Abstract
Objective
The aim of the present study was to compare the clinical outcomes and quality of life following percutaneous transforaminal endoscopic discectomy (PTED) and microscope‐assisted tubular discectomy (MTD) for lumbar disc herniation (LDH).
Methods
This study had a retrospective design. From June 2017 to June 2018, the clinical data of 120 patients with LDH treated with PTED (60 cases, PTED group) and MTD (60 cases, MTD group) were analyzed and followed up for at least 20 months. There were 59 men and 61 women. Patients were aged between 22 and 80 years. The operation time, intraoperative blood loss, incision length, frequency of intraoperative fluoroscopy, cost, hospital stay, types of herniated discs, complications, and clinical outcomes were evaluated. Clinical outcomes were assessed using the visual analog scale (VAS), the Oswestry disability index (ODI), and the modified Macnab criteria. Short‐Form 36 (SF‐36) and the EQ‐5D‐5L were used to evaluate the quality of life of patients. The data between the two groups were compared by independent sample t‐tests. Multiple comparisons between samples were analyzed by analysis of variance.
Results
Compared with the MTD group, the PTED group had shorter incision length (9.20 ± 1.19 mm vs 26.38 ± 1.82 mm), less intraoperative blood loss (18.00 ± 4.97 mL vs 39.83 ± 6.51 mL), and shorter hospital stay (5.42 ± 5.08 days vs 10.58 ± 3.69 days) (P = 0.00). PTED was much more appropriate for foraminal and extraforaminal disc herniation. The incidence of paresthesia was lower in the PTED group (6.67% vs 16.67%). At each follow up, the VAS and ODI scores of all patients were significantly improved compared with those before surgery (P = 0.00). At 3 days postoperatively, the lumbar VAS score of the PTED group was significantly lower (1.58 ± 1.00 vs 2.37 ± 1.10, P = 0.00). The excellent rate of the PTED group reached 91.67%, and that of the MTD group reached 93.33%. Compared with the preoperative SF‐36 scores for physiological function, mental health, and social function, the postoperative scores were significantly improved in both groups (P = 0.00). The EQ‐5D‐5L in the PTED group increased from 0.30 ± 0.17 before the operation to 0.69 ± 0.13 after 6 months of follow up (P = 0.00) and 0.73 ± 0.14 after 20 months of follow up. The EQ‐5D‐5L in the MTD group increased from 0.28 ± 0.17 before the operation to 0.68 ± 0.13 after a 6‐month follow up (P = 0.00), and 0.73 ± 0.12 after a 20‐month follow up.
Conclusion
Although both PTED and MTD are effective for LDH, PTED is much more appropriate for various types of LDH and has the advantages of the low incidence of low back pain, fewer complications, and early recovery.
Keywords: Clinical outcome, Lumbar disc herniation, Microscope, Percutaneous transforaminal endoscopic discectomy, Tubular
Schematic illustration
Introduction
Lumbar disc herniation (LDH) is one of the most common diseases that cause low back pain and leg pain. Both conservative and surgical treatments can be used for LDH. If conservative treatment is ineffective, surgical treatment will be considered. An 8‐year follow‐up study showed that patients undergoing surgery had greater pain relief and faster function improvement compared with conservative treatment, resulting in higher treatment satisfaction 1 .
Although traditional open lumbar discectomy has been considered the gold standard for the treatment of LDH, postoperative pain due to tissue damage in the surgery cannot be ignored 2 . With the development of minimally invasive technology, endoscopic, microscopic, and tubular technology has been widely used in the treatment of LDH, which has reduced the incidence of postoperative low back pain. Percutaneous transforaminal endoscopic discectomy (PTED) has been increasingly used for the treatment of LDH due to its good clinical efficacy, fewer complications, smaller surgical incisions, shorter hospital stay, and earlier return to daily activities 3 .
Microendoscopic discectomy (MED) is the earliest minimally invasive surgery using a tube combined with a light source to treat LDH. It was first introduced by Foley in 1997 4 . With the development of micro‐instruments and equipment, the microscope‐assisted tubular surgery for lumbar degenerative diseases has increasingly been used in the treatment of degenerative disc disease and has achieved good clinical results 5 . A recent meta‐analysis study showed that tube‐assisted discectomy, in addition to having less blood loss and shorter hospital stay, also required less postoperative analgesics compared with microdiscectomy 6 . Zhang et al. conducted a comparative study between MED and microscope‐assisted tubular discectomy (MTD) for LDH. The results showed that the two surgical methods achieved a similar treatment effect, but MTD had the advantages of short operation time and less incidence of dural tear 7 . Although many studies have compared the clinical outcomes between PTED and tubular MED 8 , 9 , to our knowledge, there have been no reports on PTED and microscope‐assisted discectomy with similar tubular retractors for symptomatic LDH.
In this study, we retrospectively compared and assessed the clinical results of PTED and MTD in LDH patients with a minimum 20‐month follow up. The major outcomes were evaluated: (i) to compare the perioperative index, including the length of incision, operation time, intraoperative bleeding loss, intraoperative fluoroscopy frequency, hospital stay, cost, type of disc herniation, and complications; (ii) to compare the clinical outcomes, such as visual analog scale (VAS) score, Oswestry disability index (ODI), and modified Macnab criteria; and (iii) to compare the quality of life, using the Short‐Form 36 (SF‐36) and the EQ‐5D‐5L.
Materials and Methods
Patients
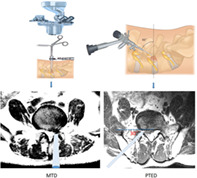
The study was performed based on the data of patients who underwent PTED or MTD (Fig. 1) for single‐segment LDH from June 2017 to June 2018. The inclusion criteria in this study were as follows: (i) patients with cauda equina syndrome; (ii) single‐segment LDH confirmed by imaging; and (iii) failure of conservative treatment. The exclusion criteria were: (i) more than two segments LDH; (ii) lumbar spondylolisthesis or instability; (iii) previous lumbar surgery, fracture, infection or tumor; and (iv) severe heart, lung and brain diseases, coagulation dysfunction, and intolerance of operation.
Fig 1.

Zista tapered retractor is 18 mm diameter at one end and 22 mm diameter at the opposite end with different length models percutaneous transforaminal endoscopic discectomy (PTED) and microscope‐assisted tubular discectomy (MTD).
According to the inclusion and exclusion criteria, 120 patients with LDH were enrolled, including 59 men and 61 women. The patients were aged was from 22 to 80 years. The patients were divided into two groups as follows: a PTED group consisting of 60 patients treated with PTED; and an MTD group consisting of 60 patients treated with MTD. The baseline data of the two groups are shown in Table 1. The surgical procedures were performed by the same senior physicians. All patients' data were obtained and collected by the same researchers. Each patient was followed up for at least 20 months.
TABLE 1.
Baseline data of patients in both groups
| PTED group | MTD group | P‐value | |
|---|---|---|---|
| Gender | 60 | 60 | 0.361 |
| Male | 27 | 32 | ‐ |
| Female | 33 | 28 | ‐ |
| Age (year) | 50.70 ± 15.20 | 53.40 ± 14.30 | 0.319 |
| Operative segment | 0.899 | ||
| L2/3 | 2 | 1 | ‐ |
| L3/4 | 3 | 3 | ‐ |
| L4/5 | 33 | 31 | ‐ |
| L5/S1 | 22 | 25 | ‐ |
| Types of herniation | 0.043 | ||
| Intraspinal canal | 54 | 60 | ‐ |
| Foraminal | 2 | 0 | ‐ |
| Extraforaminal | 4 | 0 | ‐ |
| History of disease (month) | 0.540 | ||
| ≥3 m | 45 | 42 | ‐ |
| <3 m | 15 | 18 | ‐ |
| Smoking | 0.705 | ||
| Yes | 21 | 23 | ‐ |
| None | 39 | 37 | ‐ |
| Neurologic dysfunction | 0.912 | ||
| Sensory deficits | 8 | 6 | ‐ |
| Motor deficits | 3 | 2 | ‐ |
| Body mass index | 0.898 | ||
| Normal (18.5–24.9) | 32 | 33 | ‐ |
| Overweight (25.0–29.9) | 25 | 25 | ‐ |
| Obesity (≥30.0) | 3 | 2 | ‐ |
MTD, microscope‐assisted tubular discectomy; PTED, percutaneous transforaminal endoscopic discectomy.
Surgical Operation
Percutaneous Transforaminal Endoscopic Discectomy Group
Step 1: Patients were treated with intravenous anesthesia combined with local anesthesia. The abdomen was suspended prone on the operating table. The responsible segment and puncture point were determined under C‐arm fluoroscopy.
Step 2: After routine skin disinfection, 1% lidocaine was used for anesthesia subcutaneously, fascially, muscularly, and articularly. The C‐arm guided the puncture until it reached the target position. The puncture needle tip was positioned inside the inner edge of the pedicle in anteroposterior radiographs and laterally at the upper posterior edge of the lower vertebral body. A guidewire was placed, an incision was made, and the serial soft tissue dilator was inserted. The intervertebral foramen was enlarged with a serial bone drill.
Step 3: The working tube was placed and the position was confirmed by the C‐arm. The decompression was then performed under visual control and liquid flow. The anatomical structure was identified, the nerve root was exposed, and the nucleus pulposus was removed under the endoscope system (typical example can be seen in Fig. 2).
Fig 2.
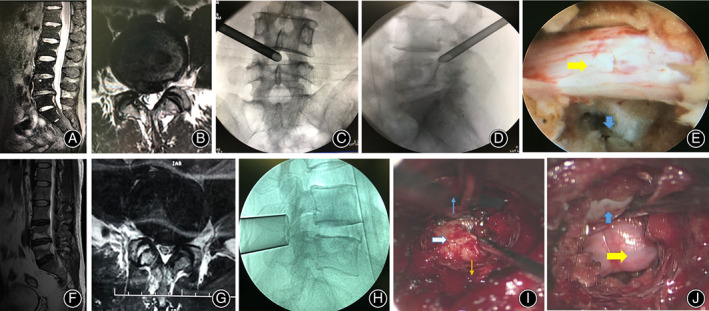
Typical cases: Percutaneous transforaminal endoscopic discectomy (PTED) group (top, female, 44 years old, L4/5 LDH) and MTD group (bottom, male, 48 years old, L4/5 LDH). (A, B) Sagittal and axial MRI before the operation. (C, D) Anterior–posterior and lateral position of the working channel. (E) Decompression. The yellow arrow indicated the nerve roots; the blue arrow indicated the intervertebral space after discectomy. (F, G) Sagittal and axial MRI before the operation. (H) The lateral view of Zista tapered retractor. (I) View under microscope. White arrow indicates protruding herniated lumbar disc; blue arrow indicates the midline of the spine; brown arrow indicates the direction of articular processes. (J) Decompression. The yellow arrow indicates the nerve root; the blue arrow indicates the dural sac.
Microscope‐Assisted Tubular Discectomy Group
Step 1: The patient was in a prone position under general anesthesia. The responsible intervertebral segment was confirmed by lateral fluoroscopy.
Step 2: After routine skin disinfection, a paramedian incision was made approximately 1 cm lateral to the midline on the symptomatic side. Sequential dilators were inserted to create a surgical pathway to the lumbar spine. An 18‐mm‐diameter Zista tapered retractor was positioned with the assistance of an operating microscope. The Zista tube was supported by a serpentine arm, which was fastened to the operating table.
Step 3: Under the microscope, the soft tissues on the surface of the lamina were resected, and the lower edges of the and part of the medial margin of the articular process were abrased. After the ligamentum flavum was removed, the dural sac and nerve root were exposed, then the protruding disc was resected. Wound drainage was applied in all cases (typical cases can be seen in Fig. 2).
Perioperative Observational Index
Length of incision, operation time, intraoperative bleeding loss, intraoperative fluoroscopy frequency, hospital stay, cost, type of disc herniation 10 , and complications (dural tear, nerve injury, and reoperation rate) were recorded and compared between the two groups.
Clinical Evaluation
The clinical outcomes were evaluated using the VAS for lumbar and lower extremity pain and the Oswestry disability index (ODI). VAS and ODI scores were recorded before and at 3 days and 3, 6, 12, and 20 months after surgery. The modified Macnab criteria were used to evaluate clinical efficacy.
Visual Analog Scale
The VAS is used to evaluate the degree of pain using a ruler, and the score is determined by measuring the distance (cm) on the 10‐cm line between the “no pain” anchor and the patient's mark, providing a range of scores from 0 to 10, where 0 means no pain and 10 represents unbearable pain. A higher score indicates greater pain intensity.
Oswestry Disability Index
The ODI is a measure to evaluate spinal disorders and to assess patient progress in clinical practice. The ODI score system includes ten sections: pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and traveling. Each section includes six statements and the total score is 5. Intervening statements are scored according to rank. The highest score is recorded if more than one box is marked in each section. If all 10 sections are completed, the score is calculated as: total scored out of total possible score × 100. If one section is not applicable, the score is calculated as: (total score/(5 × number of questions answered)) × 100%.
Evaluation of Quality of Life
The SF‐36 and the EQ‐5D‐5L 11 were used to evaluate the quality of life and health index of patients before and 3 days and 3, 6, 12, and 20 months after the operation.
Short‐Form 36
The SF‐36 questionnaire is an assessment tool for quality of life, which consists of 36 questions. Mental function, physical function, and social function are of high reliability in patients with spinal injuries. The higher the score, the better the health status.
EQ‐5D‐5L
The EQ‐5D is a preference‐based measure of health status that is widely used around the world in clinical trials, population studies, and real‐world clinical settings. The EQ‐5D is designed and developed by the EuroQol Research Foundation. The EQ‐5D‐5L descriptive system is a new version of the EQ‐5D that comprises five dimensions (mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression). Each dimension has five response levels: no problems, slight problems, moderate problems, severe problems, unable to/extreme problems. The respondent is asked to indicate his/her health state by checking the box next to the most appropriate response level for each of the five dimensions. Responses are coded as single‐digit numbers expressing the severity level selected in each dimension from 1 to 5. Therefore, the EQ‐5D‐5L health states can be summarized using a 5‐digit code. For instance, 21111 means slight problems in the mobility dimension and no problems in any of the other dimensions. Finally, the 5‐digit code was converted to the standard EQ‐5D‐5L value set by applying a formula that attaches values to each of the levels in each dimension. Details are on the website: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/.
Statistical Analysis
Statistical analysis of the data was performed using IBM SPSS 20.0 software (International Business Machines, Armonk, New York, USA). The values are described as the means ± standard deviations. The measurement data between the two groups were compared by independent sample t‐tests. Multiple comparisons between samples were analyzed by analysis of variance. The χ 2‐test or Fisher's exact probability test was used for counting data. Differences with two‐tailed P‐values <0.05 were considered statistically significant.
Results
Patients
We performed a minimum 20‐month follow‐up for 120 patients. There were no significant differences in baseline demographic characteristics, including age, gender, history of the disease, operative segment, body mass index, smoking, and neurological function, between the PTED group and the MTD group (Table 1, P > 0.05).
Surgical Operation
In this study, all patients underwent surgery successfully, and no cases were transferred to the open or other surgery. In the PTED group, the positional relationship between the herniated disc and the nerve root was observed. After decompressing the nerve root, the range of the relaxed nerve root and the rhythm of the nerve root beating with the heartbeat was observed (Fig. 2). In the MTD group, herniated discs could also be seen or probed. After decompression of the nerve root, the nerve root was observed to relax, but the pulse of the nerve root was not easy to observe (Fig. 2).
Perioperative Observational Index
The PTED group had significantly shorter incision length and hospital stay, and less blood loss, but also had more frequency of intraoperative fluoroscopy, longer operation time, and higher cost than the MTD group (Table 2, P = 0.00). Considering the type of disc herniation, PTED was appropriate for various types of disc herniation, while microscope‐assisted tubular surgery was not appropriate for foraminal and extraforaminal disc herniation (Table 1, P = 0.043).
TABLE 2.
Perioperative characteristics of patients
| PTED group | MTD group | P‐value | |
|---|---|---|---|
| Intraoperative blood loss (mL) | 18.00 ± 4.97 | 39.83 ± 6.51 | 0.00 |
| Frequency of fluoroscopy | 26.50 ± 3.06 | 2.95 ± 0.67 | 0.00 |
| Operative time (minute) | 76.17 ± 8.80 | 51.10 ± 15.83 | 0.00 |
| Cost (RMB) | 22863.87 ± 3657.35 | 18152.75 ± 2378.15 | 0.00 |
| Incision length (mm) | 9.20 ± 1.19 | 26.38 ± 1.82 | 0.00 |
| Hospital stay (days) | 5.42 ± 5.08 | 10.58 ± 3.69 | 0.00 |
MTD, microscope‐assisted tubular discectomy; PTED, percutaneous transforaminal endoscopic discectomy.
Clinical Evaluation
The postoperative scores of VAS and ODI in both groups were significantly decreased compared with those before the operation (Table 3, Figures 3, 4, 5, P = 0.00). Symptoms continued to improve at different time points after surgery in both groups. Although the VAS and ODI scores were decreased at 12‐month and 20‐month follow‐up, there were no significant differences compared with the 6‐month follow up (Figs 3 and 4). There were no significant differences between the two groups in VAS and ODI scores during different time points after surgery (Table 3, P > 0.05). The lumbar VAS score at 3 days after the operation in the PTED group was significantly lower than that in the MTD group (Table 3, P = 0.00). At the 20‐month follow up, the overall excellent rate was 91.67% in the PTED group (55/60) and 93.33% in the MTD group (56/60). There was no significant difference between the two groups (Table 3, P > 0.05).
TABLE 3.
Comparison of clinical results between two groups
| PTED group | MTD group | P‐value | |
|---|---|---|---|
| Lumbar VAS | |||
| Preoperation | 2.65 ± 2.02 | 2.88 ± 1.95 | 0.859 |
| Postoperative 3 days | 1.58 ± 1.00 | 2.37 ± 1.10 | 0.000 |
| 3‐month follow‐up | 1.23 ± 1.10 | 1.68 ± 1.07 | 0.449 |
| 6‐month follow‐up | 0.95 ± 1.02 | 1.07 ± 0.90 | 0.507 |
| 12‐month follow‐up | 0.88 ± 0.76 | 0.87 ± 0.75 | 0.904 |
| 20‐month follow‐up | 0.65 ± 0.86 | 0.80 ± 0.75 | 0.312 |
| Lower extremity VAS | |||
| Preoperation | 5.20 ± 2.02 | 5.25 ± 1.80 | 0.886 |
| Postoperative 3 days | 2.02 ± 1.07 | 2.27 ± 1.25 | 0.240 |
| 3‐month follow‐up | 1.58 ± 1.06 | 1.83 ± 0.94 | 0.175 |
| 6‐month follow‐up | 1.02 ± 0.70 | 1.18 ± 0.75 | 0.210 |
| 12‐month follow‐up | 0.77 ± 0.74 | 0.87 ± 0.77 | 0.471 |
| 20‐month follow‐up | 0.63 ± 0.64 | 0.82 ± 0.79 | 0.165 |
| ODI | |||
| Preoperation | 29.8 ± 10.27 | 31.75 ± 9.19 | 0.279 |
| Postoperative 3 days | 26.08 ± 9.57 | 28.07 ± 9.15 | 0.248 |
| 3‐month follow‐up | 19.40 ± 8.30 | 20.38 ± 8.37 | 0.520 |
| 6‐month follow‐up | 12.35 ± 6.22 | 13.20 ± 6.51 | 0.466 |
| 12‐month follow‐up | 12.50 ± 5.64 | 13.40 ± 5.38 | 0.373 |
| 20‐month follow‐up | 9.92 ± 5.49 | 11.13 ± 5.47 | 0.227 |
| Modified Macnab | 0.898 | ||
| Excellent | 48 | 47 | |
| Good | 7 | 9 | |
| Fair | 3 | ||
| Poor | 2 | 1 |
MTD, microscope‐assisted tubular discectomy; ODI, Oswestry disability index; PTED, percutaneous transforaminal endoscopic discectomy; VAS, visual analogue scale.
Fig 3.
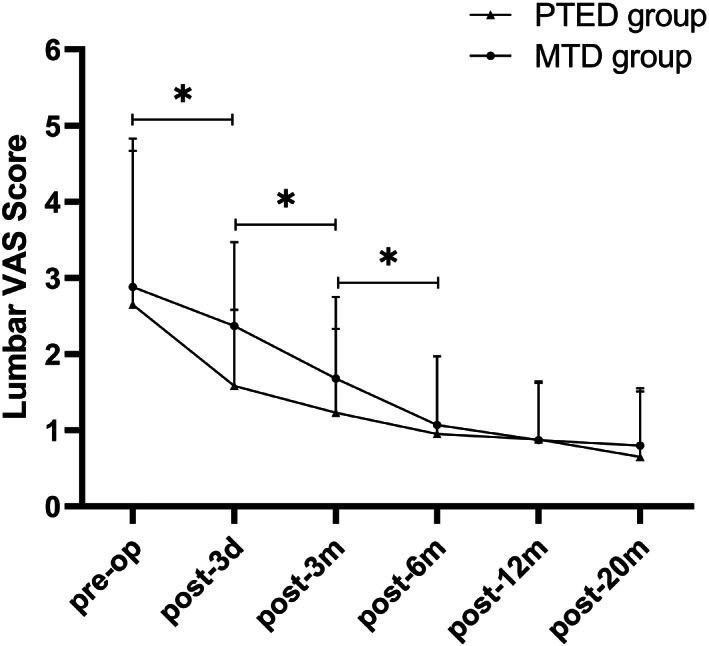
Lumbar visual analog scale (VAS) scores in both groups. The VAS scores decreased significantly during the first 6 months after surgery in both groups and continued to decline throughout the follow‐up period. * indicates P < 0.05.
Fig 4.
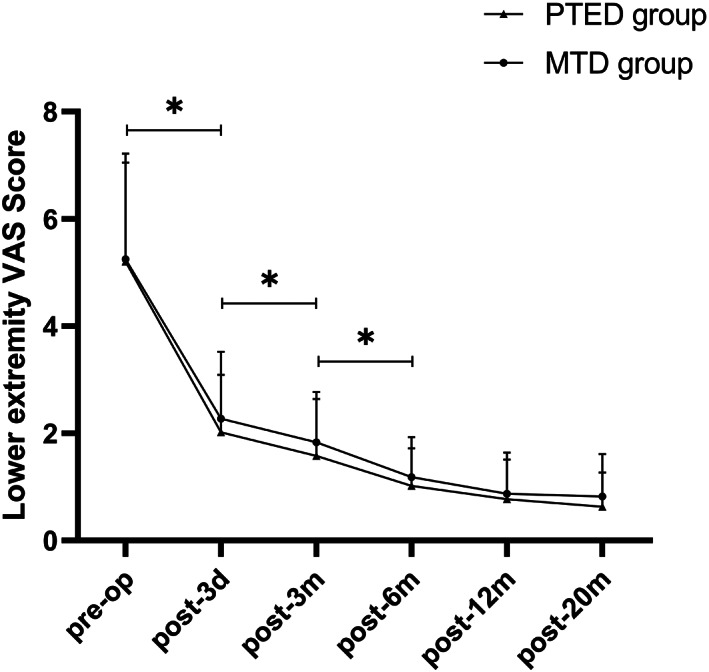
The lower extremity visual analog scale (VAS) scores in both groups. The VAS scores decreased significantly during the first 6 months after surgery in both groups and continued to decline throughout the follow‐up period. * indicates P < 0.05.
Fig 5.
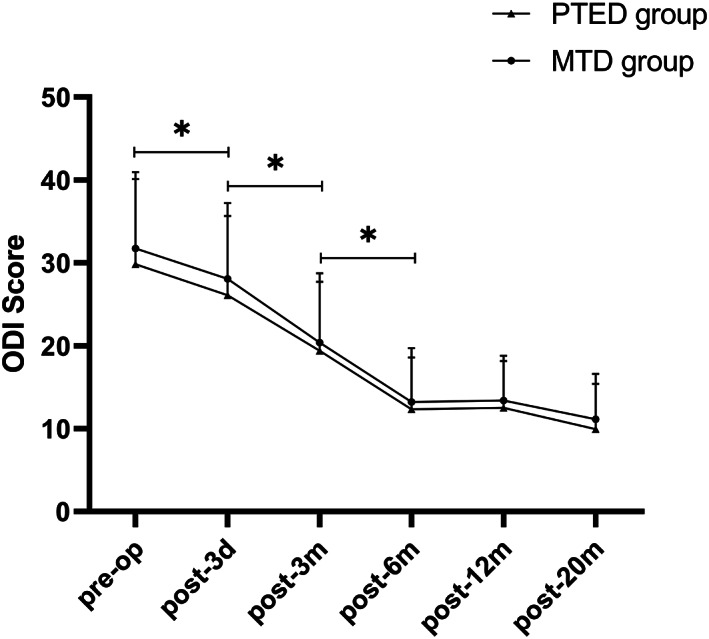
Scores of Oswestry disability index (ODI) in both groups. The ODI scores decreased significantly in the first 6 months after surgery in both groups and continued to decline throughout the follow‐up periods. * indicated P < 0.05.
Evaluation of Quality of Life
According to the SF‐36 scores, physiological function, mental health, and social function were significantly improved at different time points after surgery. The improvement of physiological function and mental health promoted the improvement of patients' social function, indicating that the overall quality of life improved significantly (Table 4, Figs 6 and 7, P = 0.00). There were no statistically significant differences in the physiological function, mental health, and social function before and after the operation (Table 4, P > 0.05) between the two groups. The results of the EQ‐5D‐5L indicated that there was no significant difference between the two groups at each follow‐up point (Table 5, P > 0.05). The EQ‐5D‐5L of the two groups gradually improved over time and reached a steady state at 6 months after surgery (Table 5, Fig. 8, P = 0.00).
TABLE 4.
Comparison of SF‐36 results between two groups
| PTED group | MTD group | P‐value | |
|---|---|---|---|
| Physical function | |||
| Preoperation | 62.41 ± 12.34 | 63.75 ± 13.14 | 0.596 |
| Postoperative 3 days | 66.08 ± 10.82 | 66.67 ± 11.34 | 0.774 |
| 3‐month follow‐up | 76.42 ± 9.16 | 77.00 ± 8.35 | 0.716 |
| 6‐month follow‐up | 82.00 ± 6.71 | 82.08 ± 6.20 | 0.944 |
| 12‐month follow‐up | 83.75 ± 6.99 | 84.08 ± 6.61 | 0.789 |
| 20‐month follow‐up | 85.00 ± 6.44 | 84.75 ± 5.71 | 0.822 |
| F | 61.121 | 61.740 | ‐ |
| P | 0.000 | 0.000 | ‐ |
| Mental health | |||
| Preoperation | 58.07 ± 12.75 | 61.07 ± 12.52 | 0.196 |
| Postoperative 3 days | 62.33 ± 11.37 | 65.20 ± 11.39 | 0.170 |
| 3‐month follow‐up | 73.33 ± 9.28 | 75.40 ± 9.37 | 0.227 |
| 6‐month follow‐up | 78.87 ± 8.27 | 80.07 ± 7.98 | 0.420 |
| 12‐month follow‐up | 80.57 ± 7.62 | 81.10 ± 6.76 | 0.686 |
| 20‐month follow‐up | 82.03 ± 7.57 | 83.17 ± 6.61 | 0.384 |
| F | 87.333 | 78.136 | ‐ |
| P | 0.000 | 0.000 | ‐ |
| Social function | |||
| Preoperation | 73.86 ± 17.42 | 75.21 ± 18.48 | 0.704 |
| Postoperative 3 days | 81.46 ± 16.35 | 82.92 ± 16.91 | 0.632 |
| 3‐month follow‐up | 99.58 ± 18.83 | 99.79 ± 17.90 | 0.951 |
| 6‐month follow‐up | 111.67 ± 14.71 | 112.08 ± 13.80 | 0.812 |
| 12‐month follow‐up | 114.17 ± 13.51 | 111.04 ± 12.83 | 0.196 |
| 20‐month follow‐up | 115.83 ± 13.59 | 113.13 ± 14.45 | 0.292 |
| F | 76.700 | 115.906 | ‐ |
| P | 0.000 | 0.000 | ‐ |
MTD, microscope‐assisted tubular discectomy; PTED, percutaneous transforaminal endoscopic discectomy.
Fig 6.
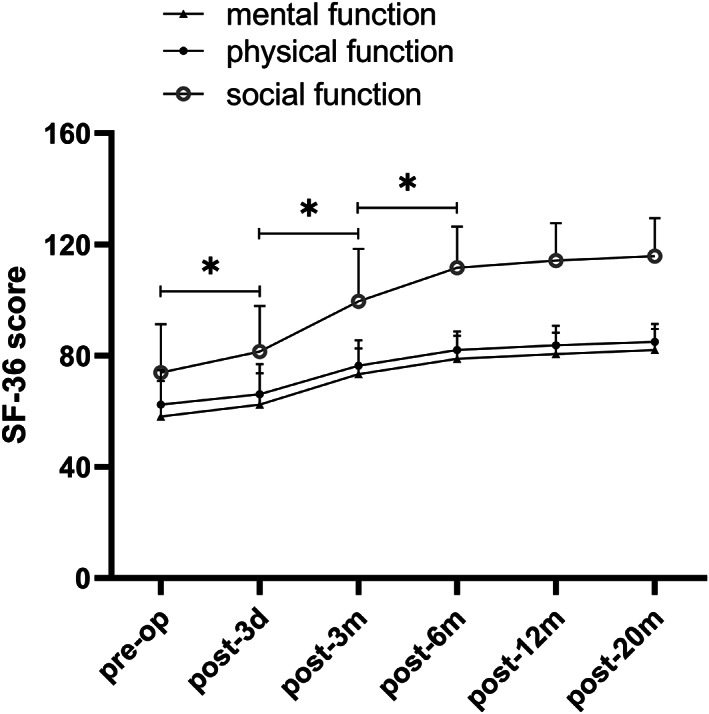
The results of the Short‐Form 36 (SF‐36) questionnaire (physical, psychological, and social scores) in the percutaneous transforaminal endoscopic discectomy (PTED) group preoperatively and at follow ups. The scores increased significantly in the first 6 months after surgery and remained stable in the following observational periods. * indicates P < 0.05.
Fig 7.
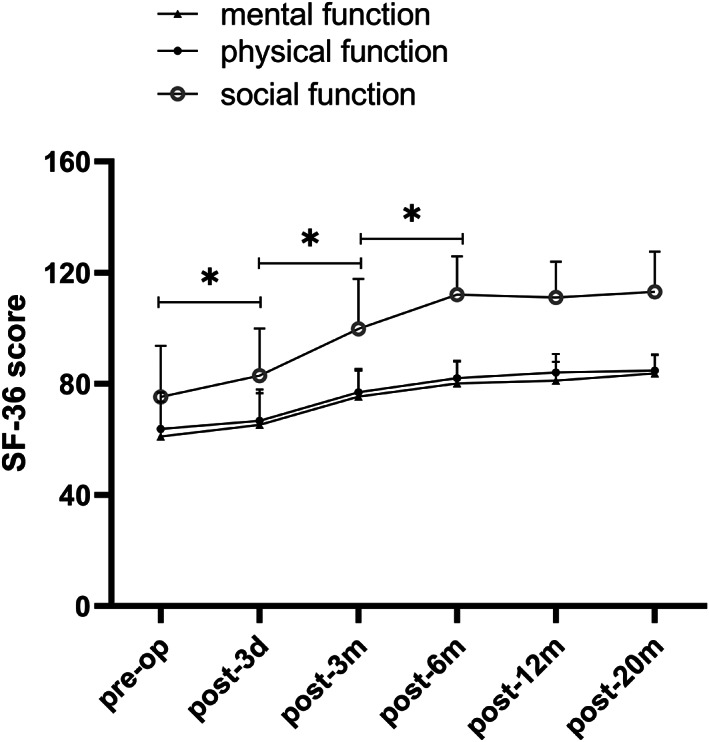
The results of the Short‐Form 36 (SF‐36) questionnaire (physical, psychological, and social scores) in the microscope‐assisted tubular discectomy (MTD) group preoperatively and at follow ups. The scores increased significantly in the first 6 months after surgery and remained stable in the following observational periods. * indicated P < 0.05.
TABLE 5.
Comparison of EQ‐5D‐5L results between two groups
| PTED group | MTD group | P‐value | |
|---|---|---|---|
| Preoperation | 0.30 ± 0.17 | 0.28 ± 0.17 | 0.538 |
| Postoperative 3 days | 0.45 ± 0.14 | 0.43 ± 0.15 | 0.430 |
| 3‐month follow‐up | 0.60 ± 0.13 | 0.57 ± 0.16 | 0.220 |
| 6‐month follow‐up | 0.69 ± 0.13 | 0.68 ± 0.13 | 0.871 |
| 12‐month follow‐up | 0.70 ± 0.11 | 0.71 ± 0.10 | 0.515 |
| 20‐month follow‐up | 0.73 ± 0.14 | 0.73 ± 0.12 | 0.934 |
| F | 89.185 | 94.213 | ‐ |
| P | 0.000 | 0.000 | ‐ |
MTD, microscope‐assisted tubular discectomy; PTED, percutaneous transforaminal endoscopic discectomy.
Fig 8.
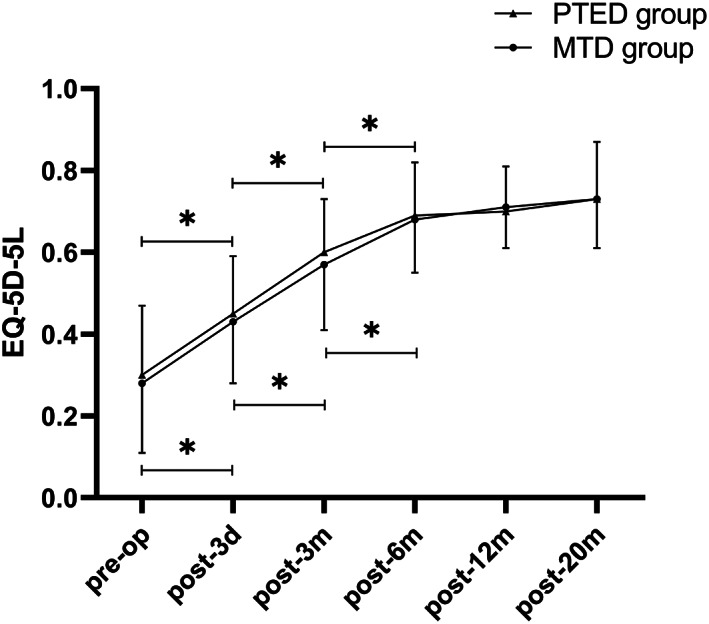
Scores of EQ‐5D‐5L in both groups. The EQ‐5D‐5L scores increased significantly in the first 6 months after surgery in both groups and continued to increase slightly in the follow‐up periods. * indicates P < 0.05.
Complications
During the surgery, dural tear occurred in 1 case (1.67%, 1/60) in the PTED group and in 2 cases (3.33%, 2/60) in the MTD group. As the rupture was small, no repairs were performed. The patients' wounds were drained after surgery and recovered well after extubation. Due to poor clinical results, 3 patients (5.00%, 3/60) in the PTED group and 2 patients (3.33%, 2/60) in the MTD group underwent secondary surgery. A total of 4 patients (6.67%, 4/60) in the PTED group and 10 (16.67%, 10/60) in the MTD group developed paresthesia. The paresthesia was significantly relieved after treatment with nerve nutrition, dehydration, and hormones.
Discussion
In the last ten years, minimally invasive techniques have been widely used in the treatment of LDH. This study confirmed that both PTED and MTD can achieve good clinical results and improve the quality of life of patients. Although more costly, PTED has advantages of shorter recovery time, fewer complications, and quicker return to normal life.
The operation time in the PTED group was significantly longer than that in MTD group, which was related to the repeated intraoperative fluoroscopy to place the endoscopic tube. In addition, the working tube of PTED is narrow, and the limited operative space further increased the operation time. Conversely, the Zista tapered retractor is 22 mm in diameter on the upper end and tapers to a diameter of 18 mm at the opposite end. It allows more free movement and angulation of the surgical tools 7 . However, the incision length, hospital stay, and intraoperative blood loss in the PTED group were significantly less than in the MTD group. These factors can help patients to get out of bed earlier, recover faster, and resume daily life sooner. However, compared with MTD, the cost of PTED was higher because of the expensive devices.
The effect on health of radiation from fluoroscopy is also a concern for surgeons and patients. The average fluoroscopic quantity in one PTED surgery was consistent with that in previous reports 12 . If no protective measures were taken, a surgeon could perform 291 PTED operations a year to reach the maximum allowable radiation absorbed dose 13 . Therefore, it is very safe to perform PTED if a surgeon adopts protective measures. For patients, protective equipment was rarely used during surgery, and studies had confirmed that the average radiation dose received during a PTED operation was 18.25 mSV, which was within a safe range 14 . Therefore, the radiation absorbed dose in a PTED was within a safe range.
Dural tears and recurrence are common complications in treating LDH. The incidence of dural tears was similar in groups A and B, which is consistent with previous reports 7 , 15 . Both the PTED and the microscope provided a clear field of view and a certain degree of magnification. In addition, the water pressure in the PTED confined the expansion of the dura mater and reduced the rate of dura mater tears, while the microscope provided a view depth to make a three‐dimensional field of view, which reduced the incidence of dural tears 7 , 15 . Although the recurrence rate in the PTED group was slightly higher than that in the MTD group, it was consistent with related reports 16 . There are many factors related to recurrence, including diabetes, weight, and age. Therefore, for such patients, the pros and cons of surgical methods must be carefully evaluated to reduce the incidence of recurrence 17 , 18 .
The type of disc herniation affected surgical choice. Both PTED and MTD were suitable for intraspinal disc herniation. Compared with MTD in a posterior approach, PTED was much more appropriate for the foraminal and extraforaminal type of LDH, because the posterior approach assisted by the tube had certain limitations. Yoshimoto reported that it was necessary to locate the tube at the junction of the articular process and the transverse process in the treatment of far lateral LDH. Although satisfying clinical results had been achieved and the stability of the spine was maintained, the operation took a long time, with an average duration of 143.9 min 19 . The treatment of far lateral LDH by the posterior approach requires resection of the articular process; otherwise, it is difficult to deal with far lateral discs 10 . Rong reported that it was not ideal to treat far lateral LDH using the posterior approach. The main reasons were related to the limited visual field caused by less facetectomy and the irritation of the nerve root caused by direct decompression of the dorsal root ganglion 10 . Resection of the articular process could easily lead to instability of the corresponding segment 20 , while PTED hardly damages the articular process joint and the posterior structure of the spine. Thus, PTED is widely used to treat the foraminal and extraforaminal types of LDH 21 , 22 .
Percutaneous transforaminal endoscopic discectomy and MTD were appropriate for all segments of the lumbar spine. However, considering the high iliac and narrow foramen of L5/S1, MTD would be a better choice for L5/S1 segment LDH. First, the interlaminar space of L5/S1 was larger than that of the other segments, and it was directly corresponding to the intervertebral disc, so it was not necessary to grind out more lamina. Second, the preganglionic distance (from the origin of exiting root to its ganglion) of L5 was the longest from L1 to L5, which decreased the incidence of ganglion injury 23 . Finally, as to the S1 root, 75% originated above and 25% at the L5/S1 disc. Furthermore, the herniated disc was mostly located at the axilla and compressed dura and nerve in the L5/S1 segment because of the proximity between the corresponding disc and nerve root 24 .
The clinical symptoms of the two groups were significantly improved after surgery. VAS and ODI scores were significantly decreased, especially in the first 6 months. The symptoms were further improved over time in the follow‐up periods and reached a steady state. Compared with the MTD group, the low back VAS score of the PTED group was significantly better at 3 days postoperatively. First, this is because the low back pain is related to postoperative incision pain. Second, the posterior approach requires a direct incision of the multifidus muscle, which causes degeneration and necrosis of muscle fibers due to abnormal loading. Finally, partial resection of the articular process would lead to injury of the posterior branch of the spinal nerve, resulting in denervation of the multifidus muscle 25 . Although there was no significant difference in the VAS score of the lower extremity, we found that there were more patients with paresthesia and other sensory abnormalities in the MTD group than in the PTED group (16.67% vs 5.7%). It had been reported that the incidence of transient sensory abnormality caused by MED is 10% (lower than 16.67%), which would be related to excessive traction of the nerve root, especially for the central LDH in our study 9 . Phan et al. (2017) report that the stimulation of bipolar electrocoagulation also leads to postoperative sensory abnormalities 26 . This symptom improved after dehydration and hormone treatment for several days. The incidence of paresthesia in the PTED group was lower because of the lateral approach and less irritation to the nerve root. The results of the SF‐36 and the EQ‐5D‐5L showed that there was no significant difference in the quality of life and health index. This showed that the temporary postoperative low back pain and paresthesia were acceptable, and the patients were satisfied with the clinical efficacy. The improvement in psychological, physical, and social functions could offset the temporary discomfort. Some studies indicated that compared with tubular discectomy (such as MED), PTED did not show superior clinical results in the treatment of LDH, especially for the central type LDH. According to Rong, PTED has a smaller operating space and limited field of view, and it is not easy to completely remove the central disc 27 . However, our results indicated that PTED was applicable for all types of LDH, associated with the characteristics of fewer complications and fast recovery, compared with MTD. The results were consistent with those reported by Liu 8 . A recent meta‐analysis of 29 randomized controlled studies involving 3146 patients showed that PTED had the highest success rate, the least complications, and a lower reoperation rate than MED in the treatment of LDH 28 .
This study has some limitations. First, this study is not a double‐blind randomized control trial. The surgeons and patients had a different understanding of the treatment and prognosis, which may affect the evaluation of results. Second, the surgeon's preference for surgical techniques may also affect the result. Finally, this study is a single‐center study, and the follow‐up time is relatively short. A high‐quality study with multi‐center and long‐term follow up is still needed to compare the clinical results of PTED and MTD.
Conclusion
Both PTED and MTD are effective treatments for LDH. Satisfactory quality of life can be achieved at 6 months after surgery. Compared with MTD, PTED is appropriate for various types of LDH and is associated with a lower incidence of postoperative low back pain, fewer complications, and quicker recovery.
Ethics Statement
The study was retrospectively designed and was approved by the institutional review board of the ethics committee of Qingdao Municipal Hospital. All procedures were performed in accordance with the Helsinki Declaration. All patients provided written informed consent.
Contributor Information
Dechun Wang, Email: dechun-w@163.com.
Mingwei Zhao, Email: 419790064@qq.com.
References
- 1. Lurie JD, Tosteson TD, Tosteson AN, et al. Surgical versus nonoperative treatment for lumbar disc herniation: eight‐year results for the spine patient outcomes research trial. Spine, 2014, 39: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choi KC, Kim JS, Lee DC, Park CK. Percutaneous endoscopic lumbar discectomy: minimally invasive technique for multiple episodes of lumbar disc herniation. BMC Musculoskelet Disord, 2017, 18: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim HS, Paudel B, Jang JS, Lee K, Oh SH, Jang IT. Percutaneous endoscopic lumbar discectomy for all types of lumbar disc Herniations (LDH) including severely difficult and extremely difficult LDH cases. Pain Physician, 2018, 21: E401–E408. [PubMed] [Google Scholar]
- 4. Foley KT, Smith MM. Micro endoscopic discectomy. Tech Neurosurg, 1997, 3: 301–307. [Google Scholar]
- 5. Parikh K, Tomasino A, Knopman J, Boockvar J, Härtl R. Operative results and learning curve: microscope‐assisted tubular microsurgery for 1‐ and 2‐level discectomies and laminectomies. Neurosurg Focus, 2008, 25: E14. [DOI] [PubMed] [Google Scholar]
- 6. Clark AJ, Safaee MM, Khan NR, Brown MT, Foley KT. Tubular microdiscectomy: techniques, complication avoidance, and review of the literature. Neurosurg Focus, 2017, 43: E7. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y, Chong F, Feng C, Wang Y, Zhou Y, Huang B. Comparison of endoscope‐assisted and microscope‐assisted tubular surgery for lumbar laminectomies and discectomies: minimum 2‐year follow‐up results. Biomed Res Int, 2019, 2019: 5321580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X, Yuan S, Tian Y, et al. Comparison of percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, and microdiscectomy for symptomatic lumbar disc herniation: minimum 2‐year follow‐up results. J Neurosurg Spine, 2018, 28: 317–325. [DOI] [PubMed] [Google Scholar]
- 9. Zhang L, Dong J, Xie P, et al. Percutaneous transforaminal endoscopic discectomy compared with microendoscopic discectomy for lumbar disc herniation: 1‐year results of an ongoing randomized controlled trial. J Neurosurg Spine, 2018, 28: 300–310. [DOI] [PubMed] [Google Scholar]
- 10. Al‐Khawaja DO, Mahasneh T, Li JC. Surgical treatment of far lateral lumbar disc herniation: a safe and simple approach. J Spine Surg, 2016, 2: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo N, Liu G, Li M, Guan H, Jin X, Rand‐Hendriksen K. Estimating an EQ‐5D‐5L value set for China. Value Health, 2017, 20: 662–669. [DOI] [PubMed] [Google Scholar]
- 12. Fan G, Han R, Gu X, et al. Navigation improves the learning curve of transforamimal percutaneous endoscopic lumbar discectomy. Int Orthop, 2017, 41: 323–332. [DOI] [PubMed] [Google Scholar]
- 13. Ahn Y, Kim CH, Lee JH, Lee SH, Kim JS. Radiation exposure to the surgeon during percutaneous endoscopic lumbar discectomy: a prospective study. Spine, 2013, 38: 617–625. [DOI] [PubMed] [Google Scholar]
- 14. Zhang M, Yan L, Li S, Li Y, Huang P. Ultrasound‐guided transforaminal percutaneous endoscopic lumbar discectomy: a new guidance method that reduces radiation doses. Eur Spine J, 2019, 28: 2543–2550. [DOI] [PubMed] [Google Scholar]
- 15. Ahn Y, Lee HY, Lee SH, Lee JH. Dural tears in percutaneous endoscopic lumbar discectomy. Eur Spine J, 2011, 20: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single‐center experience of 10,228 cases. Neurosurgery, 2015, 76: 372–380. [DOI] [PubMed] [Google Scholar]
- 17. Wu J, Zhang C, Lu K, Li C, Zhou Y. Percutaneous endoscopic lumbar reoperation for recurrent sciatica symptoms: a retrospective analysis of outcomes and prognostic factors in 94 patients. World Neurosurg, 2018, 109: e761–e769. [DOI] [PubMed] [Google Scholar]
- 18. Ikuta K, Tarukado K, Masuda K. Characterization and risk factor analysis for recurrence following microendoscopic Diskectomy for lumbar disk herniation. J Neurol Surg A, 2017, 78: 154–160. [DOI] [PubMed] [Google Scholar]
- 19. Yoshimoto M, Iwase T, Takebayashi T, Ida K, Yamashita T. Microendoscopic discectomy for far lateral lumbar disk herniation: less surgical invasiveness and minimum 2‐year follow‐up results. J Spinal Disord Tech, 2014, 27: E1–E7. [DOI] [PubMed] [Google Scholar]
- 20. Serhan HA, Varnavas G, Dooris AP, Patwadhan A, Tzermiadianos M. Biomechanics of the posterior lumbar articulating elements. Neurosurg Focus, 2007, 22: E1. [DOI] [PubMed] [Google Scholar]
- 21. Zheng C, Wu F, Cai L. Transforaminal percutaneous endoscopic discectomy in the treatment of far‐lateral lumbar disc herniations in children. Int Orthop, 2016, 40: 1099–1102. [DOI] [PubMed] [Google Scholar]
- 22. Kashlan ON, Kim HS, Khalsa SSS, et al. Percutaneous endoscopic Transforaminal approach for far lateral lumbar discectomy: 2‐dimensional operative video. Oper Neurosurg, 2020, 18: E8. [DOI] [PubMed] [Google Scholar]
- 23. Teske W, Boudelal R, Zirke S, von Schulze PC, Wiese M, Lahner M. Anatomical study of preganglionic spinal nerve and disc relation at different lumbar levels: special aspect for microscopic spine surgery. Technol Health Care, 2015, 23: 343–350. [DOI] [PubMed] [Google Scholar]
- 24. Suh SW, Shingade VU, Lee SH, Bae JH, Park CE, Song JY. Origin of lumbar spinal roots and their relationship to intervertebral discs: a cadaver and radiological study. J Bone Joint Surg, 2005, 87: 518–522. [DOI] [PubMed] [Google Scholar]
- 25. Klinger N, Yilmaz E, Halalmeh DR, Tubbs RS, Moisi MD. Reattachment of the Multifidus tendon in lumbar surgery to decrease postoperative Back pain: a technical note. Cureus, 2019, 11: e6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phan K, Teng I, Schultz K, Mobbs RJ. Treatment of lumbar spinal stenosis by microscopic unilateral laminectomy for bilateral decompression: a technical note. Orthop Surg, 2017, 9: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rong L. Percutaneous transforaminal endoscopic discectomy compared with microendoscopic discectomy for lumbar disc herniation: 1‐year results of an ongoing randomized controlled trial. J Neurosurg Spine, 2019, 26: 1–3. [DOI] [PubMed] [Google Scholar]
- 28. Feng F, Xu Q, Yan F, et al. Comparison of 7 surgical interventions for lumbar disc herniation: a network meta‐analysis. Pain Physician, 2017, 20: E863–E871. [PubMed] [Google Scholar]


