Abstract
Introduction
Ubiquitously found in all life forms, inorganic polyphosphates (polyP) are linear polymers of repeated orthophosphate units. Present in intervertebral disc tissue, polyP was previously shown to increase extracellular matrix production in nucleus pulposus (NP) cells. However, the effects of polyP on human annulus fibrosus (hAF) cell metabolism is not known.
Methods and Results
Here, hAF cells cultured in the presence of 0.5 to 1 mM polyP, chain length 22 (polyP‐22), showed an increase in glycosaminoglycan content, proteoglycan and collagen synthesis, and aggrecan and collagen type 1 gene expression. Gene expression level of matrix metalloproteinases 1 was reduced while matrix metalloproteinases 3 level was increased in hAF cells treated with 1 mM polyP. Adenosine triphosphate (ATP) synthesis was also significantly increased in hAF cell culture 72 hours after the exposure to 1 mM polyP‐22.
Conclusions
PolyP thus has both anabolic and bioenergetic effects in AF cells, similar to that observed in NP cells. Together, these results suggest polyP as a potential energy source and a metabolic regulator of disc cells.
Keywords: annulus fibrosus, disc degeneration, inorganic polyphosphate, intervertebral disc, proteoglycan matrix
The current study investigates the effect of inorganic polyphosphates (polyPs) on human annulus fibrosus (AF) cell biology. This is a first study of its kind to investigate the effect of polyPs on human AF cells. polyPs, rich in polyanhydride bonds and known donors of phosphate molecule for ATP synthesis, showed increase in matrix production and ATP generation in human AF cells and overall promoted anabolism.
1. INTRODUCTION
Low‐back pain (LBP) is a common chronic musculoskeletal disorder 1 and is a major public health problem with great socioeconomic impact worldwide, as $100 billion is spent annually in the United States alone in direct and indirect costs for LBP. 2 , 3 Intervertebral disc degeneration (IDD) is a major cause of LBP. 4 , 5 IDD etiology is complex and multifactorial, including genetic inheritance, aging, metabolic disease, and abnormal biomechanical loading of the spinal column. 6 , 7 , 8 Current clinical treatments for IDD are primarily nonoperative; consisting of analgesics and physical therapy. 9 In severe cases of IDD, if associated pain is intractable and associated with neurologic sequelae, care involves surgical interventions, such as discectomy and spine fusion surgeries, which aim to alleviate pain and restore function but does not address the underlying pathology. 10 , 11 These surgical interventions have associated morbidities and complications, such as adjacent level disc disease, which often requires revision surgeries. 12 Therefore, there is a strong need for nonsurgical therapies that both alleviate painful symptoms and restore disc structure and mechanical function by directly addressing the underlying biological causes either through retarding or reversing disc degenerative processes.
The degeneration of intervertebral discs (IVDs) leads to collapse of its structure and reduction in the intervertebral space thickness. Mostly avascular, IVDs are composed of a central proteoglycan and collagen type 2 rich region, the nucleus pulposus (NP), which is surrounded by a concentric, multi‐lamellar collagen type 1 rich annulus fibrosus (AF). The AF limits the excessive swelling and sideways displacement of the NP region during physiological loading. 13 Other studies have proposed that the process of degeneration starts in the AF region and the NP extrudes into the AF regions and starts to degenerate, eventually spreading to whole of the disc causing IVD failure. 14 , 15
IDD is influenced by limited IVD nutrient availability due to the avascular nature of disc tissue. Nutrient deprivation affects disc cell bioenergetics and is known to drive an imbalance between anabolic and catabolic processes in the extracellular matrix (ECM) of the IVD, wherein ECM anabolism is reduced while ECM catabolism is enhanced. 16 Various strategies have been employed to reversing this loss of equilibrium such as cell‐based therapies, growth factor therapy, bioactive compound therapy, etc. 17 Bioactive compounds have shown promise in the field of tissue engineering and regenerative medicine applications. Many bioactive compounds have been used for tissue engineering applications of IDD. 18 , 19 , 20 , 21 Inorganic polyphosphate (polyP) is one such bioactive compound that has recently shown promise in the field of tissue engineering and regenerative medicine applications for IVD and cartilage tissue.
PolyP is ubiquitously found in all life forms, including bacteria, fungi, amoebas, plant cells, and mammalian cells. PolyP was recently shown to be present in a variety of native mammalian tissues, including both NP and AF IVD tissue at concentrations ranging from 10 to 20 pmol Pi/mg tissue. 22 PolyPs are linear polymers of orthophosphate (PO4) residues linked by high‐energy phosphoanhydride bonds that vary in sizes ranging from a few to thousands of PO4 units. 23 , 24 PolyPs exist as granules in microbial cells, for both energy and phosphate storage. The role of polyP in mammalian cells was not known until recent studies, which reported that polyP regulates many critical mammalian processes, including bone healing, blood clotting, nerve transduction, and cancer metastasis. 25 , 26 , 27 , 28 The effects of polyP are chain length and concentration dependent. Recent studies demonstrated that polyP chain lengths 22 and 45 (polyP‐22 and polyP‐45) increased the production and accumulation of proteoglycans and downregulated matrix metalloproteinases (MMP) production in engineered NP and cartilage tissues. These results thus suggest the potential use of polyP in regenerative applications for these tissues. 21 , 29 , 30 , 31
Given the energy‐rich nature of polyPs and their presence in AF tissues, we hypothesize that polyP plays an important role in AF cell physiology, bioenergetics, and matrix homeostasis. We tested our hypothesis by treating human AF cell cultures with polyP (chain length 22, polyP‐22) and studying the effect on proteoglycan and collagen gene expression, matrix production, and AF cells bioenergetics. To our knowledge, this is the first study to evaluate the effects of polyP on human AF cells.
2. MATERIALS AND METHODS
2.1. Annulus fibrosus cell isolation and culture
Human annulus fibrosus (hAF) cells were isolated from the discarded cervical IVD tissue of 12 patients (Table 1) undergoing elective surgical procedures for degenerative cervical disc disease after consent and institutional ethics board approval. The patient demographics were average age 52.41 ± 10.78; male: female = 2:1; MRI degeneration grade 2.54 ± 0.82. The experimental protocols were approved by the human subjects Institutional Review Board at the University of Pittsburgh.
TABLE 1.
Identification of patient samples and demographics
| Pat. No | Age (y) | Gender | Diagnosis | Pfirmann grade of degeneration | Levels resected |
|---|---|---|---|---|---|
| 1 | 57 | F | Cervical stenosis | 2 | C6‐C7 |
| 2 | 51 | M | Cervical stenosis | 2 | C5‐C6; C6‐C7 |
| 3 | 65 | F | Cervical stenosis | 3 | C5‐C6 |
| 4 | 46 | F | Cervical stenosis | 3 | C5‐C6 |
| 5 | 62 | M | Cervical stenosis | 4 | C5‐C6; C6‐C7 |
| 6 | 40 | M | Cervical stenosis | 2 | C5‐C6 |
| 7 | 38 | M | Cervical stenosis | 2 | C5‐C6 |
| 8 | 57 | M | Cervical stenosis | 2 | C3‐C4 |
| 9 | 45 | M | Cervical disc displacement | 2 | C5‐C6 |
| 10 | 39 | M | Cervical stenosis | 1 | C4‐C5 |
| 11 | 60 | F | Cervical stenosis | 3 | C5‐C6 |
| 12 | 69 | M | Cervical stenosis | 3 | C5‐C6 |
AF tissue specimens from individual patients were collected (pooled if more than one level resected) without attempt made to separate inner from outer AF tissue and placed in sterile Ham's F‐12 medium (GibcoBRL, Grand Island, New York) containing 1% penicillin/streptomycin (P/S; GibcoBRL, Grand Island) and 5% fetal bovine serum (FBS; GibcoBRL, Grand Island) in the operating room. hAF cells were isolated from tissue as previously described, 32 after the tissue was diced into small pieces and sequentially digested with 0.2% Pronase (Calbiochem, EMD Millipore, San Diego, California) in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S for 1 hour, followed by 0.02% Collagenase‐P (Roche GmBH, Basel, Switzerland) in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S overnight in an incubator at 37°C and 5% CO2.
The isolated hAF cells were then seeded in T‐75 flasks at a density of 600 000 cells/flask in 5 mL of resuspension media and cultured at 37°C, 21% O2 and 5% CO2 in a humidified incubator. Cells were detached from culture flasks at 90% confluency by treating with 0.05% trypsin‐EDTA for 3 minutes (Thermo Fisher, Waltham, Massachusetts), counted, and reseeded in multi‐well cell culture plates in densities as per experimental design. Passage 1 hAF cells cultured on plates grown to 70% to 80% confluency and were treated with polyP‐22 (SPER Chemical Corporation, Clearwater, Florida) at varying concentrations (0, 0.5, and 1.0 mM) as previously tested in NP cells. 21 The highest concentration of exogenously administered polyP (1 mM) is thus approximately 1000 folds the concentration found in healthy bovine AF tissue. 22
2.2. Cell proliferation assay
Passage 1 hAF cell culture growth was measured using the CyQUANT cell proliferation reagent assay (Invitrogen, Grand Island) as per manufacturer's instructions. Passage 1 hAF Cells were seeded in 96‐well poly‐D‐lysine‐coated microplates (PerkinElmer, Hopkinton, Massachusetts) at a seeding density of 1000 cells/well in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S (100 μL media/well), cultured overnight, and then treated with different concentrations (0, 0.5, and 1.0 mM) of polyP‐22. At 48 hours the medium was removed, and the cells were incubated with CyQuant dye for 45 minutes at 37°C in a humidified incubator. The fluorescence intensity of each sample was measured using a fluorescence microplate reader (PerkinElmer).
2.3. ATPlite luminescence assay
ATP generation in passage 1 hAF cells treated with different concentrations of polyP‐22 was measured using ATPlite Luminescence Assay System (catalog number 6016941, PerkinElmer) following manufacturer's instructions. hAF cells were seeded onto a 96‐well plate at a density of 2000 cells/well and cultured for 24 hours in 100 μL of Ham's F‐12 media supplemented with 5% FBS and 1% P/S. Following day, the culture media was replaced with 100 μL fresh culture media supplemented with different concentrations of polyP‐22 (0, 0.5, and 1.0 mM) and cultured for up to 72 hours.
At each time point (1, 3, 6, 12, 24, 48, and 72 hours.), culture media was removed and 50 μL of mammalian cell lysis solution (kit component) was added to the culture wells and the culture plate shaken for 5 minutes. Then 50 μL substrate solution was added to the wells and the plate was shaken again for 5 minutes. The plate was dark adapted in the luminescence plate reader (VICTOR III Light luminescence plate reader) and luminescence was read. 33 The total DNA content (ng) of each sample well was measured using a Quant‐iT PicoGreen ds DNA Assay Kit (Invitrogen, Eugene, Oregon) and generated ATP (nM) amount was expressed normalized to total DNA content (nM/ng).
2.4. Total proteoglycan content
Passage 1 hAF cells were seeded at a seeding density of 30 000 cells/well in six‐well cell culture plates in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S (2 mL media/well). Cells were grown to 70% to 80% confluency and media was changed, then polyP‐22 was supplemented in varying concentrations (0, 0.5, and 1.0 mM). After 48 hours of polyP‐22 treatment, the cells were digested with papain digestion solution (300 mg/mL papain, 50 mM NaOAc, 5 mM EDTA, 5 mM L‐cysteine, 55 mM citric acid, and 150 mM NaCl) for 6 hours at 65°C. Total glycosaminoglycan (GAG) content was estimated by measuring the amount of sulfated GAGs as an indicator of proteoglycans in aliquots of the papain digest using the dimethylmethylene blue (DMMB) dye binding assay measured via spectrophotometry at a wavelength of 595 nm as previously described. 21 The total DNA content of each digested sample was measured using a Quant‐iT PicoGreen ds DNA Assay Kit (Invitrogen, Eugene) and GAG amount was expressed normalized to total DNA content (μg).
2.5. Proteoglycan synthesis
Passage 1 hAF cells were seeded at a seeding density of 5000 cells/well in 24‐well cell culture plates in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S (0.5 mL media/well). Cells were grown to 70% to 80% confluency and then treated with polyP‐22 supplementation in varying concentrations (0, 0.5, and 1.0 mM). At the same time 20 mCi/mL 35S‐SO4 was added for 48 hour. Proteoglycan synthesis was measured via incorporation of 35S‐SO4 as described previously. 34 Aliquots of the media were mixed with 2 mL of scintillation fluid and radioactivity quantified using a scintillation counter and normalized to DNA content (ng).
2.6. Collagen synthesis
Passage 1 hAF cells were seeded at a seeding density of 5000 cells/well in 24‐well cell culture plates in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S (0.5 mL media/well). Cells were grown to 70% to 80% confluency and then treated with polyP‐22 supplementation in varying concentrations (0, 0.5, and 1.0 mM). At the same time, 10 μCi/mL 3H‐L‐proline in 0.5 mL F‐12 was added for 48 hour. The medium was collected, and the cell samples were scraped in in homogenization buffer (0.5 mL/well) and collected and subjected to three freeze/thaw cycles. The samples were stirred at 4°C overnight, combining the medium and cell layers, then collagen synthesis was determined by 3H‐L‐proline incorporation using a modified collagenase digestion method as previously described. 35 One milliliter of the supernatant and aliquots of the pellets dissolved in 0.2 mol/L NaOH were subjected to scintillation counting to determine the collagen and noncollagen protein synthesis. 36 Raw counts per minute were normalized to DNA content (ng) determined using the Quant‐iT PicoGreen ds DNA Assay Kit (Invitrogen).
2.7. Cell immunofluorescence
Passage 1 hAF cells were seeded on eight‐well glass plates (μ‐Slide 8 Well Glass Bottom, #80827, Ibidi GmBH, Fitchburg, Wisconsin) at a seeding density of 2000cells/well in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S (0.5 mL media/well). After 24 hours, the media was removed and replaced with fresh Hams F12 supplemented with varying concentrations of polyP (0, 0.5, and 1.0 mM). After 48 hour of polyP‐22 treatment, the media was removed, and the cells were fixed in 4% paraformaldehyde (PFA) for 15 minutes. PFA was removed and cells were treated with 0.2% Triton X‐100/phosphate‐buffered saline (PBS) and 5% bovine serum albumin (BSA) to block nonspecific binding of antibodies for 15 minutes.
Cells were incubated at 4°C overnight with primary antibodies; anti‐aggrecan antibody (1:300 dilution, catalog number ab1031, EMD‐Millipore, Burlington, Massachusetts) or anti‐collagen type 1 antibody (1:200 dilution, catalog number ab34710, Abcam, Cambridge, Massachusetts), followed by incubation with Alexa fluor‐647 fluorescence conjugated secondary antibody (donkey anti‐ rabbit IgG [H + L], 1:500 dilution, Invitrogen, Carlsbad, California) for 1 hour at 37°C. The secondary antibody solution was removed, and the cells stained with DAPI (10 μg/mL) for 5 minutes. Afterwards, the wells were washed with PBS thrice for 5 minutes each. Finally, cells were mounted using ProLong Gold Antifade Mountant (#P3693, Thermo‐Fischer Scientific, Massachusetts). Samples were visualized and images were captured using a confocal fluorescence microscope (Nikon Eclipse Ts100; Nikon Instruments Inc., Melville, New York). Images used for comparison of different treatments were acquired with the same instrument settings and exposure times and were processed equivalently.
2.8. Western blot analysis
Passage 1 hAF cells were seeded at a seeding density of 30 000 cells/well in six‐well cell culture plates in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S (2 mL media/well). Cells were grown to 70% to 80% confluency, media removed and replaced with 2 mL fresh F‐12 media supplemented with polyP‐22 in varying concentrations (0, 0.5, and 1.0 mM) for 48 hour. At the end of polyP treatment, cells were lysed as per the manufacturer's instructions (RIPA lysis buffer, Sigma‐Aldrich, St. Louis, Missouri).
All the samples were then measured for total protein concentration using a BCA Protein Assay Kit (Thermo Scientific, Pittsburgh, Pennsylvania) to ensure equal loading. Loading buffer was added to 30 μg protein and separated on 10% SDS‐PAGE gels before transfer onto PVDF membranes (Bio‐Rad, Hercules, California) as previously described. 37 The membranes were probed overnight with primary antibodies reactive with aggrecan (Cat. No. ab36861, Abcam, dilution 1:500) or collagen type 1 (Cat. No. ab34710, Abcam, dilution 1:1000). The next day, the membranes were incubated with the HRP conjugated secondary antibody (goat anti‐rabbit IgG, Cat. No. 31460, Thermo‐Fischer Scientific, Burlington, Massachusetts, dilution 1:15000) for 1 hour at room temperature. The blots were then analyzed using LiCoR Odyssey imager (LI‐COR Biosciences, Lincoln, Nebraska) for visualization of the protein bands, and semi‐quantification was performed by using the software on the LiCoR Odyssey imager.
2.9. Isolation of mRNA and gene expression analysis
Passage 1 hAF cells were seeded at a seeding density of 30 000 cells/well and cultured to 70% to 80% confluency in six‐well cell culture plates in Ham's F‐12 medium supplemented with 10% FBS and 1% P/S (2 mL media/well). A low cell density was used for seeding and the cells allowed to expand before experimentation as the number of cells obtained from the human tissue was low due to the tissue amount procured. Cells were treated with fresh media containing 0, 0.5, and 1.0 mM polyP‐22. After 48 hour, hAF cells were washed with phosphate‐buffered saline (PBS−/−, calcium and magnesium free) and then lysed with RLT lysis buffer (Qiagen, Valencia, California) and RNA was isolated using RNeasy mRNA extraction kit (Qiagen Germantown, Maryland). Quantitative RT‐PCR was performed to measure relative gene expression of anabolic and catabolic genes of interest as previously described. 32 Sequence specific primers were used for aggrecan (ACAN), collagen type I (Col1A1), and matrix metalloproteinases 1 and 3 (MMP‐1 and ‐3) (Table 2). After normalization with GAPDH expression levels, the relative gene expression was reported as relative fold expression compared to control, using the ΔΔCt method. 38
TABLE 2.
Tabulation of primer sequences used in the study
| Gene | Sequence | NCBI reference |
|---|---|---|
| GAPDH |
Forward‐5′‐ACC CAC TCC TCC ACC TTT GAC Reverse‐3′‐TCC ACC ACC CTG TTG CTG TAG |
NM_001256799.2 |
| Aggrecan (ACAN) |
Forward‐5′‐AAG AAT CAA GTG GAG CCG TGT GTC Reverse‐3′‐TGA GAC CTT GTC CTG ATA GGC ACT |
NM_001135.3 |
| Collagen 1a1 (Coll1A1) |
Forward‐5′‐GGA AAC AGA CAA GCA ACC CAA ACT Reverse‐3′‐GGT CAT GTT CGG TTG GTC AAA GAT A |
XM_005257058.4 |
| MMP‐1 (MMP3) |
Forward‐5′‐CCC AAA AGC GTG TGA CAG TA Reverse‐3′‐GAG CTC AAC TTC CGG GTA GA |
NM_001145938.1 |
| MMP‐3 (MMP3) |
Forward‐5′‐CAA GGA GGC AGG CAA GAC AGC Reverse‐3′‐GCC ACG CAC AGC AAC AGT AGG |
NM_002422.5 |
2.10. Statistical analysis
All experiments were repeated a minimum of three times using independent cell preparations obtained from different donors. Each condition was done in triplicate (n = 3) unless otherwise specified. The results are expressed as mean ± SD. Two‐way analysis of variation (ANOVA) was performed using GraphPad Prism (GraphPad Software Inc., La Jolla, California) and Bonferroni's post hoc analysis was performed. Significance was assigned at P ≤ .05.
3. RESULTS
3.1. polyP‐22 increases cell proliferation of hAF cell culture
Cell proliferation is energy demanding and given the reported role of polyP as an ancient energy biomolecule we tested whether polyP influences hAF cell growth. Passage 1 hAF cells seeded in 96‐well poly‐D‐lysine‐coated microplates were evaluated for cellular growth and proliferation rates by using CytoQuant assay at time points 1, 6, 12, 24, and 48 hours. Untreated, 0.5 and 1 mM polyP‐22 treated groups all showed a trend toward increase in cellular proliferation that continued until 48‐hour time point. The 0.5 mM polyP‐22 treated cells at time points 12, 24, and 48 hours showed a higher increase in cellular proliferation compared to the untreated control cells at 1‐hour. The 1 mM polyP‐22 treated group showed significantly higher cell proliferation at 12 (*P = .044), 24 (*P = .038), and 48 (*P = .045) hours compared to untreated control cells at these individual time points (Figure 1A).
FIGURE 1.
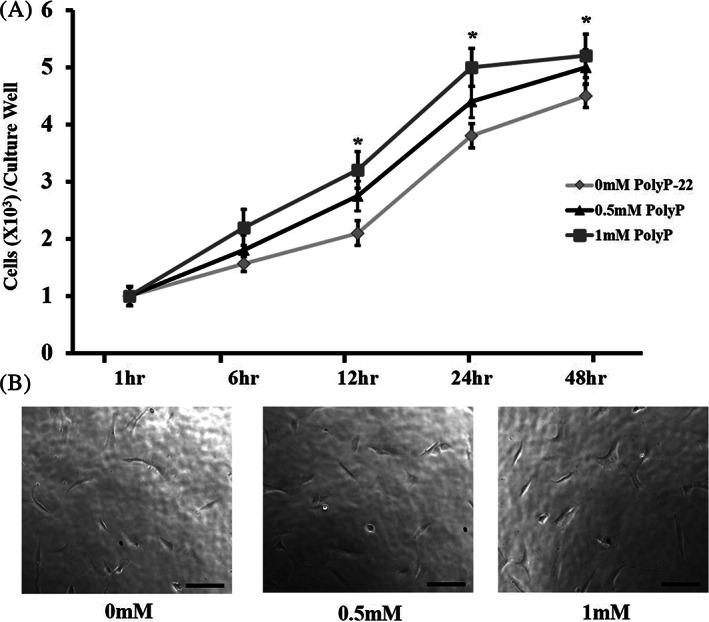
Cell proliferation (A) and (B) morphology in polyP‐22 treated (0.5 and 1 mM) and untreated groups after 48 hour in culture. *(1 mM polyP‐22) denote significance compared to untreated controls with N = 4 and significance set at P < .05. Scale bars represent 100 μm at 20X magnification
In addition, to investigate the effect of polyP‐22 treatment of hAF cell morphology, passage 1 hAF cells seeded (30 000 cells/well) in six‐well cell culture plates were imaged in bright field with an inverted microscope. At 48‐hour time point, passage 1 hAF cells 0, 0.5, and 1 mM showed no differences in cell shape and morphology. They were elongated and fibroblast like in shape (Figure 1B). Hence, polyP stimulates hAF cell proliferation without significantly impacting their cell morphology.
3.2. Increased ATP production in response to polyP treatment
The increase in hAF cell proliferation in response to polyP treatment implicates a role for polyP as an energy source as cell proliferation is an ATP requiring process and polyP has shown to be a phosphate group donor for ATP synthesis. To investigate if polyP serves as an energy provider, hAF cells treated with polyP‐22 were assessed for ATP production. The 1 mM polyP‐22 treated cells showed a significant increase in ATP production compared to untreated controls at time points 12 (*P = .002), 24 (*P = .045), 48 (*P = .02), and 72 (*P = .03) hours. At earlier time points (1, 3, and 6 hours) each of experimental groups exhibited a trend toward increased ATP production, albeit not significant. The 0.5 mM polyP‐22 treated group showed an initial ATP increase at 12‐hour time point and thereafter ATP levels showed a nonsignificant decline, possibly due to depletion of nutrients in the culture media. The 1 mM polyP‐22 treated group had a significantly higher ATP production compared to 0.5 mM polyP‐22 treated group at 72‐hour time point. (Figure 2).
FIGURE 2.
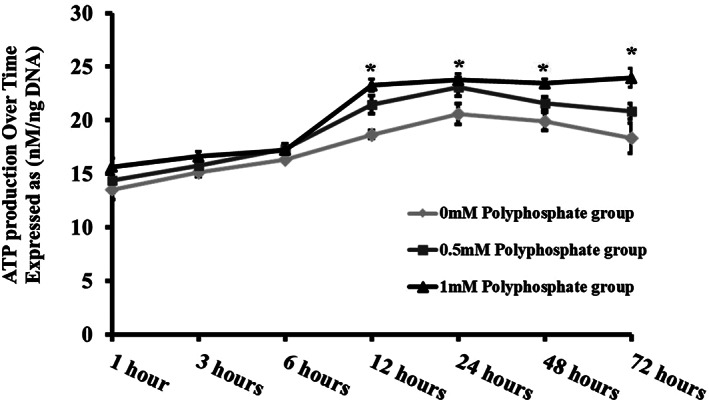
Total ATP production over various time points by untreated and polyP‐22 treated (0.5 and 1 mM) human annulus fibrosus cells after 48 hour in culture. N = 3 and significance set at P < .05 for * 1 mM polyP‐22 compared to untreated controls
3.3. polyP enhances collagen and proteoglycan matrix production in hAF cell culture
Previous studies reported that polyP enhances proteoglycan matrix production in NP cells. To demonstrate whether polyP also stimulates matrix synthesis in hAF cells, we measured total GAG production (DMMB assay), proteoglycan synthesis (35S‐SO4 assay), and collagen and protein synthesis (3H‐L‐proline assay) in hAF cells after 48‐hour treatment with polyP.
Total GAG content was significantly increased (*P = .016) in 1 mM polyP‐22 treated cells compared to untreated controls. There was an increase in total GAG in 0.5 mM polyP‐22 treatment group, but this was not significant compared to untreated controls and 1 mM polyP‐22 treated groups (Figure 3A). New proteoglycan synthesis (35S‐SO4 incorporation) significantly increased only in the 1 mM polyP‐22 treatment group compared to both untreated control (*P = .003) and 0.5 mM polyP‐22 treated groups (*P = .04) (Figure 3B). Collagen synthesis (3H‐L‐proline incorporation) significantly increased only in the 1 mM polyP‐22 treatment group compared to untreated controls (*P = .024); whereas the difference in synthesis between 0.5 mM and 1 mM polyP‐22 treatment groups was not significantly different (Figure 3C). Total protein synthesis showed an increase in 0.5 mM polyP‐22 treatment group, though the increase was not significant compared to either the untreated control or 1 mM polyP‐22 treatment groups (Figure 3D).
FIGURE 3.
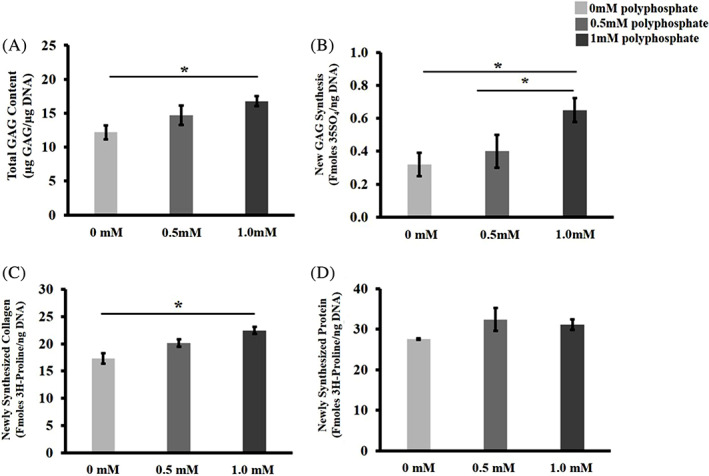
A, Total GAG accumulation, B, quantification of 35S‐SO4 incorporation into newly synthesized GAGs, C, quantification of 3H‐L‐Proline in newly synthesized collagen and D, protein in untreated and polyP‐22 treated (0.5 and 1 mM) human annulus fibrosus cells after 48 hour in culture. N = 3. * denotes significance (P < .05)
3.4. Immunocytochemistry for aggrecan and collagen type 1 expression in response to polyP‐22 treatment
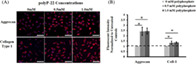
To further evaluate the stimulation of matrix production by polyP, we investigated the synthesis and distribution of proteoglycan and collagen in hAF cells by immunofluorescence using specific antibodies against aggrecan and collagen type 1 on hAF cells treated with and without polyP‐22 for 48 hours. hAF cells treated with both 0.5 mM and 1 mM polyP‐22 showed increased fluorescence intensity with fluorescent tagged secondary antibody compared to untreated control group (Figure 4A). On quantification of the fluorescence intensity, both 0.5 mM and 1 mM polyP‐22 treatment groups showed significant increases for aggrecan (both 2.4‐fold increase, *P = .003) and collagen type 1 (1.3 (*P = .046) and 1.4‐fold (*P = .01) increase respectively) compared to untreated controls. There were no differences in fluorescent intensities for aggrecan and collagen type 1 between the 0.5 mM and 1 mM polyP‐22 treatment groups (Figure 4B). The immunofluorescence results are consistent with those of matrix synthesis in suggesting that polyP acts as an anabolic agent in addition to an energy source.
FIGURE 4.
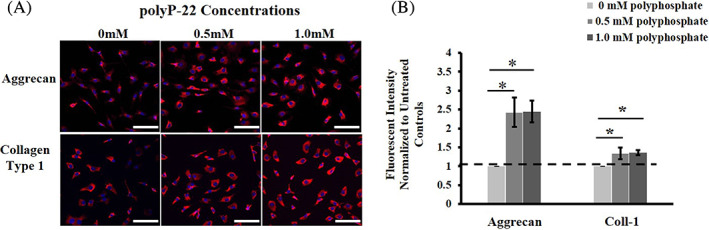
A, Immunohistochemistry showing deposition and B, quantitative analysis of aggrecan and collagen type 1 in extracellular matrix of the of untreated and polyP‐22 treated (0.5 and 1 mM) human annulus fibrosus cells after 48 h in culture. Quantification of fluorescence intensity is expressed as fold change relative to untreated control (dotted line, 0 mM polyP) set at 1. N = 3 and significance * set at P < .05 compared to untreated control. Scale bars represent 100 μm at 20X magnification
3.5. Western blot analysis for aggrecan and collagen type 1 in response to polyP‐22 treatment
To investigate the production of aggrecan and collagen, hAF treated with polyP‐22 for 48 hours, western blot analysis with specific antibodies was performed. Both the 0.5 mM and 1 mM polyP‐22 treatment groups showed increased band intensity when probed for aggrecan and collagen type 1 compared to untreated control group. Quantitative analysis showed the 1 mM polyP‐22 treatment group to have significantly higher protein expression levels for both aggrecan and collagen type 1 compared to untreated control (*P = .05 and *P = .02). The 0.5 mM polyP‐22 treatment group only showed significantly increased protein expression for collagen type 1 compared to untreated controls (*P = .03). There were no significant differences in protein expression levels for aggrecan and collage type 1 between the 0.5 mM and 1 mM polyP‐22 treatment groups (Figure 5A,B). Thus, all three independent assays, immunofluorescence, western blot, and radiolabeling, confirmed polyP acts as an anabolic agent in hAF cells in stimulating matrix production.
FIGURE 5.
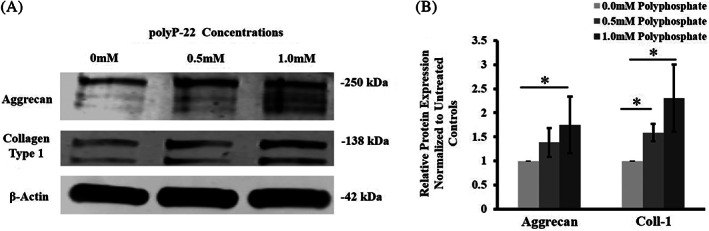
A, Western blot probing and B, quantification for aggrecan and collagen type 1 in human annulus fibrosus cell cultures treated with polyP‐22 (0.5 and 1 mM) after 48 hour in culture. Densitometry quantification of band intensity is expressed as fold change relative to untreated control (dotted line, 0 mM polyP) set at 1. N = 3 and significance * set at P < .05 compared to untreated control
3.6. Gene expression analysis in response to polyP‐22 treatment
To investigate whether polyP modulates gene expression of major matrix molecules at the transcription level in hAF cells, real‐time qRT‐PCR of selected matrix structural genes was performed 48 hours after treatment with different doses of polyP‐22. Gene expression levels of aggrecan were significantly increased in both the 0.5 and 1 mM polyP‐22 treatment groups (1.9 (*P = .01) and 2.2‐fold (*P = .002) respectively) compared to the untreated control group. The gene expression of collagen type 1 was found to be significantly increased only in the 1 mM polyP‐22 treatment group (3.1‐fold, *P = .001), whereas the 0.5 mM polyP‐22 treatment group did not show a significant increase (1.2‐fold) compared to the control group. Collagen type 1 gene expression in the 1 mM polyP‐22 group was also significantly increased compared to the 0.5 mM polyP‐22 treatment group (*P = .005).
In addition, gene expression of two key MMPs, MMP‐1 and 3, were assessed to investigate whether polyP also modulates catabolic processes in hAF cells. MMP‐1 gene expression levels in the untreated control and 0.5 mM polyP‐22 treatment groups showed no differences, whereas the 1 mM polyP‐22 treatment group showed a significant decrease in its expression levels compared to both the untreated control and 0.5 mM polyP‐22 treatment groups (5.0 (*P = .001) and 4.6‐fold (*P = .001) respectively). MMP‐3 gene expression analysis showed an increase in expression levels in both the 0.5 mM polyP‐22 (2‐fold) and 1 mM poly‐22 (2.3‐fold, (*P = .03)) treatment groups compared to the untreated control group; though only the increase with 1 mM polyP‐22 treatment was significant. The difference in expression levels between the 0.5 mM and 1 mM polyP‐22 treatment groups was not significant (Figure 6).
FIGURE 6.
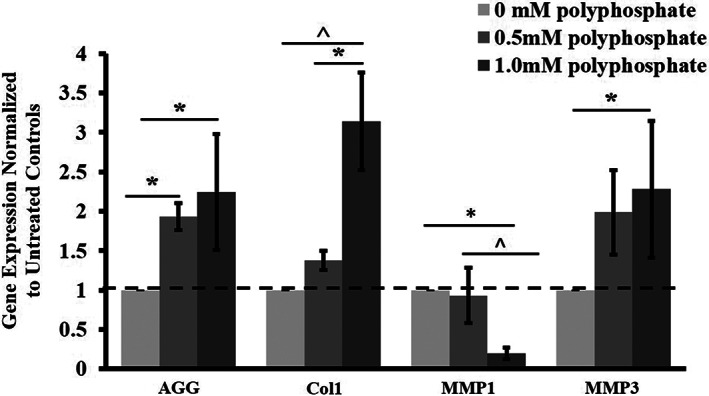
Gene expression profile in human annulus fibrosus cells treated with polyP‐22 (0.5 and 1 mM) after 48 hour in culture. Gene expression is expressed as fold change relative to untreated control (dotted line, 0 mM polyP) set at 1. N = 3 and significance * and ^ set at P < .05 compared to untreated control and 0.5 mM polyP‐22 treatment groups respectively
4. DISCUSSION
To best of our knowledge, this study is the first investigating the effects of polyP on hAF cells. The study showed that polyP has an anabolic effect on hAF cells, increasing expression and synthesis of proteoglycan and collagen. Western blot analysis showed increased accumulation of aggrecan and collagen type 1. Upregulation of aggrecan and collagen type 1 gene expression was consistent with these findings as they lead to an increase in total GAG, as well as new proteoglycan and collagen synthesis in polyP‐treated hAF cells. These results, together with the stimulatory effects on increase in ATP production and cell proliferation, suggest that polyP‐22 serves as an anabolic agent and an energy source for hAF cells.
It has been previously shown that the effect of polyP on different cell types is chain length and concentration dependent, with the specific cell types responding to a certain chain length in a specific dose and not to others. 21 , 30 , 39 , 40 , 41 St‐Pierre et al showed bovine articular chondrocytes have a maximal increase in proteoglycan accumulation in engineered cartilage constructs at chain length 45 (polyP‐45). 30 Gawri et al showed bovine NP cells to show maximum proteoglycan accumulation at chain lengths 22 and 45 (polyP‐22 and polyP‐45), and further evaluated the effects of polyP‐22 due to its relatively smaller size and potential ease of delivery to cells and tissue constructs due to smaller charge densities. 21 On the other hand, exposure to a lower or higher chain length failed to trigger an anabolic response in these two cell types. For this reason, we used polyP‐22 for our study on hAF cells. Furthermore, Gawri et al evaluated the effective dosage of polyP in NP cells ranging from 0‐1 mM and showed a maximal anabolic response at 0.5 mM dose for both chain lengths polyP‐22 and polyP‐45; thus, we evaluated the optimal dosage of polyP in AF cells by evaluating a dose curve similar to NP cells.
PolyPs have been shown to increase cellular proliferation in some cells, for example, S. cerevisiae, and inhibit it in other cell types such as MCF‐7 cancer cell line and D. discoideum. 42 , 43 , 44 , 45 In IVD tissue, NP cells showed no increase in cell proliferation compared to untreated controls over a period of 14 days in culture, 21 whereas our findings show that polyP‐22 has a stimulatory effect on human AF cell proliferation at least within first 48 hours of treatment. These differences could be due to the total duration of culture and the culture method used, that is, growth in monolayers vs 3D cultures on calcium‐polyP‐based bioscaffolds. Further studies with increased time in culture and studies with cells seeded on 3D scaffolds will aid in understanding the exact effect of polyP on AF cells cultures.
In our study, AF cells treated with poly‐22 showed an increase in aggrecan and collagen type 1 gene expression and synthesis of proteoglycans and collagen type 1 at both 0.5 mM and 1 mM doses of polyP‐22. The AF region of the IVD is typically richer in collagens than proteoglycans with AF cells producing more collagens to maintain the IVD tissue composition and physiology. We found that polyP stimulated hAF cells synthesized aggrecan in addition to collagen type 1 in a dose dependent manner. This could possibly be due to A) an inherent effect of polyP on AF cells and/or B) use of AF cells from donors with advanced grades of IVD degeneration and undergoing spinal surgery for IVD degeneration; wherein the IVD tissue loses the demarcation of NP and AF regions and the degenerating IVD has a mixed population of remnant NP and AF cells. Furthermore, since the baseline production of aggrecan is more than collagen type 1 and the morphology of the procured cells in Figure 4 is not typical of AF cells, this indicates that cells harvested from the surgical disc tissue are a mixed population of normal and degenerative AF cells, possibly with some NP crossover, resulting in mixed nonuniform phenotypes. This is a shortcoming of our study and further studies are warranted with cells from healthy human AF tissue obtained strictly from outer AF region.
With polyP treatment, MMP‐1 gene expression levels were reduced, and MMP‐3 gene expression levels were increased with the 1 mM dose. This is not necessarily unexpected as it could be an early remodeling of the newly laid down matrix by AF cells treated with polyP for 48 hours. Furthermore, MMPs are calcium and zinc ion dependent enzymes, 46 their enzymatic activities could also be regulated by polyPs since polyPs closely regulate free calcium ions concentrations. 47
In our study, after treatment with polyP‐22, the hAF cells showed increased production of ATP. Previously it has been shown that in NP cells, polyP treatment had an added anabolic effect on NP tissue constructs when cultured under near physiological hypoxic conditions. 21 Overall, the increase in matrix production in polyP‐treated hAF cells is consistent with the reported function of polyP as an energy source because matrix synthesis, like cell proliferation, is an energy‐demanding process. The increase in ATP production might have a beneficial additive effect on AF cells too as the trigger toward anabolism is an energy consuming process and these extra ATP molecules can help prevent the metabolic shift toward anaerobic respiration 48 and subsequent generation of free radicals and reactive oxygen species (ROS). This increase in ATP production with polyP treatment can be due either to polyP acting as a phosphate group donor 49 or by its regulation of mitochondrial function through the membrane permeability transition pore (mPTP). 50 , 51 Future studies are warranted to explore this effect of polyP on ATP production and upregulation of aggrecan and collagen production in response to a blockage of endogenous ATP synthesis using the ATP synthetase inhibitors 2,4‐dinitrophenol and Bz‐423.
In addition, a recent study by Bayev et al showed production and hydrolysis of inorganic polyP by F0‐F1 ATPase in mammalian mitochondria, thus providing further evidence of the involvement and modulation of polyP in mammalian cells, which could utilize polyPs as an energy source in states of increased demands such as ECM production. 52 With 1 mM dose, the trend of ATP production was upwards till 72 hours and further long‐term studies are needed to evaluate whether this trend of increased ATP production is sustained for longer time periods and how polyPs regulate the bioenergetics in AF cells. This will be of particular importance if polyP is to be used for tissue engineering and regenerative medicine applications because AF tissue generated, with or without scaffolds, will ultimately be transplanted in the native host tissue and will experience relative hypoxia as part of tissue construct acclimatization. More studies are needed to demonstrate whether treatment with polyP prevents cell death and construct rejection in the early days post‐transplantation.
PolyP has been shown to have different mechanisms of action(s) in different cell types; via activating mTOR, 44 , 53 , 54 P2Y1 receptors, 26 and FGF receptors. 55 Recently Gawri et al showed bovine articular chondrocytes to exert the action of polyP through calcium mediated signaling pathway. The mechanism of action of this pro‐anabolic effect of polyP on human AF cells still needs to be explored. Furthermore, intrinsic polyPs were found in native AF tissues, 22 indicating a possible native phosphokinase(s) present in mammalian cells generating polyPs in these tissues. Future studies will thus be conducted to decipher the mechanism of action of polyP and finding the kinase responsible for polyP physiology in AF cells.
5. CONCLUSIONS
In conclusion, this study shows that polyP has an effect on hAF cells in culture and has the potential to be developed as a potential therapeutic agent for tissue engineering applications for AF tissue regeneration. In addition to stimulating matrix production in AF cells, polyP may also have an added role as an energy source in modulating ATP production and providing an additional source of energy during induced anabolism. Further studies will elucidate these mechanism(s) and help understand the role of polyP in AF tissue physiology and pathophysiology.
CONFLICT OF INTEREST
The authors declare no competing conflicts of interest.
AUTHOR CONTRIBUTIONS
Xiangjiang Wang, Rahul Gawri, and Changbin Lei conducted the experiments and analyzed the data. Xiangjiang Wang and Rahul Gawri wrote the article. Rahul Gawri, Rita Kandel, Gwendolyn Sowa, and Nam Vo conceptualized the study. Rita Kandel, Gwendolyn Sowa, and Nam Vo helped draft the final version of the manuscript. Nam Vo secured the funding to conduct this study and confirmed the data analysis. All authors approved the draft of this manuscript.
ACKNOWLEDGMENTS
This work was made possible by R01 AG044376‐01 received by Nam Vo. Rahul Gawri received post‐doctoral fellowships from Natural Sciences and Engineering Research Council of Canada (NSERC) and The Arthritis Society of Canada (TAS). The authors would like to thank Jessa Darwin for editorial assistance on the article.
Wang X, Gawri R, Lei C, et al. Inorganic polyphosphates stimulates matrix production in human annulus fibrosus cells. JOR Spine. 2021;4:e1143. 10.1002/jsp2.1143
Funding information Foundation for the National Institutes of Health, Grant/Award Number: R01 AG044376‐01; The Arthritis Society of Canada (TAS); Natural Sciences and Engineering Research Council of Canada (NSERC)
REFERENCES
- 1. Frymoyer JW, Cats‐Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;22(2):263‐271. [PubMed] [Google Scholar]
- 2. Shvartzman L, Weingarten E, Sherry H, Levin S, Persaud A. Cost‐effectiveness analysis of extended conservative therapy versus surgical intervention in the management of herniated lumbar intervertebral disc. Spine (Phila pa 1976). 1992;17(2):176‐182. [DOI] [PubMed] [Google Scholar]
- 3. Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290(18):2443‐2454. [DOI] [PubMed] [Google Scholar]
- 4. de Schepper EI et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila pa 1976). 2010;35(5):531‐536. [DOI] [PubMed] [Google Scholar]
- 5. Torgerson WR, Dotter WE. Comparative roentgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg Am. 1976;58(6):850‐853. [PubMed] [Google Scholar]
- 6. An HS, Masuda K, Inoue N. Intervertebral disc degeneration: biological and biomechanical factors. J Orthop Sci. 2006;11(5):541‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Videman T, Battié MC, Gibbons LE, et al. Disc degeneration and bone density in monozygotic twins discordant for insulin‐dependent diabetes mellitus. J Orthop Res. 2000;18(5):768‐772. [DOI] [PubMed] [Google Scholar]
- 8. Russo F, Ambrosio L, Ngo K. The role of type I diabetes in intervertebral disc degeneration. Spine (Phila pa 1976). 2019;44(17):1177‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rainville J, Nguyen R, Suri P. Effective conservative treatment for chronic low back pain. Semin Spine Surg. 2009;21(4):257‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Sun P, Chen D, Tang L, Chen C, Wu A. Artificial total disc replacement versus fusion for lumbar degenerative disc disease: an update systematic review and meta‐analysis. Turk Neurosurg. 2018;30(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 11. Cappelletto B et al. Disc prosthesis replacement and interbody fusion in the treatment of degenerative cervical disc disease: comparative analysis of 176 consecutive cases. Eur Spine J. 2013;22(suppl 6):S894‐S899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srinivas GR, Deb A, Kumar MN, Kurnool G. Long‐term effects of segmental lumbar spinal fusion on adjacent healthy discs: a finite element study. Asian Spine J. 2016;10(2):205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sivan SS, Wachtel E, Roughley P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim Biophys Acta. 2014;1840(10):3181‐3189. [DOI] [PubMed] [Google Scholar]
- 14. Vergroesen PP et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23(7):1057‐1070. [DOI] [PubMed] [Google Scholar]
- 15. Choi YS. Pathophysiology of degenerative disc disease. Asian Spine J. 2009;3(1):39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grunhagen T et al. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(suppl 2):30‐35. [DOI] [PubMed] [Google Scholar]
- 17. O'Connell GD, Leach JK, Klineberg EO. Tissue engineering a biological repair strategy for lumbar disc herniation. Biores Open Access. 2015;4(1):431‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(suppl 4):441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petit A, Yao G, Rowas SA, et al. Effect of synthetic link N peptide on the expression of type I and type II collagens in human intervertebral disc cells. Tissue Eng Part A. 2011;17(7–8):899‐904. [DOI] [PubMed] [Google Scholar]
- 20. Gawri R et al. Best paper NASS 2013: link‐N can stimulate proteoglycan synthesis in the degenerated human intervertebral discs. Eur Cell Mater. 2013;26:107‐119. [DOI] [PubMed] [Google Scholar]
- 21. Gawri R, Shiba T, Pilliar R, Kandel R. Inorganic polyphosphates enhances nucleus pulposus tissue formation in vitro. J Orthop Res. 2017;35(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 22. Lee WD, Gawri R, Shiba T, Ji AR, Stanford WL, Kandel RA. Simple silica column‐based method to quantify inorganic polyphosphates in cartilage and other tissues. Cartilage. 2018;9(4):417‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown MR, Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci U S A. 2004;101(46):16085‐16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270(11):5818‐5822. [DOI] [PubMed] [Google Scholar]
- 25. Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103(4):903‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holmstrom KM et al. Signalling properties of inorganic polyphosphate in the mammalian brain. Nat Commun. 2013;4:1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Usui Y, Uematsu T, Uchihashi T, et al. Inorganic polyphosphate induces osteoblastic differentiation. J Dent Res. 2010;89(5):504‐509. [DOI] [PubMed] [Google Scholar]
- 28. Tsutsumi K et al. Inorganic polyphosphate enhances radio‐sensitivity in a human non‐small cell lung cancer cell line, H1299. Tumour Biol. 2017;39(6):1010428317705033. [DOI] [PubMed] [Google Scholar]
- 29. Lee WD, Gawri R, Pilliar RM, Stanford WL, Kandel RA. Sol gel‐derived hydroxyapatite films over porous calcium polyphosphate substrates for improved tissue engineering of osteochondral‐like constructs. Acta Biomater. 2017;62:352‐361. [DOI] [PubMed] [Google Scholar]
- 30. St‐Pierre JP, Wang Q, Li SQ, Pilliar RM, Kandel RA. Inorganic polyphosphate stimulates cartilage tissue formation. Tissue Eng Part A. 2012;18(11–12):1282‐1292. [DOI] [PubMed] [Google Scholar]
- 31. St‐Pierre JP, Pilliar RM, Grynpas MD, Kandel RA. Calcification of cartilage formed in vitro on calcium polyphosphate bone substitutes is regulated by inorganic polyphosphate. Acta Biomater. 2010;6(8):3302‐3309. [DOI] [PubMed] [Google Scholar]
- 32. Ngo K, Patil P, McGowan SJ, et al. Senescent intervertebral disc cells exhibit perturbed matrix homeostasis phenotype. Mech Ageing Dev. 2017;166:16‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patil P, Falabella M, Saeed A, et al. Oxidative stress‐induced senescence markedly increases disc cell bioenergetics. Mech Ageing Dev. 2019;180:97‐106. [DOI] [PubMed] [Google Scholar]
- 34. Nasto LA, Wang D, Robinson AR, et al. Genotoxic stress accelerates age‐associated degenerative changes in intervertebral discs. Mech Ageing Dev. 2013;134(1–2):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein‐2, recombinant human bone morphogenetic protein‐12, and adenoviral bone morphogenetic protein‐12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8(3):449‐456. [DOI] [PubMed] [Google Scholar]
- 36. Schmid TM, Conrad HE. Metabolism of low molecular weight collagen by chondrocytes obtained from histologically distinct zones of the chick embryo tibiotarsus. J Biol Chem. 1982;257(20):12451‐12457. [PubMed] [Google Scholar]
- 37. Ngo K, Pohl P, Wang D, et al. ADAMTS5 deficiency protects mice from chronic tobacco smoking‐induced intervertebral disc degeneration. Spine (Phila pa 1976). 2017;42(20):1521‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C[T]) method. Methods. 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 39. Smith SA, Choi SH, Davis‐Harrison R, et al. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116(20):4353‐4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hacchou Y, Uematsu T, Ueda O, et al. Inorganic polyphosphate: a possible stimulant of bone formation. J Dent Res. 2007;86(9):893‐897. [DOI] [PubMed] [Google Scholar]
- 41. Stotz SC, Scott LOM, Drummond‐Main C, et al. Inorganic polyphosphate regulates neuronal excitability through modulation of voltage‐gated channels. Mol Brain. 2014;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suess PM, Gomer RH. Extracellular polyphosphate inhibits proliferation in an autocrine negative feedback loop in dictyostelium discoideum. J Biol Chem. 2016;291(38):20260‐20269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suess PM, Watson J, Chen W, Gomer RH. Extracellular polyphosphate signals through Ras and Akt to prime dictyostelium discoideum cells for development. J Cell Sci. 2017;130(14):2394‐2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang L, Fraley CD, Faridi J, Kornberg A, Roth RA. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc Natl Acad Sci U S A. 2003;100(20):11249‐11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bru S, Martínez‐Laínez JM, Hernández‐Ortega S, et al. Polyphosphate is involved in cell cycle progression and genomic stability in Saccharomyces cerevisiae . Mol Microbiol. 2016;101(3):367‐380. [DOI] [PubMed] [Google Scholar]
- 46. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562‐573. [DOI] [PubMed] [Google Scholar]
- 47. Solesio ME, Garcia Del Molino LC, Elustondo PA, Diao C, Chang JC, Pavlov EV et al. Inorganic polyphosphate (polyP) is required for sustained free mitochondrial calcium elevation, following stimulated calcium uptake. Cell Calcium. 2020;493825(1021272):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lan R, Geng H, Singha PK, et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol. 2016;27(11):3356‐3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muller WEG et al. Polyphosphate as a donor of high‐energy phosphate for the synthesis of ADP and ATP. J Cell Sci. 2017;130(16):2747‐2756. [DOI] [PubMed] [Google Scholar]
- 50. Elustondo PA, Nichols M, Negoda A, et al. Mitochondrial permeability transition pore induction is linked to formation of the complex of ATPase C‐subunit, polyhydroxybutyrate and inorganic polyphosphate. Cell Death Discov. 2016;2:16070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seidlmayer LK, Gomez‐Garcia MR, Shiba T, et al. Dual role of inorganic polyphosphate in cardiac myocytes: the importance of polyP chain length for energy metabolism and mPTP activation. Arch Biochem Biophys. 2019;662:177‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bayev AY, Angelova PR, Abramov AY. Inorganic polyphosphate is produced and hydrolysed in F0F1‐ATP synthase of mammalian mitochondria. Biochem J. 2020;477:1515‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hassanian SM, Ardeshirylajimi A, Dinarvand P, Rezaie AR. Inorganic polyphosphate promotes cyclin D1 synthesis through activation of mTOR/Wnt/beta‐catenin signaling in endothelial cells. J Thromb Haemost. 2016;14(11):2261‐2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hassanian SM, Dinarvand P, Smith SA, Rezaie AR. Inorganic polyphosphate elicits pro‐inflammatory responses through activation of the mammalian target of rapamycin complexes 1 and 2 in vascular endothelial cells. J Thromb Haemost. 2015;13(5):860‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kawazoe Y, Katoh S, Onodera Y, Kohgo T, Shindoh M, Shiba T. Activation of the FGF signaling pathway and subsequent induction of mesenchymal stem cell differentiation by inorganic polyphosphate. Int J Biol Sci. 2008;4(1):37‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]


