Abstract
Background
The first dose of the ChAdOx1 nCoV- 19 Corona Virus Vaccine (Covishield) was administered to the eligible beneficiaries of tertiary care institute of Western Maharashtra on 16 Jan 21 and in the past three months almost 97% of the staff has been vaccinated. The present study analyses the incidence of COVID cases in the unvaccinated and vaccinated population of the institute.
Methods
All Covid 19 infections (RT-PCR positive) from 01 February 21 to 25 April 21 were included in the study and analyzed as per their vaccination status. To assess the COVID 19 transmission in contacts, Secondary Attack Rates (SAR) of the pre-vaccination period (Jun–Oct 20) was compared with the present SAR.
Results
A total of 113 cases occurred in the study period (01 Feb to 25 Apr 21). Lower number of infections were observed among the fully vaccinated as compared to partially vaccinated and non-vaccinated. The overall vaccine effectiveness was found to be 88.6% (81.55–92.37) and 44.1% (4.55–67.3) in completely and partially vaccinated individuals respectively. Hazard Ratios for getting infected dropped significantly after 28 days of the second dose. The SAR in high risk contacts (HRCs) was found to be 4.25%, which was lower than SAR (20.6%) of pre-vaccination period.
Conclusion
This is one of the earliest studies in India to report the impact of COVID-19 vaccination. The results indicate that the vaccine provides effective protection against COVID-19 infection. However, given the complex dynamics of vaccination, the role of NPIs and implementation of COVID appropriate behavior cannot be undermined.
Keywords: COVID-19 vaccination, Immunity, Secondary attack rate, Vaccine effectiveness
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic since its onset in Dec 19 had affected more than 130 million people and caused about 2.5 million deaths worldwide till 31 Mar 2021.1 The successive surge of cases across the globe has caused immense human suffering, substantial economic loss, and social mayhem. Vaccine against the SARS-COV-2 virus has been long propagated as one of the most effective weapons to combat this deadly disease, however, the recent surge of cases has evoked fresh debate regarding efficacy and protective value of the vaccine. Presently several vaccines have been authorized by different countries based on the availability, efficacy, and safety. Drugs Controller General of India (DCGI), the regulatory body of Govt of India accorded emergency use authorization to ChAdOx1 nCoV- 19 Corona Virus Vaccine (Covishield) vaccine on 01 Jan 2021 and the first dose of the vaccine was administered to the eligible beneficiaries on 16 Jan 21. Ever since the launch of the ChAdOx1 nCoV-19 Corona Virus Vaccine in India and abroad, there has been apprehension regarding the efficacy of the vaccine. The vaccine manufacturers Serum institute of India in their drug information leaflet forwarded along with the vaccine claim efficacy of 53.28% (−3.21 to 8.86) to 78.79% (37.63–92.79) depending on dose interval between the first and the second dose.2 Different Phase II/III vaccine efficacy trials in the United Kingdom, Brazil, and South Africa have also reported similar efficacy (57.4–74.0%).3 Till day India has administered approximately 100 million doses of vaccine4 and is facing worst ever surge of cases. However, experts believe that just counting the number of cases may not be a reliable marker as many positive cases may be asymptomatic or mild and a more appropriate marker for vaccine efficacy would be occurrence of death and hospitalizations among vaccinated individuals.5 Studies from the United Kingdom indicate that post-vaccination drive deaths from COVID 19 among the older population (more than 80 years) fell by 62% and by 51% among people between 65 and 79 years.6,7
To resolve the enigma surrounding the efficacy of the ChAdOx1 nCoV- 19 vaccine, there is a need to study the occurrence of COVID-19 in vaccinated and unvaccinated individuals. Our study presents the experience of a tertiary care institute with a captive population of approx. 3000 personnel with high vaccine coverage.
Material and methods
COVID-19 vaccination was offered to all 3196 employees and students of the tertiary care institute from 16 Jan 21 and were followed-up till the closing date of the study i.e. 25 Apr 21. All the cases diagnosed with SARS-CoV-2 during the study period (01 Feb 21 to 25 Apr 21) were contacted and details of vaccination, demographic profile including age, sex, occupation and whether asymptomatic or symptomatic at the time of diagnosis were obtained on a predesigned form. Date of vaccination was also crosschecked with vaccination data being maintained at the institute. All the cases were diagnosed by reverse transcriptase based real time polymerase chain reaction (RT-PCR) performed at ICMR approved laboratory. Testing was done as per the national policy in vogue, i.e. all symptomatic (ILI symptoms) cases and all asymptomatic direct and high-risk contacts of a laboratory confirmed case were tested once between day 5 and day 10 of coming into contact. Fully vaccinated was defined as those individuals who had received both doses of the ChAdOx1 nCoV- 19 Corona Virus Vaccine (Covishield) and more than 2 weeks had elapsed following the second dose.8,9 Partially vaccinated individuals were those who had taken only one dose and more than 2 weeks had elapsed after one dose till being tested positive for COVID-19 or till the results were analyzed. The non-vaccinated group included individuals who either did not receive even a single dose of the vaccine due to any reason or had received first dose within 2 weeks of testing positive for COVID-19 or till the results were analyzed. Vaccination details of the individuals who did not get infected with SARS-CoV-2 during the study period (01 Feb 21 to 25 Apr 21) were obtained from the health records of the employees maintained in the institute. The study was approved by the institutional ethical committee of the institute.
Transmission in high risk contacts (HRCs)
Fairly large number of staff of the institute is residing in dormitories with common toilets and bathrooms for each dormitory. There are 15–20 personnel residing in each dormitory. To access the COVID 19 transmission in HRCs (personnel residing in the same dormitory and sharing same washrooms), personnel sharing the dormitory with confirmed cases (RT PCR positive COVID 19 cases) were quarantined for one week and tested on day 1 and day 7 of their last contact with the primary case. The Secondary Attack Rate (SAR) in HRCs of the cases in these dormitories was compared with SAR among HRCs in the pre-vaccination period i.e. Jun–Oct 20. Although, contact tracing was done for all the cases, however, to illustrate the transmission among HRCs and to compare with pre-vaccination period, analysis of three cases which occurred among residents of the dormitories during the study period (Feb–Apr 21) was considered because both in the pre-vaccination period (Jun–Oct 20) and post-vaccination period (Feb–Apr 21) the living conditions in the dormitories have remained the same and in both the periods the HRCs of the primary case were quarantined and tested at least on day 1 and day 07 of the last contact with the primary case.
Statistical analysis
Continuous variables have been represented in terms of mean and SD and categorical variables as proportions. Cox regression was used to calculate Hazard ratios for confirmed cases by time interval after second dose of vaccination. Extracted data were entered from predesigned forms into an electronic spreadsheet (Microsoft Excel®) and analyzed using statistical software SPSS version 17.0 (SPSS, IBM, Chicago, IL, USA). Chi-square test was used to compare proportions. p-value less than 0.05 was taken as significant.
Results
A total of 113 positive cases occurred during the study period (01 Feb to 25 Apr 21). 67 (59.29%) of total cases were fully vaccinated, 27 (23.89%) were partially vaccinated and 19 (16.81%) were non-vaccinated. Demographic and other baseline characteristics of the study population are depicted in Table 1. 85 (75.21%) were symptomatic at the time of testing. 16 (84.2%) of the unvaccinated group were symptomatic whereas 49 (73.1%) of the vaccinated individuals were symptomatic.
Table 1.
Baseline Characteristics of RT PCR positive cases as per vaccination status (n = 113).
| Vaccinated (n = 67) | Partially vaccinated (n = 27) | Non vaccinated (n = 19) | |
|---|---|---|---|
| Mean Age (SD) | 32.17 (±10.28) | 33.33 (±7.39) | 33.94 (±8.87) |
| <20 yrs | 9 (13.4%) | 1 (3.7%) | 0 |
| 21–40 yrs | 41 (61.1%) | 22 (81.4%) | 16 (84.2%) |
| >40 yrs | 17 (25.3%) | 4 (14.8%) | 3 (15.7%) |
| Sex | |||
| Females | 17 (25.3%) | 4 (14.8%) | 7 (36.8%) |
| Males | 50 (74.6%) | 23 (85.1%) | 12 (63.1%) |
| Symptoms at time of test | |||
| Absent | 18 (26.8%) | 7 (25.9%) | 3 (15.7%) |
| Present | 49 (73.1%) | 20 (74.07%) | 16 (84.2%) |
| Occupation | |||
| Doctors | 22 (32.8%) | 12 (44.4%) | 8 (42.1%) |
| Paramedics | 29 (43.2%) | 7 (25.9%) | 5 (26.3%) |
| Students | 13 (19.4%) | 1 (3.7%) | 1 (5.2%) |
| Support staff | 3 (4.4%) | 7 (25.9%) | 5 (26.3%) |
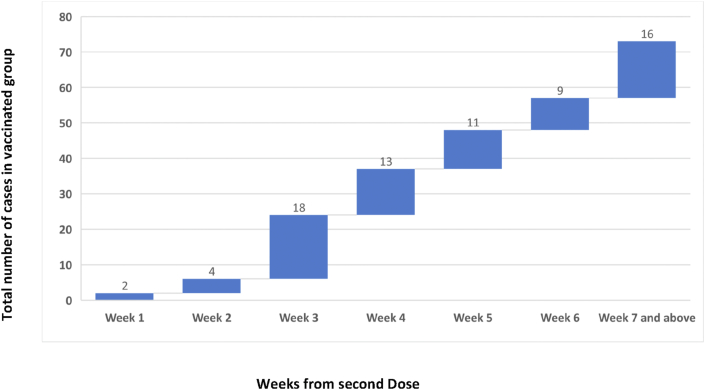
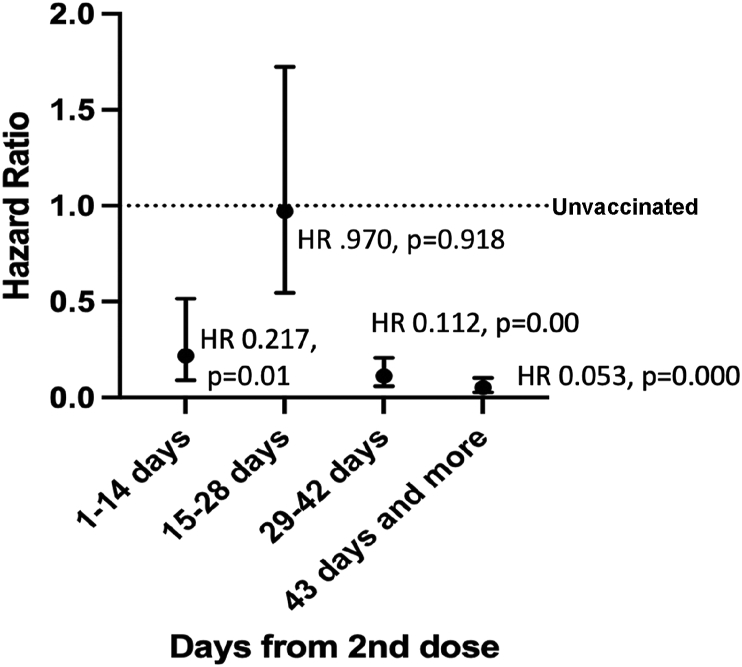
Overall, a significantly lower incidence of infection i.e. 67 out of 2863 (2.3%) was observed among the fully vaccinated individuals as compared to those among partially vaccinated 27 out of 239 (11.2%) and non-vaccinated 19 out of 94 (20.21%) (Table 2). The overall vaccine effectiveness was found to be 88.6% (81.55–92.37) in completely vaccinated and 44.1% (4.55–67.3) in partially vaccinated individuals (Table 2). Higher incidence of cases occurred among unvaccinated or partially vaccinated doctors as compared to doctors who had received both doses. Similarly, a higher incidence of infection was observed in all categories of unvaccinated staff (doctors, paramedics, medical students and support staff) as compared to completely or partially vaccinated groups. The occurrence of cases with passage of time after the second dose has been graphically depicted in Fig. 1. To further analyze the occurrence of cases with the passage of time in completely vaccinated individuals Hazard Ratios were calculated and it was observed that Hazard Ratios dropped significantly after 28 days of the second dose as compared to unvaccinated population (Fig. 2).
Table 2.
Overall vaccine effectiveness and Incidence of cases amongst vaccinated, partially vaccinated and unvaccinated groups (n = 113).
| Category | Doctors | p value | Paramedics | p value | Students | p value | Support staff | p value | Grand total | p value | Overall vaccine effectiveness (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases in Fully Vaccinated | 22/618 (3.5%) | 0.001 | 29/1021 (2.8%) | 0.001 | 13/687 (1.8%) | 0.31 | 3/537 (0.5%) | 0.001 | 67/2863 (2.3%) | 0.001 | 88.6% (81.55–92.37) |
| No. of cases in partially vaccinated | 12/41 (29.2%) | 7/109 (6.4%) | 1/6 (16.6%) | 7/83 (8.4%) | 27/239 (11.29%) | 44.1% (4.55–67.3) | |||||
| No. of cases in unvaccinated | 8/23 (34.7%) | 5/42 (11.9%) | 1/23 (4.3%) | 5/6 (83.3%) | 19/94 (20.2%) | – |
Fig. 1.
Distribution of cases per week following second dose.
Fig. 2.
Hazard ratios for PCR confirmed cases by interval after second dose of vaccine.
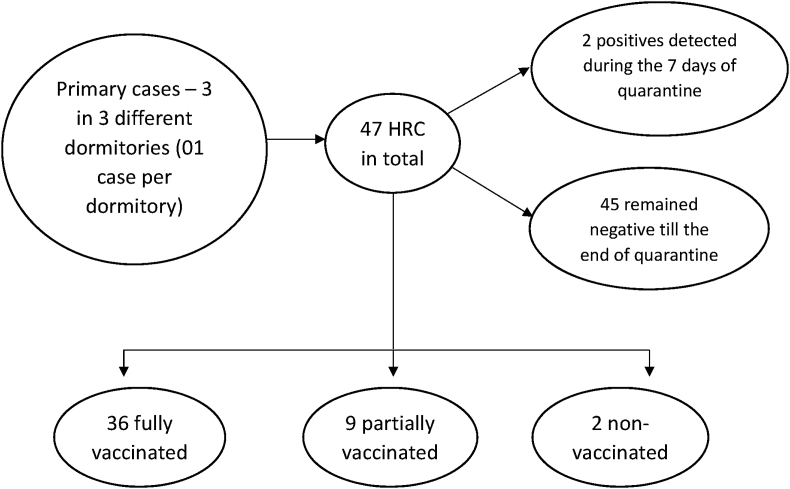
To understand the chain of transmission, the occurrence of cases in HRCs residing in the dormitories of COVID cases were analyzed. During the study period, a total of three cases were reported from three different dormitories. All 47 HRCs were quarantined in different dormitories as 3 separate cohorts (01 case was found in each dormitory) and tested on Day 1 and 7, 02 HRCs from one dormitory tested positive and both were asymptomatic, a graphical presentation has been depicted in Fig. 3. The secondary attack rate in these cohorts was found to be 4.25%, which was much lesser as compared with SAR of 21.42% in HRCs observed during the first wave of the pandemic in Pune in Jun–Oct 20 (Table 3).
Fig. 3.
Representation of cases in contacts quarantined in dormitories.
Table 3.
Secondary attack rate in pre and post-vaccination period.
| Period | Contacts tested positive | Contacts Tested Negative | Secondary Attack Rate | p Value |
|---|---|---|---|---|
| Pre-Vaccination Period | 54 | 252 | 21.42% | <0.05 |
| Post-Vaccination Period | 02 | 47 | 4.25% |
Discussion
As the onslaught of the COVID 19 pandemic continues to ravage the world, most of the countries are racing to vaccinate its population to overcome this fatal disease. However, predicaments such as the short supply of vaccines, the soaring number of cases, the emergence of new vaccine-resistant strains, doubts about the vaccine efficacy, and its subsequent side effects have hampered the progress of vaccination drive in many countries.10 COVID 19 vaccination program was started in this tertiary care institute in Western Maharashtra along with the nation on 16 Jan 21 and has achieved more than 95% vaccination coverage of Health care workers and support staff. In this study, we present the analysis of 113 cases that occurred at least two weeks after administration of the first dose i.e. from 01 Feb to 25 Apr 21 in both vaccinated and unvaccinated individuals.
In the present study, the mean age of the patients was 32 years and no difference was observed in the occurrence of cases between vaccinated, partially and unvaccinated groups as per age, gender, or place of residence. However, a higher number of cases were observed among doctors and paramedical staff (Table 1) which may be due to higher exposure rates among doctors and paramedical staff involved in direct patient care in the hospital.11
The Incidence of COVID-19 among unvaccinated/partially vaccinated was higher as compared to the incidence among the vaccinated individuals. This difference was significant and very prominent among all the categories (doctors, paramedics, medical students and support staff). The overall vaccine effectiveness was found to be 88.6% (81.55–92.37) in completely vaccinated and 44.1% (4.55–67.3) in partially vaccinated individuals. These results indicate that ChAdOx1 nCoV-19 Corona Virus Vaccine (Covishield) provides protection against the occurrence of COVID 19 infection. However, the occurrence of cases among the vaccinated persons is a matter of concern but it cannot be completely attributed to vaccine efficacy/effectiveness alone. The dynamics of vaccination and subsequent protection against SARS-CoV-2 are complex and are often influenced by age and co-morbidity-related factors, changing prevalence of infection in the community, adherence to using non-pharmaceutical interventions (NPIs), and change in attitude post-vaccination with a perception of decreased risk.10,12 ChAdOx1 nCoV-19 (AZD1222) vaccine has been found to induce Anti-spike antibody, antibody-dependent monocyte/neutrophil phagocytosis, and Cellular responses,13 however, the human factors cannot be adjusted for in a laboratory set up.
The overall vaccine efficacy of 66.7% (95% CI 57.4–74.0) has been reported in the pooled analysis of four randomized control trials for the ChAdOx1 nCoV-19 (AZD1222) vaccine, the same vaccine technology has been used in Covishield vaccine of India.3 In addition, the interval between the doses of the vaccine has been reported to influence the vaccine efficacy significantly. The vaccine efficacy has been reported to be higher in those with a longer interval between the two doses (vaccine efficacy 81.3% [95% CI 60.3–91.2] at ≥12 weeks) than in those with a short interval (vaccine efficacy 55.1% [33.0–69.9] at <6 weeks).3 As per prevailing government guidelines our study population was vaccinated with two doses at an interval of 4–6 weeks. In our study, the significant drop in the hazard ratio (HR) in the initial 14 days period after the second dose was seen may be because the individuals with recent onset of COVID symptoms tend to defer their second dose and take vaccination after the symptoms have subsided. Hence recently vaccinated individuals are less likely to be symptomatic and tested positive.14 Thereafter, there was a rise HR in 14–28 days period and finally HRs decreased significantly in post-vaccination 28 day period (Fig. 2). Thus, indicating that maximum protection from the vaccine is achieved beyond the 28 days of vaccination. In the present study, the occurrence of COVID-19 among vaccinated persons after complete vaccination, even though minuscule, can be explained with the level of vaccine efficacy offered by the Covishield vaccine, shorter dose interval, surge in cases in Maharashtra (Pune has been reporting new cases in all time record-high range of 7000–12,000/day i.e. more than double the highest number of cases reported in the first wave during Sep–Oct 2020).4 However, despite the occurrence of cases among the completely vaccinated group, the protective value of vaccine cannot be undermined, but it is also prudent to sensitize people regarding the risk of infection post-vaccination and the importance of NPIs in the prevention of the disease.
Apart from the difference in the incidence of COVID-19 among the vaccinated and unvaccinated group, we also observed a significant reduction in Secondary Attack Rate (SAR) among HRCs of the cases. During the first wave of the pandemic from Jun-Oct 2020 SAR of 21.42% was observed among HRCs of cases sharing the same living spaces (dormitories) which is much higher than SAR of 4.25% during the current second wave (Mar–Apr 2021). The SAR of the two time periods are comparable because the living conditions in the dormitories have remained the same, all the HRCs were strictly quarantined and the criteria of HRCs were the same during both the periods. The reduction in secondary attack rate by one-fifth of the current time as compared to the first wave may be an under-estimation of the impact of vaccination, given the fact that the intensity of pandemic in the current second wave is much higher than the previous first wave. This further supports the need for rapid mass vaccination of the susceptible as an effective public health strategy at the community level. However, experts from across the globe believe that the occurrence of cases may not be a reliable indicator of whether the vaccine is working as routine testing and high-risk screening would pick up asymptomatic or mild cases.6 But in our study, even after screening HRCs of cases in the three cohorts (Fig. 3) only two fresh cases were reported, and thus indicating a significantly lower SAR.
Although the present study has the unique distinction of being one of the earliest studies from the Indian subcontinent to report the impact of COVID-19 vaccination, it is not devoid of certain limitations. In addition to vaccination, we had the advantage of a captive population, NPIs and social distancing measures were also strictly implemented in our institute hence the reduction in cases cannot be attributed to vaccination alone. To tide over this limitation, the occurrence of severe disease, hospitalizations, and mortality can be considered to access the impact of vaccination. United Kingdom was one of the first countries in the world to roll out the COVID 19 vaccination program and has vaccinated more than 50% population of more than 70 years, it has reported that COVID 19 vaccination provides significant protection against severe disease, hospitalization, and death especially in the elderly population.6,7,15 Present study could not estimate the impact of the vaccination on severe disease and mortality as the majority of our population belonged to the younger age group (less than 60 Years) and no case of severe disease has occurred to date. One of the other major limitation of the study is short duration of follow-up post second dose of vaccination. The majority of the population had received their second dose by 05 Mar 21 and the surge of cases in Pune began around the third week of Mar (most of the population had completed only 14 days after second dose by the that time) hence though the results of the study are encouraging and indicate that vaccination provides protection against COVID 19 infection, more follow-up is required to further substantiate the findings of our study.
Conclusion
It is one of the earliest studies to report the impact of vaccination and the results indicate that the vaccine provides significant protection against COVID-19 infection and prevents transmission of the disease among vaccinated individuals. Hence, rapid vaccination and mass coverage of the ChAdOx1 COVID-19 vaccine seems to be an effective public health strategy to combat the COVID 19 pandemic. However, given the complex dynamics of vaccination and subsequent protection against the disease, the role of NPIs and strict implementation of COVID appropriate behavior cannot be undermined.
Disclosure of competing interest
The authors have none to declare.
Acknowledgment
We acknowledge the priceless contribution and untiring efforts of Faculty and residents of Dept. of Internal Medicine, Paediatrics, Anaesthesia, and College of Nursing of our institute for the successful conduct of the COVID 19 vaccination drive.
References
- 1.COVID 19 WHO Dashboard. Available at: https://covid19.who.int/. Accessed on 10 April 2020.
- 2.COVISHIELD – ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant) Vaccine Insert.
- 3.Voysey M., Costa Clemens S.A., Madhi S.A. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. Epub 2021 Feb 19. Erratum in: Lancet. 2021 Mar 6;397(10277):880. PMID: 33617777; PMCID: PMC7894131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID19 India Dashboard. Available at: https://www.covid19india.org/. Accessed on 10 April 2021.
- 5.Wise J. Covid-19: infections in England fall by two thirds since January. BMJ. 2021;372 doi: 10.1136/bmj.n491. [DOI] [PubMed] [Google Scholar]
- 6.Wise Jacqui. Covid-19: is vaccination roll out reducing cases and deaths in the UK? BMJ. 2021;372:n506. doi: 10.1136/bmj.n506. Available at: [DOI] [PubMed] [Google Scholar]
- 7.Bernal J.L., Andrews N., Gower C. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv preprint. 2021 doi: 10.1101/2021.03.01.21252652. Accessed on 15 April 2021. [DOI] [Google Scholar]
- 8.COVID19 Vaccine Guide for Eligible Beneficiaries. Published by MOHFW, GOI. Available online at: https://www.mohfw.gov.in/pdf/COVID19VaccinationGuideforEligibleBeneficiaries.pdf. Accessed on 15 April 2021.
- 9.Interim Public Health Recommendations for Fully Vaccinated People. CDC. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html. Accessed on 15 Apr 2021.
- 10.Moore S., Hill E.M., Tildesley M.J., Dyson L., Keeling M.J. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021;(21):S1473–S3099. doi: 10.1016/S1473-3099(21)00143-2. 00143-2. Epub ahead of print. PMID: 33743847; PMCID: PMC7972312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen L.H., Drew D.A., Joshi A.D. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. Preprint. medRxiv. 2020:2020. doi: 10.1101/2020.04.29.20084111. 04.29.20084111. Published 2020 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day Michael. Covid-19: stronger warnings are needed to curb socialising after vaccination, say doctors and behavioural scientists. BMJ. 2021;372:n783. doi: 10.1136/bmj.n783. [DOI] [PubMed] [Google Scholar]
- 13.Barrett J.R., Belij-Rammerstorfer S., Dold C. Oxford COVID Vaccine Trial Group. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2020 doi: 10.1038/s41591-020-01179-4. Epub ahead of print. PMID: 33335322. [DOI] [PubMed] [Google Scholar]
- 14.Public Health England Vaccine Effectiveness Report. Public Health England; 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/971017/SP_PH__VE_report_20210317_CC_JLB.pdf Available: [Google Scholar]
- 15.Andrews Nick, Julia Stowe, Ismael Sharif. Public Health England; 2021. Impact of COVID-19 Vaccines on Mortality in England: December 2020 to March 2021.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/977249/PHE_COVID-19_vaccine_impact_on_mortality_March.pdf Available at: [Google Scholar]