Abstract
Astrocytes play a formative role in memory consolidation during physiological conditions; when dysregulated, astrocytes release glial fibrillary acidic protein (GFAP), which has been linked with negative memory outcomes in animal studies. We examined the association between blood GFAP, memory, and white matter (WM) integrity, accounting for blood markers of AD pathology (i.e., Aβ42) and neurodegeneration (i.e., total tau; neurofilament light chain) in 114 older adults (asymptomatic, n=69; MCI/AD dementia, n=45). Higher levels of GFAP were associated with lower memory scores (p<0.0001), such that for one SD increase in mean GFAP values, the memory composite score decreased on average by 0.49 (Standard error=0.071). These results remained significant after controlling for diagnostic status and AD-related blood biomarkers. Higher GFAP was also related to lower WM integrity in regions vulnerable to AD pathology; however, WM integrity did not account for the association between GFAP and memory. Study findings suggest that higher blood levels of a marker of astrogliosis may reflect impoverished memory functions and white matter health, independent of markers of amyloid or neurodegeneration.
Keywords: astrogliosis, memory, aging, Alzheimer’s disease, astrocytes
1. Introduction
Astrocytes have been shown to play a key role in synaptic remodeling and long-term memory development, and when dysregulated, have been implicated in neurodegenerative pathological cascades. However, very few human studies have examined how astrocytic markers relate to cognition in aging adults, or have evaluated whether these markers reflect clinically meaningful information independent of indices of neuronal degeneration.
Astrocytes are abundant glial cells in the central nervous system (CNS) and communicate via calcium signaling and release of gliotransmitters (Harada et al., 2015). Despite astrocytes interacting with all cells in the CNS, their function was previously thought to be primarily tethered to neuronal structural and trophic support, maintenance of homeostatic regulatory processes, and the formation and modulation of the blood brain barrier (BBB)(Farhy-Tselnicker and Allen, 2018). Although astrocytes are considered a critical glial cell in the support of vital CNS functions, mounting evidence suggests that they may also play a more pivotal role in cognitive processes, particularly memory. Salient to their role in memory function, astrocytes project processes whose end feet engage with neurons at the pre- and post-synaptic nerve terminals, forming the tripartite synapse (Araque et al., 1999; Farhy-Tselnicker and Allen, 2018; Harada et al., 2015). In the hippocampus, astrocytic membranes encase more than 50% of synapses (Ventura and Harris, 1999), with prior work highlighting a role for astrocytes in modulating synaptic strength and long-term plasticity (Ota et al., 2013). Importantly, recent seminal studies using animal models have observed that astrocytes have the ability to modify and enhance synaptic plasticity and augment memory. Specifically, by employing optogenetic tools, investigators were able to show that astrocytic activation alone could enhance memory acquisition and allocation. Improved memory performance could not be directly attained when solely activating neuronal activity, calling into question neuron-centric views of cognition (Adamsky et al., 2018). Moreover, a recent study found that knockout mice missing the IP3R2 receptor (used specifically by astrocytes to release calcium) have disrupted astrocytic processes and perform poorly on tasks of long-term memory consolidation (Pinto-Duarte et al., 2019).
Astrocytes play a critical role in memory consolidation and retrieval during normal physiological conditions. However, in response to CNS damage, pathology, and/or immune cell activation, astrocytes undergo a process referred to as astrogliosis, which if sustained, may result in harmful effects on brain health (Sofroniew, 2014). When activated, astrocytes release glial fibrillary acidic protein (GFAP), which is an intermediate cytoskeletal protein that is posited to be a downstream biomarker of astrogliosis and a mechanistic driver of neuronal and synaptic dysfunction. Specifically, recent studies suggest that GFAP plays a modulatory role in astrocytic regulation of neurogenesis, with ablation of GFAP in animal models resulting in decreases in reactive gliosis processes and increases in hippocampal neurogenesis – both in normal conditions as well as after a sustained injury (Wilhelmsson et al., 2012).
Given astrocytes’ role in regulating synaptic transmission, it is not surprising that glial dysfunction and dysregulation of GFAP levels are also implicated in Alzheimer’s disease (AD) pathogenesis and progression (Garwood et al., 2017). Extending this work to human studies of neurodegenerative disease, elevations in GFAP have been reported in the cerebrospinal fluid (CSF)(Jesse et al., 2009), and GFAP+ hypertrophied astrocytes have been noted in the brains (Muramori et al., 1998; Sadick and Liddelow, 2019) of patients with AD relative to controls. More recently, higher blood levels of GFAP have also been linked with both early onset (Elahi et al., 2020) and late onset AD (Oeckl et al., 2019), with preliminary evidence pointing to possible specificity of elevated blood GFAP levels to AD relative to non-AD degenerative phenotypes (i.e., frontotemporal dementia) (Oeckl et al., 2019). Despite accumulating evidence that astrogliosis may underlie – or be a harbinger for AD, little is known about how astrocytic biomarkers map on to cognitive function and brain structure in aging adults and adults with clinical manifestations of AD (i.e., MCI and dementia level of severity). Epidemiological studies have demonstrated that poorer overall cognitive performance later in life is related to higher levels of GFAP measured in temporal, parietal and occipital cortices upon autopsy, even after accounting for neuritic plaques and neurofibrillary tangles (Kashon et al., 2004). Moreover, a recent observational study of GFAP and cognition in late life also suggested a possible negative association with global cognitive function (Oeckl et al., 2019). However, the assessment of specific cognitive domains using comprehensive neuropsychological measures has been lacking.
In this study, we appraised the relationship between blood levels of GFAP and verbal memory performance in a group of aging adults and adults with symptomatic AD. Given evidence from animal studies suggesting that astrogliosis may play an independent role in the maintenance of memory functions, we examined whether higher levels of blood GFAP were associated with worse memory performance, even after accounting for canonical markers of AD pathology (i.e., Aβ42) and neurodegeneration (i.e., total tau; neurofilament light chain [NfL]). Because the ratio of glia to neurons is higher in white matter, and GFAP is more highly expressed in white matter astrocytes relative to grey matter astrocytes (Bushong et al., 2002; Goursaud et al., 2009), we further evaluated whether higher levels of GFAP were associated with altered diffusion metrics in regions critical for episodic memory (e.g., fornix; cingulum-hippocampal portion) and appraised whether white matter integrity explained associations between blood GFAP levels and memory performance.
2. Methods:
2.1. Participants
A sample of 114 healthy older adults and adults with symptomatic AD were selected from the University of Colorado Alzheimer’s and Cognition Center (CUACC) database (see Table 1). All participants were enrolled in the Bio-AD study, which is a study that entails comprehensive cognitive testing, health history assessment, neurological and physical examination, and informant interview of all participants. Participants were included as healthy controls if they were community dwelling older adults with no diagnosis of MCI or Dementia and no evidence of a neurodegenerative phenotype based on a neurological exam. Adjudication of MCI due to possible AD and AD dementia was based on NIA-AA clinical criteria (Albert et al., 2011; McKhann et al., 2011), with additional categorization of atypical AD phenotypes based on published diagnostic criteria (e.g., Posterior Cortical Atrophy [PCA](Crutch et al., 2017)). Participants were excluded if they had a major psychiatric disorder, current non-AD neurological condition known to affect cognition (e.g., Parkinson’s disease; large vessel infarct; multiple sclerosis), current evidence or history in the past 2 years of a focal brain lesion, current substance abuse, significant systemic medical illness or active neoplastic disease (e.g., active cancer), significant sensory or motor deficits that would interfere with cognitive testing, or traumatic brain injury with loss of consciousness greater than 5 minutes. All participants were reviewed at a case consensus conference with a board-certified neuropsychologist, board-certified behavioral neurologist, and clinical research coordinator. A subset of cognitive measures from the research protocol were reviewed in a consensus conference and used in the adjudication of diagnosis as a clinically normal older adult, adult with MCI, or adult with AD dementia. To reduce circularity in our methodological approach, cognitive measures that were reviewed in the consensus conference for differential diagnosis were separate from those used as primary outcomes in our research study.
Table 1.
Characteristics of Participant Sample.
| Asymptomatic (N=69) | Symptomatic (N=45) | Total (N=114) | p value | |
|---|---|---|---|---|
| Age | 0.095 | |||
| Mean (SD) | 69.5 (6.4) | 71.7 (7.5) | 70.4 (6.9) | |
| Range | 53.0 – 83.0 | 54.0 – 87.0 | 53.0 – 87.0 | |
| Sex | 0.027 | |||
| Male | 21 (30.4%) | 23 (51.1%) | 44 (38.6%) | |
| Female | 48 (69.6%) | 22 (48.9%) | 70 (61.4%) | |
| Education (Years) | 0.172 | |||
| Mean (SD) | 17.1 (2.2) | 16.5 (2.5) | 16.9 (2.3) | |
| Range | 12.0 – 20.0 | 12.0 – 20.0 | 12.0 – 20.0 | |
| APOE Genotype | 0.013 | |||
| % with 1 or more E4 alleles | 25 (36.2%) | 27 (60.0%) | 52 (45.6%) | |
| Global CDR score | < 0.001 | |||
| 0 | 64 (94.1%) | 0 (0.0%) | 64 (57.1%) | |
| 0.5 | 4 (5.9%) | 34 (77.3%) | 38 (33.9%) | |
| 1 | 0 (0.0%) | 6 (13.6%) | 6 (5.4%) | |
| 2 | 0 (0.0%) | 4 (9.1%) | 4 (3.6%) | |
| MTL White Matter ROI (FA) | < 0.001 | |||
| Mean (SD) | 0.41 (0.03) | 0.38 (0.03) | 0.40 (0.03) | |
| Range | 0.32 – 0.49 | 0.31 – 0.44 | 0.31 – 0.49 | |
| AD Vulnerable White Matter ROI (FA) | < 0.001 | |||
| Mean (SD) | 0.47 (0.02) | 0.44 (0.02) | 0.46 (0.03) | |
| Range | 0.40 – 0.51 | 0.40 – 0.50 | 0.40 – 0.51 | |
| Abeta42 (pg/mL) | 0.067 | |||
| Mean (SD) | 10.2 (2.2) | 9.4 (2.4) | 9.9 (2.3) | |
| Range | 5.4 – 16.2 | 5.9 – 16.2 | 5.4 – 16.2 | |
| Tau (pg/mL) | 0.003 | |||
| Mean (SD) | 2.0 (0.5) | 2.5 (0.9) | 2.2 (0.7) | |
| Range | 1.1 – 3.8 | 1.1 – 4.9 | 1.1 – 4.9 | |
| NFL (pg/mL) | < 0.001 | |||
| Mean (SD) | 13.8 (6.7) | 21.4 (9.4) | 16.8 (8.7) | |
| Range | 5.3 – 52.1 | 7.7 – 44.1 | 5.3 – 52.1 | |
| GFAP (pg/mL) | < 0.001 | |||
| Mean (SD) | 148.1 (72.7) | 265.9 (125.6) | 194.6 (112.5) | |
| Range | 50.0 – 449.4 | 65.2 – 633.6 | 50.0 – 633.6 | |
| SENAS Memory Composite Score | < 0.001 | |||
| Mean (SD) | 0.9 (0.6) | −0.4 (0.8) | 0.4 (0.9) | |
| Range | −0.8 – 2.4 | −2.7 – 1.5 | −2.7 – 2.4 | |
| Spatial Composite Score | < 0.001 | |||
| Mean (SD) | 1.1 (0.7) | −0.3 (1.3) | 0.5 (1.2) | |
| Range | −0.7 – 2.4 | −2.9 – 2.3 | −2.9 – 2.4 | |
| Executive Composite Score | < 0.001 | |||
| Mean (SD) | 0.6 (0.5) | −0.3 (0.6) | 0.2 (0.7) | |
| Range | −0.6 – 1.7 | −1.8 – 0.7 | −1.8 – 1.7 | |
| Language/Semantic Composite Score | < 0.001 | |||
| Mean (SD) | 1.9 (0.7) | 0.8 (0.9) | 1.4 (0.9) | |
| Range | 0.4 – 3.2 | −1.9 – 3.4 | −1.9 – 3.4 | |
MTL= medial temporal lobe; WM=white matter; FA= fractional anisotropy
All participants signed informed consent approved by the University of Colorado Multiple Institutional Review Board (COMIRB). Study data were collected and managed using REDCap electronic data capture tools hosted at CU Anschutz (Harris et al., 2009).
2.2. Procedures
Participants completed cognitive testing with the Montreal Cognitive Assessment [MoCA (Nasreddine et al., 2005)] and the Spanish English Neuropsychological Assessment Scales [SENAS(Mungas et al., 2005a)]. An informant-based interview was also conducted (Clinical Dementia Rating Scale [CDR]), which was used to assess and rate functional severity.
Cognitive Assessment:
The SENAS battery was based on item response theory (IRT), and psychometrically matched measures were created across different scales, thus assuring reliability across the full ability continuum (Mungas et al., 2004; Mungas et al., 2005a; Mungas et al., 2000; Mungas et al., 2005b; Mungas et al., 2011). For the purposes of this study, IRT composite scores were used for each of the domains described below. These IRT scores do not have floor or ceiling effects and are normally distributed. IRT scores may be interpreted as unadjusted standard scores (Mean = 0; SD = 1) based on a demographically diverse sample of older adults aged 60+ (Mungas et al., 2004).
Our primary cognitive outcome was verbal episodic memory and was selected due to its mechanistic role in memory formation, which is impacted in early stages of Alzheimer’s disease (Ewers et al., 2010). It was also selected due to the primary study goal of elucidating the relationship between astrogliosis and memory performance in aging adults. The Verbal Memory IRT composite score was assessed with a multi-trial list-learning measure (5 learning trials; 15 items), incorporating both learning trials and delayed recall.
In order to delineate whether associations between the astrocytic marker and cognition were specific to memory, we also appraised secondary cognitive measures from the SENAS, including language/semantic abilities, spatial functions, and executive functions. The language/semantic knowledge IRT composite was based on scores from a nonverbal picture association measure and verbal object naming task. The Executive Function IRT Composite consisted of digit span backward, visual span backward, list sorting, and fluency. Finally, the SENAS Spatial IRT composite was based on the spatial localization scale, which evaluates the ability to perceive and reproduce two-dimensional spatial relationships. Administration procedures, measure development, and psychometric characteristics of the SENAS battery are described in detail in prior publications (Mungas et al., 2004).
Neuroimaging:
Whole brain MRI scans were obtained on a 3.0 Tesla Siemens (Iselin, NJ) Skyra scanner equipped with a 20-channel head coil. Diffusion imaging data were acquired utilizing an echo planar imaging sequence with 56 slices 2.2 mm thick (TR/TE = 8400/105 ms, matrix = 112×112) in monopolar series (B2500: 64 diffusion directions, B0 [9 averages]). Diffusion tensor imaging (DTI) data were preprocessed and analyzed using FMRIB’s Diffusion Toolbox (FDT) (Smith et al., 2006). Raw data were corrected for head movement and eddy current distortions using FDT. Brain extraction and binary brain mask creation used the b0 mean image through the FMRIB Software Library (FSL) Brain Extraction Tool (BET). Fractional anisotropy (FA) maps were created using FSL DTIFIT. All subjects’ FA data were registered using the nonlinear registration FNIRT to the IXI Aging DTI Template (Zhang et al.) masked by a study-specific average image. The mean FA and mean FA skeleton were created from the study sample. For region of interest (ROI) analyses, we employed the Johns Hopkins University ICBM-DTI-81 white matter labels (Mori et al., 2008) to label and mask areas of the white matter skeleton. Mean FA values for each white matter region was calculated using the FSL utility fslstats. To calculate a medial temporal lobe ROI, we obtained the mean FA of the following regions: hippocampal portion of the cingulum and the fornix crus. We also calculated a mean ROI of white matter regions previously shown to be vulnerable to Alzheimer’s disease pathology, composed of: hippocampal portion of the cingulum, dorsal portion of the cingulum, fornix crus, sagittal stratum, and corpus callosum. We also included a control region, which consisted of the mean FA in the corticospinal tract. The composite medial temporal lobe (MTL), AD-vulnerable, and control ROIs were calculated as the overall mean fractional anisotropy (FA) values of all the respective tracts included after averaging left and right hemispheres (where applicable).
Although FA values are the most commonly used scalar of white matter microstructure, we also examined whether alterations in non-FA diffusion metrics were associated with our primary biomarker and cognitive variables of interest. The non-FA images were projected onto the skeleton using the same parameters calculated for the FA images. Mean values for each of the target ROIs were calculated for mean diffusivity (MD), which reflects the overall – or average - motion of water molecules ((λ1 + λ2 + λ3)/3), axial diffusivity (DA), which reflects diffusion parallel to the fiber tracts (λ1), and radial diffusivity (RD), which reflects the magnitude of diffusion perpendicular to the fiber tract (mean of λ2 and λ3). Of note, prior reports suggest that RD values increase with myelin damage, and DA values are altered in the context of axonal damage (Barrick et al., 2010; Brickman et al., 2012).
Biomarker Ascertainment:
After collection, each whole blood sample was centrifuged at 1500× g for 15 min at 22°C and the plasma removed. Plasma was centrifuged at 2200x g for 10 min at 4°C and stored at −80°C.
Protein analysis of GFAP and canonical biomarkers of amyloid (Aβ42) and neurodegeneration (NfL, total tau) were measured with the Quanterix single molecule array, or SIMOA®, SR-X Analyzer system using manufacturer-supplied antibody kits. Nominal recovery for control levels remained between 111–120% with a coefficient of variation (CV) <10%. GFAP and NfL levels were available for all study participants (n=114); Aβ42 and total tau levels were available for 110 participants. We further computed the intra-individual CV utilizing the two runs for each participant and each biomarker; four participants had one laboratory value for Aβ42 and tau, and this was used in lieu of the mean replicate. We restricted the analyses to biomarkers wherein the intra-individual CV was < or = 20% (Final sample size; GFAP=114; Aβ42 =105; Total Tau = 104; NfL=114).
APOE Genotyping:
APOE ε4 genotype was performed on buffy coat samples of all participants. DNA was genotyped using a rapid allele-specific PCR methodology, adapted to Real Time PCR monitored by TaqMan probe [see full description in (Zhong et al., 2016)]. Participants were categorized using a binary variable as an APOE ε4 carrier or non-carrier.
2.3. Statistical Analysis
Demographic, cognitive, white matter, and biomarker results were compared between asymptomatic and symptomatic patients using t-tests and chi-square tests (Table 1). For formal statistical analysis, each biomarker was standardized to allow for comparison between coefficients on different biomarkers. Specifically, we subtracted the mean value from each observation and divided by the standard deviation of the variable. This permitted us to compare biomarker data in standard deviation units. For all linear regression models, Q-Q and residual plots were used to investigate assumptions. No concerning patterns were found (data not shown). There was also no visual evidence of outliers that would be influential to parametric tests in Table 1.
Primary Cognitive Analyses:
Linear regression models were used to evaluate whether GFAP was associated with memory function. We first modeled the relationship between GFAP and memory score while adjusting for demographics (i.e., age, sex, and education). We then adjusted for diagnostic status (i.e., asymptomatic versus symptomatic) to assess whether the relationship held after adjusting for AD symptomology, as well as APOE status (i.e., presence or absence of APOE ε4 allele). Given that the cell size for dementia participants was small, we elected to use a dichotomous symptom status variable, with MCI/Dementia collapsed into one group; however, for all primary analyses, we repeated the regression using traditional diagnostic severity staging (i.e., control; MCI; dementia) and CDR global score to verify that the results were maintained regardless of the type of symptom status variable selected. Additionally, to assess whether the association between GFAP and cognition was specific to memory function, we further examined associations between GFAP and other cognitive domains (i.e., executive; language/semantic knowledge; spatial) using the same modeling framework on each outcome.
To clarify whether the relationship between GFAP and memory was independent of canonical AD biomarkers, we adjusted for demographics and Aβ42, Tau, and NfL levels in the models described above. Finally, an interaction between symptom status and GFAP levels was included to identify whether associations with memory were evident or more pronounced in participants with clear symptoms of MCI or AD dementia.
Exploratory White Matter Analyses:
Given that astrocytes are more prevalent in white compared to grey matter, we examined whether mean white matter FA in a priori selected ROI’s (i.e., MTL ROI; AD vulnerable region ROI) explained the association between GFAP levels and memory function. We first examined whether there was a significant relationship between each ROI’s mean white matter FA and a) GFAP levels and b) memory function, adjusting both for demographics. Then we adjusted the regression models described above assessing the relationship between GFAP and memory by including one white matter ROI in the model – i.e., one set of models adjusted for the MTL ROI and the other adjusted for the AD vulnerability ROI. We evaluated the percentage change in the GFAP coefficient with and without adjustment for white matter ROI, as well as any changes in significance of the GFAP coefficient. We repeated these analyses using mean white matter AD, RD, and MD in selected ROI’s to determine whether non-FA diffusion metrics better explained the association between GFAP levels and memory function (results provided in supplementary tables).
All analyses were conducted using R version 4.0.2. P-values <0.05 were considered statistically significant.
3. Results:
Our participant sample was comprised of 114 aging adults (asymptomatic: healthy older adults, n=69; symptomatic: MCI and AD Dementia, n=45) whose ages ranged from 53 to 87 (mean = 70 years; see Table 1). Although the proportion of females in the asymptomatic group was higher relative to the symptomatic group (p=0.027), there were no significant differences between groups for age or education. The symptomatic (MCI/dementia) group was characterized primarily by typical, amnestic-predominant AD, although 9 participants met criteria for Posterior Cortical Atrophy syndrome. As expected, individuals in the symptomatic group performed worse on a global cognitive index (mean MoCA total score: asymptomatic = 27; symptomatic = 20; p < .001) and the primary memory outcome measure (Memory Composite; p<0.001), and had higher composite CDR scores (p<0.001). Symptomatic participants were also more likely to carry an APOE ε4 allele (p=0.001) and had higher levels of GFAP (p<0.001), NfL (p<0.001), and total tau (p=0.003), and borderline lower levels of Aβ42 (p=0.067). Participants with at least one APOE ε4 allele also demonstrated higher blood GFAP levels (controlling for demographic variables) compared to participants without an APOE ε4 allele (p=0.017).
3.1. Primary Analysis 1: Are higher levels of blood GFAP associated with worse memory performance? [FIGURE 1; TABLE 2]:
Figure 1:
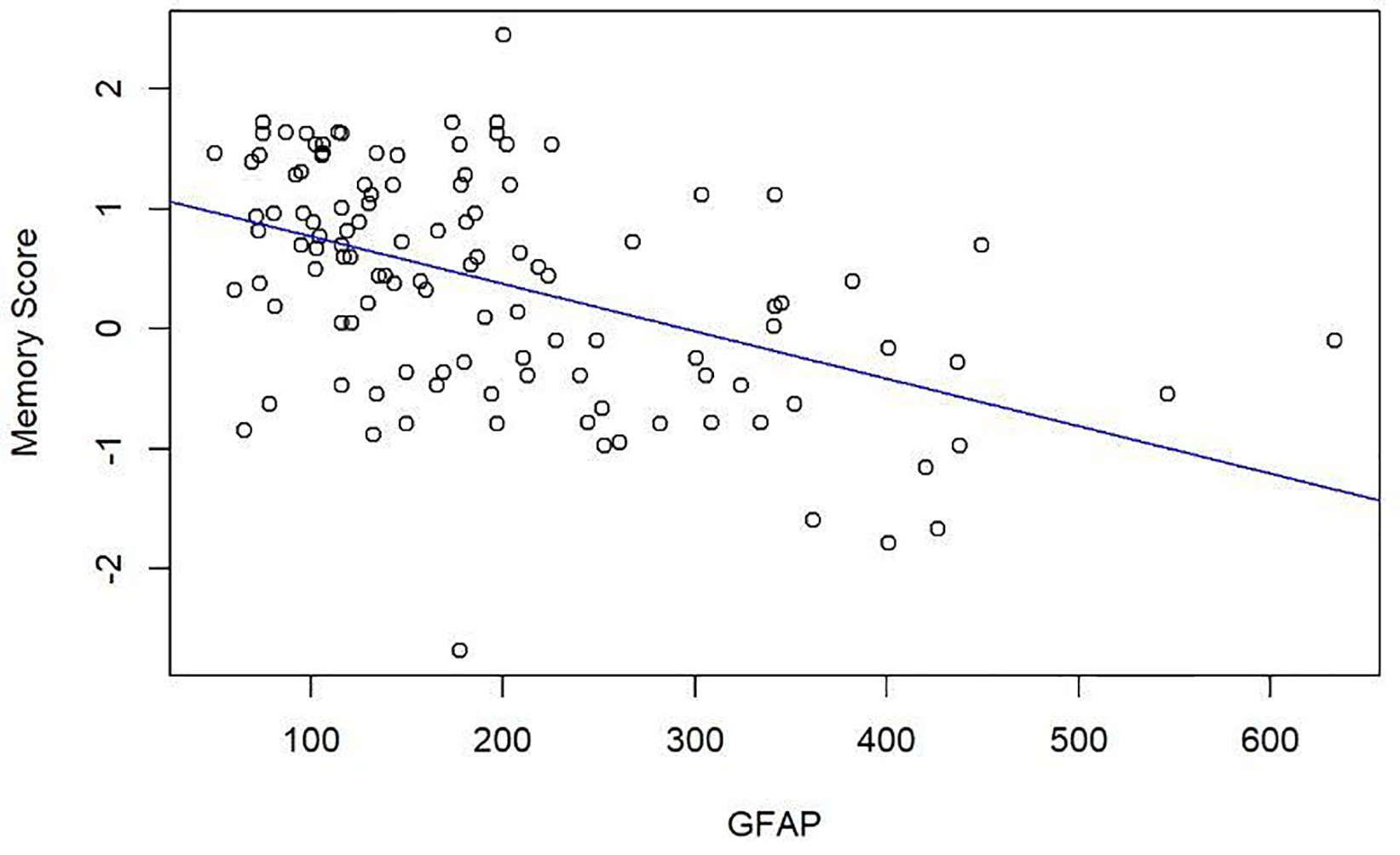
Scatterplot demonstrating the unadjusted negative association between GFAP levels (pg/mL) and SENAS Memory Composite Score.
Table 2:
Estimated (Est) regression coefficients between blood GFAP and verbal memory and non-memory cognitive domains.
| Outcome | GFAP Est. (SE)a | P-value | Adjusted GFAP Est. (SE)b | P-value |
|---|---|---|---|---|
| Verbal Memory Composite | −0.49 (0.071) | <0.0001 | −0.27 (0.074) | 0.001 |
| Spatial Composite | −0.42 (0.11) | 0.00012 | −0.094 (0.11) | 0.38 |
| Executive Composite | −0.27 (0.055) | <0.0001 | −0.11 (0.059) | 0.059 |
| Language/Semantic Knowledge Composite | −0.32 (0.085) | 0.0003 | −0.098 (0.090) | 0.28 |
Regression coefficients adjusted for age, gender and education.
Regression coefficients adjusted for age, gender, education, APOE E4 status, and symptomatic/asymptomatic.
Each row represents a separate regression analysis.
Higher levels of GFAP were associated with lower memory scores after adjusting for demographics (p<0.0001; scatterplot of unadjusted association shown in Figure 1). Specifically, for one standard deviation (SD) increase in mean GFAP values, the IRT memory composite decreased on average by 0.49 (Standard error [SE]=0.071). Based on the adjusted R2, GFAP explained an additional 25% of the variation in memory composite score.
This negative association between GFAP and memory held after accounting for both symptom and APOE status (p=0.001), although the effect size was smaller. Specifically, for every one SD increase in mean GFAP values, the memory composite score decreased by 0.27 (SE=0.074), after accounting for symptom status (i.e., absence/presence of an AD syndrome) and APOE status (i.e., absence/presence of ε4 allele). The pattern was consistent when adjusting for standard clinical severity staging (i.e., control vs. MCI vs. dementia; p=0.001) or CDR global score (p=0.0001). GFAP was significantly and negatively associated with memory in both the asymptomatic and symptomatic groups; however, the relationship between GFAP and memory did not differ based on symptom status (p=0.94).
To evaluate whether the negative association between GFAP levels and cognition was specific to memory, we further assessed non-memory cognitive domains. In models adjusting for demographics only, higher mean levels of GFAP were associated with worse scores on the spatial (p=0.0001), executive (p<0.0001), and language/semantic knowledge (p=0.0003) composites. GFAP explained a lesser amount of the variation in these measures compared to memory (12% - spatial, 15% - executive, and 10% language/semantic knowledge). The negative association between GFAP levels and non-memory cognitive domains were insignificant after adjustment for symptom and APOE status (spatial, p=0.38; language/semantic, p=0.28; Table 2), although a non-significant trend was observed for the executive composite (p=0.059). Comparable, non-significant results were noted when controlling for standard clinical severity staging rather than symptom status, with slightly smaller effect sizes (i.e., spatial, p=0.52; language/semantic, p=0.60; executive, p=0.11)
3.2. Primary Analysis 2: Are higher levels of GFAP independently associated with worse episodic memory, over and above (adjusting for) canonical measures of AD pathology and neurodegeneration?
After additionally adjusting for Aβ42, total tau, and NfL, higher GFAP levels remained significantly associated with lower memory scores (p<0.0001, see Table 3). For every standard deviation increase in mean GFAP values, memory score decreased on average by 0.47 (SE=0.089). This is a very similar association without adjustment for Aβ42, total tau, and NfL (0.47 vs. 0.49). Accounting for symptom (i.e., absence/presence of an AD syndrome) and APOE status did not alter the significance of the results (p=0.001). Moreover, the degree of attenuation was similar with and without adjustment for Aβ42, total tau, and NfL (−0.29 vs. −0.26). Finally, comparable results were noted when controlling for standard clinical severity staging and APOE status (p=0.001). These results suggest that GFAP is an independent predictor of memory performance, over and above blood-based AD-biomarkers and APOE status.
Table 3:
Estimates (Est) regression coefficients between blood GFAP, AD biomarkers and the verbal memory outcome.
| Biomarker | Est.a | Std. Error | P-value | Adj. Est.b | Std. Error | P-value |
|---|---|---|---|---|---|---|
| GFAP | −0.47 | 0.089 | <0.0001 | −0.29 | 0.086 | 0.001 |
| Abeta-42 | 0.17 | 0.081 | 0.043 | 0.03 | 0.079 | 0.71 |
| Tau | −0.046 | 0.086 | 0.60 | 0.067 | 0.081 | 0.41 |
| NFL | −0.029 | 0.093 | 0.75 | 0.026 | 0.083 | 0.76 |
Regression coefficients adjusted for age, gender and education.
Regression coefficients adjusted for age, gender, education, APOE E4 status, and symptomatic/asymptomatic.
3.3. Exploratory Analysis A: Are higher levels of GFAP associated with lower FA in medial temporal lobe tracts, and is the association between GFAP and memory explained by white matter microstructure?
To address whether alterations in white matter integrity account for the relationship between GFAP and memory scores, we first assessed potential associations between white matter ROI’s and both memory and GFAP levels (Top two panels Table 4). After controlling for demographic variables, higher levels of GFAP were significantly associated with lower memory scores (p<0.0001) and lower white matter integrity (FA) in the MTL ROI (p=0.001) and the AD vulnerability ROI (p=0.041). GFAP explains an additional 7% of the variation in the MTL ROI and only an additional 2% of the variation in the AD vulnerability ROI. Thus, GFAP is more strongly associated with the MTL ROI than the AD vulnerability ROI. In addition, poorer white matter integrity in the MTL and AD vulnerability ROI were associated with lower memory scores (p<.0001). No association between GFAP levels and the control/comparator ROI (i.e., mean FA in the corticospinal tract) was observed (p=0.62). After accounting for demographic variables and the white matter MTL ROI, higher GFAP values remained associated with lower memory scores (p<0.0001, bottom panel Table 4). For every standard deviation increase in mean GFAP values, memory score decreased on average by 0.42 (SE=0.072). After further accounting for symptom and APOE status of the patients, the relationship remained significant (p=0.002). Similar results were noted for models with AD vulnerability white matter ROI included (bottom panel Table 4). These results suggest that while white matter microstructure is associated with both GFAP levels and memory function, it does not fully account for the deleterious relationship between GFAP and memory.
Table 4.
Estimated (Est) regression coefficients investigating GFAP, white matter microstructure, and verbal memory.
| Outcome | Predictor | Est.a | Std. Error | P-value | Adj. Est.b | Std. Error | P-value |
|---|---|---|---|---|---|---|---|
| Associations between GFAP and white matter microstructure | |||||||
| MTL ROI | GFAP | −0.0094 | 0.0028 | 0.0012 | −0.0014 | 0.0032 | 0.65 |
| AD Vulnerability ROI | GFAP | −0.0050 | 0.0024 | 0.041 | 0.0015 | 0.0027 | 0.58 |
| CST ROI | GFAP | 0.0014 | 0.0028 | 0.62 | 0.0022 | 0.0035 | 0.52 |
| Associations between white matter microstructure and verbal memory | |||||||
| Verbal Memory | MTL ROI | 12.6 | 2.6 | <0.0001 | 4.8 | 2.5 | 0.053 |
| Verbal Memory | AD Vulnerability ROI | 12.8 | 3.2 | 0.00011 | 4.3 | 2.9 | 0.13 |
| Verbal Memory | CST ROI | 0.33 | 3.0 | 0.91 | 0.18 | 2.3 | 0.94 |
| Joint association between GFAP, white matter microstructure and verbal memory | |||||||
| Verbal Memory | GFAP | −0.42 | 0.0072 | <0.0001 | −0.25 | 0.077 | 0.002 |
| MTL ROI | 8.4 | 2.3 | 0.001 | 4.5 | 2.4 | 0.061 | |
| Verbal Memory | GFAP | −0.45 | 0.07 | <0.0001 | −0.26 | 0.077 | 0.001 |
| AD Vulnerability ROI | 9.3 | 2.8 | 0.001 | 4.9 | 2.7 | 0.08 | |
| Verbal Memory | GFAP | −0.50 | 0.072 | <0.0001 | −0.26 | 0.078 | 0.001 |
| CST ROI | 1.2 | 2.5 | 0.64 | 0.64 | 2.2 | 0.77 | |
Regression coefficients adjusted for age, gender and education.
Regression coefficients adjusted for age, gender, education, APOE E4 status, and symptomatic/asymptomatic.
CST= corticospinal tract
3.4. Exploratory Analysis B: Are higher levels of GFAP associated with altered Radial Diffusivity, Mean Diffusivity, and Axial Diffusivity in medial temporal lobe tracts, and is the association between GFAP and memory explained by these white matter microstructure measures?
To determine whether MD, DA, or RD better explained the association between GFAP and memory compared to FA values, we further examined potential associations between these white matter scalars and both memory and GFAP levels. As shown in the supplementary data (Top two panels, Supplementary Table 2a–c), upon controlling for demographic variables, higher levels of GFAP were significantly associated with higher RD in the MTL ROI (p=0.0070) and the AD vulnerability ROI (p=0.037), and there were non-significant trends toward higher MD in the MTL (p=0.11) and AD vulnerability (p=0.058) ROIs. In addition, higher RD values in the MTL and AD vulnerability ROI were associated with lower memory scores (p<0.001 and p=0.001, respectively), with slightly weaker, albeit significant associations also noted for MD in the same regions. No associations between GFAP levels and the control/comparator ROI (i.e., mean in the corticospinal tract) were observed, nor were there associations between DA values and either GFAP or verbal memory. After accounting for demographic variables and both the white matter RD and MD MTL ROI (in separate models), higher GFAP values remained associated with lower memory scores (p<0.001, bottom panels, Supplementary Tables 2a–c). After further accounting for symptom status and APOE status of the patients, the relationships between GFAP and memory remained significant. These results suggest that while metrics of radial diffusivity and mean diffusivity are associated with both GFAP levels and memory function, they do not fully account for the deleterious relationship between GFAP and memory. Moreover, when comparing the coefficient for GFAP, the RD adjusted analysis most closely approximated (but was not significantly stronger than) the effect sizes noted in the FA analyses.
4. Discussion:
Our study demonstrated that in a cohort of healthy older adults and adults with symptomatic AD, higher blood levels of a protein marker of astrogliosis (GFAP) were associated with worse memory performance and poorer microstructural integrity of medial temporal white matter tracts. The negative association between GFAP levels and memory performance was present across the spectrum of disease severity and was not better accounted for by APOE ε4 status or blood levels of Aβ42, total tau, or neurofilament light chain. Moreover, the relationship between GFAP and cognition appeared to be sensitive to the memory domain, with some suggestion of specificity. Given that white matter microstructure did not fully account for the deleterious association between GFAP and memory, additional mechanisms for this association should be explored.
Higher circulating levels of GFAP were sensitive to poorer verbal episodic memory function in a cohort of both asymptomatic, community dwelling older adults and adults with symptomatic Alzheimer’s disease (MCI and dementia levels of severity). Importantly, while GFAP levels were higher in symptomatic AD relative to healthy older adult participants, the relationship between GFAP and memory was not driven by or more pronounced in the AD groups. The lack of interaction with diagnosis perhaps indicates a continuum of effect on memory, arguing against prior implications that markers of astrogliosis are primarily a late stage phenomenon in disease progression (Zetterberg and Bendlin, 2020). Instead, our results suggests that elevations in GFAP levels are negatively linked with verbal memory function across the disease severity and may represent an early event in pathological aging processes. In the context of the broader literature on astrogliosis and AD pathogenesis, our study findings, while correlative, are consistent with studies indicating that elevated astrocytic proteins are related to negative aging outcomes (Oeckl et al., 2019), and are supportive of recent PET imaging studies indicating that astrocyte activation may be present prior to frank symptomology (Edison et al., 2018).
Although several human studies have examined circulating GFAP levels and both global cognitive function (Oeckl et al., 2019) and overall diagnostic status (Boon et al., 2018; Kashon et al., 2004), very few studies have examined how this proxy for astrogliosis may relate to specific cognitive domains. Our study results suggest that the adverse relationship between GFAP and cognition is most robust when examining the domain of memory, as associations with non-memory domains did not survive adjustment for diagnosis or overall severity (i.e., CDR). Preclinical animal studies highlight pivotal physiological roles for astrocytes in forming the tripartite synapse, regulating synaptic function, and modulating hippocampal-based memory consolidation (Adamsky et al., 2018; Ota et al., 2013). In the context of dysregulation, astrocytic (A1) activation has also been directly linked with hippocampal neuronal loss, memory deficits, and AD pathology in mouse models (Potter et al., 2001; Zhang et al., 2020). As such, a role for GFAP specifically in memory retention is intuitive and well-founded. Nonetheless, a recent, seminal study did not report significant associations with memory function in the context of a cohort of healthy older adults and participants with early onset and late onset AD (Elahi et al., 2020). However, there are several differences in study methodology and approach that warrant discussion and may offer insights into the discrepancy in findings. Specifically, our study utilized psychometrically-matched cognitive composite scores based on IRT methodology, which allows for a robust comparison across cognitive domains. The inclusion of these matched IRT cognitive scores is a strength of our study design, and thus may explain some differences in our results for memory vs non-memory domains. In addition, we specifically examined GFAP as a primary variable rather than a composite indicator of biofluid markers of neurodegeneration. As such, it is possible that GFAP, as a downstream indicator of astrogliosis, may reflect a stronger relationship with episodic memory than amalgamated indicators of neurodegeneration (e.g., NfL). In support of this hypothesis, our study results indicate that associations between GFAP and memory were not better accounted for by traditional blood biomarkers of amyloid and neurodegeneration. Moreover, the effect size was not appreciably altered when controlling for blood levels of Aβ42, total tau, and NfL, suggesting that the conduit by which GFAP/astrogliosis relates to memory may be an independent (and pathogenic) pathway.
Based on the prevalence of astrocytes in – and the relative specificity of GFAP to – white matter structures, we further assessed whether higher levels of GFAP mapped on to key white matter regions in the brain that are known to be involved in memory functions and are affected early in Alzheimer’s disease. Of note, although elevations in GFAP were linked with a) poorer memory and b) lower fractional anisotropy in medial temporal and AD-vulnerable white matter regions, the association between GFAP and memory was not mediated by white matter microstructure as measured by FA. Although FA is the most commonly used diffusion metric for the overall integrity of white matter microstructure, it is not necessarily specific to the types of white matter pathologies or underlying causes of altered tissue. As such, we repeated the analyses with other metrics of diffusion integrity, namely mean diffusivity, radial diffusivity (i.e., a proxy for myelin damage), and axial diffusivity (i.e., a proxy for axonal damage). When examining other diffusion metrics, similar patterns of findings to the FA analysis were observed with both radial diffusivity and mean diffusivity, with radial diffusivity showing robust and comparable effect sizes to fractional anisotropy results. No significant associations were observed between axial diffusivity metrics and either GFAP levels or verbal memory. Considering that radial diffusivity is thought to be sensitive to myelin integrity, it is possible that associations between GFAP and white matter are driven by underlying demyelination; of note, measures of RD were not more sensitive to outcomes than FA values, nor was controlling for any of the diffusion metrics (FA, RD, MD, DA) fully explanatory in the relationship between GFAP and memory. It remains possible that alterations in white matter pathways that are not fully captured by these metrics might still account for the deleterious GFAP-memory association; however, these results also suggest that other explanatory mechanisms may underlie or contribute to these associations. Current methodology and technology limits in-vivo assessment of several potential mechanisms in humans, although animal studies underscore pivotal roles for astrocytes in driving neuroinflammation and inducing amyloidogenic and excitotoxic effects on synaptic function in the context of disease states. In order to elucidate whether astrogliosis is a primary, upstream inducer of memory dysfunction in older adults, multi-modal, translational studies are needed to better understand the morphological and biochemical mechanisms of astrocyte dysfunction on cognition.
We also considered the role of APOE ε4 status on our primary outcomes, as APOE is the strongest genetic risk factor for sporadic, late onset AD and ε4 status has been linked with worse performance on episodic memory tests and altered white matter microstructure on neuroimaging (Koizumi et al., 2018; Sudre et al., 2017). ApoE ε4 is also associated with earlier and increased amyloid deposition, likely due to its catalytic effect on fibrilization of Aβ, to difficulty clearing amyloid filaments, or to functional differences in lipid biology (Chen et al., 2021; Potter and Wisniewski, 2012). Moreover, apoE in the brain is primarily expressed by astrocytes, with recent evidence suggesting that astrocytic homeostatic functions may be influenced by APOE genotype. In partial support of this complex literature, ε4 carriers demonstrated higher blood levels of GFAP in our study relative to non-carriers. Importantly, the negative association between GFAP and verbal memory remained significant after controlling for APOE status. When examining the role of APOE in exploratory white matter analyses, the effects of GFAP on diffusion metrics in key white matter ROIs were markedly reduced upon adjusting for diagnosis and gene status; however, this was driven by the effect of diagnosis rather than APOE gene status on white matter in MTL and AD vulnerable white matter regions (data not shown). Overall, our study suggests a potentially complex biological link between APOE, blood GFAP levels, and cognitive aging, wherein APOE may play a role in circulating GFAP levels, but does not fully mediate associations between GFAP and memory or GFAP and white matter integrity. Further studies are needed to clarify how astrocytic function (or glial biology more broadly) might interact with APOE to influence AD-related pathological cascades and cognitive trajectories.
When considering the mechanistic role of GFAP in cognitive aging processes, it is important to evaluate the complexity of astrocytic function in both physiological and pathological states. As noted, astrocytes interact with all cells in the CNS and are involved in a range of normal physiological activities that are critical for brain functioning, including formulation and maintenance of synapses, regulation of the BBB, provision of trophic support to neurons, and modulation of neurogenesis. Astrocytes also regulate the release of glutamate, and thus play an integral role in homeostatic functions that are necessary to prevent or mitigate hyperexcitable states. In the context of chronic CNS injury, dysregulation of these astrocytic functions may result in not only an interruption of protective, homeostatic processes, but also the activation of a cascade of pathological events that contribute to and/or worsen pathology. However, the temporal sequence of this shift in balance from beneficial to harmful effects remains markedly unclear. In particular, a recent study highlighted that GFAP-releasing, reactive astrocytes may be beneficial to amyloid clearance in early stages of disease, with ablation of these astrocytes in mice resulting in exacerbation of AD pathology (Katsouri et al., 2020).
Akin to classification systems previously used in identifying microglia, astrocytes have been proposed to have a multitude of positive and negative functions and may be characterized by their respective states (e.g., A1, A2)(Clarke et al., 2018). Nonetheless, overlap in their transcriptional signatures further highlight the functional heterogeneity and dynamic roles of astrocytes in healthy and diseased states and will require thoughtful consideration when designing future therapeutic trials. In a similar vein, while considered a surrogate and gold standard fluid biomarker for astrocyte damage, GFAP also plays complex roles in the CNS, which may vary depending on the context of its release and the duration of underlying insults (Toops et al., 2012). Thus, while we found striking negative associations between GFAP and memory across the spectrum of severity in our aging cohort, it does not necessarily mean that astrocytes play the same functional role at each stage. It will be important for future studies to evaluate the complex functional interplay between astrocytes, neurons, and other immune cells to inform our understanding of the cognitive neuroscience of immune regulation in aging and Alzheimer’s disease. Moreover, given that astrocytes may play beneficial and destructive roles in brain health, future investigations should appraise changes in both cell function and protein levels using longitudinal cognitive neuroscience paradigms to identify when and how astrocytic functions should be promoted or harnessed.
The current study has numerous strengths, including the incorporation of multiple blood-based biomarkers of AD-related pathology and astrogliosis in the context of comprehensive cognitive and neuroimaging data. Our study also utilized measures of cognition with robust psychometric properties and item response theory-based composite cores. The use of these cognitive composites in our study permitted head-to-head comparisons across neuropsychological domains. Moreover, the cognitive outcome variables used in the study were distinct from those reviewed in interdisciplinary consensus conferences to adjudicate diagnosis, thereby limiting the likelihood of circularity in findings.
Limitations to the study include the lack of CSF or PET biomarkers for AD pathology; while all participants with symptomatic AD met NIA-AA clinical criteria for MCI or dementia, it remains possible that some participants presented with AD syndromes without underlying AD pathology. While there is a growing body of literature suggesting that blood markers of AD-related pathology correlate with and may be a proxy for CNS pathology, there are still inconsistencies in study results, particularly with respect to plasma Aβ42 (Chatterjee et al., 2019), and there is limited information regarding complex, non-linear effects of blood-based biomarkers on cognitive outcomes. When examining between-group effect sizes for blood biomarkers in our study, the Cohen’s D calculated for Aβ42 was classified as small at −0.367. Cohen’s D was medium at 0.606 for Tau and large at 0.966 for NfL. The medium to large effects of tau and NfL, respectively, are in line with pathological cascades of AD, in which greater tau pathology and neurodegeneration are more proximally linked with symptom onset. Nonetheless, future studies should be conducted with mass spectrometry (Nakamura et al., 2018; Schindler et al., 2019) to confirm study findings. In addition, we incorporated blood levels of GFAP as a primary marker of CNS astrogliosis; although GFAP in blood and CSF have been shown to strongly correlate in other disease states (Abdelhak et al., 2018), peripheral biomarkers and extracellular expression of GFAP may not comprehensively reflect the CNS astrocytic milieu. Elevations of GFAP in blood suggests some degree of astrogliosis, but it is important to consider that protein levels may still be impacted by downstream processes in peripheral tissue. Finally, an additional limitation is the lack of longitudinal biomarker data on the participant sample; in order to determine whether GFAP levels dynamically change over time and elucidate their association with cognitive decline and AD progression, longitudinal appraisal is needed. This is particularly relevant given the moderate effect sizes of the cross-sectional associations between GFAP and memory; while consistent with the literature (Oeckl et al., 2019), it will be important to determine whether robust effects are noted over time.
5. Conclusions
In summary, in a cohort of aging adults and adults with symptomatic AD, higher blood levels of a marker for astrogliosis, GFAP, were related to poorer verbal episodic memory and lower white matter integrity in the medial temporal lobe and regions vulnerable to AD pathology. These findings indicate that higher levels of astrocytic markers in blood may reflect impoverished memory functions and white matter health, over and above indices of amyloid or neurodegeneration. Moreover, these results add to a burgeoning body of evidence indicating that GFAP may serve as a biomarker of astrogliosis early in pathological aging cascades.
Supplementary Material
Highlights.
Astrogliosis has been implicated in neurodegenerative pathological cascades.
Higher levels of an astrocytic marker, GFAP, were associated with worse memory
Negative links between GFAP and memory were not accounted for by Ab42, Tau, or NfL
Higher GFAP was associated with lower white matter integrity in the temporal lobe
Acknowledgements.
We would like to acknowledge the participants in this study who volunteered their time for comprehensive evaluations, blood draws, and MRI scans. We would like to acknowledge and thank Dr. Noah Johnson for his assistance with APOE genotyping. Many staff and faculty at the CU Alzheimer’s and Cognition Center (CUACC) assisted with the implementation of the study’s design, and we are grateful for the dedication of our CUACC team.
Disclosures.
This work was supported by grants from the National Institute on Aging (NIA; PI; R01 AG058772, B. Bettcher, PI), NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 (Robert Sokol, PI), NIH High-End Instrumentation Grant S10OD018435 (Tregellas, PI), and support from the State of Colorado and many generous philanthropists. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views. None of the authors have financial or personal conflicts of interest related to this work.
Financial Support
This work was supported by grants from the National Institute on Aging (NIA; PI; R01 AG058772, B. Bettcher, PI), NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 (Robert Sokol, PI), NIH High-End Instrumentation Grant S10OD018435 (Tregellas, PI), and support from the State of Colorado and many generous philanthropists.
Footnotes
Declaration of Competing Interest
None.
The data contained in this manuscript have not been submitted to or previously published in another journal, and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
This study was approved by the institutional review board at the University of Colorado Institutional Review Board (COMIRB) and all subjects provided written, IRB-approved informed consent before participating.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M, 2018. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep 8(1), 14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, London M, Goshen I, 2018. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174(1), 59–71 e14. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH, 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3), 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG, 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22(5), 208–215. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS, 2010. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage 51(2), 565–577. [DOI] [PubMed] [Google Scholar]
- Boon BDC, Hoozemans JJM, Lopuhaa B, Eigenhuis KN, Scheltens P, Kamphorst W, Rozemuller AJM, Bouwman FH, 2018. Neuroinflammation is increased in the parietal cortex of atypical Alzheimer’s disease. J Neuroinflammation 15(1), 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, Wasserman BT, Williams LM, Zimmerman ME, 2012. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiology of Aging 33(8), 1699–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH, 2002. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22(1), 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Elmi M, Goozee K, Shah T, Sohrabi HR, Dias CB, Pedrini S, Shen K, Asih PR, Dave P, Taddei K, Vanderstichele H, Zetterberg H, Blennow K, Martins RN, 2019. Ultrasensitive Detection of Plasma Amyloid-beta as a Biomarker for Cognitively Normal Elderly Individuals at Risk of Alzheimer’s Disease. J Alzheimers Dis 71(3), 775–783. [DOI] [PubMed] [Google Scholar]
- Chen Y, Strickland MR, Soranno A, Holtzman DM, 2021. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 109(2), 205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA, 2018. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A 115(8), E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, Dickerson BC, Vandenberghe R, Ahmed S, Bak TH, Boeve BF, Butler C, Cappa SF, Ceccaldi M, de Souza LC, Dubois B, Felician O, Galasko D, Graff-Radford J, Graff-Radford NR, Hof PR, Krolak-Salmon P, Lehmann M, Magnin E, Mendez MF, Nestor PJ, Onyike CU, Pelak VS, Pijnenburg Y, Primativo S, Rossor MN, Ryan NS, Scheltens P, Shakespeare TJ, Suarez Gonzalez A, Tang-Wai DF, Yong KXX, Carrillo M, Fox NC, Alzheimer’s Association I.A.A.s.D., Associated Syndromes Professional Interest A, 2017. Consensus classification of posterior cortical atrophy. Alzheimers Dement 13(8), 870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P, Donat CK, Sastre M, 2018. In vivo Imaging of Glial Activation in Alzheimer’s Disease. Front Neurol 9, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi FM, Casaletto KB, La Joie R, Walters SM, Harvey D, Wolf A, Edwards L, Rivera-Contreras W, Karydas A, Cobigo Y, Rosen HJ, DeCarli C, Miller BL, Rabinovici GD, Kramer JH, 2020. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement 16(4), 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, R JC Jr., Feldman HH, Bokde AL, Alexander GE, Scheltens P, Vellas B, Dubois B, Weiner M, Hampel H, 2010. Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging 2010/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, Allen NJ, 2018. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev 13(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood CJ, Ratcliffe LE, Simpson JE, Heath PR, Ince PG, Wharton SB, 2017. Review: Astrocytes in Alzheimer’s disease and other age-associated dementias: a supporting player with a central role. Neuropathol Appl Neurobiol 43(4), 281–298. [DOI] [PubMed] [Google Scholar]
- Goursaud S, Kozlova EN, Maloteaux JM, Hermans E, 2009. Cultured astrocytes derived from corpus callosum or cortical grey matter show distinct glutamate handling properties. J Neurochem 108(6), 1442–1452. [DOI] [PubMed] [Google Scholar]
- Harada K, Kamiya T, Tsuboi T, 2015. Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front Neurosci 9, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesse S, Steinacker P, Cepek L, von Arnim CA, Tumani H, Lehnert S, Kretzschmar HA, Baier M, Otto M, 2009. Glial fibrillary acidic protein and protein S-100B: different concentration pattern of glial proteins in cerebrospinal fluid of patients with Alzheimer’s disease and Creutzfeldt-Jakob disease. J Alzheimers Dis 17(3), 541–551. [DOI] [PubMed] [Google Scholar]
- Kashon ML, Ross GW, O’Callaghan JP, Miller DB, Petrovitch H, Burchfiel CM, Sharp DS, Markesbery WR, Davis DG, Hardman J, Nelson J, White LR, 2004. Associations of cortical astrogliosis with cognitive performance and dementia status. J Alzheimers Dis 6(6), 595–604; discussion 673–581. [DOI] [PubMed] [Google Scholar]
- Katsouri L, Birch AM, Renziehausen AWJ, Zach C, Aman Y, Steeds H, Bonsu A, Palmer EOC, Mirzaei N, Ries M, Sastre M, 2020. Ablation of reactive astrocytes exacerbates disease pathology in a model of Alzheimer’s disease. Glia 68(5), 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Hattori Y, Ahn SJ, Buendia I, Ciacciarelli A, Uekawa K, Wang G, Hiller A, Zhao L, Voss HU, Paul SM, Schaffer C, Park L, Iadecola C, 2018. Apoepsilon4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat Commun 9(1), 3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J, 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40(2), 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H, 2004. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess 16(4), 347–359. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Haan MN, Gonzalez H, 2005a. Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology 19(4), 466–475. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Marshall SC, Gonzalez HM, 2000. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology 14(2), 209–223. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C, 2005b. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic Whites. J Int Neuropsychol Soc 11(5), 620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Widaman KF, Reed BR, Tomaszewski Farias S, 2011. Measurement invariance of neuropsychological tests in diverse older persons. Neuropsychology 25(2), 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramori F, Kobayashi K, Nakamura I, 1998. A quantitative study of neurofibrillary tangles, senile plaques and astrocytes in the hippocampal subdivisions and entorhinal cortex in Alzheimer’s disease, normal controls and non-Alzheimer neuropsychiatric diseases. Psychiatry Clin Neurosci 52(6), 593–599. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, Fowler C, Li QX, Martins R, Rowe C, Tomita T, Matsuzaki K, Ishii K, Ishii K, Arahata Y, Iwamoto S, Ito K, Tanaka K, Masters CL, Yanagisawa K, 2018. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554(7691), 249–254. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4), 695–699. [DOI] [PubMed] [Google Scholar]
- Oeckl P, Halbgebauer S, Anderl-Straub S, Steinacker P, Huss AM, Neugebauer H, von Arnim CAF, Diehl-Schmid J, Grimmer T, Kornhuber J, Lewczuk P, Danek A, Consortium for Frontotemporal Lobar Degeneration G, Ludolph AC, Otto M, 2019. Glial Fibrillary Acidic Protein in Serum is Increased in Alzheimer’s Disease and Correlates with Cognitive Impairment. J Alzheimers Dis 67(2), 481–488. [DOI] [PubMed] [Google Scholar]
- Ota Y, Zanetti AT, Hallock RM, 2013. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast 2013, 185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Duarte A, Roberts AJ, Ouyang K, Sejnowski TJ, 2019. Impairments in remote memory caused by the lack of Type 2 IP3 receptors. Glia 67(10), 1976–1989. [DOI] [PubMed] [Google Scholar]
- Potter H, Wefes IM, Nilsson LN, 2001. The inflammation-induced pathological chaperones ACT and apo-E are necessary catalysts of Alzheimer amyloid formation. Neurobiology of aging 22(6), 923–930. [DOI] [PubMed] [Google Scholar]
- Potter H, Wisniewski T, 2012. Apolipoprotein e: essential catalyst of the Alzheimer amyloid cascade. Int J Alzheimers Dis 2012, 489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick JS, Liddelow SA, 2019. Don’t forget astrocytes when targeting Alzheimer’s disease. Br J Pharmacol 176(18), 3585–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzinger TLS, Xiong C, Fagan AM, Bateman RJ, 2019. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93(17), e1647–e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE, 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4), 1487–1505. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, 2014. Astrogliosis. Cold Spring Harb Perspect Biol 7(2), a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Cardoso MJ, Frost C, Barnes J, Barkhof F, Fox N, Ourselin S, Alzheimer’s Disease Neuroimaging I, 2017. APOE epsilon4 status is associated with white matter hyperintensities volume accumulation rate independent of AD diagnosis. Neurobiol Aging 53, 67–75. [DOI] [PubMed] [Google Scholar]
- Toops KA, Hagemann TL, Messing A, Nickells RW, 2012. The effect of glial fibrillary acidic protein expression on neurite outgrowth from retinal explants in a permissive environment. BMC Res Notes 5, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM, 1999. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19(16), 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson U, Faiz M, de Pablo Y, Sjoqvist M, Andersson D, Widestrand A, Potokar M, Stenovec M, Smith PL, Shinjyo N, Pekny T, Zorec R, Stahlberg A, Pekna M, Sahlgren C, Pekny M, 2012. Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells 30(10), 2320–2329. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Bendlin BB, 2020. Biomarkers for Alzheimer’s disease-preparing for a new era of disease-modifying therapies. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Rueckert D, Gee JC, A computational white matter atlas for aging with surface-based representation of fasciculi, International Workshop on Biomedical Image Registration, Lecture Notes in Computer Science ed., pp. 83–90. [Google Scholar]
- Zhang HY, Wang Y, He Y, Wang T, Huang XH, Zhao CM, Zhang L, Li SW, Wang C, Qu YN, Jiang XX, 2020. A1 astrocytes contribute to murine depression-like behavior and cognitive dysfunction, which can be alleviated by IL-10 or fluorocitrate treatment. J Neuroinflammation 17(1), 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Xie YZ, Cao TT, Wang Z, Wang T, Li X, Shen RC, Xu H, Bu G, Chen XF, 2016. A rapid and cost-effective method for genotyping apolipoprotein E gene polymorphism. Mol Neurodegener 11, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


