Abstract
Background
Efficacy of vaccines studied in clinical trial settings are likely to be different from their effectiveness in a real-world scenario. Indian Armed Forces launched its vaccine drive against COVID-19 on 16 Jan 2021. This study evaluated the effect of vaccination on mortality amongst hospitalized COVID patients.
Methods
A cross sectional study was done on all admitted moderate to severe COVID-19 patients at a designated COVID hospital in New Delhi. The primary outcome assessed the association of being fully vaccinated with mortality. Unadjusted odds ratios (OR) (with 95% CI) was performed for each predictor. Logistic regression was used for multivariable analysis and adjusted odds ratios obtained.
Results
The 1168 patients included in the study had a male preponderance with a mean age of 54.6 (± 17.51) years. A total of 266 (23%) patients were partially vaccinated with COVISHIELD® and 184 (16%) were fully vaccinated. Overall, 518 (44.3%) patients had comorbidities and 332 (28.4%) died. Among those fully vaccinated, there was 12.5% (23/184) mortality while it was 31.45 % (309/984) among the unvaccinated (OR 0.3, 95% CI 0.2 to 0.5, p<0.0001). In a logistic regression model, complete vaccination status and younger age were found to be associated with survival.
Conclusions
Vaccination with two doses of COVISHIELD® was associated with lower odds of mortality among hospitalized patients with moderate to severe COVID.
Keywords: Covid-19, Vaccination, Predictors of Mortality, Cross sectional study, COVISHIELD
Introduction
Immunity against SARS-CoV-2 virus causing COVID-19, achieved after infection, was shown to be variable in its duration and efficacy.1,2 Vaccines studied in trials were noted to achieve significant immunity in both previously infected and naïve subjects and varied from 92% for documented infection, 87% for hospitalization, and 92% for severe disease.3, 4, 5, 6, 7
The second wave of the pandemic in India, beginning March 2021, had patients presenting in larger numbers with more severe disease at hospitals. At the same time, as part of the vaccination drive that was started w.e.f 16 Jan 2021, most healthcare and frontline workers of the Indian Armed Forces had been vaccinated with COVISHIELD® (manufactured by Serum institute of India pvt ltd, Pune, India). COVISHIELD (ChAdOx1 nCoV-19) is an adenovirus vector-nonreplicating virus vaccine carrying recombinant spike protein of SARS-CoV-2. The process of vaccinating its elderly (>65yrs) clientele and those above 45 years with comorbidities was being undertaken thereafter.
Studies had shown various factors to be associated with mortality in COVID-19.8, 9, 10, 11, 12 We wanted to study the association of vaccination status with COVID-19 related mortality and hypothesized that the vaccination against SARS-COV-2 infection would be associated with a lower mortality due to COVID-19 infection in hospitalised patients.
Materials and Methods
The present record-based cross-sectional study was conducted in a 1000-bedded tertiary care hospital. A total of 2620 cases of SARS-CoV-2 infection, admitted between 01 March to 17 May 2021, comprised the population from where the study sample was selected.
Criteria for admission and discharge
Cases of RTPCR confirmed SARS-CoV-2 infection with moderate to severe illness as per the IDSA criteria for severity of COVID-19 (Resp rate >24/min and SPO2<94% on room air, at rest or after 6-min walk test) were admitted during the study period.13
Patient records were reviewed to obtain clinical and demographic data. A data extraction form was designed. The data on age, sex, comorbid conditions, and vaccination status was extracted. Dates of vaccination, symptom onset, laboratory confirmation of the diagnosis, hospital admission, and clinical outcome were recorded. The individuals were deemed partially vaccinated and fully vaccinated two weeks after the first and second dose respectively. The total duration of hospitalisation was calculated till the primary outcome (Death/Discharge). Patients were discharged at least ten days from onset of symptoms and being afebrile >72h irrespective of RT-PCR status and SPO2>94% on room air (sustained for more than 24 h) or with RT-PCR negative status with clinical recovery. Patients with RT-PCR negative status with oxygen requirement that could be met using domiciliary oxygen therapy were transferred to another center for institutional care.
Sample size estimation
To select parameters for sample size calculation, we looked at data from a similar hospital. SVBP Hospital at New Delhi, operationalised on 19 April 21 for second wave of COVID-19, was managed by Armed Forces Doctors for Civilians and had similar admission criteria as our hospital. A total of 1479 patients had been admitted till 15 May 21, of which 38.7% died. About 5% of all admissions had received 2 doses (personal communication). During the same period in New Delhi, 85% of healthcare workers and 42% of those above 45 years of age had received 1 dose of vaccine with a fourth of them (∼23%) having received two doses.14,15 In the Armed Forces, over 85% of health care workers and frontline workers had received both doses by mid May. The vaccination drive among families aged >45 years and veterans was also higher than their civilian counterparts. Therefore, we estimated that at least 10% (likely between 12 and 15%) of all admitted cases were likely to have received both doses of vaccine in our hospital. Vaccine effectiveness data with COVISHIELD had shown between 80 and 88% reduction in hospitalisation.1,16 Mortality reduction was close to 100% in the primary vaccine efficacy study.17 We estimated our sample size based on the following assumptions; namely, 10% of all admitted patients would have received two doses of vaccine and the expected overall mortality would be 25%. Those having received two doses would have 12.5% mortality (50% reduction) than unvaccinated patients. Sample size was calculated using online calculator (sample-size.net by UCSF) taking α = 0.05 and a power of 80%. The required sample size was 984 and we included 1168 patients in our study. Institutional ethical clearance was obtained.
Statistical analysis
Data was compiled in a rectangular format on spreadsheet. Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as percentages. Unadjusted odds ratios (OR) (with 95% CI) were estimated to determine the association of vaccination status, age, sex, and the presence of comorbidities with mortality. Multivariable logistic regression was done to adjust for confounding variables and adjusted OR obtained. Statistical analysis was performed on StataCorp. 2019. (Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.)
Results
The hospital saw a total of 2620 admissions during the second wave of COVID-19 from 01 Mar to 17 May 2021. Cases with milder disease (n = 561), were transferred to an isolation facility. Of the remaining, a total of 1716 patients had either died or had been discharged and 343 were still in hospital at the end of the study period. The study sample was selected randomly from the study population of 1716 patients.
Demographic characteristics
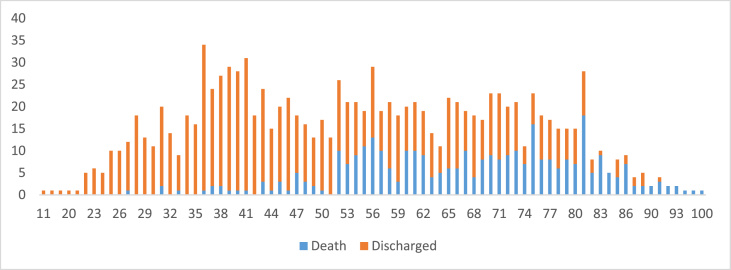
The patients included in the study comprised 1168 patients with a mean age of 54.6 (SD-17.5) years (Table 1). The age wise distribution of deaths is shown in Fig. 1. The elderly (>80 years), comprising <1% of population, accounted for 21.4% of deaths with those under 35 years (13.4%) contributed to 1.2%. There was a male preponderance (n = 783, M:F, 2:1) and 518 patients (44.3%) had one or more comorbidities. A total of 503 (43%) patients had received at least one dose of vaccine with 266 (22.8%) partially vaccinated and 184 (15.8%) fully vaccinated. The remaining 53 were considered unvaccinated as they tested positive within 14 days of receiving first dose. A higher percentage of males (21.3% vs. 4.4%) were fully vaccinated.
Table 1.
Baseline characteristics of patients included in the study.
| Patient characteristics | Died | Survived | |
|---|---|---|---|
| Numbers | 1168 | 332 (28.4%) | 836 (71.6%) |
| Sex distribution | |||
| Male | 783 | 198 (25.2%) | 585 (74.8%) |
| Female | 385 | 134 (34.8%) | 251 (65.2%) |
| Age in years, Mean (± SD) | 54.6 (±17.5) | 67.3 (±13.5) | 49.6 (±16.4) |
| Vaccination status | |||
| Not vaccinated | 718 | 218 (30.4%) | 500 (69.6%) |
| Partially vaccinated | 266 | 91 (34.2%) | 175 (65.8%) |
| Fully vaccinated | 184 | 23 (12.5%) | 161 (87.5%) |
| Comorbidities | |||
| Present | 518 | 202 (38.9%) | 316 (61.1%) |
| Absent | 650 | 130 (20%) | 520 (80%) |
Fig. 1.
Age distribution of death and discharged cases.
Outcomes
A total of 332 (28.4%) patients died. Among fully vaccinated, 12.5% (23/184) died while it was 31.4% (309/984) among unvaccinated. Being fully vaccinated was associated with lower mortality than either unvaccinated (Unadjusted OR 0.3, 95% CI 0.2 to 0.5, p < 0.0001) or partially vaccinated (Unadjusted OR 0.3, 95% CI 0.2 to 0.5, p < 0.0001) status. There was no significant difference between unvaccinated vs. partially vaccinated (Unadjusted OR 1.2, 95% CI 0.9 to 1.6, P = 0.3).
A total of 518 (44.3%) patients had comorbidities. Of them, 57.1% (296/518) had one, 24.3% (126/518) had two and 18.5% (96/518) had multiple (>2) comorbidities. Diabetes mellitus and hypertension were the commonest comorbidities present in 40.4% (209/518) and 44.2% (229/518) respectively. Other conditions included coronary artery disease (16%), hypothyroidism (12.4%), malignancies (9.8%), bronchial asthma (5.2%), chronic kidney disease (3.9%), chronic obstructive pulmonary disease (3.7%), and stroke (2.7%).
Multivariable logistic regression was done for adjustment of age, sex and co-morbidities. The adjusted odds ratio for fully vaccinated vs unvaccinated was 0.5 (95% CI 0.3–0.8, p = 0.004) while the adjusted odds ratio for partially vaccinated and un-vaccinated was 0.8 (95% CI 0.6–1.1, p = 0.1) (Table 2). In multivariable analysis in three groups (Unvaccinated, partially vaccinated, and full vaccinated), age was significantly associated with mortality with unit rise in age associated with 6% (OR 1.06; 95% CI 1.05–1.08) increase in unvaccinated/partially vaccinated and 15% (OR 1.15; 95% CI 1.1–1.2) increase in fully vaccinated persons.
Table 2.
Factors associated with mortality in Covid.
| Factors | Unadjusted OR (95% CI) | P value | Multivariable logistic regression analysis |
|
|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | |||
| Vaccination | ||||
| Not vaccinated | Reference | Reference | ||
| Partially vaccinated | 1.19 (0.88–1.61) | 0.24 | 0.77 (0.55–1.08) | 0.13 |
| Fully vaccinated | 0.33 (0.21–0.52) | <0.001 | 0.46 (0.27–0.78) | 0.004 |
| Age in years | 1.07 (1.06–1.08) | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| Sex | ||||
| F | Reference | Reference | ||
| M | 0.63 (0.48–0.83) | <0.001 | 0.83 (0.62–1.13) | 0.23 |
| Comorbidities | ||||
| No | Reference | Reference | ||
| Yes | 2.55 (1.96–3.31) | <0.001 | 1.02 (0.75–1.40) | 0.87 |
Discussion
Our study showed that being fully vaccinated and younger age were associated with lower mortality. The overall mortality amongst those who were hospitalized with severe COVID-19 was 28.4%. This was consistent with the fact that only moderate to severe patients were admitted requiring high flow oxygen or ventilation. Various studies in the literature have examined the predictors of mortality in patients with COVID-19.8, 9, 10, 11, 12 Age and comorbidities (especially diabetes mellitus and hypertension) have most commonly been shown to be associated with death.9 We noted greater mortality in those aged >65 years, and this increase was despite their vaccination status. Amongst the comorbidities, the greatest risk of increased mortality has been documented with diabetes mellitus.11 Diabetes mellitus and hypertension were the commonest comorbidities in our study. We did not analyse for individual comorbidities in our study and instead looked at the presence of comorbidities as an independent variable.
Studies have also shown that disease severity scores and the degree of the rise of inflammatory markers are associated with higher risk of mortality.18 Since our study included only patients with severe COVID-19 disease, we did not study the degree of disease severity or markers of inflammation as mortality predictors.
Most of the predictive studies had been performed when vaccination against COVID-19 had not been instituted. Earlier studies from trials and real world data has shown that vaccinated persons are less likely to have the asymptomatic or symptomatic disease and less hospitalization due to severe disease.2,6,13,19 Data obtained from the vaccine effectiveness studies for the Astra Zeneca (ChAdOx1 nCoV-19) vaccine have shown upto 80% reduction in hospitalization.16 More real-world studies were needed to assess vaccine status as a predictor of mortality.20,21 Being well powered with a large sample size, our study showed a significant association of being fully vaccinated with lower mortality status among hospitalized patients. COVISHIELD® has been shown to reduce infections by 80–94%.1,22 In present study the fully vaccinated individuals have 70% lower odds of mortality than un-vaccinated persons among hospitalized severe cases.
There are several limitations to this study as is to be expected in any cross sectional design. Though we did show association between mortality and vaccination status, causality cannot be inferred. Ideally, one needs to follow large natural cohorts of vaccinated vs unvaccinated and see actual infection/mortality rates.2,19,23 Some of the survivors might have succumbed to post COVID-19 complications at a later point in time post discharge and thus might have been misclassified. The survivors were significantly younger and had fewer comorbidities and the vaccination pattern was skewed. However, despite these limitations, the effect of vaccination status was demonstrable on multivariable logistic regression analysis.
There is a need to do further research to confirm the role of vaccination status in mitigating other predictors of mortality due to severe COVID-19. There is also the need to study the relationship between immunogenicity and vaccine effectiveness in subgroups like elderly and diabetics where mortality is the highest.
In conclusion, this is one of the earliest real life vaccine studies that showed that being fully immunized with COVISHIELD® vaccine, was associated with lower mortality among those hospitalized with severe COVID-19 infection. Findings of this study reconfirms the need to vaccinate as many persons as possible at the earliest to successfully face the future wave of COVID-19.
Disclosure of competing interest
The authors have none to declare.
References
- 1.Vasileiou E., Simpson C.R., Shi T. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet (London, England) 2021 Apr 23;397(10285) doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N., Barda N., Kepten E. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 Apr 15;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saadat S., Rikhtegaran Tehrani Z., Logue J. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA - J Am Med Assoc. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. American Medical Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebinger J.E., Fert-Bober J., Printsev I. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voysey M., Clemens S.A.C., Madhi S.A. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estiri H, Strasser ZH, Klann JG, Naseri P, Wagholikar KB, Murphy SN. Predicting COVID-19 Mortality with Electronic Medical Records. [DOI] [PMC free article] [PubMed]
- 9.Rastad H., Karim H., Ejtahed H.-S. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol Metab Syndrome. 2020 Jul 6;12(1):57. doi: 10.1186/s13098-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehraeen E., Karimi A., Barzegary A. Predictors of mortality in patients with COVID-19–a systematic review. Eur J Integr Med. 2020 Dec 1;40:101226. doi: 10.1016/j.eujim.2020.101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eumann Mesas A., Cavero-Redondo, Aparecido Sarriá Cabrera M., Maffei de Andrade S., Sequí-Dominguez I., Martínez-Vizcaíno V.I. 2020. Predictors of In-Hospital COVID-19 Mortality: A Comprehensive Systematic Review and Meta-Analysis Exploring Differences by Age, Sex and Health Conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trecarichi E.M., Mazzitelli M., Serapide F. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Sci Rep. 2020 Dec 1;10(1) doi: 10.1038/s41598-020-77641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhimraj A., Morgan R.L., Shumaker A.H. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 Apr 27 doi: 10.1093/cid/ciaa478. Epub ahead of print. PMID: 32338708; PMCID: PMC7197612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covid: Vaccination Of Frontline Workers In Delhi Below National Average: Centre [Internet]. [cited 2021 Jun 17]. Available from: https://www.ndtv.com/india-news/covid-vaccination-of-frontline-workers-in-delhi-below-national-average-centre-2442543.
- 15.42% of 45+ age vaccinated in Delhi so far, centres remain shut for 18-44 age group for fourth day - Cities News [Internet]. [cited 2021 Jun 17]. Available from: https://www.indiatoday.in/cities/delhi/story/age-vaccinated-delhi-centres-remain-shut-18-44-age-group-fourth-day-1807981-2021-05-28.
- 16.Lopez Bernal J., Andrews N., Gower C. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ (Clin Res Ed) 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro J., Dean N.E., Madewell Z.J., Yang Y., Halloran M.E., Longini I. vol. 28. 2021. pp. 70–91. (Efficacy estimates for various COVID-19 Vaccines : what we know from the literature and reports). [Google Scholar]
- 18.Tian W., Jiang W., Yao J. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–1883. doi: 10.1002/jmv.26050. John Wiley and Sons Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas E.J., Angulo F.J., McLaughlin J.M. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet (London, England) 2021 May 5;397(10287):1819. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) 2021. Evaluation of COVID-19 Vaccine Effectiveness; p. 70. [Google Scholar]
- 21.Rodrigues L.C., Smith R.G. Use of the case-control approach in vaccine evaluation: efficacy and adverse effects. Epidemiol Rev. 1999;21(1):56–72. doi: 10.1093/oxfordjournals.epirev.a017988. [DOI] [PubMed] [Google Scholar]
- 22.Hyams C., Marlow R., Maseko Z. Effectiveness of BNT162b2 and ChAdOx1nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021 Jun 23 doi: 10.1016/S1473-3099(21)00330-3. S1473-3099(21)00330-3. Epub ahead of print. Erratum in: Lancet Infect Dis. 2021 Jul 5; PMID: 34174190; PMCID: PMC8221734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossman H., Shilo S., Meir T. COVID-19 dynamics after a national immunization program in Israel. Nat Med. 2021 Jun;27(6):1055–1061. doi: 10.1038/s41591-021-01337-2. Epub 2021 Apr 19. PMID: 33875890. [DOI] [PubMed] [Google Scholar]