Abstract
Objective
Physical exercise has obvious effects on bone loss, pain relief, and improvement of bone metabolism indexes in patients with osteoporosis, but currently lacks sufficient evidence. The aim of this systematic review and meta‐analysis was to synthesize and present the best available evidence on the effectiveness and safety of exercises in the treatment of primary osteoporosis.
Methods
Publications pertaining to the effectiveness of exercise on bone mineral density (BMD), visual analog scores (VAS), and biochemical markers of bone metabolism in primary osteoporosis (POP) from PubMed, Cochrane Library, Embase, VIP, CNKI, and Wanfang Database were retrieved from their inception to April 2020.
Results
A total of 20 studies with 1824 participants were included. The results of the meta‐analysis revealed that exercise therapy for lumbar spine and femoral neck BMD is statistically different from conventional therapy (lumbar spine BMD: SMD = 0.78, 95%CI: 0.46, 1.10, P < 0.00001, I 2 = 85%; femoral neck BMD (SMD = 0.80, 95%CI: 0.34, 1.27, P = 0.0007, I 2 = 88%), exercise therapy can significantly increase the lumbar spine BMD of patients with OP, especially in lumbar spine2‐4 BMD (SMD = 0.47; 95%CI: 0.20, 0.75; P = 0.0008; I 2 = 69%). Compared with conventional treatment, kinesitherapy also has significant differences in alleviating the pain of POP patients (SMD = −1.39, 95%CI: −2.47,−0.31, P = 0.01, I 2 = 97%). Compared with conventional therapy, kinesitherapy has no significant difference in improving biochemical markers of bone metabolism such as bone glaprotein (BGP) (SMD = 2.59, 95%CI:0.90, 4.28, P = 0.003, I 2 = 98%), N‐terminal pro peptide of type I procollagen (PINP) (SMD = 0.77, 95%CI: −0.44 to 1.98, P = 0.21, I 2 = 95%), serum phosphorus (SMD = 0.04, 95%CI: −0.13, 0.22, P = 0.61, I 2 = 30%), alkaline phosphatase (ALP) (SMD = −0.08, 95%CI: −0.44, 0.27, P = 0.64, I 2 = 76%), and serum calcium (SMD = 0.12, 95%CI: −0.18, 0.43, P = 0.42, I 2 = 63%) in POP patients.
Conclusions
Kinesitherapy significantly improved lumbar spine and femoral neck BMD, and relieve the pain of patients in the current low‐quality evidence. Additional high‐quality evidence is required to confirm the effect of exercise therapy on the biochemical markers of bone metabolism in POP patients.
Keywords: Bone metabolism, Bone mineral density, Kinesitherapy, Primary osteoporosis
The results of this meta‐analysis show that different types of exercise therapies significantly improved lumbar spine and femoral neck BMD, and relieve the pain of patients in the current low‐quality evidence. Additional high‐quality evidence is required to confirm the effect of exercise therapy on the biochemical markers of bone metabolism in POP patients. Therefore, more multicenters, larger samples, long‐term, single‐blind RCTs are needed to evaluate the effects of exercise therapy on BMD, VAS and biochemical markers of bone metabolism in POP patients.
Introduction
Primary Osteoporosis (POP) is a skeletal disorder bone disease characterized by imbalanced bone metabolism, decreased bone mass, and increased risk of fractures 1 , 2 . This disease is more common in postmenopausal women, and the incidence increases with age. Approximately 200 million people around the world are affected by POP, which contributes to gradually loss of independence, produce physical pain, and bring a huge social burden 3 , 4 . The menopause of Western women generally arrives at around 50 years old, while Asian women can advance to 42 years old 5 . With the onset of menopause, the equilibrium between bone formation and bone resorption is broken in postmenopausal women due to the estrogen deficient in the body 6 , 7 . Therefore, postmenopausal women are at high risk of developing OP and at a risk for fractures due to rapid bone loss, which is particularly evident in trabecular bone 7 .
The treatment of POP mainly includes anti‐catabolic agents, bone formation and dual mechanism drugs, which by inhibiting bone resorption and reducing bone turnover, effectively maintenance of bone mineral density (BMD) and achieve better therapeutic effects, yet osteoporosis cannot be completely cured with the available drugs 8 , Drugs including estrogen, calcitonin, raloxifene and bisphosphonates are often used clinically. For patients with POP, a lasting 3 to 5 years course of anti‐catabolic treatment is common, and for patients with high‐risk osteoporotic fractures, such treatment may take up to 10 years. However, people are beginning to worry about the adverse effects of long‐term use of these drugs. According to reports, chronic use of bisphosphonates in patients with POP is responsible for musculoskeletal pain, hypocalcemia and secondary hyperparathyroidism, atypical femoral fractures, nephrotoxicity, and osteonecrosis of the jaw (ONJ) and severely inhibit bone turnover 9 , 10 , 11 , 12 , 13 , 14 . Therefore, finding a supplement strategy that with good effect, small side effects, even without side effects, and long‐term use, is critical, and it has gradually attracted the attention of researchers.
Previous studies have concluded that bone is responsive to mechanical loading, which acts on the bones through both uses muscle forces and ground reaction forces 15 . These forces increase the density and strength of bone minerals, which may be one of the main reasons that exercise can improve bone health. Because these forces have a pro‐osteogenic effect and not have adverse side effects, physical exercise is widely recommended to prevent OP 16 . Study have shown that exercising during the growth phase can increase peak bone mass, thereby reducing the risk of fractures in advanced age 16 .
Hence, the objective of this systematic review and meta‐analysis was to synthesize and present the best available evidence on the effectiveness and safety of exercises in the treatment of primary osteoporosis.
Methods
This study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis Guidelines 17 . The protocol was registered in the International Prospective Register of Systematic Reviews, PROSPERO (registration number: CRD42020175396).
Searching Strategies
We performed a literature search on Pubmed, the Cochrane Library, Embase, the China National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Science and Technology Periodical Database (VIP) from their inceptions to April 2020.The Medical Subject Headings (MeSH) and non‐MeSH used in the present study included “kinesitherapy”, “exercise”, “physical exercise”, “athletic sports”, “sport”, and “osteoporosis”, “primary osteoporosis”, “postmenopausal osteoporosis”, and “randomized controlled trial”, “controlled clinical trial”, “randomized”, “trial”. variations of different terms were used for a systematic search. The detailed search strategy for Pubmed is presented in Appendix A. Similar search combinations were used for particular databases. The reference lists of all relevant articles were reviewed to identify potential missed studies.
Inclusion and Exclusion Criteria
Eligible studies will be included if it met the following inclusion criteria: (i) patients with primary osteoporosis (there will be no restriction on sex, age, or intensity or duration of symptoms); (ii) the intervention is only any types of physical exercise or exercise combined with conventional oral medicine (referring to routine activities that enhance physical health and improve health); (iii) the control group was conventional oral drug treatment; (iv) the literature studies included were randomized controlled trials of different types of exercise in the treatment of primary osteoporosis, whether published or not. The language was limited to Chinese or English.
Exclusion criteria as follows: non‐randomized controlled trials, case series, case reports, animal experiment and crossover studies were excluded. Reviews were screened to check for potential additional studies that were not published as standalone papers.
Selecting Process
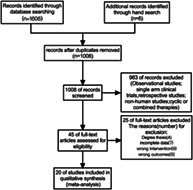
The selection process of included articles followed a consensus‐based approach. Yan Yan and Biao Tan reviewed all eligible articles separately, and the selection of included articles was based on their consensus. Fanyu Fu was invited for further consultation when there were divergences. Key design information, basic characteristics of the participants were extracted into a standardized evidence table. A total of 1612 articles were identified, of these 1008 were excluded due to duplicate data, and 559 were excluded upon title and abstract. Forty‐five articles remained for further evaluation. Finally 20 articles were included in the present meta‐analysis. The flow chart following PRISMA provided in Fig. 1.
Fig 1.
Study selection flow diagram.
Outcome Measures
Main Outcome Indicators
The main outcome we are interested in was the changes in BMD and visual analog scores (VAS) in patients with primary osteoporosis after exercise therapy.
The BMD was an absolute value, which is an important indicator of bone strength. It is expressed in grams per cubic centimeter. In clinical practice, dual‐energy X‐ray absorptiometry (DEXA) was usually used to measure bone mineral density. In the clinical practice, since the absolute values of different BMD testers are different, the T value used to determine whether the BMD is normal. T was a relative value, and its normal reference was between −1 and +1. When its lower than −2.5, it represents an abnormality.
The VAS was used for pain assessment. It is widely used in clinical practice. The basic method was to use a moving ruler with a length of about 10 cm. One side is marked with 10 scales. The two ends are respectively “0” and “10” points. Zero points means no pain, 10 points Points represent the most severe pain that is unbearable.
Secondary Outcome Indicators
Secondary outcomes were change in biochemical markers of bone metabolism (includes bone glaprotein (BGP), N‐terminal pro peptide of type I procollagen (PINP), serum phosphorus, alkaline phosphatase (ALP), serum calcium and tartrate‐resistant acid phosphatase (TRAP)).
Bone Glaprotein (BGP)
BGP is a specific non‐collagen bone matrix protein synthesized and secreted by osteoblasts in the non‐proliferative period. It is the main component of non‐collagenous protein in bone tissue. It is composed of 49 amino acids and can maintain the mineralization rate of bone, and is a functionally sensitive marker of osteoblasts. The monoclonal antibody RIA assay is usually used to detect BGP. BGP directly reflects the activity of osteoblasts and bone formation.
N‐terminal Pro Peptide of Type I Procollagen (PINP)
The type I collagen gene translates the pre‐α peptide chain in osteoblasts to form procollagen, and its N‐terminal and excess peptide chain are cut off and converted into PINP, and usually detected by immunoluminescence. It is a specific and sensitive indicator of bone formation.
Serum Phosphorus
Serum phosphorus refers to the inorganic phosphorus in human blood, which exists in the form of inorganic phosphate, such as Na2HPO4, NaH2PO4, CaHPO4, MgHPO4 and so on. Methods for detecting serum phosphorus include phosphomolybdic acid method, dye method and enzymatic method. When serum phosphorus is reduced, bone absorption can be promoted, otherwise it can promote bone formation.
Alkaline Phosphatase (ALP)
ALP is an extracellular enzyme, glycoprotein of osteoblasts. It mainly hydrolyzes phosphatase during the bone formation process to provide phosphoric acid for the deposition of hydroxyapatite. Determination of ALP is by using the phenyl disodium phosphate colorimetric method. ALP levels have a linear relationship with the activity of osteoblasts and pre‐osteoblasts, and are considered to be the most accurate markers of bone formation.
Serum Calcium
Calcium in plasma exists in two forms, ionized calcium and bound calcium, each accounting for about 50%. Serum calcium levels are related to many important functions of the human body. Serum total calcium was measured by spectrophotometry, while serum ionized calcium was measured by ion‐selective electrode method.
Tartrate‐Resistant Acid Phosphatase (TRAP)
TRAP is a single isoenzyme encoded by a gene located at P13.2–13.3 on chromosome 19. Increased TRAP may indicate primary osteoporosis, chronic renal insufficiency, metabolic bone disease, etc.; decreased TRAP may indicate hypoparathyroidism. Enzyme kinetics, electrophoresis, etc. are usually used to determine TRAP.
Data Extraction and Management
Two researchers used Endnote version X9 (Thomson Corporation, Stanford, CT, USA) to make a preliminary assessment of the title and abstract of each document in the database based on the established criteria for inclusion in the study to select eligible studies. After a preliminary evaluation, the full text of the selected literature will be evaluated, and non‐controlled studies will be excluded, no random grouping, inconsistent evaluation criteria, and similar data. To reach consensus, any screening differences that occurred during the screening study will be discussed, and if still not resolved, a third researcher will be involved. Two researchers independently extracted information from the included literature. The extracted contents included study design, random concealment and blindness, including basic information of the case, intervention methods, observation indicators and test results in the treatment group and control group. Extracted bibliographic data will be populated into a unified data statistics table. For studies that provide baseline and post‐treatment data, we will estimate the change values by the method recommended by Cochrane. If data loss occurs while screening and extracting literature data, first of all, we will actively look for the cause of the loss, and then contact the experimental research author by phone, email, etc., to retrieve the lost data. If it is impossible to retrieve the missing data, we will extract and analyze only the useful data and indicate the situation.
Quality Assessment
The scale recommended by the Cochrane Collaboration 18 was used to assess the methodological quality of identified studies as well as the risk of bias of the individual included. Two independent researchers evaluated the quality of the literature based on the Cochrane Collaboration's bias risk tool. Details are as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting. The results for each domain will be divided into three levels: low risk of bias, high risk of bias, and unclear risk of bias.
Data Synthesis and Statistical Analysis
Data analysis using RevMan version 5.3 (The Cochrane Collaboration, London, UK). Continuous outcomes were pooled to find the standard mean difference (SMD) and were accompanied by 95% confidence intervals (CIs). Categorical outcomes were pooled to find relative risks (RRs) and were accompanied by 95% CIs. I2 statistics were used to measure heterogeneity. The fixed effects model is appropriate when there is statistical heterogeneity (I2 <50%). Otherwise, the random effects model (I2 ≥ 50%) is used and publication bias is explored through funnel plot analysis.
Results
Study Characteristics
A total of 1612 studies were identified through searches, of which 1008 records remained after removal of duplicates. After screening via titles and abstracts, 45 articles remained for further evaluation. Following further evaluation, 25 articles were excluded for the following reasons: degree thesis; incomplete data; wrong intervention; wrong outcomes. Therefore, a total of 20 clinical trials published between 2003 and 2020 were included in the current meta‐analysis.
The original study included, almost all studies from China with only one from Germany 38 , the maximum number of patients included in a single study was 200, and the minimum was 30, representing data on 1824 subjects, 925 of them treated with different exercise. These exercise include aerobic, Tai Chi, Baduan Jin, Wu Qin Xi, core strength training, progressive resistance exercise, regular exercise, etc. In order to facilitate the subgroup analysis, we classified these exercise methods and divided them into traditional Chinese health exercises, aerobic exercises, core strength exercises, squaredance, mountaineering, and aerobics with other exercises. The duration of exercise in each study was different, so and the intervention period of exercise. Detailed information of the included studies is summarized in Table 1.
TABLE 1.
Characteristics of the included studies
| Reference | Region | Sample size(EG/CG) | Age (years) | Interventions | Outcomes | Blinding | Drop‐out number | |||
|---|---|---|---|---|---|---|---|---|---|---|
| EG vs. CG | Exercise time | Exercise frequency | Intervention period | |||||||
| Zhou et al. 19 | China | 82/82 |
EG: 51. 51 ± 2. 81 CG: 50. 21 ± 3. 81 |
Exercise+oral drugs vs Oral drugs | 30–60 min | Three times per week | 12 months | BMD, P, Ca, ALP | NI | NI |
| Li Rui et al. 20 | China | 26/26 |
EG: 55.46 ± 4.12 CG: 56.25 ± 3.75 |
Exercise+oral drugs vs Oral drugs | 30 min | Four times per week | 3 months | BMD, VAS, PINP, ALP, TNF‐a, TRAP, IL‐6 | NI | NI |
| Xiao et al. 21 | China | 35/35 |
EG: 58.43 ± 4.87 CG: 57.68 ± 5.29 |
Progressive resistive exercises+oral drugs vs Oral drugs | 15 times | Once per week | 6 months | VAS, PINP, TRAP | NI | NI |
| Li Ningjian et al. 22 | China | 96/96 |
EG: 64.14 ± 8.27 CG: 65.34 ± 8.13 |
Aerobic exercise+Tai Chi+oral drugs vs oral drugs | 30–60 min | Six times per week | 12 months | BMD, VAS, BGP | NI | NI |
| Kuang 23 | China | 41/41 |
EG: 68.68 ± 3.22 CG: 70.33 ± 3.34 |
Ba duan jin + oral drugs vs Oral drugs | 120 min | NI | 6 months | BMD, VAS | NI | NI |
| Li et al. 24 | China | 15/15 |
EG: 58.84 ± 4.12 CG: 58.46 ± 3.65 |
Progressive resistive exercises+oral drugs vs Oral drugs | 60 times | Once per week | 3 months | PINP, TRAP | NI | NI |
| Sun et al. 25 | China | 22/22 |
EG: 65.73 ± 2.46 CG: 65.98 ± 3.58 |
Core strength training+oral drugs vs Oral drugs | 60 min | Three times per week | 24 weeks | BMD, VAS | NI | NI |
| Qin et al. 26 | China | 25/25 | 45–60 | Squaredance+oral drugs vs Oral drugs | 30–60 min | Five times per week | NI | BMD, P, PINP, Ca, ALP | NI | NI |
| Li et al. 27 | China | 30/30 |
EG: 55.03 ± 5.71 CG: 55.10 ± 6.52 |
Wu qin xi+oral drugs vs Oral drugs | 30–60 min | Five to seven times per week | 6 months | BMD, VAS, PINP | NI | NI |
| Shen et al. 28 | China | 100/100 |
EG: 68.69 ± 5.18 CG: 69.25 ± 5.27 |
Wu qin xi+oral drugs vs Oral drugs | 45 min | Six times per week | 6 months | BGP, P, Ca, ALP, PYD/Cr | NI | 12 |
| Shen et al. 29 | China | 100/100 |
EG: 62 ± 5.0 CG: 64 ± 5.3 |
Wu qin xi+oral drugs vs Oral drugs | 45 min | Six times per week | 6 months | BMD, VAS | NI | NI |
| Shi et al. 30 | China | 40/42 |
EG: 68.69 ± 5.18 CG: 69.25 ± 5.27 |
Five elements of aerobics+oral drugs vs Oral drugs | 30‐45min | NI | 3 months | P, Ca, ALP | NI | 12 |
| Chen and Liu 31 | China | 60/60 |
EG: 64.5 ± 15.5 CG: 65.3 ± 14.7 |
Exercise training+oral drugs vs Oral drugs | 30‐50min | Once per day | NI | BMD | NI | NI |
| Liu 32 | China | 30/30 |
EG: 62.5 ± 2.45 CG: 64.5 ± 2.45 |
Ba duan jin + oral drugs vs Oral drugs | 60 min | Six times per week | 6 months | BMD | NI | NI |
| Li et al. 33 | China | 38/32 | 56.3 ± 2.1 | Exercise training+oral drugs vs Oral drugs |
Run:20min Push ups: 30 times Drafting exercise:30s Abdominal muscle isometric exercise:30s |
Once every four days | 6 months | BMD | NI | NI |
| Song 34 | China | 20/20 |
EG: 62.67 ± 11.23 CG: 63.81 ± 13.07 |
Tai Chi+oral drugs vs Oral drugs | 60 min | Six times per week | NI | BMD, VAS, BGP, P, Ca, ALP | NI | NI |
| Liu et al. 35 | China | 36/32 | 56 0.3 ± 2.1 | Exercise training+oral drugs vs Oral drugs |
Run:20min Push ups: 30 times Drafting exercise:30s Abdominal muscle isometric exercise:30s |
Once every three days | 6 months | BMD | NI | NI |
| Zhu et al. 36 | China | 48/48 | 67.1 ± 7.2 | outdoor exercise+oral drugs vs Oral drugs | 30–60 min | Three to five times per week | 12 months | BMD, BGP | NI | NI |
| Gong et al. 37 | China | 22/22 |
EG: 61.25 ± 6.90 CG: 62.14 ± 7.03 |
Mountaineering+oral drugs vs Oral drugs | 90‐120min | Five to seven times per week | 12 months | BMD | NI | NI |
| Wolfgang Kemmler et al. 38 | Germany | 59/41 |
EG: 55.1 ± 3.4 CG: 55.9 ± 3.1 |
Exercise training+oral drugs vs Oral drugs | 85–95 min | Four times per week | 14 months | BMD | NI | NI |
ALP, alkaline phosphatase; BMD, bone mineral density; Ca, calcium; CG, control group; EG, exercise group; IL‐6; interleukin‐6; BGP, bone glaprotein; NI, no information; P, phosphorus; PINP, N‐terminal pro peptide of type I procollagen; PYD/Cr, pyridinoline/creatinine; TNF‐a, tumor necrosis factor‐a; TRAP, tartrate resistant acid phosphatase; VAS, visual analog scale.
Risk of Bias Assessment
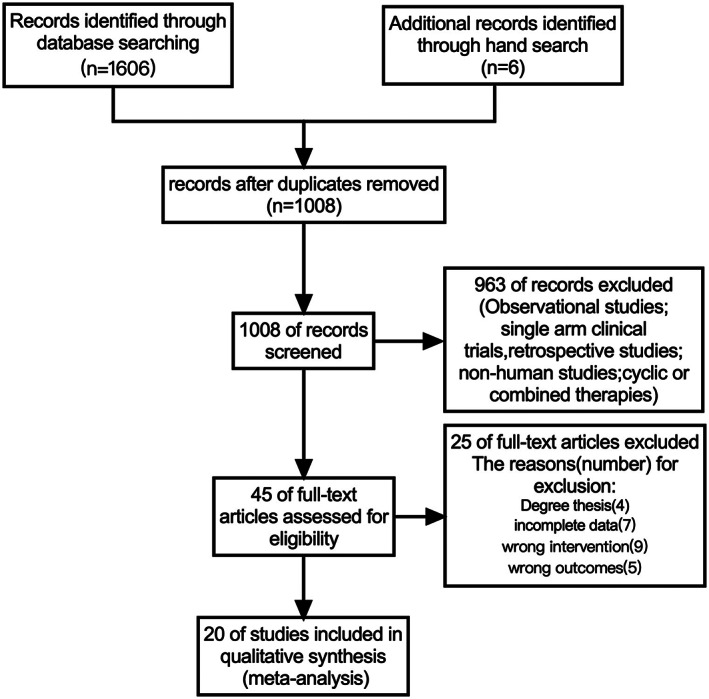
In general, the included studies had a substantial risk of bias. As shown in Fig. 2, all of the RCTs provide the generation of random sequences. Seven trials involve allocation concealment. However, the blind intervention associated with the intervention exercises cannot be implemented blindly. The blinding of outcome assessment of all studies was unclear. There were no dropouts indicated or explanations for withdrawal in the 20 studies.
Fig 2.
Risk of bias summary for each included study.
Results of Meta‐Analysis
The results of the Meta analysis with six outcome indicators show a high degree of heterogeneity among the studies (I2 values are all over 70%), In order to explore the possible causes of heterogeneity, according to some research characteristics (different types of exercise) that may cause heterogeneity, this study subgroup analyzes the six outcome indicators of lumbar spine BMD, Femoral Neck BMD, VAS, BGP, PINP and serum calcium.
Meta‐Analysis of Lumbar Spine Bone Mineral Density (BMD)
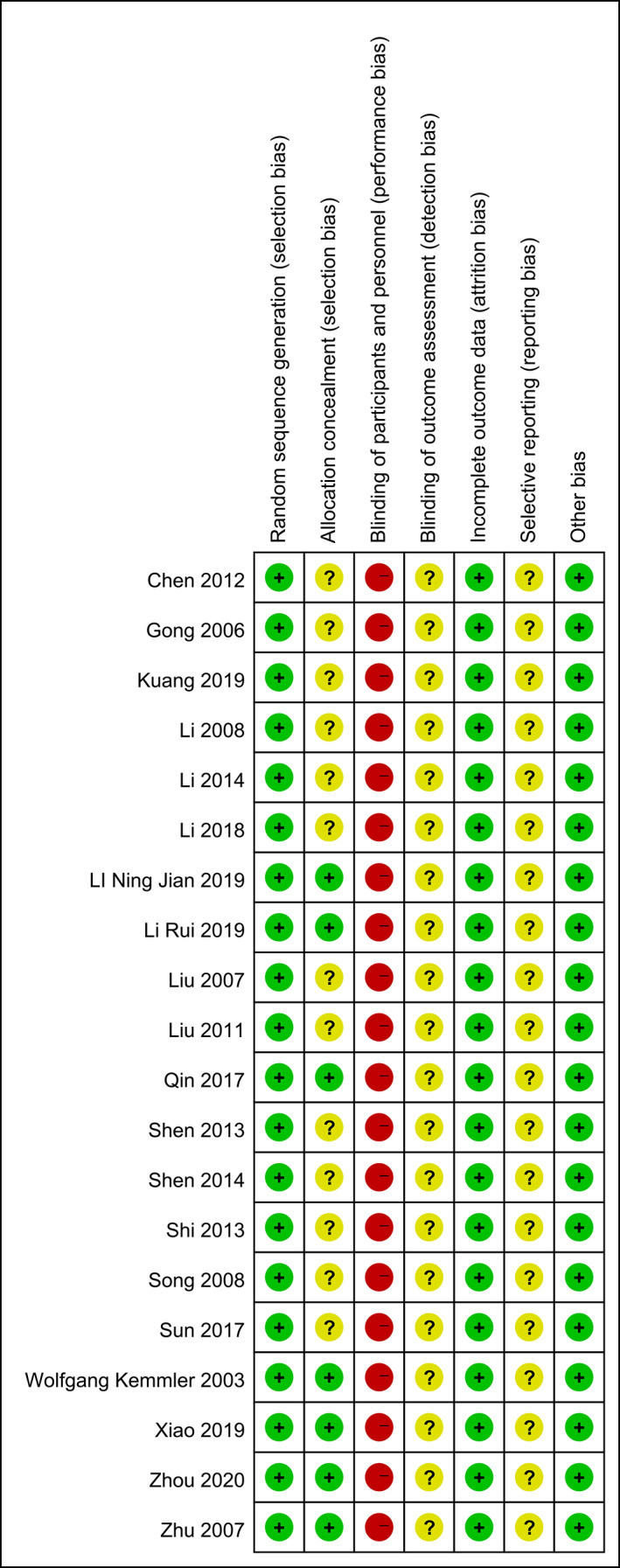
Thirteen trials 19 , 22 , 23 , 25 , 26 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 involving 1130 participants compared effect of exercises with conventional treatment on lumbar spine BMD. The meta‐analysis revealed a significant antiosteoporosis effect on lumbar Spine BMD (SMD = 0.70; 95%CI: 0.37, 1.02; P < 0.00001) but with high heterogeneity (I 2 = 85%), the results of subgroup analysis show that the same type of exercise group has low heterogeneity (Fig. 3), funnel plot of publication bias showed in Fig. 4.
Fig 3.
Forest plot of effects of exercises on lumbar spine BMD.
Fig 4.
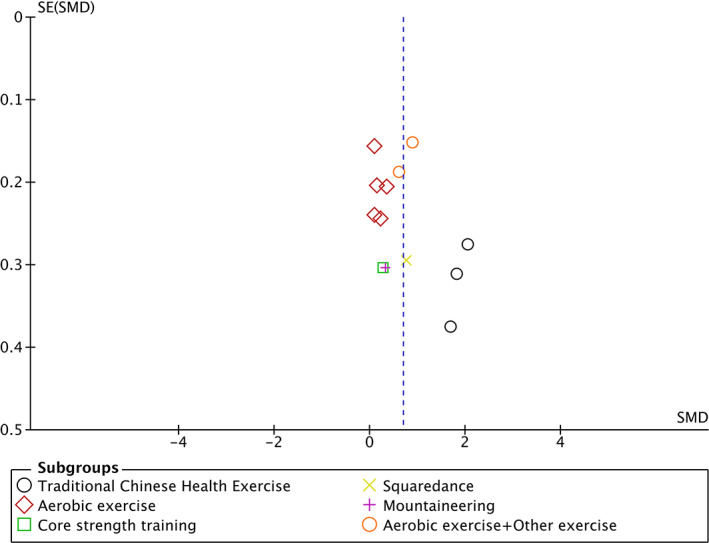
Funnel plot of publication bias.
Meta‐Analysis of Lumbar Spine2‐4 Bone Mineral Density (BMD)
Seven trials 19 , 22 , 25 , 26 , 29 , 37 , 38 involving 782 participants compared effect of exercises with conventional treatment on lumbar spine2‐4 BMD. The meta‐analysis revealed a significant antiosteoporosis effect on lumbar spine2‐4 BMD (SMD = 0.47; 95%CI: 0.20, 0.75; P = 0.0008; I 2 = 69%), The results of subgroup analysis show that the same type of exercise group has low heterogeneity (Fig. 5).
Fig 5.
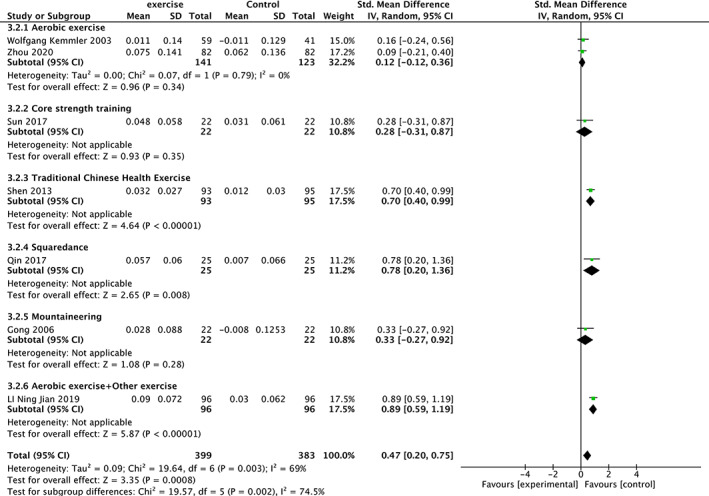
Forest plot of effects of exercises on lumbar spine2‐4 BMD.
Meta‐Analysis of Femoral Neck Bone Mineral Density (BMD)
Nine trials 19 , 20 , 22 , 25 , 26 , 32 , 34 , 37 , 38 involving 746 participants compared effect of exercises with conventional treatment on femoral neck BMD. The meta‐analysis revealed a significant antiosteoporosis effect on femoral neck BMD (SMD = 0.80, 95%CI: 0.34, 1.27, P = 0.0007) but with high heterogeneity (I 2 = 88%). It showed low heterogeneity(I 2 = 24%) after the sensitivity analysis when three studies were removed 26 , 32 , 34 . The results of subgroup analysis show that the same type of exercise group has low heterogeneity (Fig. 6).
Fig 6.
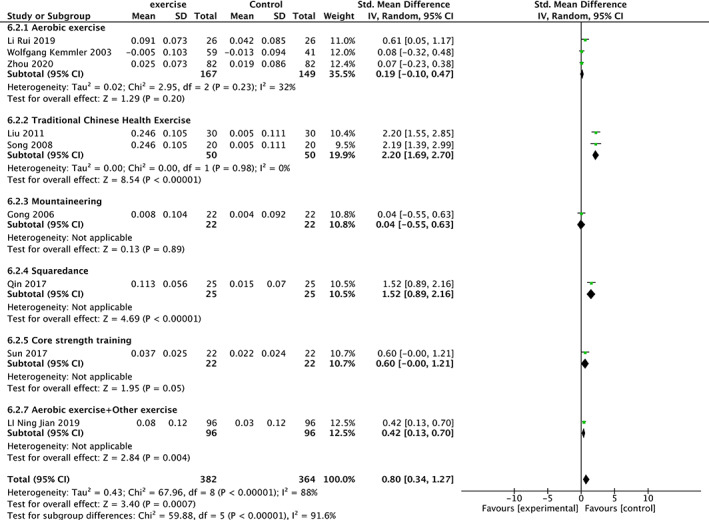
Forest plot of effects of exercises on Femoral Neck BMD.
Meta‐Analysis of Visual Analog Scores (VAS)
Eight trials 20 , 21 , 22 , 23 , 25 , 27 , 29 , 34 involving 720 participants compared effect of exercises with conventional treatment on VAS. The meta‐analysis showed a significant reduction in VAS score (SMD = −1.39; 95%CI: −2.47,−0.31; P = 0.01) but with high heterogeneity (I 2 = 97%). It showed low heterogeneity (I 2 = 31%) after the sensitivity analysis when two studies were removed 22 , 25 . The results of subgroup analysis show that the same type of exercise group has low heterogeneity (Fig. 7).
Fig 7.
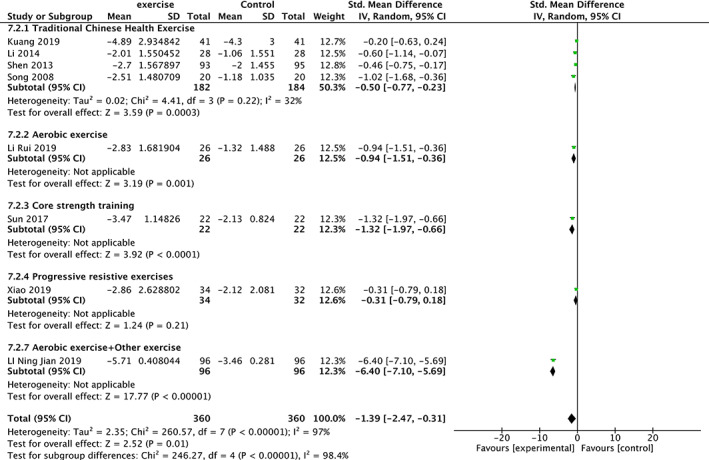
Forest plot of effects of exercises on VAS level.
Meta‐analysis of Biochemical Markers of Bone Metabolism
Meta‐analysis of Bone Glaprotein (BGP)
Four trials 22 , 28 , 34 , 36 involving 516 participants compared effect of exercises with conventional treatment on BGP. The meta‐analysis showed that BGP was not significantly increased (SMD = 2.59, 95%CI: 0.90,4.28, P = 0.003) but with high heterogeneity (I 2 = 98%). It showed low heterogeneity(I 2 = 0%) after the sensitivity analysis when one study was removed 36 . The results of subgroup analysis show that the same type of exercise group has low heterogeneity (Fig. 8).
Fig 8.
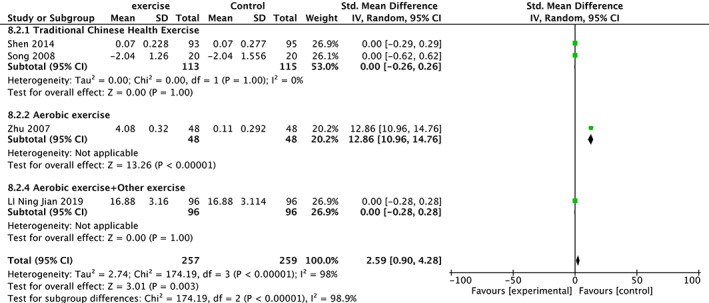
Forest plot of effects of exercises on BGP level.
Meta‐analysis of N‐Terminal Pro Peptide of Type I Procollagen (PINP)
Five trials 20 , 21 , 24 , 26 , 27 involving 254 participants compared effect of exercises with conventional treatment on N‐terminal pro peptide of type I procollagen (PINP). The meta‐analysis showed that PINP was not significantly increased (SMD = 0.77, 95%CI: −0.44,1.98, P = 0.21) but with high heterogeneity (I 2 = 95%). It showed low heterogeneity (I 2 = 0%) after the sensitivity analysis when one study was removed 26 . The results of subgroup analysis show that the same type of exercise group has low heterogeneity (Fig. 9).
Fig 9.
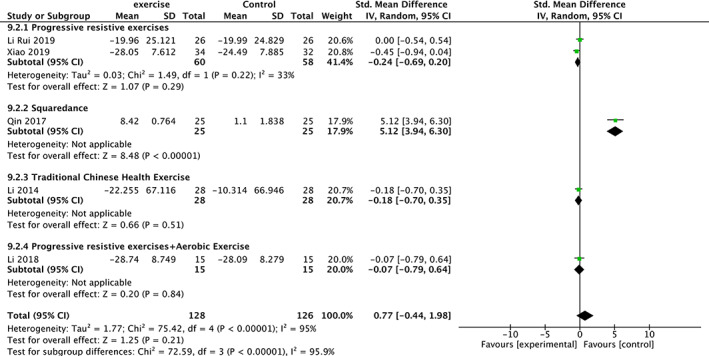
Forest plot of effects of exercises on PINP level.
Meta‐analysis of Serum Phosphorus
Five trials 19 , 26 , 28 , 30 , 34 involving 524 participants compared effect of exercises with conventional treatment on serum phosphorus. The meta‐analysis showed that serum phosphorus was not significantly increased (SMD = 0.04, 95%CI: −0.13,0.22, P = 0.61, I 2 = 30%) (Fig. 10).
Fig 10.
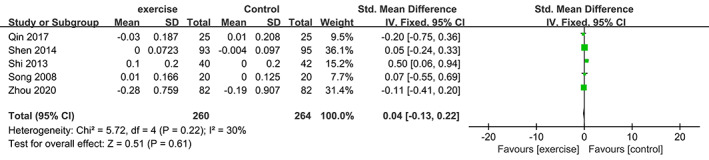
Forest plot of effects of exercises on serum phosphorus level.
Meta‐analysis of Alkaline Phosphatase (ALP)
Six trials 19 , 20 , 26 , 28 , 30 , 34 involving 576 participants compared effect of exercises with conventional treatment on alkaline phosphatase (ALP). The meta‐analysis showed that ALP was not significantly increased (SMD = −0.08, 95%CI: −0.44,0.27, P = 0.64) but with high heterogeneity (I 2 = 76%). It showed low heterogeneity(I 2 = 0%) after the sensitivity analysis when one study was removed 19 . The results of subgroup analysis show that the same type of exercise group has low heterogeneity (Fig. 11).
Fig 11.
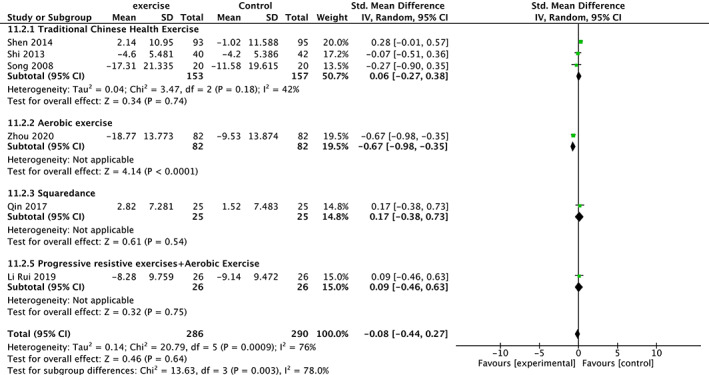
Forest plot of effects of exercises on ALP level.
Meta‐analysis of Serum Calcium
Five trials 19 , 26 , 28 , 30 , 34 involving 524 participants compared effect of exercises with conventional treatment on serum calcium. The meta‐analysis showed that serum calcium was not significantly increased (SMD = 0.12, 95%CI: −0.18,0.43, P = 0.42) but with high heterogeneity (I 2 = 63%). It showed low heterogeneity(I 2 = 0%) after the sensitivity analysis when two studies were removed 19 , 28 . The results of subgroup analysis show that the same type of exercise group has low heterogeneity (Fig. 12).
Fig 12.
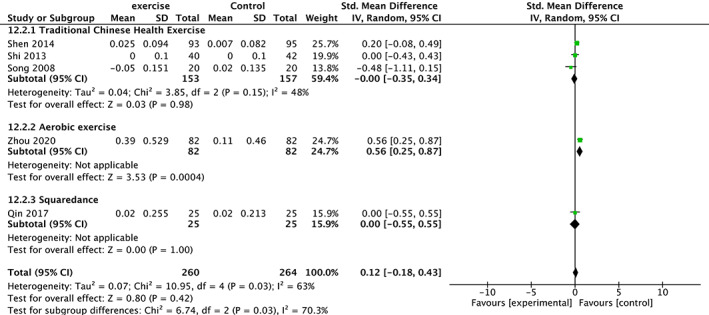
Forest plot of effects of exercises on serum calcium level.
Meta‐analysis of Tartrate‐Resistant Acid Phosphatase (TRAP)
Three trials 20 , 21 , 24 involving 148 participants compared effect of exercises with conventional treatment on TRAP. The meta‐analysis showed that the TRAP value of the exercise group was significantly reduced (SMD = −0.93, 95% CI: −1.27 to 0.59, P < 0.00001, I 2 = 0%) (Fig. 13).
Fig 13.
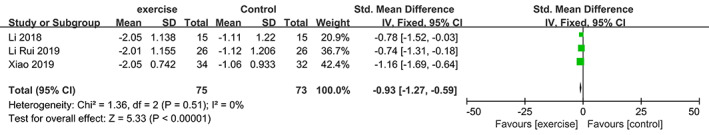
Forest plot of effects of exercises on TRAP level.
Discussion
POP is a global health problem that is getting more and more attention. The population it mainly affects is postmenopausal women and the elderly. In addition, POP is usually taken the blame for physical weakness, increased risk of falls, a large number of illnesses, deaths and decreased quality of life 39 . The current treatment for POP can be divided into two categories: nonpharmacological therapy and pharmacological treatment, which nonpharmacological therapy includes keep a healthy diet, prevent falls and regular physical exercise, pharmacological treatment involves calcium, vitamin D, and drugs that can activate bone tissue (such as anti‐resorption agents, bone forming agents and Mixtures) 40 . A recently updated Endocrine Society Clinical Practice Guideline also recommends romosozumab as a pharmacological treatment for POP 41 . However, as far as we know, whether it is calcium or bisphosphonates or selective estrogen receptor modulators, including the recently introduced denosumab, corresponding adverse events have been reported 42 . In addition, exercise as a nonpharmacological therapy without adverse effects is considered important for maintaining bone health, people with OP are strongly recommended to participate in various forms of exercise regularly 43 . Several studies have confirmed that exercise can increase the BMD of the femoral neck and lumbar spine in elderly patients with OP 44 , 45 .
This review is a relatively comprehensive systematic review and meta‐analysis over the past two years to evaluate the effects of different types of exercise therapy on BMD of lumbar spine and femoral neck, VAS scores and biochemical markers of bone metabolism in patients with POP from RCTs. Compared with some previous systematic reviews that only focus on whether exercise can improve the function 46 or BMD 47 of patients with POP, this systematic review is more comprehensive. The most important thing is to add bone metabolism indicators as one of the outcome indicators. This study involved 20 RCTs that included a total of 1824 subjects with POP. The outcome measure primarily consisted of BMD, VAS, and biochemical markers of bone metabolism. The results of the meta‐analysis showed that exercise therapy for lumbar spine and femoral neck BMD is statistically different from conventional drug therapy, exercise therapy can significantly increase the lumbar spine BMD of patients with OP, especially in lumbar spine2‐4 BMD. This result is also similar to the previously reported meta‐analysis 47 . But, according to the guidelines 48 , the changes in bone turnover markers predate BMD, and BMD should be measured one year after the start of treatment. However, in the studies we included, there are twelve RCTs 20 , 21 , 23 , 24 , 25 , 27 , 28 , 29 , 30 , 32 , 33 , 35 that took less than 1 year to measure BMD, and even three months 20 , 24 , 30 . Compared with conventional drug treatment, kinesitherapy also has significant differences in alleviating the pain (evaluation index: VAS score) of POP patients. Although the results of the meta‐analysis show that exercise therapy has a positive effect on reducing the pain of POP patients, its mechanism of action needs further research.
However, we failed to find that compared with conventional drug therapy, kinesitherapy has significant difference in improving biochemical markers of bone metabolism such as BGP, serum phosphorus, and serum calcium in POP patients, this may indicate that exercise therapy and conventional drug therapy have little difference in the impact of POP patients on biochemical markers of bone metabolism. This conclusion seems to be similar to previous animal experiments 49 , but at the same time we have also seen reports that exercise is helpful for bone metabolites 50 . Due to the limitation of the outcome indicators of the original study, the bone resorption markers recommended by the guidelines, such as serum C‐terminal telopeptide of type 1 collagen (CTX), were not reported in the original study, so analysis could not be performed. Only three studies reported on the tartrate‐resistant acid phosphatase (TRAP) index, Although this marker showed positive significance in the exercise group, it is still not convincing overall. Therefore, we believe that the existing RCTs do not have much reference to authoritative guidelines in the design of the scheme, resulting in some important outcome indicators not being included in the research scheme.
The results of the subgroup analysis show that different types of exercise may be the reason for the higher heterogeneity, and the results show that the traditional Chinese healthy exercise (Tai Chi, Baduan Jin, Wu Qin Xi, etc.) may be better in improving osteoporosis. But this needs to be confirmed by higher‐quality clinical studies.
A total of 20 RCTs were included in this review, which showed that kinesitherapy had a favorable effect on improving BMD and alleviating pain in POP patients. Nevertheless, the interpretation and generalization of this systematic review and meta‐analysis are subject to some limitations. According to the Cochrane Collaboration's tool, low‐quality evidence, which included studies with a high risk of bias, resulted in a high heterogeneity of the meta‐analysis results and favored the positive effect of exercises on BMD and VAS in patients with POP. Although most RCTs report the random sequences generation (only one RCT did not report), these RCTs lacking detailed descriptions of randomization, which result in selection bias. The performance bias was high since the blinding of participants and personnel was not implemented. Although three trial reported withdrawal and dropout, an intention‐to‐treat analysis was not performed in the data analysis phase for which attrition bias was inevitable. The results of this meta‐analysis show that different types of exercise therapies significantly improved lumbar spine and femoral neck BMD, and relieve the pain of patients in the current low‐quality evidence. Additional high‐quality evidence is required to confirm the effect of exercise therapy on the biochemical markers of bone metabolism in POP patients. Therefore, more multicenters, larger samples, long‐term, single‐blind RCTs are needed to evaluate the effects of exercise therapy on BMD, VAS and biochemical markers of bone metabolism in POP patients.
Supporting information
Appendix A. Search strategy used in PubMed database.
Acknowledgments
All listed authors have each made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; participated in drafting the manuscript or revising it critically for content, and have approved the final version of the submitted manuscript.
Grant Sources: This study was supported by funding for the following topics: National Natural Science Foundation of China (No.81873322);National key research and development program (NO.2018YFC1704703).
Disclosure: The authors declare that they have no conflicts of interest.
Contributor Information
Biao Tan, Email: tan520vip@sina.cn.
Weiheng Chen, Email: drchenweiheng@bucm.edu.cn.
Haijun He, Email: drhjhe@126.com.
References
- 1. Marcus R. Post‐menopausal osteoporosis. Best Pract Res Clin Obstet Gynaecol, 2002, 16: 309–327. [DOI] [PubMed] [Google Scholar]
- 2. Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol, 2013, 9: 575–583. [DOI] [PubMed] [Google Scholar]
- 3. Lin JT, Lane JM. Osteoporosis: a review. Clin Orthop Relat Res, 2004, 425: 126–134. [PubMed] [Google Scholar]
- 4. Riggs BL, Melton LJ 3rd. The prevention and treatment of osteoporosis. N Engl J Med, 1992, 327: 620–627. [DOI] [PubMed] [Google Scholar]
- 5. Wylie‐Rosett J. Menopause, micronutrients, and hormone therapy. Am J Clin Nutr, 2005, 81: 1223s–1231s. [DOI] [PubMed] [Google Scholar]
- 6. Seeman E. Pathogenesis of bone fragility in women and men. Lancet, 2002, 359: 1841–1850. [DOI] [PubMed] [Google Scholar]
- 7. Riggs BL, Khosla S, Atkinson EJ, Dunstan CR, Melton LJ 3rd. Evidence that type I osteoporosis results from enhanced responsiveness of bone to estrogen deficiency. Osteoporos Int, 2003, 14: 728–733. [DOI] [PubMed] [Google Scholar]
- 8. Agnusdei D, Crepaldi G, Isaia G, et al. A double blind, placebo‐controlled trial of ipriflavone for prevention of postmenopausal spinal bone loss. Calcif Tissue Int, 1997, 61: 142–147. [DOI] [PubMed] [Google Scholar]
- 9. Akehurst R, Brereton N, Ariely R, et al. The cost effectiveness of zoledronic acid 5 mg for the management of postmenopausal osteoporosis in women with prior fractures: evidence from Finland, Norway and The Netherlands. J Med Econ, 2011, 14: 53–64. [DOI] [PubMed] [Google Scholar]
- 10. Hadji P, Ziller V, Gamerdinger D, et al. Quality of life and health status with zoledronic acid and generic alendronate–a secondary analysis of the rapid onset and sustained efficacy (ROSE) study in postmenopausal women with low bone mass. Osteoporos Int, 2012, 23: 2043–2051. [DOI] [PubMed] [Google Scholar]
- 11. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone, 2007, 41: 122–128. [DOI] [PubMed] [Google Scholar]
- 12. Saag K, Lindsay R, Kriegman A, Beamer E, Zhou W. A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone, 2007, 40: 1238–1243. [DOI] [PubMed] [Google Scholar]
- 13. Shane E. Evolving data about subtrochanteric fractures and bisphosphonates. N Engl J Med, 2010, 362: 1825–1827. [DOI] [PubMed] [Google Scholar]
- 14. Woodis CB. Once‐yearly administered intravenous zoledronic acid for postmenopausal osteoporosis. Ann Pharmacother, 2008, 42: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 15. Yokota H, Leong DJ, Sun HB. Mechanical loading: bone remodeling and cartilage maintenance. Curr Osteoporos Rep, 2011, 9: 237–242. [DOI] [PubMed] [Google Scholar]
- 16. Cheung AM, Giangregorio L. Mechanical stimuli and bone health: what is the evidence? Curr Opin Rheumatol, 2012, 24: 561–566. [DOI] [PubMed] [Google Scholar]
- 17. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ, 2015, 350: g7647–g7672. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ, 2011, 343: d5928–d5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou L, Ren ZZ, Zhang Z, Tian Y. Analysis of exercise therapy on prevention and treatment of osteoporosis in postmenopausal women and its effect on bone density. Matern Child Health Care China, 2020, 35: 1270–1273 (in Chinese). [Google Scholar]
- 20. Li R, Yang Z, Han W, et al. Effects of combined exercise therapy on bone metabolism in patients with postmenopausal osteoporosis. Int J Orthop, 2019, 40: 52–56+62 (in Chinese). [Google Scholar]
- 21. Xiao B, Sun PC, Sun C, Huo HL, Wang Y. The auxiliary improvement effect of progressive resistance training in postmenopausal women with osteoporosis. J Cervicodynia Lumbodynia, 2019, 40: 372–374 (in Chinese). [Google Scholar]
- 22. Li NJ, Hua B, ZL WG, et al. The effect of exercise rehabilitation along with bisphosphonata treatment on the clinical efficay of postmenopausal osteoporosis. Chin Prev Med, 2019, 20: 1097–1100 (in Chinese). [Google Scholar]
- 23. Kuang X‐w. Observation on the curative effect of simplified Baduanjin in preventing and improving primary osteoporosis in the elderly. Chine Community Doctors, 2019, 35: 186–188 (in Chinese). [Google Scholar]
- 24. Li LJ, Bie MB, Zhang ZX, Wang Y, Tang DS. Effects of progressive resistance exercises on bone metabolic markers in patients with postmenopausal osteoporosis. Chin J Bone Tumor Bone Dis, 2018, 7: 77–80 (in Chinese). [Google Scholar]
- 25. Sun ZC, Ou YG, Gu XM, et al. Effects of core strength training on related factors of fall risk and bone mineral density of patients with postmenopaisal osteoporosis. J Hebei Med Univ, 2017, 38: 1300–1304 +1314 (in Chinese). [Google Scholar]
- 26. Qin JZ, Rong XX, Zhu GX, Jiang Y. The effects of square dancing on bone mineral density and bone turnover markers in patients with postmenopausal osteoporosis. Chin J Osteoporos, 2017, 23: 43–46 +50 (in Chinese). [Google Scholar]
- 27. Li JW, Pan DQ, He KH, Shi XL. Understanding of clinical application of modified five‐bird game in the prevention and treatment of primary type I osteoporosis. Chin J Osteoporos, 2014, 20: 920–923 (in Chinese). [Google Scholar]
- 28. Shen MR, Feng YJ, Wei WW, et al. Effect of HUA Tuo's frolics of five animals on the patients with senile osteoporosis. Chin J Tradit Chin Med Pharm, 2014, 29: 895–897 (in Chinese). [Google Scholar]
- 29. Shen MR, Feng YJ, Wang T, et al. Effect of HUA Tuo's frolics of five animals on the bone mineral density of lumbar vertebrae in senile patients with osteoporosis. Chin J Osteoporos, 2013, 19: 271–274 (in Chinese). [Google Scholar]
- 30. Shi D, Shi X, Li FL, Ren JB, Gu LJ, et al. Clinical effect of exercise therapy on the patients with osteoporosis. Chin J Osteoporos, 2013, 32: 872–874 (in Chinese). [Google Scholar]
- 31. Chen HT, Liu HJ. Influence research of the movement intervention to seIlile osteoporosis. Chin Mod Med, 2012, 19: 66–67 (in Chinese). [Google Scholar]
- 32. Liu XJ. Effect of Baduanjin exercise on bone mineral density in patients with primary osteoporosis. Bus J, 2011, 39: 161–162 (in Chinese). [Google Scholar]
- 33. Li HB, Mei Q, Yin XL, Chang R. Clinical observation on the effect of exercise and osteopeptide preparation on osteoporotic bone density. J HeNan Med College Staff and Wokers, 2008, 20: 446–447 (in Chinese). [Google Scholar]
- 34. Song Y. Effects of Taijiquan exercise on bone density and bone metabolism of primary osteoporosis sufferers. J Phys Educ, 2008, 11: 106–108 (in Chinese). [Google Scholar]
- 35. Liu HQ, Qin JJ, Liu HJ. The effect of comprehensive exercise therapy on bone mineral density in postmenopausal osteoporosis. J Trad Chin Orth Traum, 2007, 19: 14 +82 (in Chinese). [Google Scholar]
- 36. Zhu HL, Liu XQ, Xia Q. Effect of exercise on bone mass and metabolism of aged osteoporosis patients. Maternal and Child Health Care of China., 2007, 22: 1251‐1252 (in Chinese). [Google Scholar]
- 37. Gong XH, Yan FZ, Zhang HX. Effect of climbing hills on bone mass of postmenopause patients with osteoporosis. Mod J Integr Trad Chin West Med, 2006, 19: 2611–2612 (in Chinese). [Google Scholar]
- 38. Kemmler W, Engelke K, Weineck J, Hensen J, Kalender WA. The Erlangen fitness osteoporosis prevention study: a controlled exercise trial in early postmenopausal women with low bone density‐first‐year results. Arch Phys Med Rehabil, 2003, 84: 673–682. [DOI] [PubMed] [Google Scholar]
- 39. Lai CL, Tseng SY, Chen CN, et al. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin Interv Aging, 2013, 8: 1603–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maeda SS, Lazaretti‐Castro M. An overview on the treatment of postmenopausal osteoporosis. Arq Bras Endocrinol Metabol, 2014, 58: 162–171. [DOI] [PubMed] [Google Scholar]
- 41. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab, 2020, 105: 587–594. [DOI] [PubMed] [Google Scholar]
- 42. Body JJ, Bergmann P, Boonen S, et al. Extraskeletal benefits and risks of calcium, vitamin D and anti‐osteoporosis medications. Osteoporos Int, 2012, 23: S1–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giangregorio LM, Papaioannou A, Macintyre NJ, et al. Too fit to fracture: exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos Int, 2014, 25: 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Angın E, Erden Z, Can F. The effects of clinical pilates exercises on bone mineral density, physical performance and quality of life of women with postmenopausal osteoporosis. J Back Musculoskelet Rehabil, 2015, 28: 849–858. [DOI] [PubMed] [Google Scholar]
- 45. Shanb A‐SA, Youssef EF, El‐Barkouky MG, Kamal RM, Tawfick AM. The effect of magnetic therapy and active exercise on bone mineral density in elderly women with osteoporosis. J Musculoskelet Res, 2012, 15: 65–74. [Google Scholar]
- 46. Varahra A, Rodrigues IB, Mac Dermid JC, Bryant D, Birmingham T. Exercise to improve functional outcomes in persons with osteoporosis: a systematic review and meta‐analysis. Osteoporos Int, 2018, 29: 265–286. [DOI] [PubMed] [Google Scholar]
- 47. Zhao R, Zhang M, Zhang Q. The effectiveness of combined exercise interventions for preventing postmenopausal bone loss: a systematic review and meta‐analysis. J Orthop Sports Phys Ther, 2017, 47: 241–251. [DOI] [PubMed] [Google Scholar]
- 48. Expert Panel on Musculoskeletal Imaging , Ward RJ, Roberts CC, et al. ACR appropriateness criteria® osteoporosis and bone mineral density. J Am Coll Radiol, 2017, 14: S189–S202. [DOI] [PubMed] [Google Scholar]
- 49. Vrbanac Z, Brkljaca Bottegaro N, Skrlin B, et al. The effect of a moderate exercise program on serum markers of bone metabolism in dogs. Animals (Basel), 2020, 10: 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Armamento‐Villareal R, Aguirre L, Waters DL, Napoli N, Qualls C, Villareal DT. Effect of aerobic or resistance exercise, or both, on bone mineral density and bone metabolism in obese older adults while dieting: a randomized controlled trial. J Bone Miner Res, 2020, 35: 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Search strategy used in PubMed database.


