Abstract
Background
Antibody response to SARS-CoV may be estimated to give trends and patterns emerging in a population during an evolving epidemic. The novel coronavirus has opened a new chapter in the history of pandemics and understanding the disease epidemiology.
Methods
The study was a cross-sectional descriptive study. Institutional Ethical clearance and informed consent were taken for participation in the study. The study population included all personnel reporting to the institute for training courses, permanent posting or joining back from leave during the study period of 2 months (16 June to 16 August 2020). The sample size was calculated assuming the prevalence of COVID-19 to be 1% with the absolute precision of 0.5% and 5% level of significance, and finite correction for population size of 500, and the calculated sample size was 377. Inclusion criteria were all personnel reporting to the institute from different states and districts. Exclusion criteria-Any personnel reported for a short visit of lesser than 14 days. Demographic details and details of any likely exposure to a confirmed COVID-19 case were noted. A blood sample was collected, and serological tests were done using ErbaLisa COVID-19 IgG kit by Calbiotech, as per the manufacturer's instructions.
Results
Overall seropositivity of IgG COVID-19 antibodies was 7.5% (31/413) (95% CI: 5.3–10.4%). Study population (n = 413) comprised of an adult population in the age range of 21 years–53 years, and the mean age was 31.4 years (SD = 6.2 years).
Conclusion
As the personnel joining the institute have come from various parts of the country the study provides an estimation of antibodies against COVID-19.
Keywords: Antibody, Pandemic, Epidemiology, Serology
Introduction
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has captured global attention and has become a worldwide pandemic.1 Due to its rapid spread and easy transmissibility, there are over 32 million positive cases and nearly a million deaths already.2 The World Health Organisation (WHO) India situation report as of 21 Sep 2020 reported 5,400,619 confirmed cases and 86,752 deaths.3 Being a novel virus, research is lacking about its immunobiology and more studies are needed.4 WHO has emphasised research on this novel pathogen to chart out a roadmap for the pandemic, including population serosurveys.5 Seroepidemiological surveys give an insight into the humoral antibody response of the population and predict trends.6,7
According to the WHO, around eighty percent of the new cases are mild or asymptomatic.8,9 The detection of such cases in the community is important in preventing the spread of the virus.10 Currently, the methodology for identifying the at-risk individual is by self-declaration, wherein the person gives a history of having had an exposure to or contact with a COVID positive case and is then screened for symptoms or via contact tracing. However, asymptomatic individuals may go undetected, unaware of infection as they may have developed only mild symptoms or none. However, they may have shed the virus even before symptom onset.11
The current diagnosis of COVID-19 infection relies on molecular techniques such as the reverse transcription real-time polymerase chain reaction (rRT-PCR).12 While molecular assays are the gold standard for detecting acute infections, serological assays such as the Enzyme-linked Immunosorbent Assay (ELISA) can detect antibodies indicating past infection or prior exposure to the virus.13 In the early stages post symptom onset, IgG ELISA detection is low (4%–57%), it increases in the intermediate stage (54%–88%), and after 14 days, it is between 91% and 100%.14 Thus, serological studies conducted on a mass scale for antibody detection may provide information regarding disease prevalence in a population.15 This may be used to assess the immune response and the herd immunity of a population.16 The Spike (S) protein and the Nucleocapsid (N) protein of the virus may be targeted by these antibody assays, primarily as they are conserved in nature.17
The indigenous serological assay recommended by the Indian Council of Medical Research (ICMR) is the ELISA-based IgG kit developed by the National Institute of Virology (NIV), which detects IgG antibodies two weeks post-infection.18 Antibody surveillance studies and serosurveys strengthen public health mitigation measures and have to be conducted during the changing landscape of an evolving pandemic.19 A serosurvey conducted during the early months of the pandemic by ICMR reported that 0.73% of the surveyed population had antibodies to SARS-CoV-2.20 However, as the pandemic spreads across the subcontinent and lockdown restrictions ease in the country, surveys will have to be done in the community, as well as in targeted populations to map out the changing trends and extent of infection.21
Being a central teaching institute gave the unique opportunity to estimate the antibody response in individuals, mostly healthcare workers who returned to the institute post lockdown from various parts of the country. Hence this sero-surveillance study using ELISA IgG is an assessment of seropositivity in this unique cohort.
Materials and methods
This study was a cross-sectional descriptive study. Institutional ethical clearance was taken. Informed consent was taken for participation in the study. The study population included all personnel reporting back to the institute post lockdown. The duration of the study was two months (16 June to 16 August 2020). The sample size was calculated assuming the prevalence of COVID-19 in the general population to be 1% with the absolute precision of 0.5% and 5% level of significance and finite correction for the population of 500; the study sample size was calculated as 377. The inclusion criteria were all personnel reporting to institute from outstation/different states and districts. Exclusion criteria were any personnel reporting for a visit shorter than 14 days. We were able to recruit 413 individuals. Data collection was done as per the WHO recommended protocol.22 All personnel on joining the institute were asked to fill in their demographic details and self-declare exposure to a confirmed COVID-19 case in the past. All the personnel were placed in quarantine as per the existing quarantine protocols, and if any person was found to have any Influenza-like illness (ILI) symptoms, he/she was immediately tested for COVID-19 by RT-PCR. Blood sample collection for serology was done on the samples collected from all the personnel as per the instructions after the completion of 14 days of quarantine. The ErbaLisa COVID-19 IgG kit by Calbiotech, an indirect qualitative/semiquantitative ELISA based on the recombinant spike subunit antigen was used in the study and the manufacturer's instructions were followed. The sensitivity and specificity of the kit is 98.0% and 99.0%. The optical density (OD) was read at 450 nm using an ELISA reader, and a cut off value was calculated, which was the ratio of the calibrator reading multiplied by the Calibrator factor (CF) included in the kit. The antibody (Ab) index was calculated for each sample by dividing the sample reading by the cutoff value. The Ab index interpretation was; no detectable IgG antibody to SARS Co V 2 if Ab index was <0.9, a borderline positive if Ab index 0.9–1.1 and positive >1.1.
Statistical analysis: Data were summarised as numbers, percentages, mean and standard deviation (SD) as applicable. Odds Ratio with 95% CI was used to compare variables. A P-value of less than 0.05 was considered significant. SPSS ver 21 was used to analyse data.
Results
The study population (n = 413) comprised of an adult population in the age range of 21–53 years, and the mean age was 31.4 years (SD = 6.2 years). The mixed study cohort comprised of doctors-164 (39.7%), paramedics-143 (34.6%) and general duty staff- 106 (25.6%). The overall IgG antibodies for SARS-CoV-2 in this asymptomatic cohort were found to be 7.5% (31/413) (95% CI: 5.3–10.4%). The study participants travelled to the central academic institution by different modes after the lockdown was lifted; 192 (46.5%) travelled by air, 114 (27.6%) by road, and 107 (25.9%) by train. The demographic details of the seropositive individuals are as shown in Table 1. As shown in Table 1, along-with the seropositivity percentages for demographic variables, odds ratios were calculated to assess the increase or decrease in odds of seropositivity among various categories in relation to the reference category (denoted as 1) of the variable, while the range of 95% Confidential Interval provided the dispersion of seropositivity odds ratio i.e., an estimate of precision.
Table 1.
Sociodemographic profile of SARS COv antibody-positive study subjects (n = 31).
| S No. | Variable | Total N | Positive (%) | OR (95% CI) | p-value |
|---|---|---|---|---|---|
| 1. | Overall | 413 | 31 (7.5%) | ||
| 2. | Gender | ||||
| Female | 49 | 04 (8.2%) | 1 (Reference) | ||
| Male | 364 | 27 (7.4%) | 0.9 (0.3–2.7) | 0.8 | |
| 3. | Age Groups | ||||
| 20–30 yrs | 232 | 16 (6.9%) | 1 (Reference) | ||
| 31–40 yrs | 141 | 13 (9.2%) | 1.4 (0.6–2.9) | ||
| 41–50 yrs | 35 | 02 (5.7%) | 0.8 (0.2–3.7) | ||
| 51–60 yrs | 05 | 0 (0%) | – | 0.7 | |
| 4. | Profession | ||||
| Paramedical | 143 | 09 (6.3%) | 1 (Reference) | ||
| Doctors | 164 | 13 (7.9%) | 1.28 (0.5–3.0) | ||
| General duty | 106 | 09 (8.5%) | 1.38 (0.5–3.6) | 0.8 | |
| 5. | Mode of Travel | ||||
| Train | 107 | 05 (4.7%) | 1 (Reference) | ||
| Air | 192 | 14 (7.3%) | 1.6 (0.5–4.6) | ||
| Road | 114 | 12 (10.5%) | 2.4 (0.8–7.2) | 0.2 | |
| 6. | States | ||||
| Delhi | 18 | 03 (16.7%) | 1 (Reference) | ||
| Haryana | 28 | 03 (15%) | 0.9 (0.17–5.2) | ||
| Maharashtra | 72 | 09 (12.5%) | 0.7 (0.17–2.9) | ||
| Uttar Pradesh | 54 | 04 (7.4%) | 0.4 (0.05–2.7) | ||
| Madhya Pradesh | 20 | 02 (7.4%) | 0.4 (0.07–2.4) | ||
| Other (1 or less case) | 221 | 10 (4.5%) | 0.2 (0.05–9.7) | 0.1 | |
Genderwise, females had slightly higher seropositivity (8.2%) than males, with odds of seropositivity among males 10% lesser than that of females. Age group-wise highest seropositivity was observed between 31 and 40 years age group (9.2%) with odds of seropositivity 1.4 times that of reference category (20–30 years). Travelling by road increased seropositivity (10.5%) with odds of seropositivity 2.4 times that of the reference category (travel by train). Profession-wise nonmedical staff had slightly higher seropositivity (8.5%) with odds of seropositivity 1.38 times that of the reference category (paramedical staff). The seropositivity was highest among individuals who had returned from Delhi (16.7%), and when Delhi was taken as reference, the odds of seropositivity was found to be 10% lesser for Haryana, 30% lesser for Maharashtra, 60% lesser for Uttar Pradesh and Madhya Pradesh and 80% lesser for all other States. However, the difference in odds ratio among various categories of the above-mentioned demographic variables was not found to be statistically significant (p > 0.05).
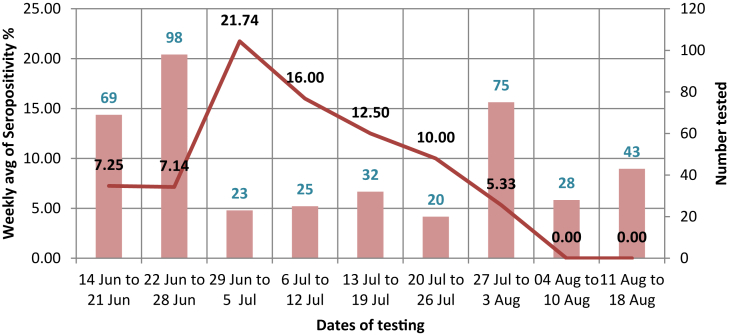
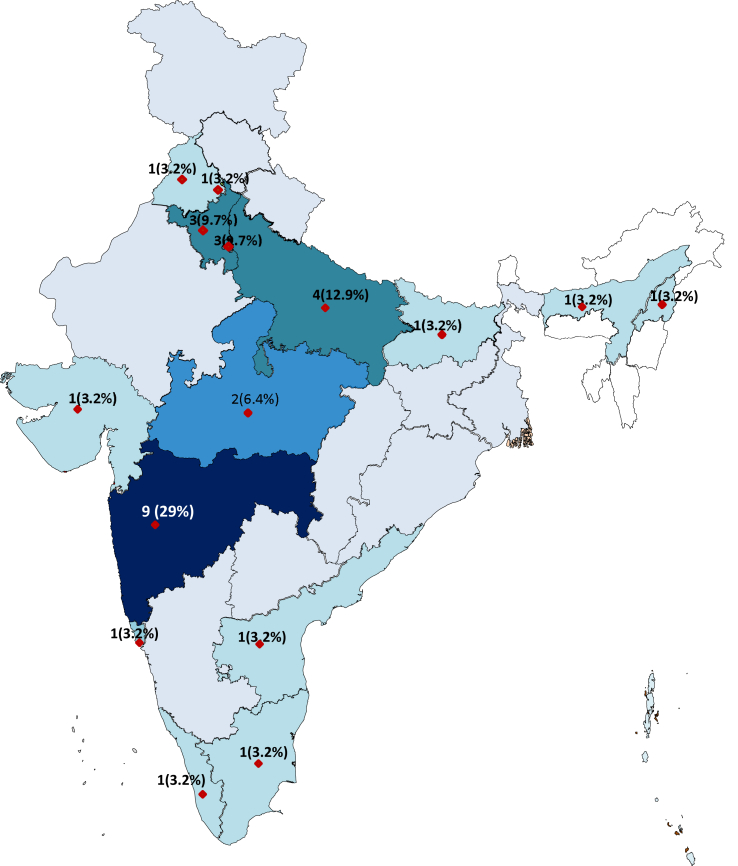
The week-wise distribution of the cases was as shown in Fig. 1. The weekly average seropositivity percentage and the individuals tested were plotted, and the week-wise distribution of cases starting from 14 June–18 Aug 2020 was included. The percentage weekly average seropositivity was highest for week 29 June–5 July 2020 with the rate of 21.74 and showed a gradual decrease into the month of August when we stopped recruiting participants for the study and adopted the changed institutional protocol of RTPCR for screening the individuals and sending those even with mild symptoms post quarantine to the flu clinic. The state-wise distribution of seropositive cases is as depicted in the map in Fig. 2. The individuals joining the institute back after the lockdown belonged to 28 States/UTs, and seropositives were reported from persons belonging to 15 States/UTs. The States shown in darker shade had a higher number of seropositives. These were the five states of Delhi (03), Haryana (03), Uttar Pradesh (04), Madhya Pradesh (02), and Maharashtra (09). These contributed to 67.7% (21) of total (31) seropositive cases, while they represented only 41% of those tested. Among States, the persons who travelled back from Delhi, Haryana and Maharashtra had the highest seropositivity i.e., 16.7%, 15% and 12.5%, respectively. For comparison among variables within various categories, the baseline reference value of the variable against which comparisons were made was shown to have an Odds Ratio (OR) value of 1. The difference in OR within various categories was not found to be statistically significant (p > 0.05).
Fig. 1.
Week-wise distribution of cases.
Fig. 2.
Seropositive (% of total positives) in various states of India in our study cohort.
A quantitative assessment of the ELISA values among seropositive persons showed that all (100%) seropositive females had an ELISA value of ≥2 while among males, only 33.3% seropositives had an ELISA value of ≥ 2. The difference was found to be statistically significant (p = 0.01). Age group-wise, the odds of having ELISA value ≥ 2 was 50% lesser among persons who were more than 30 years of age in reference to persons in the age category of 20–30 years; however, the difference was not statistically significant (p = 0.3) (Table 2).
Table 2.
Seropositivity value as per gender and age group.
| S No | Variable | Value =>2 | OR (95% CI) | p-value |
|---|---|---|---|---|
| 1. | Gender | |||
| Female | 04 (100%) | 0.01 | ||
| Male | 09 (33.3%) | – | ||
| 2. | Age Gp | |||
| 20–30 yr | 08 (50%) | 1(Reference) | 0.3 | |
| >30 yr | 05 (33.3%) | 0.5 (0.1–2.2) | ||
P value 0.01 is significant.
Discussion
The seropositivity reported in our study was 7.5%. The press release by ICMR in early May 2020 reported a seroprevalence of 0.73 percent among adults in India. A low seroprevalence at that time could be due to India being in the early stages of the pandemic with a sizeable adult population not having been exposed, and thus, susceptible. However, this data was expected to change with time. ICMR COVID Study groups sampled large asymptomatic cohorts and reported seropositivity from Jan–Apr 202023 and May–June 2020.24 A study from West Bengal conducted in the last week of July and 1st week of August 2020 among 458 asymptomatic general population reported 19 asymptomatic individuals to be IgG seropositive for SARS-CoV-2.25 Results of studies from Germany,26 Italy,27 England28 and Geneva29 reported prevalence rates of 1.6%, 3.4%, 6% and 6.4%, respectively. However, whether the data from all these studies is comparable depends on variables like sample collection, the methodology of testing, data analysis, and interpretation.
The study cohort of this study travelled back to the institute and was required to be in quarantine for 14 days. The seropositivity seen in the study group was for the months from June to August. The months of June and July represent the pandemic in an exponential phase throughout the country. The data from Pune city during that time estimated the seroprevalence in five high-incidence, hot spot areas to be 51.5% (CI: 49.1–53.9%).30 However, the results of various studies may not be comparable due to the different assays/formats used for antibody testing.
The present study provided seropositivity in a cohort who were health care workers coming from urban areas to the central institute in Pune. All participants had a history of long-distance travel after the opening of lockdown for joining the institute and hailed from cities where prevalence was higher during the early and mid of the year. We observed in our study that the percentage of individuals from various states/UT correlated with the incidence of COVID-19 cases in those States/UTs as reported by ICMR studies during that period. Delhi, Haryana, and Maharashtra had an early rise of COVID-19 cases in the country, which also got reflected in our study.
Seroprevalence surveys are of utmost importance to assess the proportion of the population that has already developed antibodies against the virus and might potentially be protected against subsequent infection.31 Serological tests for COVID mainly target IgG, IgM, and IgA for detection. Studies reveal that most patients seroconvert within 7–14 days of infection.32 Although IgG levels drop by eight weeks after symptom onset, it persists in the body for a longer time and is responsible for long-term immune memory. Recovered patients have high spike-specific IgG antibody levels. IgA levels may also remain high and correlate with disease severity.33 On testing for humoral antibody response by a study conducted in China using an ELISA based on the recombinant viral nucleocapsid protein on 208 plasma samples from 82 confirmed and 58 probable cases reported median duration of IgM and IgA antibody detection was 5 (IQR, 3–6) days, while IgG was detected 14 (IQR, 10–18) days after symptom onset, with a positive rate of 85.4%, 92.7% and 77.9%, respectively. The detection efficiency of IgM ELISA was higher than that of qPCR after 5.5 days of symptom onset;34 however, our cohort was an asymptomatic cohort who had no symptoms and were apparently healthy.
Current evidence suggests that most PCR confirmed SARS-CoV-2 infected patients seroconverted in 2 weeks of disease onset.35 One study reported that IgM was present even up to a month post-infection, whereas it was seen that SARS-CoV-2-specific IgG antibodies were detected early by the 4th day post-infection and peaked around the 17th day. It has also been reported that IgG has persisted up to 90 days post-infection.36 A study demonstrating the longitudinal profile of both antibodies in a population of 63 COVID-19 patients showed no specific chronological order in terms of IgM and IgG seroconversion. Synchronous or asynchronous seroconversion may occur.37 It has also been revealed that a higher level of IgG and IgM has been correlated with increasing severity.38 In contrast to other flu-like infections such as influenza, instead of IgG1, IgG3 appears to be the dominant IgG subtype during SARS-CoV-2 infection.39 A study of 23 patients demonstrated that in both severe and mild COVID-19 cases, the antibodies peak around day 10 and persist through day 25 post-symptom onset. In this cohort, IgM peaked after IgG in 53% of cases.40
The serological tests available for SARS-CoV-2 are ELISA, Electrochemiluminescence immunoassay (ECLIA), Chemiluminescent immunoassay (CLIA), Western Blot, Immunofluorescence assays and virus neutralisation assays, and studies have compared them.41, 42, 43, 44 Some studies have demonstrated that though ELISA had a high sensitivity (85–100%), false-positive results due to common conserved antigens of Coronavirus species resulted in lower specificity.45 Antibody profiling also helps to estimate the ideal time for the collection of plasma for the development of neutralizing monoclonal antibodies. These studies shall be more useful if conducted in targeted populations.46 IgG antibodies against SARS-Cov-2 will establish the true prevalence of disease and a more accurate national denominator for the number of people infected, thus determining the case fatality rate. Serological assays need to have a high specificity (>97%) as a test with low specificity will have a higher rate of false-positive results. A study from Kerala, India, performed antibody testing to estimate the seroprevalence of SARS-CoV-2 antibodies in asymptomatic HCWs;47 however, they found only three positive individuals of the total tested. The inclusion of large demographic features will help in setting a strong basis for understanding transmission and aspects of herd immunity. SARS-CoV-2 serosurveys show the changing trends with time, and mass-level serological testing at a population level shall help to determine whether the economy is ready to go back to normal.48 Our study represents a unique, mixed cohort of asymptomatic individuals and estimates the proportion of IgG antibodies in them.
Conclusion
The study provides an estimation of antibodies against COVID-19 from various states of the country to help in epidemiological assessment, demographic profiling, and understanding the immunological responses to COVID-19.
Disclosure of competing interest.
The authors have none to declare.
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asia S-WHO . 2020. India Situation. [Google Scholar]
- 4.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of covid-19 - studies needed. N Engl J Med. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 5.WHO . 2020. A Coordinated Global Research Roadmap. [Google Scholar]
- 6.Koopmans M., Haagmans B. Assessing the extent of SARS-CoV-2 circulation through serological studies. Nat Med. 2020;26(8):1171–1172. doi: 10.1038/s41591-020-1018-x. [DOI] [PubMed] [Google Scholar]
- 7.Denning D.W., Kilcoyne A., Ucer C. Non-infectious status indicated by detectable IgG antibody to SARS-CoV-2. Br Dent J. 2020;229(8):521–524. doi: 10.1038/s41415-020-2228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothe C., Schunk M., Sothmann P. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO . 2020. Coronavirus Disease COVID-2019. [DOI] [Google Scholar]
- 10.Huff Hanalise V., Singh Avantika. 2019. Asymptomatic transmission during the COVID-19 pandemic and implications for public health strategies; pp. 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolai L.A., Meyer C.G., Kremsner P.G., Velavan T.P. Asymptomatic SARS coronavirus 2 infection: invisible yet invincible. Int J Infect Dis. 2020;100:112–116. doi: 10.1016/j.ijid.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udugama B., Kadhiresan P., Kozlowski H.N. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 13.Petherick A. vol. 395. Elsevier Ltd; 2020. (Developing Antibody Tests for SARS-CoV-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shey M.S., Schmidt B.-M., Wiysonge C.S. Antibody tests for diagnosing COVID-19: how relevant are they? Pan Afr Med J. 2020;37(suppl 1):19–22. doi: 10.11604/pamj.supp.2020.37.1.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter A.K., Hegde S.T. The important role of serology for COVID-19 control. Lancet Infect Dis. 2020;20(July):758–759. doi: 10.1093/nsr/nwaa036.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidiq Z., Hanif M., Dwivedi K.K., Chopra K.K. Benefits and limitations of serological assays in COVID-19 infection. Indian Joural Tuberc. 2020 doi: 10.1016/j.ijtb.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81(104260):1–4. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ICMR ICMR advises States to conduct sero-survey to measure Coronavirus exposure in the population using IgG ELISA Test. Dep Heal Res Indian Counc Med Res. 2020:1–4. doi: 10.1016/0140-6736(90)92794-I. [DOI] [Google Scholar]
- 19.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. SSRN Electron J. 2020:1–22. doi: 10.2139/ssrn.3546052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ICMR . 2020. Updates on COVID-19 ICMR’ S Sero-Surveillance Study Reveals Only 0.73 % of the Sample Population Infected with COVID-19. [Google Scholar]
- 21.Gupta M., Mohanta S.S., Rao A. Transmission Dynamics of the COVID-19 Epidemic in India and Modelling Optimal Lockdown Exit Strategies. medRxiv. 2020:1–20. doi: 10.1101/2020.05.13.20096826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO, World Health Organization Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection. World Heal Organ. 2020;(March):1–19. [Google Scholar]
- 23.Study Group ICMR COVID Laboratory surveillance for SARS-CoV-2 in India: performance of testing & descriptive epidemiology of detected COVID-19, January 22 - April 30, 2020. Indian J Med Res. 2020;76(11):1532–1539. doi: 10.4103/ijmr.IJMR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Study Group ICMR COVID Prevalence of SARS-CoV-2 infection in India: findings from the national serosurvey, May-June 2020. Indian J Med Res. 2020;152:48–60. doi: 10.4103/ijmr.IJMR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satpati P.S., Sarangi S.S., Gantait K.S. Sero-surveillance (IgG) of SARS-CoV-2 Among Asymptomatic General Population of Paschim Medinipur, West Bengal, India. medRxiv. 2020 doi: 10.1101/2020.09.12.20193219. [DOI] [Google Scholar]
- 26.Korth J., Wilde B., Dol S. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128(104437):1–4. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fusco F.M., Pisaturo M., Iodice V. COVID-19 among healthcare workers in a specialist infectious diseases setting in Naples, Southern Italy: results of a cross-sectional surveillance study. J Hosp Infect. 2020;105:596–600. doi: 10.1016/j.jhin.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulikakos D. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. J Clin Virol. 2020;129(104545) doi: 10.1515/cclm-2020-0443. [DOI] [PubMed] [Google Scholar]
- 29.Stringhini S., Wisniak A., Piumatti G. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SARS-CoV-2/Pune Serosurvey/Technical Report/16-Aug 2020 Epidemiological and Serological Surveillance of COVID-19 in Pune City. 2020. [DOI] [Google Scholar]
- 31.Lee C.Y.P., Lin R.T.P., Renia L., Ng L.F.P. Serological approaches for COVID-19: epidemiologic perspective on surveillance and control. Front Immunol. 2020;11(April):1–7. doi: 10.3389/fimmu.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew D., Giles J.R., Baxter A.E. Deep immune profiling of COVID-19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. bioRxiv Prepr Serv Biol. 2020;8511:1–28. doi: 10.1101/2020.05.20.106401. [DOI] [Google Scholar]
- 33.Adams E.R., Ainsworth M., Anand R. Antibody testing for COVID-19: a report from the national COVID scientific advisory panel. Wellcome Open Res. 2020;5:139. doi: 10.12688/wellcomeopenres.15927.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L., Ren L., Yang S. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020;71(15):778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan W., Lu Y., Zhang J. Viral Kinetics and Antibody Responses in Patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.24.20042382. [DOI] [Google Scholar]
- 36.Lee Y.-L., LiaoL C.H. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect. 2020;(81):e55–e58. doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azkur A.K., Akdis M., Azkur D. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy Eur J Allergy Clin Immunol. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okba N.M.A., Müller M.A., Li W. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26(7):1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bettencourt Paulo, Catarina Fernandes A.G., Alvelos A.A.M. Qualitative serology in patients recovered from SARS CoV 2 infection. J Infect. 2020;(81):e120–e121. doi: 10.1016/j.jinf.2020.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song J.W., Zhang C., Fan X. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zainol Rashid Z., Othman S.N., Abdul Samat M.N., Ali U.K., Wong K.K. Diagnostic performance of COVID-19 serology assays. Malays J Pathol. 2020;42(1):13–21. [PubMed] [Google Scholar]
- 42.Lisboa Bastos M., Tavaziva G., Abidi S.K. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020:370. doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J.-L., Tseng W.-P., Lin C.-H. Four point-of-care lateral flow immunoassays for diagnosis of COVID-19 and for assessing dynamics of antibody responses to SARS-CoV-2. J Infect. 2020 doi: 10.1016/j.jinf.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman Brandi, Lester Sandra, Mills Lisa. Validation of a SARS-CoV-2 Spike Protein ELISA for Use in Contact Investigations and Sero- Surveillance. bioRxiv. 2020:1–12. doi: 10.1101/2020.04.24.057323. [DOI] [Google Scholar]
- 45.Zhong L., Chuan J., Gong B. Detection of serum IgM and IgG for COVID-19 diagnosis. Sci China Life Sci. 2020;63(5):777–780. doi: 10.1007/s11427-020-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theel E.S., Slev P., Wheeler S., Couturier M.R., Wong S.J., Kadkhoda K. The role of antibody testing for SARS-CoV-2: is there one? J Clin Microbiol. 2020;58(8):1–7. doi: 10.1128/JCM.00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar A., Sathyapalan D., Ramachandran A., Subhash K., Biswas L., Beena K.V. SARS-CoV-2 antibodies in healthcare workers in a large university hospital, Kerala, India. Clin Microbiol Infect. 2020;(xxxx):9–11. doi: 10.1016/j.cmi.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray Sougat, Chawla Naveen, Gupta Ayon, Kumar Maramaraj Kiran, Kumar Sanjeev, Anand Kavita Bala. Return to work strategy with antibody-based tests in COVID19: an observational study from a metropolitan area, India. J Mar Med Soc. 2020:1–5. doi: 10.4103/jmms.jmms. [DOI] [Google Scholar]