, J = 7.2 Hz, Ar–H), 7.52–7.57 (m, 3H, Ar–H), 7.67–7.71 (m, 4H, Ar–H), 7.73 (s, 1H, CH), 7.83 (s, 1H, Ar–H), 8.03 (d, 1H(35%), J = 6.8 Hz, Ar–H), 8.06 (d, 1H(65%), J = 7.2 Hz, Ar–H), 11.46 (d, 1H(61%), J = 4.0 Hz, N–H), 11.56 (d, 1H(39%), J = 4.0 Hz, N–H), 13.88 (s, 1H(53%), N–H), 14.23 (s, 1H(47%), N–H). 13C NMR (DMSO-d6, 100 MHz) δ: 51.3 (CH2), 122.9 (Ar–C), 124.0 (Ar–C), 124.6 (Ar–C), 125.8 (Ar–C), 127.1 (Ar–C), 127.7 (Ar–C), 128.0 (Ar–C), 128.6 (Ar–C), 129.4 (Ar–C), 129.9 (Ar–C), 131.7 (Ar–C), 132.4 (Ar–C), 132.8 (Ar–C), 134.2 (Ar–C), 134.5 (Ar–C), 134.8 (Ar–C), 138.7 (2 Ar–C), 139.7 (Ar–C), 142.7 (Ar–C), 149.5 (=CH), 170.9 (C=O); EI-MS (m/z): 440 (7 %), 326 (11), 280 (100), 217 (22).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-(3-methoxybenzylidene)acetohydrazide (5h)
White powder; m.p. 225 °C; FT-IR (KBr) vmax/(cm−1) : 3715, 3298, 1694, 1606, 1328, 1157 cm−1;1H NMR (DMSO-d6, 400 MHz) δ: 3.73 (s, 3H, O-CH3), 4.23 (s, 2H(53%), N-CH2), 4.29 (s, 2H(47%), N-CH2), 6.94 (d, 1H, J = 5.6 Hz, Ar–H), 7.00 (s, 1H, Ar–H), 7.25 (t, 2H, J = 7.6 Hz, Ar–H), 7.47–7.69 (m, 3H, Ar–H), 7.74 (s, 1H, CH), 7.77–7.89 (m, 4H, Ar–H), 8.00 (d, 1H(33%), J = 7.2 Hz, Ar–H), 8.06 (d, 1H(67%), J = 7.6 Hz, Ar–H), 11.25 (d, 1H(61%), J = 6.0 Hz, N–H), 11.35 (d, 1H(39%), J = 6.4 Hz, N–H), 14.25 (s, 1H(53%), N–H), 14.26 (s, 1H(47%), N–H); 13C NMR(DMSO-d6, 100 MHz) δ: 48.7 (OCH3), 51.7 (CH2), 111.4 (Ar–C), 116.7 (Ar–C), 122.8 (Ar–C), 124.3 (Ar–C), 125.7 (Ar–C), 127.6 (Ar–C), 128.1 (Ar–C), 128.7 (Ar–C), 129.5 (Ar–C), 129.7 (Ar–C), 130.9 (Ar–C), 132.5 (Ar–C), 132.7 (Ar–C), 134.1 (Ar–C), 134.4 (Ar–C), 134.7 (Ar–C), 135.0 (Ar–C), 135.4 (Ar–C), 137.8 (Ar–C), 139.5 (Ar–C), 142.6 (=CH), 161.4 (Ar–C–OCH3), 168.9 (C=O); EI-MS (m/z): 521 (1 %, M+), 326 (5), 280 (100), 217 (12).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-(2,3,4-trimethoxybenzylidene) acetohydrazide (5i)
Off white powder; m.p. 205 °C; FT-IR (KBr) vmax/(cm−1) : 3619, 3250, 1682, 1588, 1324, 1156 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ: 3.76 (s, 9H, O-CH3), 4.19 (s, 2H(53%), N-CH2), 4.25 (s, 2H(47%), N-CH2), 6.76 (d, 1H, J = 8.8 Hz, Ar–H), 6.78 (s, 1H, CH), 7.54–7.87 (m, 6H, Ar–H), 7.93 (s, 1H, Ar–H) 7.99–8.06 (m, 2H, Ar–H), 11.24 (s, 1H(72%), N–H), 11.31 (s, 1H(28%), N–H), 13.88 (s, 1H(53%), N–H), 14.25 (s, 1H(47%), N–H). 13C NMR (DMSO-d6, 100 MHz) δ: 50.1 (2 × OCH3), 51.7 (CH2), 51.8 (OCH3), 106.7 (2 × Ar–C), 112.5 (Ar–C), 122.7 (Ar–C), 124.4 (Ar–C), 125.9 (Ar–C), 127.8 (Ar–C), 128.3 (Ar–C), 128.7 (Ar–C), 129.4 (Ar–C), 129.8 (Ar–C), 132.5 (Ar–C), 134.2 (Ar–C), 134.5 (Ar–C), 134.7 (Ar–C), 135.1 (Ar–C), 135.5 (Ar–C), 137.7 (Ar–C), 142.5 (Ar–C), 149.7 (=CH), 161.1 (2 × Ar–C), 168.7 (C=O); EI-MS (m/z): 516 (1 %, M+), 461 (14), 326 (18), 280 (38), 217 (11).
2-[3-(3-chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-(3,4,5-trimethoxybenzylidene) acetohydrazide (5j)
Off white powder; m.p. 243 °C; FT-IR (KBr) vmax/(cm−1) : 3618, 3230, 1692, 1604, 1323, 1121 cm−1;1H NMR: (DMSO-d6, 400 MHz) δ: 3.75 (s, 9H, O–CH3), 4.24 (s, 2H(53%), N–CH2), 4.29 (s, 2H(47%), N–CH2), 6.79 (s, 2H, Ar–H), 6.84 (s, 1H, CH), 7.45–7.70 (m, 3H, Ar–H), 7.77–7.86 (m, 4H, Ar–H), 8.01 (d, 1H(42%), J = 6. 8 Hz, Ar–H), 8.06 (d, 1H(58%), J = 6.8 Hz, Ar–H), 11.09 (s, 1H(72%), N–H), 11.26 (s, 1H(28%), N–H), 13.84 (s, 1H(53%), N–H), 14.24 (s, 1H(47%), N–H); 13C NMR(DMSO-d6, 400 MHz) δ: 49.9(2 × OCH3), 51.5 (CH2), 51.7 (OCH3), 113.7 (Ar–C), 115.9 (Ar–C), 122.6 (Ar–C), 124.5 (Ar–C), 125.8 (Ar–C), 127.6 (Ar–C), 128.4 (Ar–C), 128.9 (Ar–C), 129.5 (Ar–C), 129.8 (Ar–C), 132.4 (Ar–C), 132.7 (Ar–C), 134.3 (Ar–C), 134.5 (Ar–C), 134.8 (Ar–C), 135.0 (Ar–C), 135.5 (Ar–C), 137.5 (Ar–C), 142.7 (Ar–C), 149.8 (=CH), 151.7 (Ar–C), 168.4 (C=O); EI-MS (m/z): 583 (1, M + 2), 581 (3 %, M+), 461 (13), 326 (5), 280 (100), 217 (14).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-(2-nitrobenzylidene)acetohydrazide (5k)
Pale yellow powder; m.p. 263 °C; FT-IR (KBr) vmax/(cm−1) : 3737, 3226, 1688, 1588, 1322, 1156 cm−1;1H NMR (DMSO-d6, 400 MHz) δ: 4.24 (s, 2H(54%), N–CH2), 4.30 (s, 2H(46%), N–CH2), 7.48–7.69 (m, 6H, Ar–H), 7.76–7.88 (m, 4H, CH, Ar–H), 7.97–8.07 (m, 2H, Ar–H), 8.15 (s, 1H, Ar–H) 11.55 (s, 1H(70%), NH), 11.68 (s, 1H(30%), NH), 13.91 (s, 1H(53%), NH), 14.27 (s, 1H(47%), NH); 13C NMR (DMSO-d6, 100 MHz) δ: 51.8 (CH2), 122.7 (Ar–C), 124.0 (Ar–C), 124.7 (Ar–C), 125.7 (Ar–C), 127.7 (Ar–C), 128.0 (Ar–C), 128.3 (Ar–C), 128.7 (Ar–C), 129.4 (Ar–C), 129.8 (Ar–C), 131.7 (Ar–C), 132.5 (Ar–C), 132.8 (Ar–C), 134.1 (Ar–C), 134.6 (Ar–C), 134.8 (Ar–C), 135.5 (Ar–C), 135.9 (Ar–C), 137.4 (Ar–C), 139.7 (Ar–C), 142.6 (Ar–C), 148.8 (=CH), 171.1 (C=O); EI-MS (m/z):536 (2 %, M+), 534 (4 %, M+), 326 (22), 280 (100), 217 (24).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-(3-nitrobenzylidene)acetohydrazide (5l)
Yellow powder; m.p. 203 °C; FT-IR (KBr) vmax/(cm−1) : 3723, 3319, 1689, 1599, 1328, 1158 cm−1;1H NMR (DMSO-d6, 400 MHz) δ: 4.22 (s, 2H(53%), N-CH2), 4.28 (s, 2H(47%), N-CH2), 7.47 (d, 2H, J = 7.2 Hz, Ar–H), 7.51–7.59 (m, 3H, Ar–H), 7.70 (s, 1H, Ar–H),7.78–7.85 (m, 4H, Ar–H), 8.01 (s, 1H, C–H), 8.05 (d, 1H(60%), J = 8.0 Hz, Ar–H), 8.07 (d, 1H(40%), J = 7.6 Hz, Ar–H), 8.25 (s, 1H, Ar–H), 11.65 (s, 1H(74%), NH), 11.75 (s, 1H(36%), NH), 13.74 (s, 1H(53%), NH), 14.03 (s, 1H(47%), NH); 13C NMR(DMSO-d6, 100 MHz) δ: 51.7 (CH2), 122.5 (Ar–C), 124.1 (Ar–C), 124.6 (Ar–C), 125.8 (Ar–C), 127.7 (Ar–C), 128.1 (Ar–C), 128.4 (Ar–C), 128.8 (Ar–C), 129.3 (Ar–C), 129.7 (Ar–C), 131.9 (Ar–C), 132.4 (Ar–C), 132.6 (Ar–C), 134.2 (Ar–C), 134.5 (Ar–C), 134.7 (Ar–C), 135.4 (Ar–C), 135.8 (Ar–C), 137.5 (Ar–C), 139.8 (Ar–C), 142.7 (Ar–C), 149.9 (=CH), 170.9 (C=O); EI-MS (m/z): 418 (2 %), 416 (9), 326 (1), 280 (100), 217 (9).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-[4-nitrobenzylidene]acetohydrazide (5m)
Light yellow powder; m.p. 273 °C; FT-IR (KBr) vmax/(cm−1) : 3748, 3321, 1688, 1587, 1327, 1155 cm−1;1H NMR (DMSO-d6, 400 MHz) δ: 4.19 (s, 2H(53%), N-CH2), 4.25 (s, 2H(47%), N-CH2), 7.28 (t, 2H, J = 9.4 Hz, Ar–H), 7.55–7.63 (m, 4H, Ar–H), 7.71 (s, 1H, Ar–H), 7.76–7.83 (m, 4H, Ar–H), 8.01 (s, 1H, C–H), 8.05 (d, 1H(44%), J = 7.6 Hz, Ar–H), 8.07 (d, 1H(66%), J = 7.6 Hz, Ar–H), 11.56 (s, 1H(61%), N–H), 11.66 (s, 1H(39%), N–H), 13.91 (s, 1H(53%), N–H), 14.36 (s, 1HB(47%), N–H); 13C NMR (DMSO-d6, 100 MHz) δ: 51.7 (CH2), 122.7 (Ar–C), 124.3 (Ar–C), 124.8 (Ar–C), 125.7 (Ar–C), 127.7 (Ar–C), 128.3 (Ar–C), 128.5 (Ar–C), 128.7 (Ar–C), 129.5 (Ar–C), 129.9 (Ar–C), 132.4 (Ar–C), 132.7 (Ar–C), 134.3 (Ar–C), 134.5 (Ar–C), 134.8 (Ar–C), 135.0 (Ar–C), 135.7 (Ar–C), 135.9 (Ar–C), 137.6 (Ar–C), 139.7 (Ar–C), 142.5 (Ar–C), 151.3 (=CH), 170.0 (C=O); EI-MS (m/z): 536 (6 %, M+), 416 (4), 326 (24), 280 (100), 217 (31).
4-[(2-(2-(3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo [4,3-c][1,2]thiazin-4(2H)-yl)acetyl)hydrazono)methyl]benzoic acid (5n)
Off white powder; m.p. 247 °C; FT-IR (KBr) vmax/(cm−1) : 3723, 3316, 3074, 1733, 1696, 1602, 1325, 1158 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ: 4.26 (s, 2H, N-CH2), 7.47–7.66 (m, 5H, CH, Ar–H), 7.82–8.04 (m, 8H, Ar–H), 11.41 (s, 1H(67%), NH), 11.51 (s, 1H(33%), NH), 13.07(s, 1H, OH), 13.90 (s, 1H(55%), NH), 14.26 (s, 1H(45%), NH); 13C NMR (DMSO-d6, 100 MHz) δ: 51.7 (CH2), 122.7 (Ar–C), 124.3 (Ar–C), 124.7 (Ar–C), 125.6 (Ar–C), 127.5 (Ar–C), 128.1 (Ar–C), 128.5 (Ar–C), 128.8 (Ar–C), 129.4 (Ar–C), 129.8 (Ar–C), 130.7 (Ar–C), 132.2 (Ar–C), 132.5 (Ar–C), 134.2 (Ar–C), 134.6 (Ar–C), 134.8 (Ar–C), 135.1 (Ar–C), 135.6 (Ar–C), 135.9 (Ar–C), 137.5 (Ar–C), 139.6 (Ar–C), 142.7 (=CH), 167.3 (C=O), 171.1 (COOH); EI-MS (m/z): 415 (4 %), 326 (5), 280 (52), 217 (10), 76(100).
2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazin-4(2H)-yl]-N′-[4-(dimethylamino)benzylidene] acetohydrazide (5o)
Lemon yellow powder; m.p. 269 °C; FT-IR (KBr) vmax/(cm−1) : 3747, 3322, 1685, 1598, 1322, 1154 cm−1;1H NMR (DMSO-d6, 400 MHz) δ: 2.92 (s, 6H, N–CH3), 4.18 (s, 2H(53%), N–CH2), 4.24 (s, 2H(47%), N–CH2), 6.61–6.64 (m, 2H, Ar–H), 7.32, 7.34 (dd, 1H, J1 = 4.6 Hz, J2 = 3.2 Hz, Ar–H), 7.50–7.89 (m, 10H, Ar–H), 7.99 (d, 2H(40%), J = 7.6 Hz, Ar–H), 8.05 (d, 2H(60%), J = 8.0 Hz, Ar–H), 10.91 (s, 1H(67%), NH), 10.92 (s, 1H(36%), NH), 13.87 (s, 1H(50%), NH), 14.22 (s, 1H(50%), NH); 13C NMR(DMSO-d6, 100 MHz) δ: 41.7(2CH3), 51.5 (CH2), 115.6(2 × Ar–C), 122.5 (Ar–C), 124.3 (Ar–C), 124.7 (Ar–C), 125.8 (Ar–C), 127.3 (Ar–C), 127.5 (Ar–C), 128.1 (Ar–C), 128.7 (Ar–C), 129.1 (Ar–C), 129.5 (Ar–C), 129.8 (Ar–C), 131.9 (Ar–C), 132.4 (Ar–C), 134.4 (Ar–C), 134.7 (Ar–C), 135.1 (Ar–C), 137.5 (Ar–C), 139.8 (Ar–C), 142.4 (Ar–C), 151.9(=CH), 168.9 (C=O); EI-MS (m/z): 536 (2 %, M + 2), 534 (6, M+), 414 (13), 326 (3), 280 (35), 217 (12), 77(100).
X-ray crystallographic studies
A colorless prismatic crystal of 5d was used for data collection. The crystals were coated with Paratone 8277 oil (Exxon) and mounted on a glass fiber. All measurements were made on a Nonius Kappa CCD diffractometer with graphite monochromated Mo-Kα radiation. Details of crystal data and structure refinement have been provided in Table 2. The data were collected at a temperature of 173(2) K using ω and φ scans, corrected for Lorentz and polarization effects and for absorption using multi-scan method (Hooft, 1998; Otwinowski and Minor, 1997). The structures were solved by the direct methods (Altomare et al., 1993) and expanded using Fourier techniques (Beurskens et al., 1994). The nonhydrogen atoms were refined anisotropically. The final cycle of full-matrix least-squares refinement using SHELXL97 (Sheldrick, 2008) converged with unweighted and weighted agreement factors, R and wR = 0.0488 and 0.0919 (all data) for 5d, and goodness of fit, S = 1.079 and 1.085, respectively. The weighting schemes were based on counting statistics and the final difference Fourier maps were essentially featureless. The figures were plotted with the aid of ORTEP-3 (Farrugia, 1997).
Table 2.
Anti-HIV-1 and cytotoxic studies of 2-[5,5-dioxido-3-(3′-chlorophenyl)pyrazolo[4,3-c][1,2]benzothiazin-4(2H)-yl]-N′-[arylmethylidene] acetohydrazides (5a–o)
| Sr. no. | Compound | R | Anti-HIV-1 activity in PBM cells (μM) | Cytotoxicity (IC50, μM) | |||
|---|---|---|---|---|---|---|---|
| EC50 | EC90 | PBM | CEM | Vero | |||
| 1. | 1 | >100 | >100 | >100 | >100 | 54.8 | |
| 2. | 2 | >100 | >100 | >100 | 26.7 | >100 | |
| 3. | 3 | 20.0 | 73.7 | 57.3 | 22.4 | 40.6 | |
| 4. | 4 | >100 | >100 | >100 | >100 | >100 | |
| 5. | 5a | 4-F | 9.2 | 26.7 | 20.0 | 31.6 | 45.7 |
| 6. | 5b | 3-Cl | >10 | >10 | 36.3 | 31.6 | 16.4 |
| 7. | 5c | 4-Cl | 4.0 | 27.1 | 12.8 | 2.5 | 19.2 |
| 8. | 5d | 3-Br | >10 | >10 | 22.9 | 12.9 | >100 |
| 9. | 5e | 4-Br | 5.2 | 22.2 | 66.7 | 5.0 | 46.5 |
| 10. | 5f | 2,4-Cl2 | 19.6 | 76.3 | 80.7 | 13.8 | 84.8 |
| 11. | 5g | 2,6-Cl2 | 12.5 | 41.5 | 52.5 | 12.5 | 60.5 |
| 12. | 5h | 3-MeO | >10 | >10 | 19.0 | 31.6 | 22.3 |
| 13. | 5i | 2,3,4(MeO)3 | 4.2 | 23.8 | 21.8 | 31.6 | 29.0 |
| 14. | 5j | 3,4,5(MeO)3 | 5.8 | 13.5 | 11.0 | 31.6 | 14.3 |
| 15. | 5k | 2-NO2 | >100 | >100 | >100 | >100 | >100 |
| 16. | 51 | 3- NO2 | 92.4 | >100 | >100 | 47.3 | 27.0 |
| 17. | 5m | 4- NO2 | 7.5 | 20.4 | 16.7 | 2.8 | 21.2 |
| 18. | 5n | 4-COOH | 8.3 | 23.0 | 17.2 | 13.4 | 18.2 |
| 19. | 5o | 4-N(CH3)2 | 5.8 | 13.3 | 23.7 | 2.5 | 10.4 |
| 20. | AZT | 0.0033 | 0.031 | >100 | 14.3 | 56.0 | |
All experiments were conducted in replicate
Anti-HIV-1 assay
The assay was performed as described by Schinazi et al. 1990 with modifications (Schinazi et al., 1990). The percent of control was calculated, and then, the median effective concentration (EC50) was determined using the reported method (BeleN′kii and Schinazi, 1994). The results are expressed in Table 2.
Cytotoxicity assay
Human PBM, CEM and VERO cells were cultured in 96-well plates (5 × 104 cells per well) along with increasing concentrations of the test compound (Stuyver et al., 2002). Cell viability was measured after 5-day incubation period using the Cell Titer 96 Aqueous One Solution cell proliferation assay (Promega, Madison, WI) by incubating in an incubator at 37 °C with 5 % CO2 for human PBM cells. The results are summarized in Table 2.
Results and discussion
Chemistry
N-3′-Chlorophenacylsaccharine (1) was prepared by the condensation of saccharine with 3′-chlorophenacyl bromide. It was subjected to Gabriel–Coleman rearrangement to obtain (3-chlorophenyl)(4-hydroxy-1,1-dioxido-2H-benzo[e] [1,2]thiazin-3-yl)methanone (2) which was reacted with methyl chloroacetate to get methyl 2-[3-(3-chlorobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl]acetate (3). Treatment of 3 with hydrazine monohydrate resulted in the formation of 2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo-[e]pyrazolo[4,3-c][1,2]thiazin-4(2H)-yl)]acetohydrazide (4) which was condensed with a series of benzaldehydes to get 2-[3-(3-chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2] thiazin-4(2H)-yl]-N′-arylmethyleneacetohydrazides (5a–o) (Scheme 1).
Spectroscopic tools were effectively utilized for the characterization of products. 2-[3-(3-Chlorophenyl)-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-4(2H)-yl)]acetohydrazide (4) was found to exist as a tautomeric mixture (Fig. 2) in solution. The signal at δ 8.97 and δ 4.48 can be attributed to NH and NH2 proton of CONHNH2 group. The two singlets at δ 13.87 and δ 14.23 could be assigned to NH proton of pyrazole moiety in both tautomers A (53 %) and B (47 %). Because of these two forms, the indicated proton (starred proton in Fig. 2) appeared as two doublets at δ 7.97 and δ 8.02 for A and B, respectively, in the ratio of 53:47. The two singlets at δ 4.06 and δ 4.17 probably appeared because of CH2 protons in 4A and 4B. All other protons appeared as two multiplets at δ 7.59–7.64 and δ 7.79–7.83. In the mass spectrum of 4, the M+ and M + 2 can be observed at m/z 403 and 405 in the ratio of 3:1.
Fig. 2.
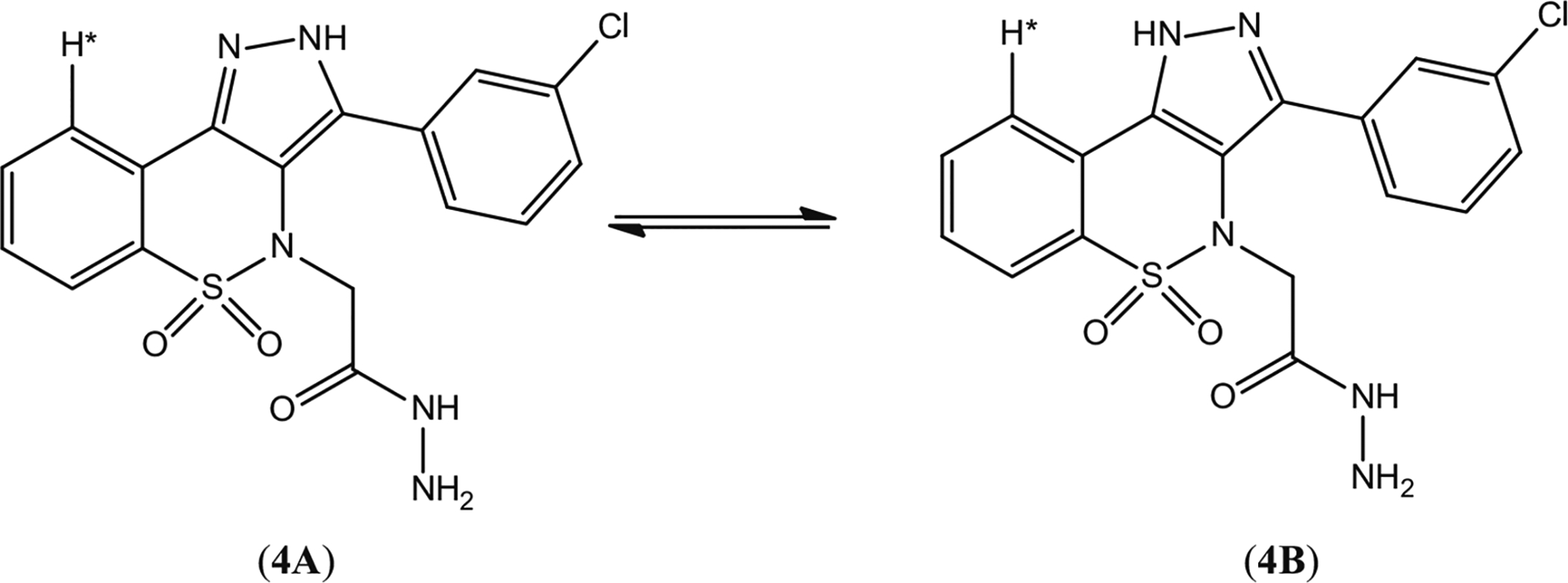
Structures of tautomeric isomers 4A and 4B of precursor, pyrazolobenzothiazine-based acetohydrazide
All hydrazones (5a–5o) were found to exist as inseparable mixtures of tautomeric as well as re and si forms, as indicated by so-called fractional splitting of signals due to NH protons and protons in their vicinity. The absence of NH2 signals in these compounds confirmed their formation. The mass spectral data were also found in complete agreement with the proposed structures. Moreover, the X-ray crystal structure (Fig. 3) confirmed the formation of hydrazones of compound 5d as a representative of this series.
Fig. 3.
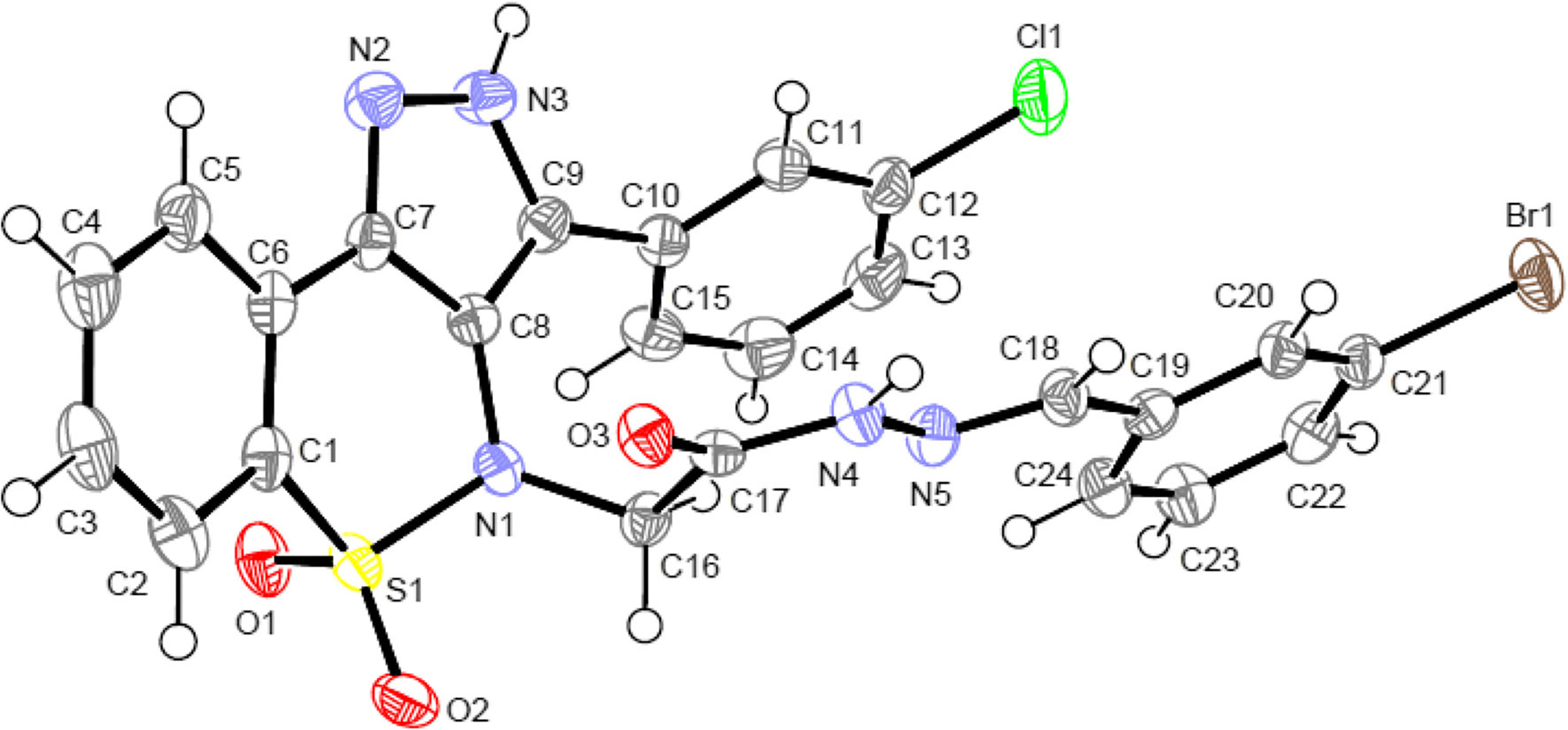
Molecular structure of 5d with the atom numbering scheme. Displacement ellipsoids are drawn at the 50 % probability level. H atoms are presented as small spheres of arbitrary radius
X-ray structure studies
The molecular structure of compound 5d is shown in Fig. 3, and the crystal data and structure refinement parameters are listed in Table 1. The heterocyclic thiazine ring adopts a twist chair conformation with atoms S1 and C1 displaced by 0.760(6) and 0.226(8) Å, respectively, from the mean plane formed by the remaining ring atoms (N1//C6–C8). The mean planes of the benzene rings C1–C6 and C10–C15 make dihedral angles 12.1(2)° and 56.4(2)°, respectively, with the mean plane of the pyrazolyl ring (N2/N3/C7/C8/C9). The acetamide chain (O3/N4/N5/C17/C18) linking is essentially planar (rms deviation 0.0098 Å) and forms dihedral angles with the mean planes of the ring C19–C24 10.4(2)°.
Table 1.
Crystal data and structure refinement parameters of compound 5d
| Parameter | Value |
|---|---|
| Compound | 5d |
| CCDC no. | 881,054 |
| Moiety formula | C24 H17 Br Cl N5 O3 S |
| Formula mass (g mol−1) | 570.85 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Unit cell dimensions | |
| a (Å) | 7.132(2) |
| b (Å) | 21.825(8) |
| c (Å) | 15.238(5) |
| β (°) | 99.144(18) |
| V (Å3) | 2341.7(13) |
| θ Ranges for data collection (°) | 3.11–25.00 |
| Z | 4 |
| ρcalc. (g cm−3) | 1.619 |
| F(000) | 1152 |
| Crystal size (mm) | 0.08 × 0.06 × 0.04 |
| T (K) | 173(2) |
| Index ranges | h: −8–8; k: −25–20; l: −18–18 |
| Total data | 5272 |
| Independent reflections | 4100 [R(int) = 0.035] |
| Completeness to θ = 27.5° | 99.1 % |
| Absorption correction | Multi-scan method |
| Max. and min. transmission | 0.924 and 0.857 |
| Data/restraints/parameters | 4100/0/316 |
| Final R indices[I >/2σ(I)] | R1 = 0.0302; wR2 = 0.0761 |
| Goodness of fit on F2 | 1.09 |
| Largest diff. peak and hole | 0.34 and −0.39 e.Å−3 |
Pharmacology
Anti-HIV activity
Compounds 5a–o appeared as good anti-HIV agents, and among fifteen compounds in this series, thirteen exhibited good activity with EC50 values <20 μM. Compound 5c proved to be the most active compound with EC50 value of 4 μM, but it is highly toxic to all the cell lines used. It is believed that further structural modifications could be carried out on the basis of the data obtained for these compounds to get worthwhile molecules regarding anti-HIV-1 activity (Table 2).
Cytotoxicity studies
Compounds were screened for their cytotoxicity in human PBM, CEM and Vero cells to determine their spectrum of toxicity. CEM cells are a line of lymphoblastic cells originally derived from a child with acute lymphoblastic leukemia, whereas Vero cells are derived from African green monkey kidney cells.
The precursor hydrazide (4) is nontoxic to all the cell lines used; however, it is inactive against HIV-1. Similarly, compound 5k showed no toxicity in any of the cell system and has exhibited no inhibition of HIV replication. However, 5e, 5f, 5g and 5l were not toxic in PBM cells (Table 2).
Conclusion
In summary, we herein report the anti-HIV-1 activity of new acetohydrazides based on pyrazolobenzothiazine ring system. Thirteen compounds were active against HIV-1 with EC50 values less than 20.0 μM, which indicates the potential of these compounds as anti-HIV-1 agents. Among these twenty compounds, 5c and 5i were the potent anti-HIV-1 agents with EC50 values <5.0 μM. Most of the active compounds were found highly toxic to the PBM, CEM and Vero cell lines except 5f and 5g, which were toxic to only CEM cell line. On these grounds, the structural modification of these compounds is expected to result in more potent candidates of anti-HIV drugs.
Acknowledgments
The authors (MA, ZK, SA and MAM) are grateful to Higher Education Commission, Pakistan, and Institute of Chemistry, University of the Punjab, Lahore, for financial assistance. We are also thankful to International Centre for Chemical and Biological Sciences, HEJ Research Institute of Chemistry, University of Karachi, Karachi, for spectral measurements. This work was also supported in part by NIH grant 2P30-AI-050409 and the Department of Veterans Affairs (RFS), USA. The X-ray coordinate of the compounds has been deposited at Cambridge Crystallographic Data Centre with CCDC numbers 881055.
Footnotes
We dedicate the research article to Prof. Dr. Hamid Latif Siddiqui (Late) who was our beloved teacher (ZK, SA and MA) and he passed away on July 26, 2011.
References
- Abbas EH, Farghaly T (2010) Synthesis, reactions, and biological activity of 1,4-benzothiazine derivatives. Monatsh Chem 141:661–667 [Google Scholar]
- Ahmad M, Aslam S, Bukhari MH, Montero C, Detorio M, Parvez M, Schinazi RF (2014) Synthesis of novel pyrazolobenzothiazine 5,5-dioxide derivatives as potent anti-HIV-1 agents. Med Chem Res 23:1309–1319 [Google Scholar]
- Altomare A, Cascarano M, Giacovazzo C, Guagliardi A (1993) SIR92. J Appl Cryst 26:343 [Google Scholar]
- Aslam S, Ahmad M, Athar M, Ashfaq UA, Gardiner JM, Montero C, Detorio M, Parvez M, Schinazi RF (2014a) Synthesis, molecular docking and antiviral screening of novel N′-substitutedbenzylidene-2-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c] [1,2]thiazin-1(4H)-yl)acetohydrazides. Med Chem Res 23: 2930–2946 [Google Scholar]
- Aslam S, Ahmad M, Zia-Ur-Rehman M, Montero C, Detorio M, Parvez M, Schinazi RF (2014b) Synthesis and anti-HIV-1 screening of novel N′-(1-(aryl)ethylidene)-2-(5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-4(1H)-yl)acetohydrazides. Arch Pharm Res 37:1380–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam S, Zaib S, Ahmad M, Gardiner JM, Ahmad M, Hameed A, Furtmann N, Gütschow M, Bajorath J, Iqbal J (2014c) Novel structural hybrids of pyrazolobenzothiazines with benzimidazoles as cholinesterase inhibitors. Eur J Med Chem 78:106–117 [DOI] [PubMed] [Google Scholar]
- Balfour J, Fitton A, Barradell L (1996) Lornoxicam. Drugs 51:639–657 [DOI] [PubMed] [Google Scholar]
- Barazarte A, Camacho J, Domínguez J, Lobo G, Gamboa N, Rodrigues J, Capparelli MV,Álvarez-Larenav Á, Andujar S, Enriz D, Charris J (2008) Synthesis, antimalarial activity, structure–activity relationship analysis of thieno-[3,2-b]benzothiazine S, S-dioxide analogs. Bioorg Med Chem 16:3661–3674 [DOI] [PubMed] [Google Scholar]
- Belen’kii MS, Schinazi RF (1994) Multiple drug effect analysis with confidence interval. Antivir Res 25:1–11 [DOI] [PubMed] [Google Scholar]
- Beurskens PT, Admiraal G, Beurskens G, Bosman WP, De Gelder R, Israel R and Smits JMM (1994) The DIRDIF-94 program system, Technical Report of the Crystallography Laboratory, University of Nijmegen, The Netherlands [Google Scholar]
- Clercq ED (2007) The design of drugs for HIV and HCV. Nat Rev Drug Discov 6:1001–1018 [DOI] [PubMed] [Google Scholar]
- Coughlin SA, Danz DW, Robinson RG, Klingbeil KM, Wentland MP, Corbett TH, Waud WR, Zwelling LA, Altschuler E, Bales E, Rake JB (1995) Mechanism of action and antitumor activity of (S)-10-(2,6-dimethyl-4-pyridinyl)-9-fluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyridol [1,2,3-de]-[1,4]benzothiazine-6-carboxylic acid (WIN 58161). Biochem Pharmacol 50:111–122 [DOI] [PubMed] [Google Scholar]
- El Safadi Y, Vivet-Boudou V, Marquet R (2007) HIV-1 reverse transcriptase inhibitors. Appl Microbiol Biotechnol 75:723–737 [DOI] [PubMed] [Google Scholar]
- Farrugia LJJ (1997) Appl Cryst 30:565 [Google Scholar]
- Gannarapu MR, Vasamsetti SB, Punna N, Royya NK, Pamulaparthy SR, Nanubolu JB, Kotamraju S, Banda N (2014) Synthesis of novel 1,2-benzothiazine 1,1-dioxide-3-ethanone oxime N-aryl acetamide ether derivatives as potent anti-inflammatory agents and inhibitors of monocyte-to-macrophage transformation. Eur J Med Chem 75:143–150 [DOI] [PubMed] [Google Scholar]
- Hadadzadeh H, Salimi M, Weil M, Jannesari Z, Darabi F, Abdi K, Khalaji AD, Sardari S, Ahangari R (2012) The piroxicam complex of copper(II), trans-[Cu(Pir)2(THF)2], and its interaction with DNA. J Mol Struct 1022:172–180 [Google Scholar]
- He Y, Kuang Y, Chen F, Wang S, Ji L, De Clercq E, Balzarini J, Pannecouque C (2005) Nonnucleoside HIV-1 reverse transcriptase inhibitors, part 4[1]. Synthesis and anti-HIV activity of N-1-β-carbonyl-6-naphthyl-methyl analogues of HEPT. Monatsh Chem 136:1233–1245 [Google Scholar]
- Hooft R (1998) Collect. Nonius B V, Delft [Google Scholar]
- Jochmans D (2008) Novel HIV-1 reverse transcriptase inhibitors. Virus Res 134:171–185 [DOI] [PubMed] [Google Scholar]
- Kamel MM, Ali HI, Anwar MM, Mohamed NA, Soliman AM (2010) Synthesis, antitumor activity and molecular docking study of novel Sulfonamide-Schiff’s bases, thiazolidinones, benzothiazinones and their C-nucleoside derivatives. Eur J Med Chem 45:572–580 [DOI] [PubMed] [Google Scholar]
- Katzmann W (1990) Tenoxicam. Drug Invest 2:31–37 [Google Scholar]
- Khalid Z, Siddiqui HL, Ahmad M, Bukhari I (2010a) 2-[2-(3-Chlorophenyl)-2-oxoethyl]-1,2-benzisothiazol-3(2H)-one 1,1-dioxide. Acta Cryst E 66:o617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid Z, Siddiqui HL, Ahmad M, Aslam S, Parvez M (2010b) 3-(3-Chlorobenzoyl)-4-hydroxy-2H-1,2-benzothiazine 1,1-dioxide. Acta Cryst E 66:o995 [Google Scholar]
- Kukhanova MK (2012) Anti-HIV nucleoside drugs: a retrospective view into the future. Mol Biol 46:768–779 [PubMed] [Google Scholar]
- Mehellou YDCE (2010) Twenty-six years of anti-HIV drug discovery: where do we stand and where do we go? J Med Chem 53:521–538 [DOI] [PubMed] [Google Scholar]
- Meng G, Liu Y, Zheng A, Chen F, Chen W, De Clercq E, Pannecouque C, Balzarini J (2014) Design and synthesis of a new series of modified CH-diarylpyrimidines as drug-resistant HIV non-nucleoside reverse transcriptase inhibitors. Eur J Med Chem 82:600–611 [DOI] [PubMed] [Google Scholar]
- Olkkola K, Brunetto A, Mattila M (1994) Pharmacokinetics of oxicam nonsteroidal anti-inflammatory agents. Clin Pharmacokinet 26:107–120 [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W (1997) Methods Enzymol 276:307–326 [DOI] [PubMed] [Google Scholar]
- Peng H, Liang Y, Chen L, Fu L, Wang H, He H (2011) Efficient synthesis and biological evaluation of 1,3-benzenedicarbonyl dithioureas. Bioorg Med Chem Lett 21:1102–1104 [DOI] [PubMed] [Google Scholar]
- Reed C, Daar E (2006) Novel antiretroviral agents in HIV therapy. Curr Infect Dis Rep 8:489–496 [DOI] [PubMed] [Google Scholar]
- Schinazi RF, Sommadossi JP, Saalmann V, Cannon DL, Xie MY, Hart GC, Smith GA, Hahn EF (1990) Activities of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother 34:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick GM (2008) Acta Cryst A 64:112–122 [DOI] [PubMed] [Google Scholar]
- Stuyver LJ, Lostia S, Adams M, Mathew SJ, Pai SB, Grier J, Tharnish MP, Choi Y, Chong Y, Choo H, Chu KC, Otto JM, Schinazi RF (2002) Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob Agents Chemother 46:3854–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivelan S, Deeksha TN, Arun S, Machiraju PK, Gundla R, Sinha BN, Jagarlapudi SARP (2011) Virtual screening studies on HIV-1 reverse transcriptase inhibitors to design potent leads. Eur J Med Chem 46:851–859 [DOI] [PubMed] [Google Scholar]
- Valuev-Elliston VT, Ivanov AV, Orlinson BS, Gerasimov EN, Brunilina LL, Kochetkov SN, Novakov IA, Navrotskii MB (2012) Synthesis and biological activity of new 6-benzylisocytosine derivatives: non-nucleoside HIV-1 reverse transcriptase inhibitors. Pharm Chem J 46:397–401 [Google Scholar]
- Xu Z, Ba M, Zhou H, Cao Y, Tang C, Yang Y, He R, Liang Y, Zhang X, Li Z, Zhu L, Guo Y, Guo C (2014) 2,4,5-Trisubstituted thiazole derivatives: a novel and potent class of non-nucleoside inhibitors of wild type and mutant HIV-1 reverse transcriptase. Eur J Med Chem 85: 27–42 [DOI] [PubMed] [Google Scholar]
- Zięba A, Latocha M, Sochanik A (2013) Synthesis and in vitro antiproliferative activity of novel 12(H)-quino[3,4-b][1,4]benzothiazine derivatives. Med Chem Res 22:4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]


