Summary
Considering its worldwide abundance, cellulose can be a suitable candidate to replace the fossil oil‐based materials, even if its potential is still untapped, due to some scientific and technical gaps. This work offers new possibilities demonstrating for the first time the ability of a cerato‐platanin, a small fungal protein, to valorize lignocellulosic Agri‐food Wastes. Indeed, cerato‐platanins can loosen cellulose rendering it more accessible to hydrolytic attack. The cerato‐platanin ThCP from a marine strain of Trichoderma harzianum, characterized as an efficient biosurfactant protein, has proven able to efficiently pre‐treat apple pomace, obtaining a sugar conversion yield of 65%. Moreover, when used in combination with a laccase enzyme, a notable increase in the sugar conversion yield was measured. Similar results were also obtained when other wastes, coffee silverskin and potato peel, were pre‐treated. With respect to the widespread laccase pre‐treatments, this new pre‐treatment approach minimizes process time, increasing energy efficiency.
The cerato‐platanin ThCP from a marine strain of Trichoderma harzianum is able to efficiently pre‐treat three Agro‐food wastes endowed with different lignin and carbohydrate contents. ThCP and a Pleurotus ostreatus laccase synergistically work in the pre‐treatment process, allowing an almost complete sugar recovery from the three Agro‐food wastes.

Introduction
The continuous increase in energy consumption needs for effective alternatives to gradually replace the fossil fuels, as encouraged by the EU Bioeconomy Strategy. The valorization of inexpensive, abundant, and renewable biomass wastes could provide benefits reducing both fossil fuel demands and environmental concerns.
In this regard, the possibility to recover fermentable sugars from lignocellulosic biomass has long been deemed as a sustainable alternative to obtain biofuels and other biomaterials from different microorganisms (Chohan et al., 2020; Hyväkkö et al., 2020). Moreover, lignocellulosic raw materials, including forest residues, agricultural wastes and agri‐food wastes (AFW), are the most abundant source of organic material in the world (Giacobbe et al., 2020).
In this direction, some hurdles still exist, such as physico‐chemical, structural and compositional factors hindering the hydrolysis of cellulose present in biomasses, beyond other economic aspects (Kahn et al., 2020). A necessary step is the removal or reduction of any obstacle to hydrolysis, in order to maximize the yields of fermentable sugars from cellulose or hemicellulose (Mosier et al., 2005). This step is still a matter of investigation, since an effective pre‐treatment able to combine environmental sustainability, sugar recovery and cost‐effectiveness is still missing (Fillat et al., 2017). A number of physical and chemical pre‐treatment methods have been tested; however, they are often expensive, high‐energy demanding and cause loss of carbohydrates and/or formation of unfavourable by‐products. Laccases do the lion's share of the biological pre‐treatment approaches, since they have been used in the valorization of many lignocellulosic biomasses alone, or in mixture with other ligninolytic enzymes and in combination with physical and chemical treatment methods (Giacobbe et al., 2020).
In the last years, cerato‐platanin family proteins have proven able to weaken filter paper, acting similarly to another class of fungal proteins, the expansins (Luti et al., 2019). Cerato‐platanin is small, cysteine‐rich, non‐catalytic fungal proteins (Gaderer and Bonazza, 2014). Their structure shows a double‐ψ β‐barrel structurally related to the expansin domain D1 (De Oliveira et al., 2011). Their physiological role is still a matter of debate, indeed they are reported to act both as virulence factor and as elicitors, even if other study suggests that their main biological functions are also related to other, more general aspects of fungal growth (Djonovic et al., 2007).
Biochemical analysis of the properties of cerato‐platanins showed that they have both carbohydrate‐binding/carbohydrate‐loosening properties (Baccelli et al., 2014), and the ability to self‐assemble and change the polarity of surfaces (Frischmann et al., 2013).
The ability to weaken cellulose derived substrates in the absence of hydrolytic activity has been reported till now for several cerato‐platanins, such as CP from Ceratocystis platani, Pop1 from Ceratocystis populicola (Baccelli et al., 2014), MpCP2 from Moniliophthora perniciosa (Barsottini et al., 2013), FgCPPs from Fusarium graminearum (Quarantin et al., 2019) and recently for ThCP from Trichoderma harzianum (Pitocchi et al., 2020). Luti and coworkers carried out an analysis of site‐directed mutants of CP from C. platani and figured out that it weakly binds cellulose while performing its non‐lytic loosening, like other single domain expansin‐like proteins, and its expansin‐like activity depends on its net charge (Luti et al., 2020). This ability is potentially very interesting for biotechnological applications, since cerato‐platanin may contribute to the valorization of lignocellulosic waste materials, but we did not find out any ongoing efforts in this respect yet.
The cerato‐platanin ThCP was recently isolated from T. harzianum grown on oil as sole carbon source and characterized as an efficient biosurfactant protein (Pitocchi et al., 2020). This work explores for the first time the potential of a cerato‐platanin in the valorization of lignocellulosic biomasses promoting enzymatic attack of cellulose. Three AFWs, apple pomace (AP), coffee silverskin (CS) and potato peels (PP) endowed with different lignin and carbohydrate contents and already used in biorefinery approaches, were successfully pre‐treated with ThCP confirming that cerato‐platanins could play an important role in the future of waste valorization circle.
Results and discussion
Valorization of Apple Pomace (AP)
ThCP was produced in the presence of lampante oil as sole carbon source and was purified by simple ultrafiltration to remove high molecular weight proteins (> 30 kDa), and then concentrated and dialyzed. As a matter of fact, only one band was observed when this sample was loaded on SDS‐PAGE (Fig. 1A).
Fig. 1.
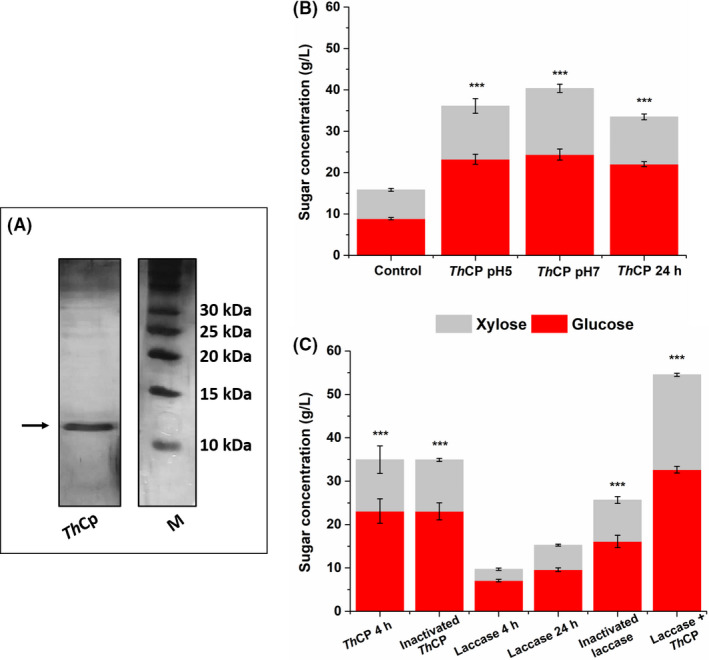
(A) SDS‐PAGE of the cerato‐platanin ThCP purified from T. harzianum used for the pre‐treatment. (B) Glucose and xylose concentration obtained after 72 h enzymatic hydrolysis by commercial enzymes of pre‐treated Apple Pomace biomass. ThCP (4 × 10−6 M) pre‐treatment was performed for 4 h at pH 5 and 7 and for 24 h at pH5. BSA (4 × 10−6 M) was used as control (4 h at pH 5). (C) Glucose and xylose concentration obtained after enzymatic hydrolysis of pre‐treated Apple Pomace biomass by cellulolytic commercial enzymes. ThCP pre‐treatment (4 h), laccase pre‐treatment (24 h) and laccase+ ThCP pre‐treatment (24 h) were followed by 6 h inactivation at 50°C. Results of two technical replicates of at least three biological replicates were analysed. The mean values were compared to the control and considered significant when P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001) according to Student's t‐test.
The purified ThCP was firstly tested on AP, considering that it shows a high sugar content (52%) with a high percentage (16%) of soluble carbohydrates, along with a low lignin content (18%) (Giacobbe et al., 2018a). With the aim to set up the process conditions avoiding intermediate steps between pre‐treatment and hydrolysis, the effect of time, temperature and pH were considered, taking into account that cellulolytic enzymes are reported to efficiently work at 50°C and pH 5 (Procentese et al., 2017). The solubility of ThCP was evaluated by measuring the protein concentration at different temperatures: ThCP was soluble at 30°C for at least 4 h, while its concentration was quickly reduced about one fourth at 40°C and 50°C, therefore, the temperature of 30°C was selected for the pre‐treatment. As for the pH, two different pHs were tested: 7.0, the pH of the solution containing ThCP, and 5.0, the pH needed for the enzymatic hydrolysis. After a 4h ThCP pre‐treatment, the saccharification experiments were carried out for 72 h, obtaining the release of about 40 gl−1 of total sugars (corresponding to 65% sugar yield) at both pH 5.0 and 7.0, indicating that this pH variation did not affect the pre‐treatment step (Fig. 1B). Increasing the duration of the ThCP pre‐treatment did not increase the efficiency of the process. Considering these results, the selected pre‐treatment conditions were 4 h at 30°C, at pH 5.0, thus avoiding the need of buffer exchange.
These results demonstrate for the first time that the action of ThCP in loosening cellulose increases its availability for the hydrolysis by a tailored mix of hydrolytic enzymes in AFW. Giacobbe et al. (2018b) reported a very efficient AP pre‐treatment with laccase after a 24 h process at 28°C followed by a 6 h inactivation step at 50°C. Indeed, when the laccase pre‐treatment was 4 h long (the same duration of cerato‐platanin pre‐treatment, a negligible amount of sugars was released (Fig. 1C). Moreover, the laccase thermal inactivation is necessary to maintain cellulase activity, while the thermal inactivation is not necessary when the cerato‐platanin was used to pre‐treat AP.
A mix of ThCP and laccase in a pre‐treatment process was used (Fig. 1C), in order to evaluate their synergistic action, setting conditions suitable to highlight the contribution of both proteins (5 U of laccase, 24 h pre‐treatment followed by 6 h inactivation at 50°C). A very relevant sugars conversion yield (95%) was achieved (Table 1), thanks to the action of laccase in lignin removal and of cerato‐platanin in loosening cellulose, making cellulose biomass fully accessible to cellulases.
Table 1.
Lignin removal of pre‐treated biomass waste as percentage respect to control samples. Results of two technical replicates of at least three biological replicates were analysed.
| AFW | Pre‐treatment | Lignin removal (%) | Sugar conversion yield (%) |
|---|---|---|---|
| AP | ThCP | 5 | 65 ± 2 |
| Laccase | 15 | 56 ± 7 | |
| Laccase + ThCP | n. a. | 95 ± 1 | |
| CS | ThCP | 11 | 54 ± 2 |
| Laccase | 25 | 42 ± 2 | |
| Laccase + ThCP | n. a. | 99 ± 1 | |
| PP | ThCP | 15 | 59 ± 4 |
| Laccase | 28 | 45 ± 6 | |
| Laccase + ThCP | n. a. | 94 ± 2 |
n. a., not analysed.
To further analyse the process, Klason‐lignin (KL) content was evaluated after pre‐treatment with cerato‐platanin or laccase enzyme. As expected, the reduction of lignin content (5% decrease) was negligible when the cerato‐platanin was used, while a higher decrease of lignin content (15%) was observed after laccase pre‐treatment (Table 1).
Process optimization
With the aim to optimize the conditions of cerato‐platanin pre‐treatment and to improve the sugar yield from the biomasses, different experiments were performed. Table 2 reports results obtained after ThCP pre‐treatment and enzymatic hydrolysis at higher biomass loading, compared with those obtained using laccase alone and in combination with the cerato‐platanin. Albeit an increased amount of released sugars was obtained at higher biomass loading (15% w/v), the sugar conversion yield was lower. Moreover, a further decrease was observed at 20% (w/v) biomass loading, leading to an amount of released sugars comparable to that obtained at 10% (w/v) biomass loading (Table 2). Since high‐water absorption at such high biomass loading was observed, the lower availability of free water can affect the protein efficiency. At all the tested biomass loadings, the combined action of cerato‐platanin and laccase is very effective.
Table 2.
Sugar conversion yield obtained after enzymatic hydrolysis by commercial enzymes of AP biomass pre‐treated at different biomass loading. Results of two technical replicates of at least three biological replicates were analysed.
| Sugar conversion yield (%) | ||||
|---|---|---|---|---|
| 10% AP | 15% AP | 20% AP | ||
| ThCP | 65 ± 2 | 50 ± 1 | 33 ± 1 | |
| Laccase | 56 ± 7 | 40 ± 1 | 27 ± 1 | |
| Laccase + ThCP | 95 ± 1 | 69 ± 1 | 46 ± 1 | |
In addition, in order to assess the reusability of the proteins in the pre‐treatment process, several successive cycles of pre‐treatment were performed recovering the soluble part and adding it to fresh biomass. After each pre‐treatment step, the insoluble recovered biomass underwent the enzymatic hydrolysis (Fig. 2A). After four cycles with cerato‐platanin, a sugar conversion yield of 45% was still obtained (Fig. 2B), while laccase ability dropped already at the second cycle (Fig. 2C).
Fig. 2.
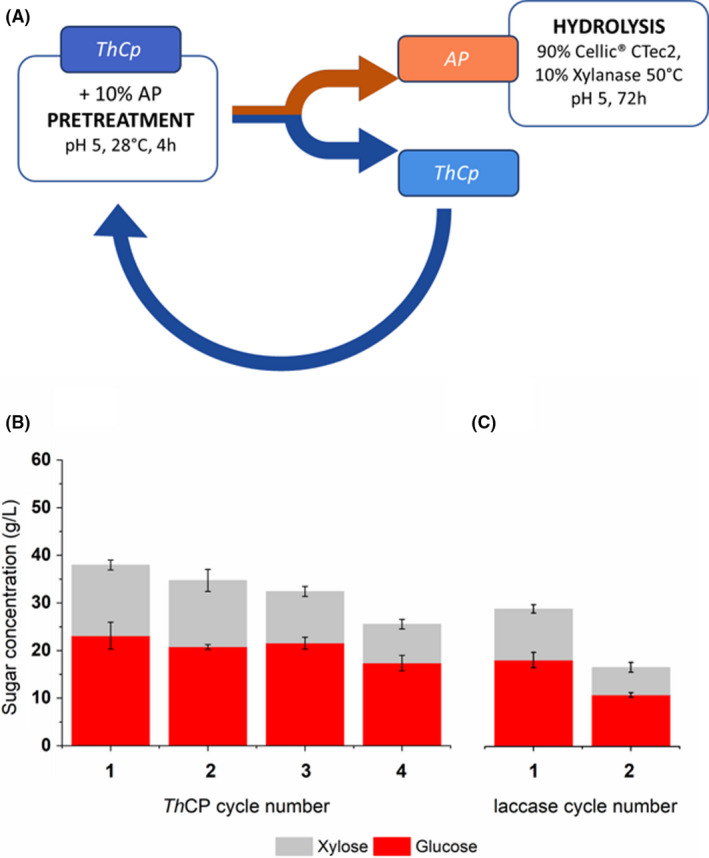
(A) Scheme of the recycling protocol. Glucose and xylose concentration obtained after enzymatic hydrolysis of Apple Pomace biomass pre‐treated by cerato‐platanin (B) or laccase (C). Results of two technical replicates of at least three biological replicates were analysed.
The amount of cerato‐platanin recovered up to the third cycle is 75% compared to the initial concentration, while it decreased up to 50% after four cycles. On the other hand, laccase activity decreased to 40% in the second cycle.
Protein production optimization
Different culture conditions were tested to increase protein yield production growing the fungus T. harzianum in rich media. In the condition reported above (§ 2.2 Production of cerato‐platanin), an increase of about five‐fold mycelium biomass, and about two‐fold total protein production was obtained in half time, moreover, several protein bands were evident in the sample loaded on SDS‐PAGE (Fig. 3A).
Fig. 3.
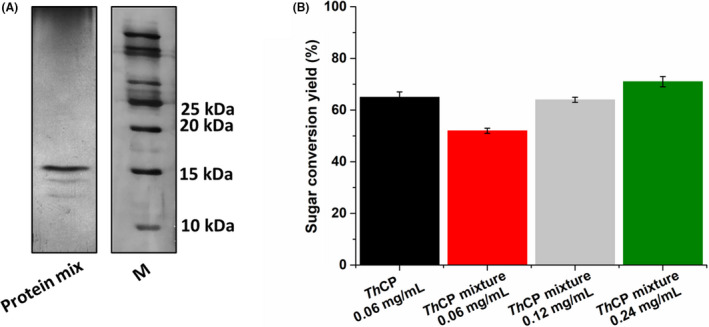
(A) SDS‐PAGE of T. harzianum protein mixtures used for the pre‐treatment. (B) Sugar conversion yields (%) obtained after enzymatic hydrolysis by commercial enzymes of Apple Pomace biomass pre‐treated with purified ThCP and ThCP in mixture at different total protein amount. Results of two technical replicates of at least three biological replicates were analysed.
Nonetheless, different amounts of these secreted proteins were used for AP pre‐treatment. A yield comparable to that obtained with the purified ThCP was achievable when the protein amounts of the secreted proteins were doubled (Fig. 3B), and a slight increase of the sugar yield conversion was obtained after further doubling the protein amount.
These results indicate that the use of this rich medium to produce the protein does not confer a real advantage to the process, therefore, other strategies to increase the sustainability of the process must be explored, for example, ThCP recombinant expression.
Valorization of other agri‐food wastes
To test the versatility of the process, cerato‐platanin was also tested using two other biomasses, CS and PP, endowed with a lower sugar and a higher lignin content with respect to AP. In particular, PP showed the highest lignin content (33%) also characterized by the presence of suberin, a very complex polymer (Liang and McDonald, 2014).
As already observed for AP, the cerato‐platanin pre‐treatment of CS and PP allowed a good sugar release (Fig. 4), even if lower compared to that obtained with AP, as a consequence of their higher lignin content. Analysis of Klason‐lignin (KL) content and sugar yield (Table 1) suggests that the presence of lignin barrier is not the only obstacle to the accessibility of cellulose by cellulolytic enzymes, and other side effects must be taken into account.
Fig. 4.
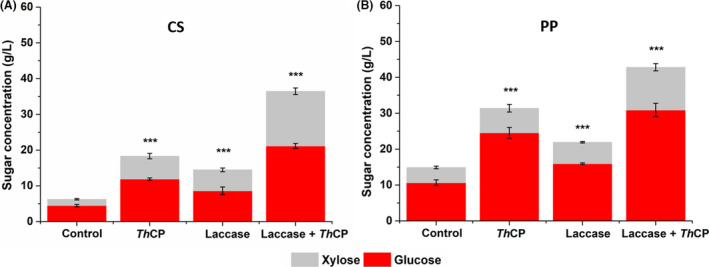
Glucose and xylose concentration obtained after enzymatic hydrolysis by commercial enzymes of pre‐treated Coffee Silverskin (A) and Potato Peels (B) biomass. ThCP (4 × 10−6 M) pre‐treatment was performed for 4 h at pH 5. BSA (4 × 10−6 M) was used as control. Laccase and laccase+ ThCP pre‐treatment was performed for 24 h followed by 6 h inactivation at 50°C. Results of two technical replicates of at least three biological replicates were analysed. The mean values were compared to the control and considered significant when P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001) according to Student's t‐test.
It is worth noting that 99% and 94% sugar conversion yield was achieved when the combined action of laccase enzyme and cerato‐platanin was tested on CS and PP respectively (Table 1).
The sugar conversion yield obtained after cerato‐platanin pre‐treatment is comparable or higher to that reported on the same biomasses by Giacobbe et al. (2018b) and by Hijosa‐Valsero et al. (2018a) (Hijosa‐Valsero, 2017; Hijosa‐Valsero et al., 2018a, 2018b,) after enzymatic and physico‐chemical pre‐treatments respectively. Moreover, the combination of cerato‐platanin and laccase allowed a significant improvement of these results, demonstrating that the action of cerato‐platanin is very efficient and versatile.
Conclusion
Recent findings suggest that surfactants supplementation during saccharification is an effective strategy to achieve higher saccharification yields, since they can reduce non‐specific binding of enzymes on lignin (Singhania et al., 2017), promoting physico‐chemical and biological delignification and enzyme recovery (Agrawal et al., 2017). However, the mechanism of action of surfactants for improved saccharification is far to be well understood.
The biosurfactant properties recently demonstrated for ThCP (Pitocchi et al., 2020) can explain its ability to loosen cellulose rendering it more accessible to hydrolytic attack. This work offers new possibilities to improve the biological pre‐treatment of lignocellulolytic biomasses. the herein developed process ensures high chemical efficiency and minimizes operative time, increasing energy efficiency.
Experimental procedures
Materials
Reagents were purchased from Sigma‐Aldrich Corp. (St. Louis, MO) unless otherwise specified. The commercial enzymes used in this study were cellulolytic enzyme cocktail Cellic®CTec2 (kindly supplied by Novozyme); endo‐1,4‐β‐Xylanase M1 from Trichoderma viride (Megazyme, Bray, Ireland); α‐amylase from Bacillus licheniformis (Megazyme). Dry apple pomace (AP) was provided by Muns Agroindustrial S.L. (Lleida, Spain). Coffee silverskin (CS) was kindly provided by Illy S.p.A (Trieste, Italy). Fresh potato peel (PP) containing 76.94% moisture was kindly provided by Aperitivos Gus S.L. (Riego de la Vega, León, Spain). The supplied biomasses were oven‐dried, milled and stored as specified by Giacobbe et al. (2018b).
Production of cerato‐platanin
Trichoderma harzianum MUT 290 was maintained through periodic transfer on agar plate at 28°C, using XNST30 agar medium (3 g l−1 malt extract; 3 g l−1 yeast extract; 30 g l−1 NaCl; 10 g l−1 glucose; 5 g l−1 peptone; 15 g l−1 agar).
Protein production and isolation was performed as described elsewhere (Pitocchi et al., 2020). Mycelium plugs (10 mm diameter) cut from the actively growing colonies were pre‐inoculated in 100 ml flasks containing 50 ml of ONR7 mineral medium (30 g of NaCl, 3.98 g of Na2SO4, 1.3 g of HEPES {4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid}, 0.72 g of KCl, 0.27 g of NH4Cl, 47 mg of Na2HPO4, 83 mg of NaBr, 2.6 mg of NaF, 31 mg of NaHCO3, 27 mg of H3B03, 24 mg of SrCI2·6H20, 11.18 g of MgCl2·6H2O, 1.46 g of CaCl2·2H2O and 2.0 mg of FeCl2·4H20 per litre of distillate water) supplemented with 10 g l−1 glucose, 2 g l−1 peptone, and incubated at 28°C for 3 days. Then, 50 ml of these pre‐inoculum were inoculated in 1 l flasks containing ONR7 mineral medium (500 ml) supplemented with 1% v/v lampante oil and 0.1% v/v Tween 80. Lampante oil is used to avoid corrosion of industrial and laboratory machines. Flasks were incubated in the dark at 28°C for 14 days, then mycelia were separated from the culture broth using a Whatman 3MM paper filters (Termo Fischer Scientific, Rodano (MI), Italy). Culture broth was filtered using Stericup GP vacuum filtration system (0.22 µm pore size, Merck KGaA, Darmstadt, Germany), and ThCP was concentrated by air bubbling, using a Waring blender. The formed foam was separated, collected and loaded in an Amicon Ultrafiltration cell equipped with a 30 kDa cut‐off PES Millipore Ultrafiltration Disc (Merck KGaA, Darmstadt, Germany). The ultrafiltrate, containing low molecular weight proteins (<30 kDa), was concentrated using a 3 kDa cut‐off disc and dialyzed against 10 mM sodium phosphate buffer pH 7.0.
Another culture condition was tested with the aim to increase protein yield, using a rich medium (1 g l−1 yeast extract, 1 g l−1 of peptone, 1 g l−1 gelatine, 5 g l−1 glucose, 30 g l NaCl). Flasks were incubated in the dark at 28°C for 7 days and mycelia were separated from the culture broth using a Whatman 3MM paper filters (Termo Fischer Scientific). Then proteins were overnight precipitated by the addition of (NH4)2SO4 up to 50% saturation at 4°C and centrifuged at 10 000 rpm for 20 min. The ammonium sulphate precipitate was resuspended in 10 mM sodium phosphate buffer (pH 7.0) and extensively dialysed against the same buffer, using regenerated Cellulose dialysis tube with 3.5 kDa cut‐off (Spectrum Spectra/Por, Thermo Fischer Scientific).
Production of laccase
The Pleurotus ostreatus laccases adopted in this study was the recombinant POXA1b expressed in Pichia pastoris and secreted in the cultural broth as reported in Pezzella et al. (2017). The broth was ultrafiltered and dialyzed towards 50 mM Tris HCl pH8, using Centricon (Merck KGaA, Darmstadt, Germany), cut‐off 10 kDa. Laccase activity against ABTS was assayed as reported by (Macellaro et al., 2014).
Determination of protein concentration
Protein concentration was determined with the Pierce Protein Assay (Thermo Fischer Scientific, Rodano (MI), Italy) using Bovine Serum Albumin (BSA) as standard.
Pre‐treatment experiments
ThCP pre‐treatments were carried out at 10% (w/v) of AP and CS, at 30°C for 4 and 24 h in a total volume of 2.5 ml at two different pHs, pH 5.0 in 50 mM sodium citrate and 7.0 in 50 mM sodium phosphate. In the case of PP, due to its high‐water absorption, the pre‐treatments were carried at 5% (w/v).
Laccase pre‐treatments were carried out at 10% (w/v) of AP and CS at 28°C for 4 and 24 h in a total volume of 2.5 ml followed by an inactivation step of 6 h at 50°C.
Trials at 15 and 20%w/v AP were also performed. The reuse of cerato‐platanin and laccase enzyme was tested: after biomass pre‐treatment, the solution was centrifuged, and the supernatant transferred to another 10% biomass waste.
About 0.06 mg ml−1 (4 × 10−6 M) of ThCP and 5 U of POXA1b were used for each‐pre‐treatment experiment. Control assays were performed under the same conditions using BSA (0.24 mg ml−1, 4 × 10−6 M). Results represent the mean ± the SD of at least three independent samples.
Klason lignin evaluation
The Klason lignin content of treated and untreated biomass waste was determined according to NREL LAPs (Sluiter et al., 2012). Percentage of lignin reduction with respect to untreated samples was reported.
Enzymatic hydrolysis and sugar concentration
Enzymatic hydrolysis of pre‐treated biomasses was carried out in the presence of 0.5 mM sodium azide to prevent microbial and fungal growth. According to Giacobbe et al. (2018b), the hydrolytic enzymatic cocktail was composed by 90% of Cellic® CTec2 and 10% of xylanase for AP and CS, while in the case of PP, the enzyme mixture contained 80% of amylase, 10% of Cellic® CTec2 and 10% of xylanase.
Samples were collected after 72 h, cooled on ice, centrifuged at 16 500 × g for 30 min at 4°C and the amount of sugars released was determined and quantified in the recovered supernatant, by high‐performance liquid chromatography (HPLC) using a system 7Q (Dionex, California, USA), equipped with an anionic exchange column (Carbopac PA‐100) and a pulsed electrochemical detector. Glucose and xylose were separated with 16 mM sodium hydroxide at a flow rate of 0.25 ml min−1 and identified by the respective standards. Fucose was used as internal standard. The total amount of reducing sugars was determined using a Dionex CarboPac MA1 column with pulsed amperometric detection. Standard sugar solutions were prepared separately by dissolving different concentration of glucose, xylose, fructose, arabinose, mannose and sucrose.
The saccharification yields, that are means of three replicates, were expressed as percentages with respect to the sugar content of the pre‐treated material before the hydrolysis using the formula (Santhi et al., 2014):
Statistics
The data reported were statistically validated using the Student's t‐test comparing the sugar concentration obtained after pre‐treatment with control BSA and that obtained after pre‐treatment with cerato‐platanin and/or laccase. The significance of the differences between the values was calculated using a two‐tailed Student's t‐test. A P‐value of < 0.05 was considered significant.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors thank Prof. Antonio Marzocchella, Department of Chemical, Materials and Production Engineering of the University of Naples Federico II for kindly making available coffee silverskin; Dr. Rebeca Díez‐Antolínez and Dr. María Hijosa‐Valsero of the Centre of Biofuels and Bioproducts. Agricultural Technological Institute of Castilla y León, Spain, for kindly making available apple pomace and potato peels.
Microb. Biotechnol. (2021) 14(4), 1699–1706
Funding Information
No funding information provided.
References
- Agrawal, R. , Satlewal, A. , Kapoor, M. , Mondal, S. , and Basu, B. (2017) Investigating the enzyme‐lignin binding with surfactants for improved saccharification of pilot scale pretreated wheat straw. Bioresour Technol 224: 411–418. [DOI] [PubMed] [Google Scholar]
- Baccelli, I. , Luti, S. , Bernardi, R. , Scala, A. , and Pazzagli, L. (2014) Cerato‐platanin shows expansin‐like activity on cellulosic materials. Appl Microbiol Biotechnol 98: 175–184. [DOI] [PubMed] [Google Scholar]
- Barsottini, M.R.D.O. , De Oliveira, J.F. , Adamoski, D. , Teixeira, P.J.P.L. , Prado, P.F.V. , Tiezzi, H.O. , et al. (2013) Functional diversification of cerato‐platanins in Moniliophthora perniciosa as seen by differential expression and protein function specialization. Mol Plant‐Microbe Interact 26: 1281–1293. [DOI] [PubMed] [Google Scholar]
- Chohan, N.A. , Aruwajoye, G.S. , Sewsynker‐Sukai, Y. , and Gueguim Kana, E.B. (2020) Valorisation of potato peel wastes for bioethanol production using simultaneous saccharification and fermentation: process optimization and kinetic assessment. Renew Energy 146: 1031–1040. [Google Scholar]
- De Oliveira, A.L. , Gallo, M. , Pazzagli, L. , Benedetti, C.E. , Cappugi, G. , Scala, A. , et al. (2011) The structure of the elicitor Cerato‐platanin (CP), the first member of the CP fungal protein family, reveals a double ψβ‐Barrel fold and carbohydrate binding. J Biol Chem 286: 17560–21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djonovic, S. , Vargas, W.A. , Kolomiets, M.V. , and Horndeski, M. (2007) A proteinaceous Elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol 145: 875–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillat, Ú. , Ibarra, D. , Eugenio, M. , Moreno, A. , Tomás‐Pejó, E. , and Martín‐Sampedro, R. (2017) Laccases as a potential tool for the efficient conversion of Lignocellulosic biomass: a review. Fermentation 3: 17–47. [Google Scholar]
- Frischmann, A. , Neudl, S. , Gaderer, R. , Bonazza, K. , Zach, S. , Gruber, S. , et al. (2013) Self‐assembly at air/water interfaces and carbohydrate binding properties of the small secreted protein EPL1 from the fungus Trichoderma atroviride. J Biol Chem 288:4278–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaderer, R. , Bonazza, K. , and Seidl‐Seiboth, V. (2014) Cerato‐platanins: a fungal protein family with intriguing properties and application potential. Appl Microbiol Biotechnol 98: 4795–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe, S. , Pezzella, C. , Lettera, V. , Sannia, G. , and Piscitelli, A. (2018a) Bioresource Technology Laccase pretreatment for agrofood wastes valorization. Bioresour Technol 265: 59–65. [DOI] [PubMed] [Google Scholar]
- Giacobbe, S. , Pezzella, C. , Lettera, V. , Sannia, G. , and Piscitelli, A. (2018b) Laccase pretreatment for agrofood wastes valorization. Bioresour Technol 265: 59–65. [DOI] [PubMed] [Google Scholar]
- Giacobbe, S. , Pezzella, C. , Sannia, G. , and Piscitelli, A. (2020) Old enzymes at the forefront of Lignocellulosic waste valorization. In Laccases in Bioremediation and Waste Valorisation. Cham, Switzerland: Springer Nature, pp. 57–78. 10.1007/978-3-030-47906-0_3 [DOI] [Google Scholar]
- Hijosa‐Valsero, M. , Cambronero, J.G. , García, A.I.P. , and Antolínez, R.D. (2018a) Biobutanol production from coffee silverskin. Microb Cell Fact 17: 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijosa‐Valsero, M. , Paniagua‐García, A.I. , and Díez‐Antolínez, R. (2018b) Industrial potato peel as a feedstock for biobutanol production. N Biotechnol 46: 54–56. [DOI] [PubMed] [Google Scholar]
- Hijosa‐Valsero, M. , Paniagua‐García, A.I. , and Díez‐Antolínez, R. (2017) Biobutanol production from apple pomace: the importance of pretreatment methods on the fermentability of lignocellulosic agro‐food wastes. Appl Microbiol Biotechnol 101: 8041–8052. [DOI] [PubMed] [Google Scholar]
- Hyväkkö, U. , Maltari, R. , Kakko, T. , Kontro, J. , Mikkilä, J. , Kilpeläinen, P. , et al. (2020) On the effect of hot‐water pretreatment in sulfur‐free pulping of aspen and wheat straw. ACS Omega 5: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, A. , Moraïs, S. , Chung, D. , Sarai, N.S. , Hengge, N.N. , Kahn, A. , et al. (2020) Glycosylation of hyperthermostable designer cellulosome components yields enhanced stability and cellulose hydrolysis. FEBS J 287: 4370–4388. [DOI] [PubMed] [Google Scholar]
- Liang, S. , and McDonald, A.G. (2014) Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J Agric Food Chem 62: 8421–8429. [DOI] [PubMed] [Google Scholar]
- Luti, S. , Bemporad, F. , Vivoli Vega, M. , Leri, M. , Musiani, F. , Baccelli, I. , and Pazzagli, L. (2020) Partitioning the structural features that underlie expansin‐like and elicitor activities of cerato‐platanin. Int J Biol Macromol 165: 2845–2854. [DOI] [PubMed] [Google Scholar]
- Luti, S. , Sella, L. , Quarantin, A. , Pazzagli, L. , and Baccelli, I. (2019) Twenty years of research on cerato‐platanin family proteins: clues, conclusions, and unsolved issues. Fungal Biol Rev 34: 13–24. [Google Scholar]
- Macellaro, G. , Pezzella, C. , Cicatiello, P. , Sannia, G. , and Piscitelli, A. (2014) Fungal laccases degradation of endocrine disrupting compounds. Biomed Res Int 2014: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier, N. , Wyman, C. , Dale, B. , Elander, R. , Lee, Y.Y. , Holtzapple, M. , et al. (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96: 673–686. [DOI] [PubMed] [Google Scholar]
- Pezzella, C. , Giacobelli, V.G. , Lettera, V. , Olivieri, G. , Cicatiello, P. , Sannia, G. , and Piscitelli, A. (2017) A step forward in laccase exploitation: recombinant production and evaluation of techno‐economic feasibility of the process. J Biotechnol 259: 175–181. [DOI] [PubMed] [Google Scholar]
- Pitocchi, R. , Cicatiello, P. , Birolo, L. , Piscitelli, A. , Bovio, E. , Varese, G.C. , and Giardina, P. (2020) Cerato‐platanins from marine fungi as effective protein biosurfactants and bioemulsifiers. Int J Mol Sci 21: 2913–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procentese, A. , Raganati, F. , Olivieri, G. , Elena Russo, M. , and Marzocchella, A. (2017) Pre‐treatment and enzymatic hydrolysis of lettuce residues as feedstock for bio‐butanol production biomass and bioenergy pre‐treatment and enzymatic hydrolysis of lettuce residues as feedstock for bio‐butanol production. Biomass Bioenerg 96: 172–179. [Google Scholar]
- Quarantin, A. , Castiglioni, C. , Schäfer, W. , Favaron, F. , and Sella, L. (2019) The Fusarium graminearum cerato‐platanins loosen cellulose substrates enhancing fungal cellulase activity as expansin‐like proteins. Plant Physiol Biochem 139: 229–238. [DOI] [PubMed] [Google Scholar]
- Satheeja Santhi, V. , Bhagat, A.K. , Saranya, S. , Govindarajan, G. , and Jebakumar, S.R.D. (2014) Seaweed (Eucheuma cottonii) associated microorganisms, a versatile enzyme source for the lignocellulosic biomass processing. Int Biodeterior Biodegrad 96: 144–151. [Google Scholar]
- Singhania, R.R. , Patel, A.K. , Saini, R. , and Pandey, A. (2017) 5‐Industrial enzymes: β‐glucosidases. In Current Developments in Biotechnology and Bioengineering. Amsterdam, Netherlands: Elsevier, pp. 103–125. 10.1016/B978-0-444-63662-1.00005-1 [DOI] [Google Scholar]
- Sluiter, A.D. , Hames, B. , Ruiz, R. , and Scarlata, C. (2012) Determination of Structural Carbohydrates and Lignin in Biomass Laboratory Analytical Procedure (LAP). Natl Anal Energy Lab.


