ABSTRACT
Obesity is a modifiable risk factor in the development of type 2 diabetes mellitus (T2DM), with the prevalence of both increasing worldwide. This trend is associated with increasing mortality, cardiovascular risk and healthcare costs. An individual's weight will be determined by complex physiological, psychological and societal factors. Assessment by a skilled multidisciplinary team will help identify these factors and will also support screening for secondary causes, assessing cardiovascular risk and identifying sequelae of obesity.
A range of treatment options are available for people with obesity and T2DM, including low-calorie diets, medications and bariatric surgery. People should be carefully counselled and personalised care plans developed. Bariatric surgery is an under-utilised resource in this context.
Obesity should also be considered when choosing medical therapy for T2DM. Common diabetes medications may lead to weight gain whereas others (such as glucagon-like peptide-1 agonists and sodium-glucose cotransporter-2 inhibitors) support weight loss.
Bariatric surgery improves obesity-related complications and all-cause mortality. Diabetes remission is possible after surgery and is recommended by National Institute for Health and Care Excellence in individuals with a body mass index of >35 kg/m2 and recent onset T2DM.
KEYWORDS: diabetes, obesity, bariatrics, GLP-1, total diet replacement
Key points
Being overweight or obese is a key modifiable risk factor in the development of diabetes, with 90% of patients with diabetes being classified as overweight or obese.
A multidisciplinary approach to management is recommended and assessment beyond BMI using obesity staging is valuable.
Low-calorie diet programmes have been shown to support remission of diabetes and are currently being piloted throughout the UK.
Medications used in the management of diabetes can have a positive, negative or neutral effect on weight. Potent weight loss maybe seen with newer agents.
Bariatric surgery is a safe, effective therapy but is underutilised for people with type 2 diabetes mellitus and obesity.
Introduction
Obesity and type 2 diabetes mellitus (T2DM) are closely linked and are increasing in prevalence worldwide. Both chronic conditions have multisystem impact and are associated with increased mortality and cardiovascular risk.1 Individuals from non-White communities and those living in deprived areas are disproportionately affected.2 These associations were clearly highlighted during the recent COVID-19 pandemic.3
Obesity is a key modifiable risk factor for the development of diabetes, with 90% of adults with T2DM classified as overweight or obese. There is an estimated threefold increase in the development of diabetes associated with being overweight and a 7-fold increase in those with obesity. Current models predict 9.5% of the adult population will have diabetes by 2030 and a third of this increase can be directly attributable to obesity. By 2050, the cost to the NHS of overweight- and obesity-related morbidity is estimated to be £9.7 billion, with wider society costs reaching almost £50 billion.4
Pathophysiology of obesity and T2DM
The mechanisms linking obesity and T2DM are complex and still being understood, but likely involve a combination of:
An approach to the patient with obesity and diabetes
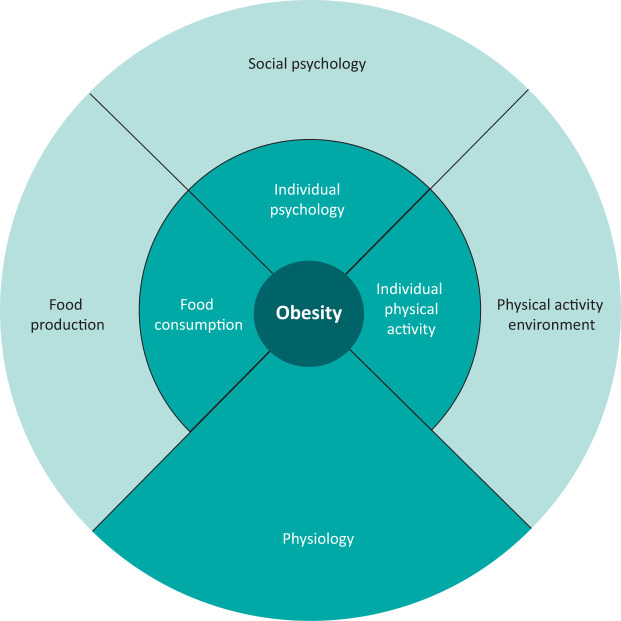
An individual with T2DM and obesity may present to a range of specialties. Typically, recurrent patterns of dieting and weight regain are described (Box 1). As with obesity management in any context, this should be explored in a non-judgmental way and in the context of expected physiological changes which accompany dieting.8 It is also worth considering the many complex factors that contribute to obesity (Fig 1) alongside the stigmatisation and social isolation often experienced by patients.9
Box 1.
Challenges in weight modification: perspectives
|
Fig 1.
A simplified version of the full generic obesity system map clusters.9
Consideration of context will support the design of an effective and individualised care plan. Motivational interviewing and SMART goal setting are useful techniques. SMART goals are built around the following criteria: specific, measurable, achievable, relevant and time-bound. Assessment beyond body mass index (BMI) is also valuable; for example, King's Obesity Staging Criteria (Table 1) supports conversations around how obesity is affecting an individual.10 It may also form a useful framework to discuss intervention. A complete assessment of obesity requires a skilled multidisciplinary team.
Table 1.
Modified King's Obesity Staging Criteria9
| Stage 0 ‘Normal health’ |
Stage 1 ‘At risk of disease’ |
Stage 2 ‘Established disease’ |
Stage 3 ‘Advanced disease’ |
|
|---|---|---|---|---|
| Airways | Normal | Snoring | Required CPAP | Cor pulmonale |
| Body mass index | <35 | 35–40 | 40–60 | >60 |
| Cardiovascular | <10% risk | 10–20% risk | Heart disease | Heart failure |
| Diabetes | Normal | Impaired fasting glucose | Type 2 diabetes | Uncontrolled type 2 diabetes |
| Economic | Normal | Expensive travel/clothes | Workplace discrimination | Unemployed due to obesity |
| Functional | Can manage three flights of stairs | Manages one or two flights of stairs | Requires walking aids or wheelchair | Housebound |
| Gonadal | Normal | PCOS | Infertility | Sexual dysfunction |
| Health perceived | Normal | Low mood or QoL | Depression or poor QoL | Severe depression |
| Body image | Normal | Dislikes body | Body image dysphoria | Eating disorder with purging (drug use or vomiting) |
CPAP = continuous positive airway pressure; PCOS = polycystic ovary syndrome; QoL = quality of life.
Clinical assessment
Detailed history taking and evaluation is described elsewhere.11 Aspects to consider include:
age of onset of excess weight
where onset was as a young child, whether other traits are present suggestive of genetic syndromes
family history of obesity and its pattern, especially if severe obesity is dichotomously present with normal weight
pattern of weight gain, noting periods of acceleration or weight loss and their relation to health or life events
intake of alcohol or other highly calorific liquids
success and failure of previous attempts at losing weight.11
National Institute for Health and Care Excellence (NICE) guidelines (CG189) also provide further advice on the assessment of obesity.12 During examination of a person with diabetes and obesity, aspects to consider include cardiovascular risk, secondary obesity (including genetic causes and endocrinopathies such as Cushing's syndrome) and sequelae (eg osteoarthritis and sleep apnoea). A thorough assessment, combined with a sensitive approach which considers context, provides the foundation to discuss intervention.
Lifestyle interventions
Individualised advice on diet and physical activity, combined with a personalised diabetes care plan, underpins all approaches. The Look AHEAD Trial compared a 4-year intensive programme (including lifestyle counsellor, dietary interventions, portion-controlled meal plans, physical activity and behavioural modification techniques) with a diabetes support/education (DSE) group and usual medical care.13 The intensive intervention group showed mean weight loss at 1 year of –8.6% versus –0.7% in the DSE group. This was sustained over 4 years with a mean weight loss of –6.15% and –0.88% in the intervention and DSE groups, respectively. In a separate study, an intensive diet intervention soon after diagnosis was shown to improve glycaemic control.14 Unfortunately, weight maintenance has been reported to be a challenge following lifestyle-induced weight loss.15
Low-calorie diets
T2DM has often been described as a chronic and progressive condition. The landmark Diabetes Remission Clinical Trial (DiRECT) demonstrated, however, that diabetes remission was possible through a low-calorie total diet replacement programme.16
The control group received best-practice diabetes care while the intervention group received a structured weight management programme and total diet replacement (825–853 kcal/day) for 12–20 weeks. This was followed by stepped food reintroduction over 2–8 weeks and support for weight maintenance. At 24 months, 36% of intervention participants achieved remission of diabetes (defined by HbA1c <48 mmol/mol on no diabetic medications). The mean change in body weight between control and intervention group was –5.4 kg. Remission was closely related to weight loss, with 64% of those with at least 10 kg weight loss achieving diabetes remission. Sustained remission at 2 years for more a third of people with T2DM has been reported. Based on this evidence, the NHS Low Calorie Diet Programme is currently being piloted throughout the UK and may be effectively delivered in a primary care setting for people with obesity.17
In clinical practice, screening, engagement and specialist support are important elements for success of a total diet replacement programme. Close review of glycaemic control and diabetes ‘deprescribing’ are required. Behavioural support and self-monitoring are valuable components. Often people report appreciating the opportunity to ‘reset’ their relationship with food and eating; one patient commented ‘I’m currently on a total diet replacement (TDR) which means I don’t have to think about food choices’.
Recent evidence suggests that the prevention of weight regain following TDR may require the addition of components such as intensive exercise.18
Medical therapy
Prescribing options for T2DM and obesity have widened substantially in recent years. Large-scale trials demonstrate significant weight loss as well as cardiovascular benefits with agents such as glucagon-like peptide-1 agonists and sodium-glucose cotransporter-2 inhibitors. American Diabetes Association / European Association for the Study of Diabetes guidance reflects this and advocates treating T2DM with consideration for comorbidities such as obesity.19
When prescribing for a person with T2DM and obesity, it is useful to articulate that the agent is only a tool within a multicomponent treatment strategy. Full counselling on expected benefits, side effects and cessation criteria should be provided. This is particularly pertinent for women of childbearing age.
Challenges in prescribing include weight gain associated with certain agents and also with glycaemic improvement. Medications licensed for use in T2DM, mechanisms and overall weight effect are summarised in Table 2.19
Table 2.
Type 2 diabetes mellitus mediations and their overall weight effect
| Drug | Mechanism of action | Weight effect |
|---|---|---|
| Medications with a weight neutral or weight loss effect | ||
| Alpha-glucosidase inhibitors (acarbose) | Delays breakdown of polysaccharides by blocking gut enzymes and decreasing postprandial glucose spike | 0 kg to –0.2 kg |
| Biguanides (metformin) | Improves peripheral insulin resistance and normalises hepatic glucose output | 0 kg to –3.8 kg |
| DDP-4 inhibitors | Inhibits DPP4, the enzyme which inactivates GLP-1, thereby extending the metabolic effects of GLP-1 | 0 kg to –0.4 kg |
| GLP-1 agonists | Acts as GLP-1 mimetic. GLP1:
|
–1.3 kg to –7.2 kg |
| SGLT2 inhibitors | Inhibits glucose reabsorption in proximal tubule of the kidney and cause excess urinary glucose excretion; note risk of euglycaemic diabetic ketoacidosis | –1.5 kg to –2.4 kg |
| Medications with a weight gain effect | ||
| Insulin analogues, isophane insulin or animal insulin | Perform the same as human insulin | +3.9 kg to +5.0 kg |
| Sulphonylurea | Acts as an insulin secretagogue on receptors within beta-cells, increasing insulin secretion | +1.6 kg to +2.6 kg |
| Thiazolidinediones | Activates PPAR-G receptors to decrease insulin resistance and increase glucose uptake in cells
Activating of PPAR-G cells on adipocytes can stimulate adipogenesis |
+4.2 kg to +4.8 kg |
GLP-1 = glucagon-like peptide-1; PPAR-G = peroxisome proliferator-activated receptor gamma; SGLT2 = sodium-glucose cotransporter-2.
Orlistat is licensed in the management of obesity and NICE recommends its prescription as part of an overall plan for managing obesity in those with BMI >30 kg/m2 or >28 kg/m2 with other associated risk factors. Side effects are often poorly tolerated, however.
Surgical therapy
Bariatric surgery is an effective and durable treatment for severe obesity, linked to improvements in obesity-related comorbid conditions as well as all-cause mortality. A systematic review and metanalysis of 621 studies with 135,246 patients undergoing a range of bariatric procedures reported an overall weight loss of 38.5 kg or 55.9% excess body weight loss.20 78.1% of diabetic patients reached remission and a further 8.5% had an improvement in their diabetic control. A low prevalence of relapse has been reported in those who achieve remission.21
Given the factors associated with diabetes remission, NICE recommend expedited bariatric surgery for people with BMI of 35 kg/m2 or over who have recent onset T2DM (<10 years). Those with BMI 30–34.9 kg/m2 and recent onset T2DM should also be considered for assessment. Lower BMI cut-offs (reduced by 2.5 kg/m2) are recommended for people of Asian origin.
Unfortunately, less than 1% of people who are eligible are referred for and receive this effective and safe intervention.22
While surgery is typically safe in carefully selected and prepared individuals, complications including dumping syndrome and vitamin deficiencies are recognised.
Conclusion
A range of options is available for the treatment of diabetes and obesity, with significant advances made in recent years. In order to provide the most effective treatment plan, it is important to assess and communicate the risks of obesity to an individual in a manner they understand. It is also vital to appreciate the barriers they face. Glycaemic improvement and diabetes remission are realistic goals and people should be empowered to seek out such options when they are ready to do so.
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world -a growing challenge. N Engl J Med 2007;356:213–5. [DOI] [PubMed] [Google Scholar]
- 2.Marmot M, Goldblatt P, Allen J, et al. Fair Society, Healthy Lives (The Marmot Review). Department for International Development, 2010. www.gov.uk/research-for-development-outputs/fair-society-healthy-lives-the-marmot-review-strategic-review-of-health-inequalities-in-england-post-2010 [Google Scholar]
- 3.Taher N, Huda MSB, Chowdhury TA. COVID-19 and diabetes: what have we learned so far? Clin Med 2020;20:e87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatineau M, Hancock C, Holman N, et al. Adult obesity and type 2 diabetes. Public Health England, 2014. [Google Scholar]
- 5.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 2014;7:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day C, Bailey CJ. Obesity in the pathogenesis of type 2 diabetes. Br J Diabetes Vasc Dis 2011;11:55–61. [Google Scholar]
- 7.All-party Parliamentary Group on Obesity . The current landscape of obesity services. APPG, 2018. www.rcpch.ac.uk/sites/default/files/2018-05/report_appg_obesity_2018.pdf [Google Scholar]
- 8.MacLean PS, Blundell JE, Mennella JA, Batterham RL. Biological control of appetite: a daunting complexity. Obesity 2017;25:S8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandenbroeck P, Goosens J, Clemens M. Tackling Obesities: Future Choices – Obesity System Atlas. Government Office for Science, 2007. www.gov.uk/government/collections/tackling-obesities-future-choices [Google Scholar]
- 10.Aasheim ET, Aylwin SJB, Radhakrishnan ST, et al. Assessment of obesity beyond body mass index to determine benefit of treatment. Clin Obes 2011;1:77–84. [DOI] [PubMed] [Google Scholar]
- 11.Crane JD, McGowan BM. Clinical assessment of the patient with overweight or obesity. In: Sbraccia P, Finer N. (eds), Obesity. Endocrinology. Springer, 2017. [Google Scholar]
- 12.National Institute for Health and Care Excellence . Obesity: identification, assessment and management: Clinical guideline [CG189]. NICE, 2014. www.nice.org.uk/guidance/cg189. [PubMed] [Google Scholar]
- 13.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the early ACTID randomised controlled trial. Lancet 2011;378:129–39. [DOI] [PubMed] [Google Scholar]
- 15.Mann T, Tomiyama AJ, Westling E, et al. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol 2007;62:220–33. [DOI] [PubMed] [Google Scholar]
- 16.Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–55. [DOI] [PubMed] [Google Scholar]
- 17.Jebb SA, Astbury NM, Tearne S, Nickless A, Aveyard P. Doctor referral of overweight people to a low-energy treatment (DROPLET) in primary care using total diet replacement products. BMJ Open 2017;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide or both combined. N Engl J Med 2021:384:1719–30. [DOI] [PubMed] [Google Scholar]
- 19.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–56. [DOI] [PubMed] [Google Scholar]
- 21.Conte C, et al. Diabetes remission and relapse after bariatric surgery; a nationwide population-based study. Obes Surg 2020;30:4810–20. [DOI] [PubMed] [Google Scholar]
- 22.Desogus D, Menon V, Oyebode O. An examination of who is eligible and who is receiving bariatric surgery in England: secondary analysis of the health survey for England dataset. Obesity Surg 2019;29:3246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]