Dear Editor,
Coronavirus disease 2019 (COVID-19) has affected the world in an unprecedent manner. Countries across the globe are making efforts to vaccinate their vulnerable population against this disease. Development of COVID-19 vaccines and processes for their mandatory regulatory approval have been fast-tracked keeping in view of urgent requirement of these vaccines to contain the pandemic.1 India has given emergency approval and rolled out two vaccines—ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant) (COVISHIELD) and whole-virion inactivated SARS-CoV-2 vaccine BBV152 (COVAXIN) for vaccination of its health care workers and frontline workers.2 At present, data on adverse events after immunisation (AEFI) following COVID-19 vaccination are limited.3 Hence, we carried out this study to determine incidence and risk factors of systemic AEFI reported following first dose of ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant).
This study was conducted among Armed Forces Medical Services healthcare workers (HCW) deployed in Northern India, who took first dose of ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant) voluntarily in 14 vaccination centres in January–February 2021. Data regarding their age, COVID-19 infection in the past and other comorbidities was also obtained through a structured questionnaire before vaccination. These health workers were given 0.5 ml of vaccine intramuscularly in deltoid region and observed for 30 min in vaccination centres. Thereafter, these vaccine recipients were asked to report to officer in charge vaccination centre in case they develop AEFI symptoms, as per COVID-19 vaccination operational guidelines.3 Data obtained from all vaccination centres was collated and summarised by mean, standard deviation and proportions. Incidence proportion and relative risk of any AEFI with 95% confidence interval (CI) were calculated by log binomial regression. Variables with p-value < 0.1 in bivariable analysis were included for multivariable regression to estimate adjusted relative risk. We also carried out sensitivity analysis by excluding one vaccination site at a time from the analysis to assess the robustness of association of risk factors with AEFI. R software ver 3.6.1 was used for statistical analysis. Informed consent was taken from the study participants and the study was approved by the institutional ethics committee.
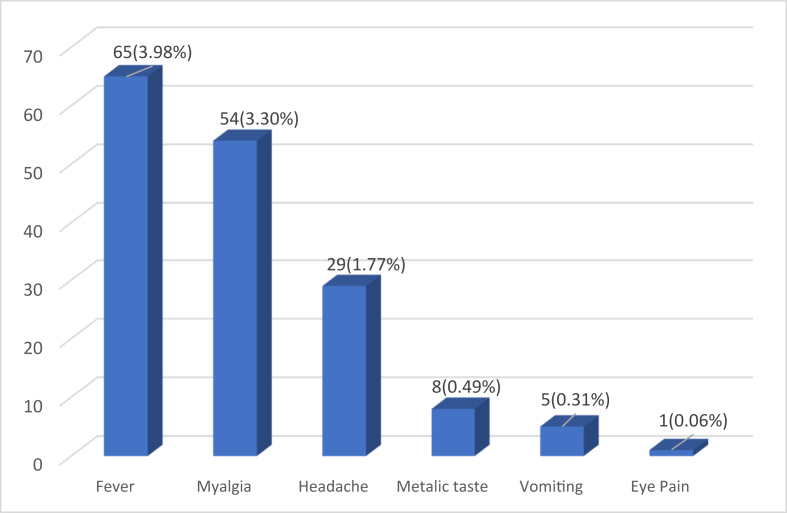
A total of 1634 HCW were given first dose of ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant). Their mean (SD) age was 32.04 (7.84) years and only 68 (4.16%) out of them were females. Study participants consisted of 106 (6.5%) doctors, 734 (44.9%) paramedical staff and 794 (48.6%) administrative and support staff. About 105 vaccine recipients reported at least one AEFI symptoms following COVID-19 vaccination (incidence proportion 6.4%, 95% CI: 5.3%, 7.7%). All AEFI reported were minor which were managed by tablet paracetamol and subsided after 1–2 days. Fever (65, 3.98%) was the most commonly reported AEFI followed by myalgia (54, 3.30%) (Fig. 1). About 48 (2.94%) study participants reported 2 or more AEFI, most common being fever with myalgia (19, 1.16%) and fever with headache (18, 1.10%). No severe or serious AEFI was reported among vaccine recipients. Incidence of systemic AEFI reported in our study is lesser than reported in phase 1/2 clinical trial of this vaccine4, as we have used passive surveillance to monitor AEFI as per Government of India's policy on COVID-19 vaccine AEFI surveillance3 as compared with active surveillance used in clinical trials. In our study, incidence of reported AEFI was higher among female HCW, doctors, younger HCW and those who had previous COVID-19 infection (Table 1). Pre-existing comorbidities were not found to be associated with AEFI. We observed that after adjusting for other variables, previous COVID-19 infection (aRR = 2.40, 95% CI: 1.48, 3.91) and female sex (aRR = 2.24, 95% CI: 1.22, 4.09) were significant independent risk factors for any systemic AEFI reported after COVID-19 vaccination. In sensitivity analysis also, previous COVID-19 infection and female sex were found to be consistently associated with reported AEFI.
Fig. 1.
Systemic AEFI reported after first dose of ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant).
Table 1.
Risk factors of reported AEFI after COVID-19 vaccination.
| Risk Factors | Total Number | No. Reporting AEFI (%) | Relative Risk (95% CI) | p value | Adjusted Relative Risk (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Previous COVID-19 infection | ||||||
| No | 1529 | 88 (05.8%) | Ref | <0.001 | Ref | <0.001 |
| Yes | 105 | 17 (16.2%) | 2.81 (1.74, 4.55) | 2.40 (1.48, 3.91) | ||
| Sex | ||||||
| Male | 1566 | 92 (05.9%) | Ref | <0.001 | Ref | 0.009 |
| Female | 68 | 13 (19.1%) | 3.25 (1.92, 5.52) | 2.24 (1.22,4.09) | ||
| Type of HCW | ||||||
| Paramedical & Support staff | 1528 | 90 (05.9%) | Ref | <0.001 | Ref | 0.072 |
| Doctors | 106 | 15 (14.2%) | 2.40 (1.44, 4.00) | 1.70 (0.95, 3.02) | ||
| Co-morbidities | ||||||
| No | 1601 | 104 (06.5%) | Ref | 0.441 | ||
| Yes | 33 | 1 (03.0%) | 0.47 (0.06, 3.24) | |||
| Age (per year) | 0.98 (0.95, 1.00) | 0.075 | 0.98 (0.95, 1.00) | 0.104 | ||
Most of these AEFI symptoms reported were related to vaccine reactogenicity, which is mediated by pyrogenic cytokines such as interleukin-1 (IL-1), IL-6, prostaglandin-E2 and tumour necrosis factor–alpha (TNF-α), released due to activation of immune response on vaccination. Some vaccines are known to cause increased postvaccination titres in those with evidence of prior infection as well as more systemic reactions after repeat doses due to induction of pre-existing immunity.5 In our study also, incidence of AEFI reported among those with previous COVID-19 infection was higher than other health workers even after adjusting for their sex, age and profession. Higher incidence of AEFI after first dose of vaccine among previously infected COVID-19 cases has also been reported in a study by Krammer et al6 (mRNA vaccines) as well as from ZOE COVID Symptom Study7 being conducted in the United Kingdom (mRNA and ChAdOx1 nCoV-19 vaccine). Studies on mRNA vaccines by Saadat et al8 and Krammer F et al6 have brought out that the antibody titers in COVID-19 recovered vaccinees is 10–20 times higher than other vaccine recipients, which can be responsible for higher incidence of AEFI in this group of individuals.
We observed higher incidence of reported AEFI among female and younger age group vaccine recipients. These findings are consistent with the results of study by CDC in USA9 (mRNA vaccines) and Jayadevan et al10 in India (ChAdOx1 nCoV- 19 vaccine and BBV152/COVAXIN), which have also reported that women are more likely to report AEFI after COVID-19 vaccination. Studies done on AEFI of other vaccines5,11,12 had also documented higher rate of AEFI among females. The difference can be due to genetic factors as well as due to hormones which are known to influence cytokine levels and immune response to vaccination. It has been reported that women tend to produce higher neutralizing titres after vaccination as compared with men.12 Similarly, studies have found that young people are more likely to report AEFI due to higher immune response.10,12 Older people are known to have lower levels of CRP, IL-10 and IL-6 after vaccination, which can explain lesser systemic adverse events in them.5 In view of these findings, we recommend that further studies on immune response and AEFI after COVID-19 vaccination may be carried out in these high-risk groups.
Disclosure of competing interest
The authors have none to declare.
References
- 1.Grigoryan L., Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol. 2020;50:101422. doi: 10.1016/j.smim.2020.101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministry of Health & Family Welfare. Precautions and Contraindications for COVID-19 vaccination (cited 2021 March 28): Available from: https://www.mohfw.gov.in/pdf/LetterfromAddlSecyMoHFWregContraindicationsandFactsheetforCOVID19vaccines.PDF.
- 3.Ministry of Health & Family Welfare . 2020 Dec 28. COVID-19 Vaccines Operational Guidelines.https://www.mohfw.gov.in/pdf/COVID19VaccineOG111Chapter16.pdf Available from: [Google Scholar]
- 4.Folegatti P.M., Ewer K.J., Aley P.K. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020 Aug 15;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hervé C., Laupèze B., Del Giudice G., Didierlaurent A.M., Da Silva F.T. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4(1):1–11. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krammer F., Srivastava K., the PARIS team. Simon V. medRxiv [Preprint]; 2021. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA Vaccine. [cited 2021 March 30] [DOI] [Google Scholar]
- 7.ZOE Global COVID symptom study. Vaccine after effects more common in those who already had COVID. (Internet). London: King's College [cited 2021 March 29]. https://covid.joinzoe.com/post/vaccine-aftereffects-more-common-in-those-who-already-had-covid.
- 8.Saadat S., Zahra R.T., Logue J. medRxiv[Preprint]; 2021. Single Dose Vaccination in Healthcare Workers Previously Infected with SARS-CoV-2. [cited 2021 March 28]. [DOI] [Google Scholar]
- 9.Gee J., Marquez P., Su J. First month of COVID-19 vaccine safety monitoring — United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayadevan R., Shenoy R., Anithadevi T.S. medRxiv [Preprint]; 2021. Survey of Symptoms Following COVID-19 Vaccination in India. [cited 2021 March 30]. [DOI] [Google Scholar]
- 11.Beyer W.E., Palache A.M., Kerstens R., Masurel N. Gender differences in local and systemic reactions to inactivated influenza vaccine, established by a meta-analysis of fourteen independent studies. Eur J Clin Microbiol Infect Dis. 1996;15(1):65–70. doi: 10.1007/BF01586187. [DOI] [PubMed] [Google Scholar]
- 12.Potluri T., Fink A.L., Sylvia K.E. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPG Vaccines. 2019;4(29) doi: 10.1038/s41541-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]