Abstract
Background
The immune response after SARS-CoV-2 is complex and may be affected by severity of the disease, race, etc. The present study was conducted to assess the serial antibody response among the COVID-19 patients admitted in the hospital.
Methods
The study was conducted between July and October 2020 in a dedicated COVID-19 hospital. All consented patients underwent serial testing of antibodies using a rapid chromatographic immunoassay-based qualitative IgG/IgM kit every third day until their discharge or death. The data about age, sex, severity of disease, length of stay, onset of symptoms, date of molecular testing were also collected. Appropriate statistical tests were used.
Results
The mean age of 1000 COVID-19 patients was 47.5 ± 17.9 years. Out of the total, 687 (68.7%) were males. With respect to severity, 682 (68.2%) were asymptomatic/mild, 200 (20%) were moderate and 118 (11.8%) were severe cases. The seroconversion percentage increased from 12.8% to 97.9% and 16.3% to 80.9% for IgG and IgM respectively in 21 days. The median time for seroconversion was 10 days (IQR:6–12 days) for IgG and eight days (IQR: 6–11 days) for IgM. At the time of discharge (median nine days), detectable IgG and IgM antibodies were present in 502 (52.46%) and 414 (43.26%) participants respectively. Seroconversion was associated with days after the symptoms, increasing severity of the disease and the presence of co-morbidity.
Conclusion
Seroconversion increased during the period of observation. The severe/moderate cases of COVID-19 tend to have an early seroconversion as compared to the asymptomatic/mild cases. Only half of the patients were seroconverted at discharge.
Keywords: COVID-19, SARS-CoV-2, Serial antibody, Seroconversion, COVID-19 hospital
Introduction
As of March 17, 2021, 119,960,700 confirmed cases and 2,656,822 deaths due to coronavirus disease-19 (COVID-19) were reported globally.1 The understanding of the novel COVID-19 disease regarding agent characteristics and infectivity has increased over the past year. However, the immunological response to the infection has remained elusive. There are still gaps in scientific knowledge about the antigenicity of the novel Severe Acute Respiratory Syndrome -2 (SARS-CoV-2) strain, innate and adaptive human immune response to the infection, antibody response, protection from reinfection, role of cytokine induction, etc.2
The Spike (S) and Nucleocapsid (N) are the main immunogens amongst SARS-CoV-2 structural proteins.3 The antibodies against S and N proteins have correlated well with neutralising antibodies.4 SARS-CoV-2 antibodies have enabled researchers to examine naturally acquired immunity to COVID-19. There were reports published highlighting that a small proportion of COVID-19 cases may not develop antibodies.5 The second round of national serosurvey conducted in India showed that nearly 30% of the positive cases do not have antibodies.6 Another study showed that 85% of the COVID-19 patients had antibodies at 20.5 days.7 On the other hand, a study conducted in the medical OPD of a tertiary care hospital found that nearly 20% of the patients in OPD with no suspicion of COVID-19 had antibodies.8 The immune correlates of infection are complex and depend on viral loads.9 Further, The serological response may also vary based on ethnicity or race.10
Detection of antibodies not only helps in identification of the current or past infections but is also essential for serosurveillance. Understanding the kinetics of immunity have widespread application in clinical case management and public health policy decision making especially for vaccination. Hence, the study was conducted to assess the seroconversion after SARS-CoV-2 infection among hospitalized COVID-19 patients.
Material and methods
The study was conducted in a dedicated COVID-19 hospital in the northern part of India. The hospital was constructed to address the surge in COVID cases at a national level and was temporary in nature. It was a dedicated facility with 500 beds for COVID-19 patients. Only cases with a confirmed diagnosis of COVID-19 using molecular diagnostic (Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) or Rapid Antigen Test) were admitted to the hospital. The study design was a longitudinal follow-up study. The study was carried out from July 2020 to October 2020. The exclusion criteria included contraindication for phlebotomy and existing coagulation disorders.
Dates pertaining to date of admission, onset of symptoms, molecular testing, contact details and reason for testing were collected from patient case sheets. The clinical severity was classified as mild, moderate, and severe as per the Indian Council of Medical Research (ICMR) guidelines.11 The patients were tested for antibodies on admission and then every third day till the patient's discharge or death. The criteria for discharge was according to the revised policy of the ICMR dated May 8, 2020.12 The patients were discharged on 10th day if asymptomatic or resolution of clinical symptoms and ability to maintain oxygen saturation for three days. The institution's ethical committee approved the study. Written informed consent was obtained from the patients or their legally authorised representatives. Specimen collection of venous blood for antibody testing was banded together with other investigations.
Rapid Antibody Detection kit, STANDARD Q COVID-19 IgM/IgG was used. The test was a rapid chromatographic immunoassay for qualitative detection of specific antibodies to SARS-CoV-2 present in human serum, plasma, or blood. It detects antibodies produced against Nucleocapsid (N) protein. According to the manufacturer, the kit's sensitivity and specificity were 99.1% and 95.1%, respectively, 14 days after symptom onset.13
The data were collated in MS Excel. The continuous variables were defined as mean and standard deviation while the categorical variables were defined as numbers and percentages. Contingency tables were made. Logistic regression was conducted for analyzing various factors associated with seroconversion. A new variable was created by adding the number of days of symptoms before hospitalization and the number of days of hospital stay to adjust for the days post onset of symptoms (POS). The findings of the first 80 COVID-19 cases have been published earlier.14 These 80 COVID-19 cases were excluded from the present analysis. Data analysis was performed using StataCorp, 2019, Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. A p-value of less than 0.05 was taken as significant.
Results
A total of 1011 COVID-19 patients were enrolled for the study. However, the final analysis was done for 1000 patients as samples could not be obtained for 11 patients. A total of 4119 serological tests for IgM and IgG were conducted. The mean age of patients was 47.5 ± 17.9 years (median: 48 years; Interquartile range (IQR): 33–61.5 years). Out of the 1000 participants, 687 (68.7%) were male. As per severity, 682 (68.2%) were asymptomatic/mild, 200 (20%) were moderate, and 118 (11.8%) were severe cases. The mean time of molecular testing was 5.2 ± 3.1 days (Median: 2 days; Range: 0–20 days) after the onset of symptoms. The average length of hospital stay for the patients was 9.6 ± 5.1 days (median: 9 days; IQR 8–10 days). The mean days for admission after the onset of symptoms was 4.3 ± 4 days (median days: 4; IQR 2–7 days). Mean duration post onset of symptoms to outcome (discharge/death) was 14 ± 6.6 days. Severe cases had a longer duration from symptoms to outcome as compared to moderate and asymptomatic/mild and this difference was statistically significant (p < 0.001) (Table 1). The median time for seroconversion for IgM and IgG was eight days (IQR: 6–11days) and 10 days (IQR: 6–12days) respectively POS.
Table 1.
Clinico-social profile (N = 1000).
| Characteristic | Total | Asymptomatic/Mild | Moderate | Severe | P value |
|---|---|---|---|---|---|
| Age Mean (SD) | 47.5 (17.5) | 42.9 (17.5) | 54.6 (13.9) | 62.4 (13.1) | <0.001c |
| Sex N (%)a | 0.3d | ||||
| Female | 313 (31.3) | 223 (32.7) | 54 (27) | 36 (30.5) | |
| Male | 687 (68.7) | 459 (67.3) | 146 (73) | 82 (69.5) | |
| Days from onset of symptoms Mean (SD) | 14 (6.6) | 12.1 (4.2) | 16.4 (5.8) | 21.1 (11) | <0.001c |
| Co-morbidities N (%)a | |||||
| Yes | 472 (47.2) | 253 (37.1) | 133 (66.5) | 86 (72.9) | |
| No | 528 (52.8) | 429 (62.9) | 67 (33.5) | 32 (27.1) | <0.001d |
| Antibody test conducted at | |||||
| Admissionb | 1000 | 682 (68.2) | 200 (20) | 118 (11.8) | <0.001d |
| 03 days | 977 | 676 (69.2) | 197 (20.1) | 104 (10.6) | <0.001d |
| 06 days | 929 | 651 (70) | 192 (20.7) | 86 (9.3) | <0.001d |
| 09 days | 749 | 512 (68.4) | 161 (21.5) | 76 (10.1) | <0.001d |
| 12 days | 239 | 90 (37.7) | 82 (34.3) | 67 (28) | <0.001d |
| 15 days | 110 | 18 (16.4) | 39 (35.5) | 53 (48.2) | <0.001d |
| 18 days | 66 | 5 (7.5) | 19 (28.8) | 42 (63.6) | <0.001d |
| 21 days | 47 | 1 (2.1) | 11 (23.4) | 35 (74.5) | <0.001d |
SD- Standard deviation.
column percentages.
row percentages.
Anova test.
Chi-square test.
Seroconversion of antibodies against SARS-CoV-2 in COVID-19 patients
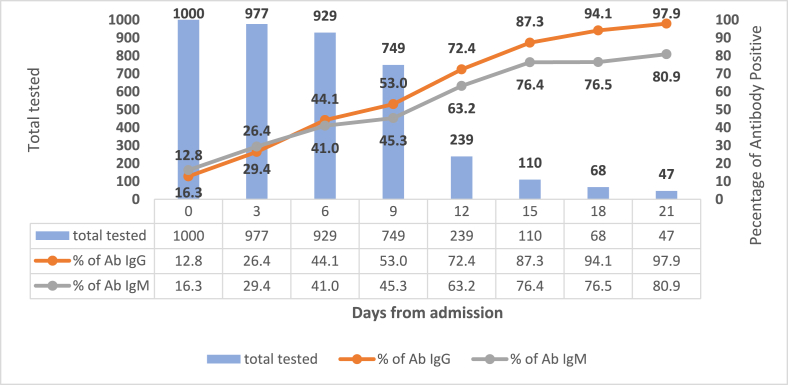
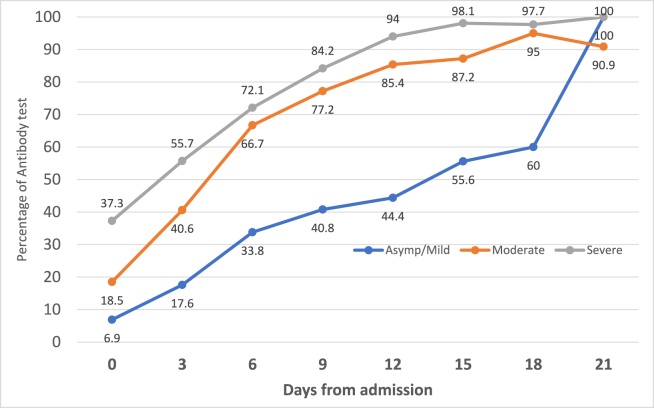
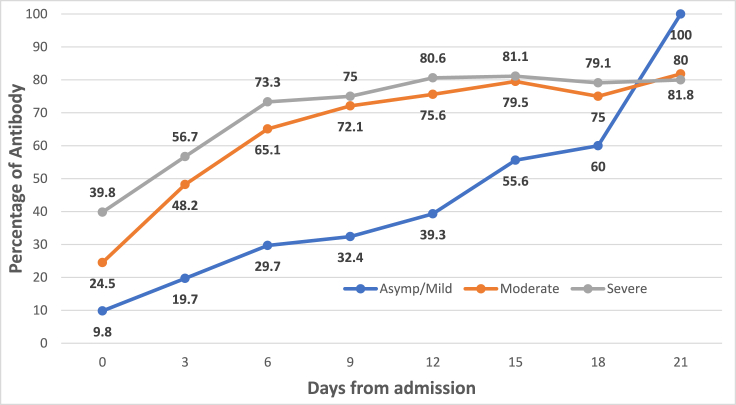
Fig. 1 shows the IgG and IgM antibody detection among COVID-19 cases from day 0 (Day of admission) to day 21. The proportion of seroconversion among IgG increased from 12.8% to 97.9%, whereas of IgM, it increased from 16.3% to 80.9%. The IgG and IgM antibody detection according to clinical condition is given in Fig. 2, Fig. 3 respectively. The antibodies (IgG and IgM) among moderate and severe cases were higher in proportion than in mild cases on the 18th day (p value < 0.001). However, between moderate and severe cases, there was no significant difference in the percentages of antibody detection.
Fig. 1.
Kinetics of antibody response after Hospital Admission (N = 1000).
Fig. 2.
Antibody IgG percentage according to clinical condition (N = 1000).
Fig. 3.
Antibody IgM percentage according to clinical condition (N = 1000).
Factors associated with seroconversion at discharge
A total of 957 patients were tested before discharge, out of which 502 (52.46%: 95% CI 49.2–55.7%) had IgG seroconversion, and 414 (43.26%: 95% CI 40–46.4%) had IgM seroconversion. IgG seroconversion at discharge was associated with increase in age, increase in the number of days POS, presence of co-morbidities and increasing clinical severity in bivariate (unadjusted) as well as multivariable (adjusted) analyses. The IgM seroconversion at discharge was associated with male sex, increase in duration of days from onset of symptoms, increase in clinical severity, and co-morbidity in bivariate and multivariable analyses (Table 2). Male sex was associated with increased odds for formation of IgM but not IgG antibody. The Increase in severity and presence of co-morbidity were associated with an increase in the odds ratio for seroconversion at discharge even after adjustment for duration of symptoms, for both IgG and IgM antibody. With each passing day, the odds of IgG and IgM seroconversion increased by 10% and 5%, respectively. The presence of comorbid conditions increased the odds of seroconversion among discharged patient by 1.9 times for IgG and 2.1 for IgM even after adjustment for age, sex, POS duration and clinical severity.
Table 2.
Association of antibody formation with clinical condition (N = 957).
| IgG Antibody | Unadjusted Odds ratio | 95% CI | Adjusted Odds ratio | 95% CI |
|---|---|---|---|---|
| Age | 1.03 | 1.03–1.04∗∗∗ | 1.01 | 1.00–1.02∗ |
| Sex | ||||
| F | Reference | Reference | Reference | Reference |
| M | 1.04 | 0.8–1.4 | 1.13 | 0.8–1.5 |
| Days from onset of symptoms | 1.2 | 1.1–1.2∗∗∗ | 1.1 | 1.07–1.1∗∗∗ |
| Clinical Severity | ||||
| Mild | Reference | Reference | Reference | Reference |
| Moderate | 5.4 | 3.7–7.9∗∗∗ | 2.9 | 2–4.4∗∗∗ |
| Severity | 12.7 | 5.9–26.3∗∗∗ | 3.5 | 1.6–7.9∗∗ |
| Co-morbidity |
3.1 |
2.4–4∗∗∗ |
1.9 |
1.3–2.6∗∗∗ |
| IgM Antibody | ||||
| Age | 1.03 | 1.02–1.04∗∗∗ | 1.0 | 0.9–1.01 |
| Sex | ||||
| F | Reference | Reference | Reference | Reference |
| M | 1.3 | 1.01–1.8∗ | 1.5 | 1.1–2.1∗ |
| Days from onset of symptoms | 1.1 | 1.07–1.1∗∗∗ | 1.05 | 1.03–1.08∗∗∗ |
| Clinical Severity | ||||
| Mild | Reference | Reference | Reference | Reference |
| Moderate | 5.2 | 3.7–7.4∗∗∗ | 3.3 | 2.3–4.8∗∗∗ |
| Severity | 4.6 | 2.8–7.5∗∗∗ | 1.9 | 1.01–3.4∗ |
| Co-morbidity | 2.9 | 2.2–3.8∗∗∗ | 2.1 | 1.5–2.9∗∗∗ |
∗<0.05, ∗∗<0.01, ∗∗∗<0.001.
Discussion
The study assessed the antibody response of the COVID-19 patients during their hospitalisation. A total of 1000 COVID-19 patients were observed from the time of their admission till their discharge or death. There were more male patients than female patients. A study conducted on 11,263 COVID-19 patients in the western part of India also showed male preponderance of cases.15 At national level also, the male patients made of 60% of all reported COVID-19 cases.16 The average time of hospitalisation of COVID-19 patients in the study was shorter than that in China (median: 14 days; IQR 10–19 days) but longer than in the Western world (median 5 days; IQR 3–9 days).17 This is primarily due to differences in discharge policies. While patients in India are discharged after 10 days of symptom onset or an afebrile period of three days (whichever is later) without the requirement of a negative RT-PCR, China followed a discharge policy of two negative tests within a gap of 24 h and European Union had a discharge policy of three days asymptomatic period, or presence of IgG antibody or laboratory evidence of SARS-CoV-2 clearance in respiratory samples.18,19
A study conducted on 14,618 hospitalized patients in China observed an interval time of three to 10.5 days for admission to hospital after onset of symptoms, which was similar to our study.20 However, we did not find any study from India to compare these figures.
In the present study, the median time for seroconversion for IgM and IgG was eight days (IQR: 6–11 days) and 10 days (IQR: 6–12 days) respectively POS. A study conducted on 173 COVID-19 patients observed median seroconversion at 12 days and 14 days for IgM and IgG respectively, with less than 40% seroconversion in the first week. This increased to 79.8% for IgG and 94.3% for IgM 15 days POS.21 Another study on 41 COVID-19 patients observed the median seroconversion time of 11 days (8–28 days) and 14 days (8–28 days) for IgG and IgM antibody respectively.22 A study conducted among 88 admitted COVID-19 patients in China showed that seroconversion started on day five and showed a steep rise in IgG levels as compared to IgM.23 We too observed a steep rise in proportion among whom IgG seroconversion took place. The findings from our study of early and steep seroconversion among severe cases is similar to that of other studies.22, 23, 24, 25 In another hospital-based study among 285 hospitalized patients in China, the proportion of patients with positive virus-specific IgG reached almost 100% 17–19 days POS. The proportion of patients with positive virus-specific IgM peaked at 94.1% about 20–22 days after symptom onset.26 These findings are similar to our findings but we could follow up only 47 patients till 21 days.
As discharge policies are country specific and differ within countries at different times, factors associated with current discharge policy were also studied. We found that 52.46% were seroconverted for IgG and 43.26% for IgM at discharge. The findings that seroconversion at discharge was associated with severity of disease at presentation even after adjustment for age, sex, length of stay is similar to other studies.25,27 The association of age and days POS, similar to the present study have also been observed.28 We also found that COVID-19 cases with co-morbidity are more likely to have antibodies even when adjusted to other variables. A cohort study conducted on 509 COVID cases in which 139 (27.3%) were diabetics concluded that diabetics had same humoral response as compared to non-diabetics.29 The association between specific co-morbid conditions and seroconversion may further be explored using appropriate study designs.
The present study, however has certain limitations. The quantitative analysis of antibodies could not be done. Second, the sensitivity and specificity of the kit was based on 14 days POS whereas the testing was done earlier than 14 days too. Third, the serology was done only for N protein of the virus. The antibodies against other viral proteins like ORF8, Spike protein and Neutralising antibodies were not carried out. Co-morbidities were analysed as present or not, the effect of different or multiple co-morbidities was not studied. We could not follow up with the participants for development of antibodies after their discharge from the hospital.
Conclusion
In summary, we observed that seroconversion increased with increased duration of observation. Severe/moderate cases of COVID-19 tended to have higher percentage of, and early seroconversion than asymptomatic/mild cases. Only half of the patients were seroconverted at discharge and co-morbid conditions were positively associated with seroconversion.
Disclosure of competing interest
The authors have none to declare.
Acknowledgements
We are grateful to all the patients and the health care workers of the COVID-19 hospital. We are also grateful to the O/o DGAFMS for providing all antibody testing kits during the entire study.
References
- 1.WHO Coronavirus (COVID-19) Dashboard [Internet]. [cited 2021 Mar 17]. Available from: https://covid19.who.int.
- 2.Percivalle E., Cambiè G., Cassaniti I. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Eurosurveillance. 2020 Jun 18;25(24) doi: 10.2807/1560-7917.ES.2020.25.24.2001031. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7315724/ [Internet] [cited 2021 Mar 18]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galipeau Y., Greig M., Liu G., Driedger M., Langlois M.-A. Humoral responses and serological assays in SARS-CoV-2 infections. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.610688. https://www.frontiersin.org/articles/10.3389/fimmu.2020.610688/full [Internet] [cited 2021 May 24] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dogan M., Kozhaya L., Placek L. SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun Biol. 2021 Jan 29;4(1):1–13. doi: 10.1038/s42003-021-01649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J., Chen C., Li Q., Cai P., Wang Z., Wang L. COVID-19 confirmed patients with negative antibodies results. BMC Infect Dis. 2020 Sep 22;20(1):698. doi: 10.1186/s12879-020-05419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murhekar M.V., Bhatnagar T., Selvaraju S. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health. 2021 Jan 27 doi: 10.1016/S2214-109X(20)30544-1. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(20)30544-1/abstract [Internet] [cited 2021 Jan 30];0(0). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettencourt P., Fernandes C., Gil A., Almeida A., Alvelos M. Qualitative serology in patients recovered from SARS CoV 2 infection. J Infect. 2020 Aug;81(2):e120–e121. doi: 10.1016/j.jinf.2020.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray A., Singh K., Chattopadhyay S. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in hospitalized patients at a tertiary referral center in North India. Infectious Diseases (except HIV/AIDS) 2020 Aug http://medrxiv.org/lookup/doi/10.1101/2020.08.22.20179937 [Internet] [cited 2021 Mar 18]. Available from: [Google Scholar]
- 9.Sui Y., Bekele Y., Berzofsky J.A. Potential SARS-CoV-2 immune correlates of protection in infection and vaccine immunization. Pathogens. 2021 Jan 30;10(2) doi: 10.3390/pathogens10020138. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7912691/ [Internet] [cited 2021 May 24] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurupati R., Kossenkov A., Haut L. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct pre-vaccination gene expression profiles in blood. Oncotarget. 2016 Aug 30;7(39):62898–62911. doi: 10.18632/oncotarget.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Updated Clinical Management Protocol for COVID 19 dated 03072020.pdf [Internet]. [cited 2021 Mar 18]. Available from: https://www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID19dated03072020.pdf.
- 12.Revised Discharge Policy for COVID19.pdf [Internet]. [cited 2021 May 6]. Available from: https://www.mohfw.gov.in/pdf/ReviseddischargePolicyforCOVID19.pdf.
- 13.Products - STANDARD Q COVID-19 IgM/IgG Duo [Internet]. [cited 2021 Mar 23]. Available from: http://sdbiosensor.com/xe/product/7662.
- 14.Yadav A.K., Ghosh S., Kotwal A., Kumar S., Bobdey S. Serial antibody response among hospitalized coronavirus disease 2019 cases in India. Med J Armed Forces India. 2020 Nov 26 doi: 10.1016/j.mjafi.2020.09.010. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7690321/ [Internet] [cited 2021 Mar 23]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kansara N., Nandapurkar A.B., Maniyar R., Yadav A.K. Prediction of mortality by age and multi-morbidities among confirmed COVID-19 patients: secondary analysis of surveillance data in Pune, Maharashtra, India. Indian J Publ Health. 2021 Jan 1;65(1):64. doi: 10.4103/ijph.IJPH_1096_20. [DOI] [PubMed] [Google Scholar]
- 16.Dashboard: National Centre for Disease Control (NCDC) [Internet]. [cited 2021 May 25]. Available from: https://ncdc.gov.in/dashboard.php.
- 17.Rees E.M., Nightingale E.S., Jafari Y. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020 Sep 3;18(1):270. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.https://www.ecdc.europa.eu.Discharge.criteria.for.confirmed.COVID-19.cases.pdf [accessed March 27, 2021].
- 19.Tian M., Long Y., Hong Y., Zhang X., Zha Y. The treatment and follow-up of “recurrence” with discharged COVID-19 patients: data from Guizhou, China. Environ Microbiol. 2020 Jul 6 doi: 10.1111/1462-2920.15156. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7361525/ [Internet] [cited 2021 May 25]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faes C., Abrams S., Van Beckhoven D., Meyfroidt G., Vlieghe E., Hens N. Time between symptom onset, hospitalisation and recovery or death: statistical analysis of Belgian COVID-19 patients. Int J Environ Res Publ Health. 2020 Oct;17(20) doi: 10.3390/ijerph17207560. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7589278/ [Internet] [cited 2021 May 6]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Nov 19;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu J., Wu C., Li X. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Apr 27 doi: 10.1093/cid/ciaa489. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7197626/ [Internet] [cited 2021 Mar 23]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong W.-H., Zhao R., Zhou J.-B. Serologic response to SARS-CoV-2 in COVID-19 patients with different severity. Virol Sin. 2020 Jul 23:1–6. doi: 10.1007/s12250-020-00270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Guo X., Xin Q. Neutralizing antibody responses to severe Acute respiratory Syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020 Nov 15;71(10):2688–2694. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young M.K., Kornmeier C., Carpenter R.M. IgG antibodies against SARS-CoV-2 correlate with days from symptom onset, viral load and IL-10. medRxiv. 2020 Dec 7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7743087/ [Internet] [cited 2021 Mar 20]; Available from: [Google Scholar]
- 26.Long Q.-X., Liu B.-Z., Deng H.-J. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020 Jun;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Zhang L., Sang L. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020 Oct 1;130(10):5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein S.L., Pekosz A., Park H.-S. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020 Nov 2;130(11):6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampasona V., Secchi M., Scavini M. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020 Dec 1;63(12):2548–2558. doi: 10.1007/s00125-020-05284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]