Abstract
Objective
To investigate the clinical effect of mouse nerve growth factor (mNGF) and methylcobalamin (MeCbl) for the treatment of lumbar disk herniation (LDH) with foot drop.
Methods
A total of 46 patients suffering from LDH with foot drop who underwent transforaminal lumbar interbody fusion (TLIF) surgery in our department from January 2015 to December 2017 were retrospectively analyzed. We divided these patients into two groups according to the different postoperative treatment which independently selected by patients after signing informed consent form: one group of 25 patients was treated with MeCbl alone (Group MeCbl), the other group of 21 patients was treated with a combination of mNGF and MeCbl (Group MeCbl+mNGF). Patient demographics, the visual analogue scale (VAS) scores, sensory and muscular strength improvement statistics at 1 week, 4 weeks, 12 weeks, and 12 months postoperatively were recorded. Motor/sensory deficits, sciatica and overall neurological outcome after treatment of MeCbl alone and combination of mNGF and MeCbl were retrospectively analyzed.
Results
The follow‐up ranged between 12 and 42 months (mean 20.8 months). There were no significant differences between these two groups of patients with respect to sex ratio, age, smoking, diabetes, disease course, section of protruding disc(s), muscular strength of foot dorsiflexion or preoperative visual analogue scale (VAS) score (P > 0.05). The VAS scores of Group MeCbl+mNGF were significantly lower than Group MeCbl at 1 week, 4 weeks, 12 weeks, and 12 months postoperatively (4.32 ± 0.75 vs 5.25 ± 0.79,2.65 ± 0.48 vs 3.42 ± 0.52, 1.72 ± 0.36 vs 2.45 ± 0.39, 1.12 ± 0.22 vs 1.52 ± 0.24, P < 0.05). The effective rates of sensory improvement were significantly higher in Group MeCbl+mNGF compared with Group MeCbl at 12‐week/12‐month follow‐up time point (90.48% vs 52.00%,95.24% vs 68.00%, P < 0.05). The effective rate of muscular strength improvement of the two groups did not differ significantly at 1 week after surgery but exhibited statistically significant differences at subsequent time points (61.90% vs 32.00%, 76.19% vs 44.00%, 80.95% vs 48.00%, P < 0.05).
Conclusions
Application of mNGF had clinical effects on promoting the recovery of neurological function in patients suffering from LDH with foot drop.
Keywords: Foot drop, Lumbar disc herniation, Methylcobalamin, Mouse nerve growth factor
Application of mNGF in combination with MeCbl had clinical effects on promoting the recovery of neurological function in patients suffering from LDH with foot drop.

Introduction
Foot drop, or palsy of tibialis anterior muscular is a complicated syndrome, which is clinically characterized by the difficulty or disability of foot dorsiflexion. It results from many different disorders, including spinal cord lesions, brain diseases, and lumbar degeneration diseases 1 , 2 . In the cases with foot drop caused by lumbar degeneration diseases, lumbar disc herniation (LDH) accounts for a large proportion 1 , 2 , 3 . LDH with foot drop is a particular type of lumbar degeneration disease. Although it is clinically rare compared with other complications as a result of LDH, accounting for 2.5% to 3% of all cases of lumbar disc herniation 1 , 2 , it still induces claudication symptoms and consequently influences the quality of life of patients. The occurrence of foot drop in patients with LDH indicates the severe nerve root compression and huge nucleus pulposus prolapse. Conservative therapies for this type of lumbar disc herniation alone are generally ineffective and difficult to achieve satisfactory outcomes; instead, surgery is recommended to completely relieve the compression of damaged nerve roots in many studies 3 , 4 . However, surgery for lumbar disc herniation with sciatica symptoms has been shown improved outcomes profoundly 4 , the complete remission of foot drop resulted from peripheral nerve dysfunction is extremely difficult to achieve due to the severe impairment of neurological function. Meanwhile, the routine drug treatment plan after surgery varies according to experience of different clinicians and an appropriate medication scheme remains unknown. Summarily, there exists a demand to undergo the relevant further study in the optimized combination of surgery and drug treatment to this particular disorder.
Nerve growth factor is a bioactive polypeptide. It is a regulatory factor of nerve cell growth that exhibits the dual biological functions of neuronal maintenance and the promotion of neurite growth 8 . We have previously used mouse nerve growth factor (mNGF) to treat patients with spinal cord injury and have demonstrated that mNGF shows good efficacy in the recovery of the spinal cord injury 12 . However, few clinical reports have examined the use of mNGF for the treatment of lumbar disc herniation with sciatica, particularly in cases that involve foot drop. Methylcobalamin (MeCbl) is the activated form of vitamin B12, and exerts neuronal protection by promoting regeneration of injured nerves and antagonizing glutamate‐induced neurotoxicity as an auxiliary agent 4 . The effects of MeCbl on recovery of peripheral neural injuries have been reported in many studies 5 , 6 , 7 . In the current study, we retrospectively analyzed patients administrated with mNGF/ MeCbl suffering from LDH with foot drop who underwent surgery in our department between January 2015 and December 2017. In particular, by comparing the clinical efficacy of a treatment that combined mNGF and MeCbl with which of MeCbl alone among these patients, the potential clinical applications of mNGF for the treatment of lumbar disc herniation with foot drop were investigated.
Accordingly, the objectives set to this current retrospective study are as follows: (i) statistically summarize the demographics and clinical characteristics of patients with foot drop due to LDH; (ii) investigate the potential therapeutic effects and efficacy of mNGF in treating LDH with foot drop particularly; and (iii) hopefully assist clinicians to select a treatment scheme with better neurological outcome in patients with LDH especially represented by foot drop.
Materials and Methods
Inclusion Criteria
Between January 2015 and December 2017, a total of 989 patients with lumbar disc herniation underwent surgery in our department. Our inclusion criterion was as follows: (i) age ranged from 35 to 55 years old and diagnosed with LDH by computed tomography (CT) and magnetic resonance imaging (MRI); (ii) patients suffered from foot drop and the course of low back pain and/or sciatica ranged from 6 to 24 months; (iii) patients treated with combination of mNGF and MeCbl or MeCbl alone after surgery; (iv) patients could be observed the recovery of neurological function by the specific methods; and (v) indicators observed could be retrospectively collected and statistically analyzed.
Exclusion Criteria
Our exclusion criterion was as follows: (i) took other medications known to improve the nerve function before surgery or during the follow‐up; (ii) took physical exercise known to improve the nerve function before surgery or during the follow‐up; (iii) with any other nerve system disease which could induce the similar symptom; and (iv) with severe heart/liver/kidney diseases or allergic to the medications.
General Data
Finally, an IRB approved retrospect cohort including 46 cases with complete data among the patients were analyzed in this study. Before surgery, these patients had experienced unilateral or bilateral pain, numbness, hypoaesthesia in their lower extremities and foot drop. Most of the patients were also hampered by lower back pain and limited waist mobility (38 cases). A small number of these patients also experienced urinary retention, saddle paraesthesia and other symptoms of cauda equina syndrome (six cases). Computed tomography (CT) and magnetic resonance imaging (MRI) indicated that the patients suffered from the protrusion or even the prolapse of lumbar intervertebral discs, which caused the unilateral or bilateral compression of nerve roots. Each patient was treated with MeCbl from Eisai Corporation (Suzhou, China) to improve the nerve function routinely the next day after the surgery. Whether the patients were treated with mNGF from Sinobioway Corporation (Xiamen, China) depends on the patients' willing.
All persons gave their informed consent prior to their inclusion in the study. Details that might disclose the identity of the subjects under study should be omitted. Our study protocol has been approved by the ethics committee of Zhongshan Hospital, Fudan University.
Treatment
The objective of surgery is decompression, discectomy and stabilization. The patients in both groups underwent laminoplasty and transforaminal lumbar interbody fusion (TLIF) with internal fixation operated by the same team in our department. The next day after surgery, patients in Group MeCbl+mNGF were treated with a combination of 9000 IU of mNGF and 500 μg of MeCbl by intramuscular injection, whereas patients in Group MeCbl were intramuscularly injected with 500 μg of MeCbl alone. Both groups were injected once daily over a four‐week course of treatment.
Outcome Measures
General Demographics
Factors including sex ratio, age, smoking, diabetes, disease course, section of protruding disc(s), muscular strength of foot dorsiflexion, preoperative visual analogue scale (VAS) score, effective rates of sensory improvement were collected and measured. The presence or absence of postoperative infection and other complications, such as long‐term deficiencies in interbody fusion or fractures related to internal fixation, were also recorded.
Muscular Strength
Muscular strength of foot dorsiflexion was defined as determining muscular strength of the tibialis anterior using a manual muscle test and by characterizing it according to the Medical Research Council scale into 6 grades 2 . To be specific, grade 0 indicates no contraction of tibialis anterior; grade 1 indicates slight contraction of tibialis anterior is observed, but no joint motion of ankle; grade 2 indicates patients can invert and dorsiflex ankle with gravity eliminated through full range of motion; grade 3 indicates patient can invert and dorsiflex ankle against gravity through partial range of motion; grade 4 indicates patients can dorsiflex and invert ankle against gravity and moderate resistance; grade 5 indicates patients can dorsiflex and invert ankle against gravity and full resistance.
Visual Analogue Scale (VAS) Score
Visual analogue scale (VAS) score was a routine measurement of pain intensity which is scored from 0 to 10 according to the patient's subjective sense of pain. It is measured with the distance(mm) on the 10 cm line between the “no pain” anchor and the patient's mark 16 .
Neurological Function Assessment
The recovery of neurological function was evaluated by the following criteria. A highly effective recovery was defined as a case in which there was a significantly improvement in sensory disturbance, a total recovery from foot drop, or two or more levels of improvement in tibialis anterior muscular strength by the manual muscle test. An effective recovery was defined as a case in which there was a partial improvement in sensory disturbance, a partial recovery from foot drop, and an improvement in tibialis anterior muscular strength by no more than two levels. An ineffective recovery was defined as a case in which sensory disturbance was unimproved and there was no tibialis anterior muscular strength recovery.
Statistical Analysis
The IBM SPSS Statistics 19.0 (SPSS Inc., Chicago, United States) was used for data analysis, and continuous data were expressed as means ± standard deviation (SD). Continuous data from the two groups of patients were compared using the independent samples t‐test, and discrete data were compared using the chi‐square test. Repeated measures analyses of variance were used to compare patients' indicators at each time point before and after surgery. The least significant difference (LSD) t‐test was used for within‐group pairwise comparisons. P < 0.05 was regarded as a significant difference.
Results
General Results
A total of 46 patients were divided into two groups according to the postoperative treatment (Table 1). In particular, one group of patients was treated with MeCbl alone (Group MeCbl, consisting of 25 cases), whereas the other group of patients was treated with a combination of mNGF and MeCbl (Group MeCbl+mNGF, consisting of 21 cases). There were no significant differences between these two groups of patients with respect to sex ratio, age, smoking, diabetes, disease course, section of protruding disc(s), muscular strength of foot dorsiflexion or preoperative visual analogue scale (VAS) score (P > 0.05). Thus, these two groups were comparable and our study was a non‐random retrospective analysis.
TABLE 1.
Patient characteristics of the two groups (mean ± SD; P < 0.05 was identified with significant difference; n = number)
| Group | MeCbl | (n = 25) | MeCbl+mNGF | (n = 21) | Statistic value | P value | |
|---|---|---|---|---|---|---|---|
| Sex (male/female) | 12/13 | 11/10 | <0.001 | 0.979 | |||
| Age (year) | 49.65 ± 12.34 | 50.15 ± 12.80 | −0.107 | 0.846 | |||
| Smoking (Y/N) | 12/13 | 11/10 | <0.001 | 0.979 | |||
| Diabetes (Y/N) | 5/20 | 4/17 | 0.007 | 0.935 | |||
| Course of low back pain and sciatica (month) | 18.85 ± 4.28 | 18.66 ± 4.83 | −0.086 | 0.932 | |||
| Course of foot drop (week) | 2.40 ± 0.50 | 2.57 ± 0.51 | 1.151 | 0.256 | |||
| The segment of the lumbar disc herniation (n) | L3 /4 | 1 | 1 | 0.038 | 1.000 | ||
| L4/5 | 11 | 9 | |||||
| L5/S1 | 7 | 6 | |||||
| L3/4/5 | 3 | 2 | |||||
| L4/5/S1 | 3 | 3 | |||||
| Muscular strength of foot dorsiflexion (n) | grade 0 | 2 | 2 | 0.024 | 0.988 | ||
| grade 1 | 6 | 5 | |||||
| grade 2 | 17 | 14 | |||||
All patients underwent follow‐up via outpatient for a period of 12–42 months, with a mean follow‐up duration of 20.8 months.
Visual Analogue Scale (VAS) Scores
For both groups of patients, the VAS scores at 1 week, 4 weeks, 12 weeks, and 12 months after treatment significantly differed from pre‐treatment VAS scores (P < 0.05) (Fig. 1). Furthermore, the VAS scores of Group MeCbl+mNGF were significantly lower than the VAS scores of Group MeCbl at 1 week (4.32 ± 0.75 vs 5.25 ± 0.79, P < 0.05), 4 weeks (2.65 ± 0.48 vs 3.42 ± 0.52, P < 0.05), 12 weeks (1.72 ± 0.36 vs 2.45 ± 0.39, P < 0.05), and 12 months (1.12 ± 0.22 vs 1.52 ± 0.24, P < 0.05).
Fig 1.

VAS scores of Group MeCbl+mNGF were significantly lower than that of Group MeCbl at 1 week (4.32 ± 0.75 vs 5.25 ± 0.79, P < 0.05), 4 weeks (2.65 ± 0.48 vs 3.42 ± 0.52, P < 0.05), 12 weeks (1.72 ± 0.36 vs 2.45 ± 0.39, P < 0.05), and 12 months (1.12 ± 0.22 vs 1.52 ± 0.24, P < 0.05). *Significant when P < 0.05.
Neurological Function Recovery
In both groups, all of the patients with urinary retention (three patients in Group MeCbl+mNGF and three patients in Group MeCbl) recovered normal urinary function by 4 weeks after treatment. Higher effective rates of sensory improvement were observed in Group MeCbl+mNGF than in Group MeCbl and exhibited statistically significant differences at 1 week (52.38% vs 24.00%, P < 0.05), 4 weeks (76.19% vs 44.00%, P < 0.05), 12 weeks (90.48% vs 52.00%, P < 0.05), and 12 months (95.24% vs 68.00%, P < 0.05) after treatment (Fig. 2).
Fig 2.
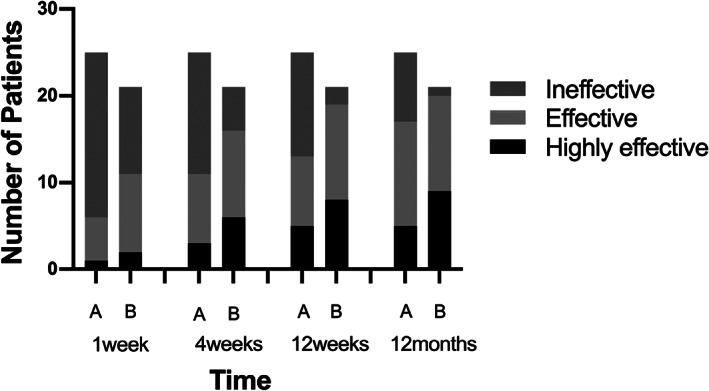
Distribution of neurological recovery level in patients in two groups. More significant sensory improvements were found in patients in Group MeCbl+mNGF at 1 week, 4 weeks, 12 weeks, and 12 months after treatment (A = MeCbl, B = mNGF+MeCbl).
Muscular Strength Recovery
More significant muscular strength improvements were found in patients in Group MeCbl+mNGF at 4 weeks, 12 weeks, and 12 months after treatment (Fig. 3), and the effective rate of muscular strength improvement of the two groups of patients did not differ significantly at 1 week after surgery (14.29% vs 12.00%, P > 0.05) but showed significant differences at 4 weeks (61.90% vs 32.00%, P < 0.05), 12 weeks (76.19% vs 44.00%, P < 0.05) and 12 months (80.95% vs 48.00%, P < 0.05) after treatment (Table 2).
Fig 3.

Distribution of muscular strength recovery level in patients in two groups. More significant muscular strength improvements were found in patients in Group MeCbl+mNGF at 4 weeks, 12 weeks, and 12 months after treatment (A = MeCbl, B = mNGF+MeCbl).
TABLE 2.
A comparison of the effective rates (%) of neurological improvement for the two groups of patients at 1 week, 4 weeks, 12 weeks, and 12 months after treatment (P < 0.05 was identified with significant difference)
| Group | 1 week after treatment | 4 weeks after treatment | 12 weeks after treatment | 12 months after treatment | ||||
|---|---|---|---|---|---|---|---|---|
| Effective rate of sensory improvement | Effective rate of muscular strength improvement | Effective rate of sensory improvement | Effective rate of muscular strength improvement | Effective rate of sensory improvement | Effective rate of muscular strength improvement | Effective rate of sensory improvement | Effective rate of muscular strength improvement | |
| MeCbl (n = 25) | 24.00 (6) | 12.00 (3) | 44.00 (11) | 32.00 (8) | 52.00 (13) | 44.00 (11) | 68.00 (17) | 48.00 (12) |
| mNGF+MeCbl (n= 21) | 52.38 (11) | 14.29 (3) | 76.19 (16) | 61.90 (13) | 90.48 (19) | 76.19 (16) | 95.24 (20) | 80.95 (17) |
| Pearson chi‐square | 3.946 | 0.053 | 4.878 | 4.114 | 7.980 | 4.878 | 5.381 | 5.319 |
| P | 0.047* | 0.819 | 0.027* | 0.043* | 0.004* | 0.027* | 0.020* | 0.021* |
*P < 0.05.
Complications
No patients died during the follow‐up period. Two patients of the Group MeCbl+mNGF had minor rashes and itch. Rashes and itch recovered after symptomatic treatment, which did not affect the following treatment. The surgical incisions of patients in both groups exhibited primary healing without infections or other complications. Interbody fusion was achieved in all patients by 6 to 12 months after surgery (with a mean duration of 8 months), and stable internal fixation without fracture occurred in all cases.
Discussion
It is essential for the recovery of neurological function of the patients suffering from lumbar disc herniation with foot drop to relieve the decompression of the nerve roots 17 , 18 . The occurrence of foot drop indicates the severe nerve root compression and huge nucleus pulposus prolapse 18 , 19 , 20 . Before surgery, CT and MRI showed that the patients suffered from the protrusion or even the prolapse of lumbar intervertebral discs. The prolapse of lumbar discs may indicate segmental instability and to relieve that situation, surgical intervention especially decompression and TLIF is recommended by many surgeons with exactly proven effects nowadays 1 , 3 , 19 . Nevertheless, the effective recovery from foot drop is still a tremendous task even after those who underwent surgery.
MeCbl, a traditional neurotrophic drug, is a coenzyme form of vitamin B12 4 . MeCbl facilitates the repair of damaged nerve tissue by enhancing nerve tissue metabolism and promoting the synthesis of not only lecithin, the major component of myelin, but also various nucleic acids and proteins of nerve cells, including axonal proteins 4 , 5 , 6 . MeCbl has been widely used for the treatment of nerve injury and has demonstrated good clinical efficacy. The use of neurotrophic drugs is an essential aspect of the recovery. Nerve growth factor (NGF) is a bioactive polypeptide and the first neurotrophic factor to be discovered. It is a regulatory factor of nerve cell growth that exhibits the dual biological functions of neuronal maintenance and the promotion of neurite growth 8 . NGF causes the enlargement of sensory and sympathetic neurons, accelerates mitosis, increases the number of nerve cells, and induces the directional growth of nerve fibers. In addition, it improves neuronal survival, stimulates the development of neuronal soma and dendrites, enhances the density of nerve fibers in targeted areas, and exerts a protective effect on injured neurons 9 , 13 , 14 . The functions of NGF include accelerating the regeneration of peripheral nerves and promoting the repair of damage to the central nervous system and/or peripheral nerves 10 , 11 . The mNGF is a bioactive protein extracted from the submandibular glands of mice. This protein has a relative molecular mass of 26.5 × 103 and is more than 90% homologous to human NGF. In the human nervous system, mNGF exhibits neurotrophic effects on normal cells and promotes the recovery of injured nerves. It has been widely used for the treatment of central nervous system injuries and achieved good clinical effects. Thus, mNGF is an ideal drug for the treatment of central nervous system damage. Moreover, earlier mNGF administration is associated with more rapid recovery and greater efficacy 9 . In Zeng's retrospective study, they used mNGF combined with methylprednisolone sodium succinate (MP) to treat acute spinal cord injury and cauda equina injury. The improvement rates of neurological function of combined group were significantly higher than those of patients treated with MP alone 15 . However, few clinical reports have examined the use of mNGF for the treatment of lumbar disc herniation with sciatica, particularly in cases that involve motor dysfunction, such as foot drop. Given the good clinical effect of mNGF in the treatment of spinal cord injury, we investigated the effects of a combination of mNGF and MeCbl on the treatment of patients suffering from lumbar disc herniation with foot drop. Each patient was treated with MeCbl to improve the nerve function routinely postoperatively in our institute. From our point of view, the patient who suffer from the nerve injury is treated without any neurotrophic drugs, which may violate ethic. Therefore, in the retrospective study there were no patients treated only with surgery.
Our study showed that the combination of mNGF and MeCbl had good clinical efficacy, and no specific complications were observed among these patients. Our study suggests that mNGF may safely be used to treat lumbar disc herniation with foot drop. At each time point after surgery, the VAS scores of Group MeCbl+mNGF were significantly lower than the VAS scores of Group MeCbl, indicating that treatment with a combination of mNGF and MeCbl was more effective than treatment with MeCbl alone. Follow‐up of the recovery of neurological function produced the following results. (i) At 1 week after surgical treatment, the effective rates of sensory improvement for Group MeCbl+mNGF and Group MeCbl were 52.38% and 24%, respectively. Thus, greater improvement was observed in Group MeCbl+mNGF than in Group MeCbl, and the difference in the improvement rates of these two groups was statistically significant. The effective rates of muscular strength improvement for Group MeCbl+mNGF and Group MeCbl were 14.29% and 12%, respectively. Although this rate was greater for Group MeCbl+mNGF than for Group MeCbl, the difference in rates between the two groups was not statistically significant. These results indicate that even in the early stages of the treatment process, the use of mNGF for the treatment of lumbar disc herniation with foot drop markedly improved patients' sensory and motor function and their quality of life. Earlier mNGF treatment was associated with more rapid recovery. During early stages of mNGF treatment, the recovery of sensory function was more pronounced than the recovery of motor function. (ii) By the end of a four‐week treatment with a neurotrophic drug regimen, patients in both groups with urinary retention had recovered normal urinary function. There was no difference between two groups about the urinary retention. At this time, the effective rates of sensory improvement for Group MeCbl+mNGF and Group MeCbl were 76.19% and 44%, respectively, and the effective rates of muscular strength improvement for Group MeCbl+mNGF and Group MeCbl were 61.9% and 32%, respectively. Both of these rates of improvement were significantly higher for Group MeCbl+mNGF than for Group MeCbl. These results indicate that although MeCbl treatment produced good recovery of neurological function among patients suffering from lumbar disc herniation with foot drop, the treatment that combined mNGF and MeCbl exhibited superior efficacy. The effects of mNGF and MeCbl may combine in a synergistic manner to facilitate the recovery of patients' neurological function. (iii) At 12 weeks and 12 months after surgery, greater effective rates of sensory improvement and muscular strength improvement were observed for Group MeCbl+mNGF than for Group MeCbl. These results demonstrated that the examined neurotrophic drugs produced persistent long‐term effects that caused the increasingly apparent recovery of neurological function over time.
The current study revealed that a treatment that combined mNGF and MeCbl exhibited good clinical efficacy for the treatment of lumbar disc herniation with foot drop. Among patients suffering from lumbar disc herniation with foot drop, this treatment promoted the recovery of neurological function and improved quality of life; thus, this approach demonstrates clinical applicability.
However, it is of great significance to note limitations about the present study. First, due to the lack of randomization and its retrospective nature, the study we analyzed was only a process of hypothesis‐generating, further prospective cohort study and randomized controlled trials are needed to make more convincing results. Additionally, we were unable to set an agent‐free group as the blank controlled group which might violate the ethics, so our results are insufficient with comparison. Besides, the data of electromyography and other specific examination is missing in the period of follow‐up.
Conclusions
Application of mNGF in combination with MeCbl had clinical effects on promoting the recovery of neurological function in patients suffering from LDH with foot drop. Yet, a prospective randomized clinical trial is needed to confirm the conclusion.
Grant Sources: The study was funded by the following found program: Program for Outstanding Medical Academic Leader of Shanghai (LJ10017), Shanghai International Science and Technology Partnership Program (11540702700).
Disclosure: The authors declare that they have no conflict of interest. The authors did not have any conflict of interest with the pharmaceutical factory in production of mNGF.
The manuscript has been read and approved by all the authors and that the requirements for authorship as stated earlier in this document have been met and that each author believes that the manuscript represents honest work.
Reference
- 1. Iizuka Y, Iizuka H, Tsutsumi S, et al. Foot drop due to lumbar degenerative conditions: mechanism and prognostic factors in herniated nucleus pulposus and lumbar spinal stenosis. J Neurosurg Spine, 2009, 10: 260–264. [DOI] [PubMed] [Google Scholar]
- 2. Aono H, Iwasaki M, Ohwada T, et al. Surgical outcome of drop foot caused by degenerative lumbar diseases. Spine (Phila Pa 1976), 2007, 32: 262–266. [DOI] [PubMed] [Google Scholar]
- 3. Takaishi Y, Okada M, Fujiwara D, Uyama A, Kondoh T, Arai A. Surgical results of lumbar degenerative disease with foot drop. No Shinkei Geka, 2019, 47: 851–857. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Nataraj A. Foot drop resulting from degenerative lumbar spinal diseases: clinical characteristics and prognosis. Clin Neurol Neurosurg, 2014, 117: 33–39. [DOI] [PubMed] [Google Scholar]
- 5. Zhang M, Han W, Hu S, Xu H. Methylcobalamin: a potential vitamin of pain killer. Neural Plast, 2013, 2013: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spence JD. Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementia and stroke. Nutr Res, 2016, 36: 109–116. [DOI] [PubMed] [Google Scholar]
- 7. Thakkar K, Billa G. Treatment of vitamin B12 deficiency‐methylcobalamine? Cyancobalamine? Hydroxocobalamin?‐clearing the confusion. Eur J Clin Nutr, 2015, 69: 1–2. [DOI] [PubMed] [Google Scholar]
- 8. Aloe L. Rita Levi‐Montalcini: the discovery of nerve growth factor and modern neurobiology. Trends Cell Biol, 2004, 14: 395–399. [DOI] [PubMed] [Google Scholar]
- 9. Bennet MR, Gibson WG, Lemon G. Neuronal cell death, nerve growth factor and neurotrophic models: 50 years on. Auton Neurosci, 2002, 95: 1–23. [DOI] [PubMed] [Google Scholar]
- 10. Chen ZY, Chai YF, Cao L, et al. Glial cell line‐derived neurotrophic factor enhances axonal regeneration following sciatic nerve transection in adult rats. Brain Res, 2001, 902: 272–276. [DOI] [PubMed] [Google Scholar]
- 11. Huang F, Liu Z, Liu H, et al. GM1 and NGF modulate Ca2 + homeostasis and GAP43 mRNA expression in cultured dorsal root ganglion neurons with excitotoxicity induced by glutamate. Nutr Neurosci, 2007, 10: 105–111. [DOI] [PubMed] [Google Scholar]
- 12. Lin H, Li C, Jiang YQ, et al. Combination of mouse nerve growth factor and mecobalamin for treatment of incomplete spinal cord injury without high‐dose methylprednisolone therapy. Chin J Orthop Trauma, 2012, 14: 207–210. [Google Scholar]
- 13. Micera A, Puxeddu I, Aloe L, et al. New insights on the involvement of nerve growth factor in allergic inflammation and fibrosis. Cytokine Growth Factor Rev, 2003, 14: 369–374. [DOI] [PubMed] [Google Scholar]
- 14. Wild KD, Bian D, Zhu D, et al. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther, 2007, 322: 282–287. [DOI] [PubMed] [Google Scholar]
- 15. Zeng Y, Xiong M, Yu H, et al. Clinical effect of methylprednisolone sodium succinate and mouse nerve growth factor for injection in treating acute spinal cord injury and cauda equina injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2010, 24: 1208–1211. [PubMed] [Google Scholar]
- 16. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short‐form McGill pain questionnaire (SF‐MPQ), chronic pain grade scale (CPGS), short form‐36 bodily pain scale (SF‐36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res (Hoboken), 2011, 63: 240–252. [DOI] [PubMed] [Google Scholar]
- 17. Macki M, Lim S, Elmenini J, Fakih M, Chang V. Clinching the cause: a review of foot drop secondary to lumbar degenerative diseases. J Neurol Sci, 2018, 395: 126–130. [DOI] [PubMed] [Google Scholar]
- 18. Liu K, Zhu W, Shi J, et al. Foot drop caused by lumbar degenerative disease: clinical features, prognostic factors of surgical outcome and clinical stage. PLoS One, 2013, 8: e80375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macki M, Syeda S, Kerezoudis P, Gokaslan ZL, Bydon A, Bydon M. Preoperative motor strength and time to surgery are the most important predictors of improvement in foot drop due to degenerative lumbar disease. J Neurol Sci, 2016, 361: 133–136. [DOI] [PubMed] [Google Scholar]
- 20. Ma J, He Y, Wang A, et al. Risk factors analysis for foot drop associated with lumbar disc herniation: an analysis of 236 patients. World Neurosurg, 2018, 110: 1017–1024. [DOI] [PubMed] [Google Scholar]


