Spray‐induced gene silencing (SIGS) using topical dsRNA applications has risen as a promising, target‐specific, and environmentally‐friendly disease management strategy against phytopathogenic fungi. We demonstrated that minicell‐encapsulated dsRNA (ME‐dsRNA) halted gray mold disease progression in strawberries. ME‐dsRNAs offer a platform that can readily be translated to large‐scale production and deployed in open‐field applications to control gray mold in strawberries.
Summary
Spray‐induced gene silencing (SIGS) using topical dsRNA applications has risen as a promising, target‐specific, and environmentally friendly disease management strategy against phytopathogenic fungi. However, dsRNA stability, efficacy, and scalability are still the main constraints facing SIGS broader application. Here we show that Escherichia coli‐derived anucleated minicells can be utilized as a cost‐effective, scalable platform for dsRNA production and encapsulation. We demonstrated that minicell‐encapsulated dsRNA (ME‐dsRNA) was shielded from RNase degradation and stabilized on strawberry surfaces, allowing dsRNA persistence in field‐like conditions. ME‐dsRNAs targeting chitin synthase class III (Chs3a, Chs3b) and DICER‐like proteins (DCL1 and DCL2) genes of Botryotinia fuckeliana selectively knocked‐down the target genes and led to significant fungal growth inhibition in vitro. We also observed a compensatory relationship between DCL1 and DCL2 gene transcripts, where the silencing of one gene upregulated the expression of the other. Contrary to naked‐dsRNAs, ME‐dsRNAs halted disease progression in strawberries for 12 days under greenhouse conditions. These results elucidate the potential of ME‐dsRNAs to enable the commercial application of RNAi‐based, species‐specific biocontrols comparable in efficacy to conventional synthetics. ME‐dsRNAs offer a platform that can readily be translated to large‐scale production and deployed in open‐field applications to control grey mould in strawberries.
Introduction
Each year, grey mould disease caused by the fungal pathogen Botryotinia fuckeliana (anamorph: Botrytis cinerea) leads to $10 to $100 billion of global agricultural losses (Petrasch et al., 2019). Predominant control strategies for grey mould disease heavily rely on chemical fungicidal products (Fernández‐Ortuño et al., 2015; Rupp et al., 2017). The frequent and prophylactic use of these chemical fungicides has led to ecotoxicological harm, human health risk and resistance development in fungal pathogens (Weber, 2011; Amiri et al., 2013; Konstantinou et al., 2015; Panebianco et al., 2015). These repercussions evidence the urgent need for alternative biocontrols that are effective and environmentally sustainable.
RNAi is a post‐transcription gene regulation mechanism that is present in all known eukaryotes. The cellular RNAi machinery is initiated by double‐stranded RNAs (dsRNAs) that are initially processed into small interfering RNAs (siRNAs) by Dicer and Dicer‐Like (DCL) proteins and eventually leads to the degradation of target mRNAs through the action of the RNA‐induced silencing complex (RISC). RNAi‐based genetic transformation technology has widely been utilized to control several insect pests, and diseases, in what is collectively coined as ‘host‐induced gene silencing’ (HIGS) (Fire et al., 1998; Baulcombe, 2015; Wang et al., 2016). In HIGS, gene(s) essential for the development, survival, and virulence of the pathogen or the host plant's susceptibility is being inactivated by gene silencing, leading to improved resistance against invading pathogens. For instance, the expression of dsRNAs targeting DCL1/2 or Target of the Rapamycin (TOR) (Xiong et al., 2019) genes of B. fuckeliana significantly suppressed grey mould disease progression in transgenic Arabidopsis, potato and tomato plants. However, technical limitations of generating stable transformed plants and the growing public concerns surrounding the cultivation of genetically modified (GM) crops restrict the broad application of HIGS technology to a majority of horticultural crops. Recently, spray‐induced gene silencing (SIGS), which involves the exogenous application of dsRNAs and siRNAs has emerged as an appealing alternative to HIGS, as it does not incorporate foreign genes in the treated species and transgenerational inheritance of silencing constructs is unlikely (Wang et al., 2017; Mcloughlin et al., 2018a; Islam and Sherif, 2020). The exogenous application of dsRNA or siRNA to reduce B. fuckeliana infection has already been reported in many pathobiological systems (Wang et al., 2016; Mcloughlin et al., 2018b). However, due to limited field stability and high production cost, sprayable dsRNA biofungicides remain only conceptually conceivable, and not yet practically feasible at a large scale (Wang et al., 2016; Mitter et al., 2017). To address the applicability issues associated with sprayable dsRNAs, layered double hydroxides (LDH) based nanoparticle, termed “BioClay” has been suggested and utilized as a carrier for dsRNAs to improve their field stability and effectiveness against plant virus (Mitter et al., 2017). Despite the demonstrated efficacy of BioClay and other similar technologies, the current production schemes are largely dependent on in vitro transcription kits that are vastly expensive, and largely inapplicable to large‐scale production schemes. In an attempt to address these issues, the present work describes a dsRNA bioproduction platform that is based on a bacterial minicell carrier system, referred to here as minicell‐encapsulated dsRNA (ME‐dsRNA).
The minicell technology is a novel platform for the single step production and encapsulation of dsRNA to enable efficient and scalable RNAi‐based solutions. It is a fermentation technology in which the microbe first produces dsRNA then produces minicells; resulting in minicell‐encapsulated dsRNA. This technology combines the production element and delivery element of biofungicides. The environment in which it is applied degrades unformulated dsRNA quickly which reduces its viability as a biofungicides. As a result, all RNA based biofungicides require formulation (Taning et al., 2020). This formulation adds an extra layer in the process of creating an RNA based biofungicides. The minicell technology encapsulates its RNA as a method of formulation. The novelty of the system and its advantage over existing formulation technologies, including BioClay (Mitter et al., 2017) and chitosan/dsRNA nanoparticles (Zhang et al., 2010), comes from the platform technology’s capability of both production and encapsulation, while the previously mentioned formulation technologies rely on different production sources for dsRNA. Furthermore, we demonstrated that minicell encapsulation protects the dsRNA from the RNase A degradation, inhibits B. fuckeliana mycelial growth, silence sequence‐specific genes of B. fuckeliana, and most importantly ME‐dsRNAs confer extended protection to strawberries against B. fuckeliana under greenhouse conditions.
Results and discussion
Minicells are bacterially derived achromosomal microparticles, generally produced as a result of aberrant cell divisions. Similar to the parental cells, minicells contain membranes, ribosomes, RNA and proteins; but unlike normal cells, they cannot divide or grow (Farley et al., 2016). This unique feature has led to the development of minicells as human therapeutic delivery vesicles for drugs (MacDiarmid et al., 2007), vaccines (Carleton et al., 2013), and siRNAs (MacDiarmid et al., 2009). Deletions of the E. coli cell cycle‐related genes minCDE produce a large number of intact and stable minicells (Hale et al., 1983; MacDiarmid et al., 2007). We generated an E. coli mutant (minCDE and rnc) that produces a large number of minicells with compromised RNase‐III activity (Fig. 1A), thus allowing the expression of dsRNAs (Takiff et al., 1989). We transformed this E. coli mutant with DNA constructs to express dsRNAs targeting B. fuckeliana genes that are essential for pathogenicity. The first group of genes we used are the B. fuckeliana RNAi‐ machinery related genes, DCL1 (ME‐DCL1) and DCL2 (ME‐DCL2). Recently it has been demonstrated that DCL1 and DCL2 are involved in the synthesis of fungal siRNA‐effectors to suppress plant immunity and facilitate grey mould disease progression (Wang et al., 2016). The second group of genes we targeted are cell wall integrity‐related genes, including two isoforms of chitin synthase class III; Chs3a (ME‐CHS3a) and Chs3b (ME‐CHS3b1 and ME‐CHS3b2). Chitin is the rigid carbohydrate polymer that constitutes a main structural element of the cell wall of fungi. In B. fuckeliana, seven different classes of the chitin synthase genes have been identified, with duplications present in class III (Chs3a and Chs3b). Deletions of Chs3a significantly reduce the virulence and radial growth of B. fuckeliana (Soulie et al., 2006).
Fig. 1.
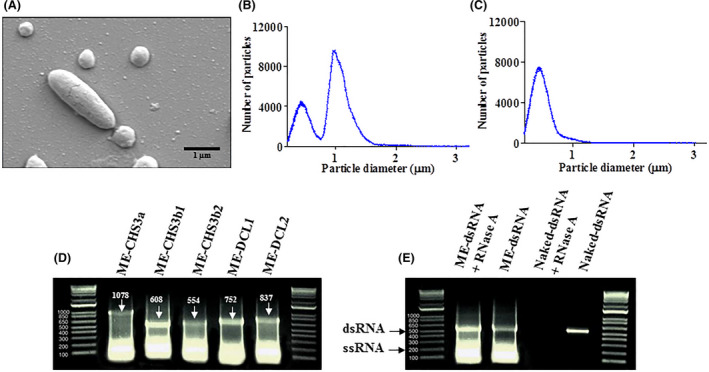
Characterization, purification, and stability of minicell‐encapsulated dsRNAs (ME‐dsRNAs).
A. Scanning electron microscopy of bacterial parental and minicells.
B and C. Purification of minicells (B); the first peak of the multisizer data represents the minicells and second peak is for the E. coli cells (C); following the purification the parental cells peak was disappeared.
D. The sequence length of dsRNAs molecules encapsulated into minicells.
E. Treatment of ME‐ and naked‐dsRNA (CHS3b2) with the RNase A. The treated and untreated ME‐ dsRNA were extracted prior to gel electrophoresis.
The transformed E. coli mutant was cultured in batch phase, induced for dsRNA expression and processed via differential centrifugation. Minicell budding from transformed bacteria are depicted in a SEM micrograph in Fig. 1A. The recovery of purified minicells by centrifugation was verified with dynamic light scattering, where centrifugation yields a single peak of approximately 400nm, representing the minicells as illustrated in Fig. 1B and C. The dsRNA from the pure minicell solution was extracted and size‐verified by gel electrophoresis (Fig. 1D). Using this platform, we were able to produce more than 100 mg/l minicell‐encapsulated dsRNA in four different bioreactors (Table S1). The ability of minicells to protect dsRNAs from RNase A degradation was confirmed in vitro (Fig. 1E). Compared to naked‐dsRNAs, minicells were resistant to RNase A treatment and yielded an intact dsRNA band on a native agarose gel, consistent with untreated (no RNase) ME‐dsRNA. (Fig. 1E). These results indicate that the RNase cannot access dsRNA that is minicell‐encapsulated. The naked‐dsRNA was degraded beyond the limit of detection given the same RNase A treatment, while the non‐treated naked‐dsRNA band was visualized using the same method indicating that the RNase A was responsible for the degradation. Of note, there was a slight and unexpected increase in the intensity of the dsRNA band with RNAse treatment (Fig. 1E), possibly reflecting the non‐linearity of the native agarose gel or could be a simple variation of RNA amounts during gel loading, or increased RNA recovery during extraction.
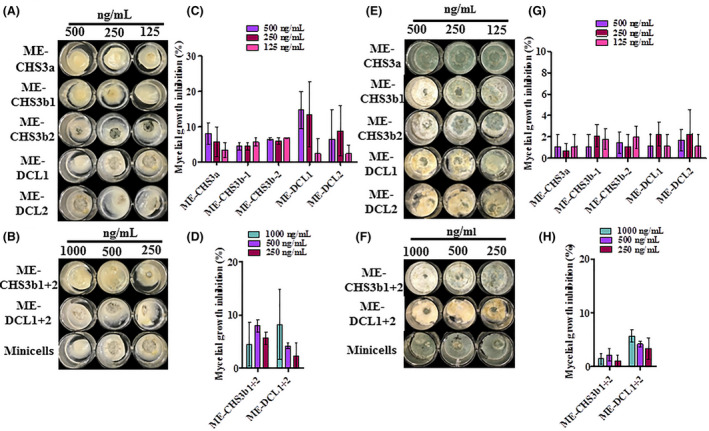
The potential of ME‐dsRNAs to function as an effective biofungicide was examined in vitro by exposing actively growing fungal mycelia of B. fuckeliana to ME‐dsRNAs. The treatment of ME‐dsRNA individually targeting DCL1 (ME‐DCL1) or DCL2 (ME‐DCL2) did not lead to any significant mycelial growth inhibition (Fig. 2A). These results are in agreement with what has previously been reported by Wang et al., 2016, where mutations in DCL1 or DCL2 did not compromise the virulence or growth of B. fuckeliana, whereas the fungus double mutant dcl1 + 2 showed reduced virulence. In line with this finding, the combined application of ME‐dsRNA targeting DCL11 and DCL2 at 1000 ng/ml inhibited fungal growth at 72 h post‐treatment (hpt) (Fig. 2B, and Fig. S1). Our results further support their finding that silencing of both DCL1 and DCL2 is required to inhibit B. fuckeliana mycelial growth. In contrast, when naked‐dsRNAs targeting both DCL1 and DCL2 were used, no significant mycelial growth inhibition was observed (Fig. S2). A previous research by Nerva et al. (2020) indicated that 500 ng naked‐dsRNA should be applied every 12 h to the B. fuckeliana culture media to achieve mycelial growth inhibition, otherwise dsRNA degradation by the nuclease and/or the availability of the dsRNA for siRNA biogenesis could limit the long‐lasting effects of naked‐dsRNAs. In the present study, we showed that a single ME‐dsRNA application (1000 ng/ml) was significantly effective compared to empty minicells for as long as 72 hpt. This remarkable efficacy of ME‐dsRNA could be explained, at least partially, by the ability of minicells to protect dsRNAs from enzymatic degradation (MacDiarmid et al., 2009) as demonstrated above (Fig. 1E).
Fig. 2.
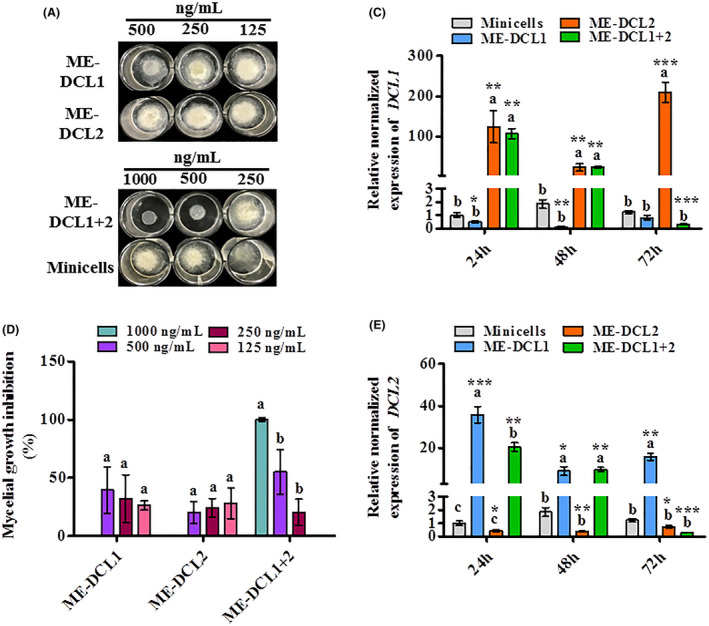
Effects of the minicell‐encapsulated dsRNAs (ME‐dsRNAs) on mycelial growth and silencing of respective RNAi‐machinery related‐ genes in Botryotinia fuckeliana at different time points.
A. Inhibition of fungal growth in response to ME‐dsRNAs targeting RNAi‐machinery related‐ genes dicer‐like protein 1 (DCL1), and dicer‐like protein 2 (DCL2) of B. fuckeliana at different concentrations.
B. Mycelial growth inhibition rate in response to ME‐DCL1 and ME‐DCL2.
C and D. Relative normalized expressions of the B. fuckeliana genes in responses to ME‐DCL1 and ME‐DCL2 (E); DCL1, (F); DCL2. Data represents the mean ± SEM. Bars labelled with different letters are significantly different at P < 0.05 according to Duncan’s multiple range test. Asterisks indicate significant differences between minicells and ME‐dsRNA‐treated sample at each time point according to t‐test; *P < 0.05, **P < 0.01, ***P < 0.001.
The robust efficacy of ME‐DCL1 and ME‐DCL2 was also confirmed at the molecular level using qRT‐PCR (Fig. 2C and D). Of note, our data not only showed a significant reduction in DCL1 and DCL2 transcript levels when the respective minicells were used, but we also observed a potential compensatory relationship between DCL1 and DCL2 expression. The downregulation of DCL1 transcripts, via ME‐DCL1 treatment, was paralleled with an upregulation of DCL2 transcripts, and vice versa (Fig. 2C and D). Although this is the first study to report such expression patterns in the Botrytis species, the functional redundancy of DICERs and DCLs has previously been reported in Arabidopsis, Drosophila and Neurospora crassa (Catalanotto et al., 2004; Czech et al., 2008; Wang et al., 2015). The potential compensatory relationship between DCL1 and DCL2 genes and its implications in dsRNA‐based management of B. fuckeliana will be the focus of our future studies. The combination of ME‐DCL1 + 2 treatment, resulted in a spike in expression of both genes at the 24 hpt, followed by a gradual, yet significant, decline in transcript levels of both genes at 48 and 72 hpt (Fig. 2C and D). This finding is consistent with the results in Fig. 2A and B, where the combined, but not the single application, of ME‐DCL1 and 2 led to a complete inhibition of the mycelial growth of B. fuckeliana.
As shown in Fig. 3A and B, ME‐dsRNAs targeting the chitin synthase 3a (Chs3a) and 3b (Chs3b) genes showed significant antifungal activity, comparable to efficacy observed using DCL1/2 gene targets, as discussed above. Both ME‐CHS3a and ME‐CHS3b2, applied at 500 ng/ml, showed significant antifungal activity, and the combination of the two constructs targeting Chs3b (CHS3b1 + 2 at 1000 ng/ml) completely inhibited the mycelial growth (Fig. 2B, Fig. S3). To further validate these findings at the molecular level, the relative expression of target genes was evaluated by qRT‐PCR, using B. fuckeliana β‐actin and Tubulin genes as housekeeping genes and an empty minicell treatment as a negative control. Similar to DCL1 & 2, the application of ME‐CHS3a led to an intial induction of Chs3a transcription at 24 hpt, before being suppressed at later times (Fig. 3C), suggesting the existence of a feedback loop to confront potential exposures to dsRNA molecules such as those of mycoviruses; however, further studies are needed to support this conclusion. Treatments ME‐CHS3b1 + 2 significantly reduced the relative transcript level of Chs3a and Chs3b in fungal mycelia, at 72 (hpt). The reduction in Chs3a observed in response to ME‐CHS3b2 and ME‐CHS3b1 + 2 at 72 hpt (Fig. 3C and D), could be explained by the high degree of similarities (60.5%) between Chs3a and Chs3b gene homologs (Choquer et al., 2004). Interestingly, it has previously been reported that Chs3b transcripts were not detected in the mycelium of B. fuckeliana using the semi‐quantitative RT‐PCR approach (Choquer et al., 2003). Likewise, we observed significant reduction in Chs3b transcript levels in responses to ME‐CHS3b1 + 2 treatment, however, the level of the Chs3b transcripts was generally low as indicated by the high Cq values (i.e. 35 out of 40 PCR cycles). These datasets together suggest that ME‐dsRNA targeting the Chs3b gene of B. fuckeliana suppressed both Chs3a and Chs3b, and the simultaneous suppression of both genes of chitin synthase class III might be crucial for the complete inhibition of B. fuckeliana mycelial growth.
Fig. 3.
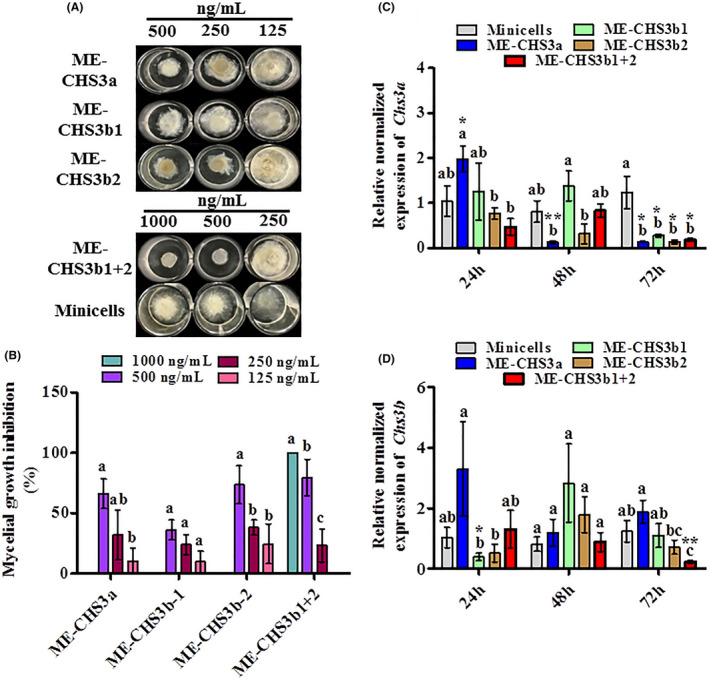
Suppression of mycelial growth and silencing of the respective genes in responses to minicell‐encapsulated dsRNAs (ME‐dsRNAs) targeting cell wall integrity‐related genes of Botryotinia fuckeliana.
A. Inhibition of fungal growth in response to ME‐dsRNAs targeting cell wall integrity‐related genes chitin synthase 3a (Chs3a), and chitin synthase 3b (Chs3b) of B. fuckeliana at different concentrations.
B. Mycelial growth inhibition rate in response to ME‐dsRNAs.
C and D. Relative normalized expressions of the B. fuckeliana genes in responses to ME‐CHS3a, ME‐CHS3b1 and ME‐CHS3b2 (C); Chs3a, (D); Chs3b. Data represent the mean ± SEM. Bars labelled with different letters are significantly different at P < 0.05 according to Duncan’s multiple range test. Asterisks indicate significant differences between minicells and ME‐dsRNA‐treated sample at each time point according to t‐test; *P < 0.05, **P < 0.01, ***P < 0.001.
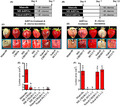
ME‐dsRNAs, targeting different B. fuckeliana genes, were next tested for their efficacy as a topical spray application on fruit‐bearing strawberries under greenhouse conditions. The initial in vivo experiment investigated the bio‐fungicidal capacity of naked‐ or ME‐dsRNA when applied 1h before B. fuckeliana inoculation (Fig. 4A, and Figs S4–S6). ME‐CHS3b1 + 2, ME‐DCL1 + 2 and naked‐CHS3b1 + 2 completely inhibited disease progression, while ME‐CHS3a and naked‐DCL1 + 2 showed minimal fungal growth (Fig. 4C and E). The second experimental strategy investigated the short‐term efficacy (3–7 days post‐treatment) of exogenous dsRNAs (Fig. 4B), as field stability is reported as a major limitation for RNAi‐based fungicides (Gan et al., 2010; Wang et al., 2016; Mitter et al., 2017). For this experiment, ME‐, naked‐dsRNAs or empty minicells were applied 7 days prior to inoculation of B. fuckeliana, and disease progression was analysed at 5 days post‐inoculation (DPI) (Fig. 4B, and Figs S7 and S8). After the analysis of two separate experiments (Fig. 4B, D, E, and Figs S7 and S8), we can conclude that while naked‐dsRNAs failed to provide disease protection, showing a similar level of disease progression as the negative control, ME‐dsRNAs provided complete protection, with no visible signs of grey mould (Fig. 4D and F). These results demonstrate that ME‐dsRNAs can extend the protection window against grey mould diseases by 5–12 days compared to naked‐dsRNAs.
Fig. 4.
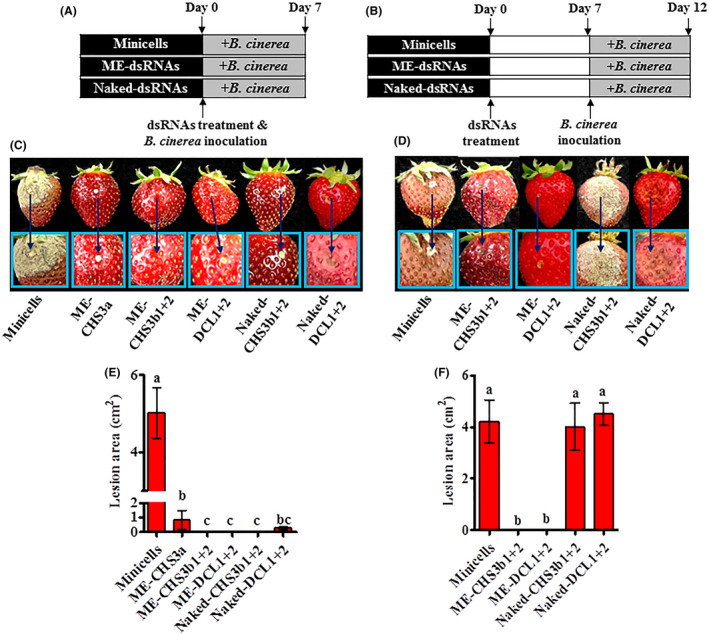
The effectiveness of topically applied minicells‐encapsulated dsRNAs (ME‐dsRNAs) and naked‐dsRNAs in inhibiting grey mould diseases under greenhouse conditions.
A. Schematic representation of the time points of ME‐dsRNAs and naked‐dsRNA applications on strawberry fruit 1 h prior to inoculation, and B, 7 days prior to B. fuckeliana inoculation and the sampling time of the strawberry fruits from the strawberry plants for testing disease severity.
C and E. Images showing ME‐dsRNAs and naked‐dsRNA sprayed onto fruits 1 h prior to B. fuckeliana inoculations reducing grey mould disease symptoms and lesion diameter when compared with the application of empty minicells.
D and F. Images showing ME‐dsRNA sustained protections against grey mould disease compared to naked‐dsRNAs and the disease lesion diameter on treated fruits at 12 days post‐treatment of ME‐dsRNA. Data represent the mean ± SEM. Bars labelled with the different letters are significantly different at P < 0.05 according to Duncan’s multiple range test.
The target‐specific inhibition by ME‐dsRNAs was confirmed in vitro, by experimental results showing that ME‐dsRNAs for B. fuckeliana failed to inhibit the mycelial growth of two other fruit‐rotting fungal pathogens, Alternaria alternata (Fig. 5A–D), and Penicillium expansum (Fig. 5E–H), which represents a major advantage of the ME–dsRNA as a promising new class of selective biofungicides. Interestingly, there is more than 77% similarity between the dsRNA sequence of B. fuckeliana’s chitin synthase 3a gene and its nearest homolog in P. expansum (XM_016744173.1), yet no mycelial growth inhibition was observed when P. expansum was treated with ME‐CHS3a. Taken together with Figs 2 and 3, these results validate the efficacy of ME‐dsRNAs for specific gene silencing of essential B. fuckeliana genes and in vitro fungal inhibition.
Fig. 5.
The mycelial growth inhibitory activity of the minicells‐encapsulated dsRNAs (ME‐dsRNAs) targeting various genes of B. cinerea on two other fruit rot causing fungi. Different concentrations of ME‐dsRNAs effects on mycelial growth of (A, B) Alternaria alternata, and (E, F) Penicillium expansum at 72 h after treatment. The mycelial growth inhibition rate of (C, D) Alternaria alternata, and (G, H) Penicillium expansum. Data represent the mean ± SEM. Bars labelled with different letters are significantly different at P < 0.05 according to Duncan’s multiple range test.
This study presents a robust, scalable platform for producing ME‐dsRNAs using a prokaryotic expression system that sufficiently addresses the major shortcomings of exogenous dsRNA‐based biofungicides; especially those related to stability, efficacy and scalability. This synthetic biology platform has the potential to be incorporated into commercial disease management programs against B. fuckeliana and other economically important phytopathogenic fungi. Investigating and understanding the mechanisms of ME‐dsRNA interactions with pathogenic fungi is a core focus of our ongoing current and future research. We envision the use of minicell‐based RNAi technology in integrated pest/disease management programs for controlling pests, viruses, and other fungal pathogens, in a sustainable way that addresses public concerns around GMOs and overuse of synthetic chemicals.
Conflict of interest
The authors declare the following competing interests: AgroSpheres, INC., an early stage AgTech company that is active in developing commercial minicell‐encapsulated products, contributed to sponsoring this research.
Supporting information
Table S1. RNA production data from four different bioreactors.
Table S2. Specific primers used for qRT‐PCR analysis.
Fig. S1. The Botryotinia fuckeliana mycelial growth inhibitory activity of the minicells‐encapsulated dsRNAs targeting DCL1 and DCL2 genes of B. fuckeliana at 72 hours after treatment. (a) ME‐DCL1, (b) ME‐DCL2, and (c) ME‐DCL1+2. R: Biological replication; ME: empty‐Minicells.
Fig. S2. The Botryotinia fuckeliana mycelial growth inhibitory activity of the minicells‐encapsulated dsRNAs (ME‐dsRNAs; ME‐DCL1 and ME‐DCL2) targeting both DCL1 and DCL2 genes of B. fuckeliana at 72 hours after treatment. R: Biological replication.
Fig. S3. The Botryotinia fuckeliana mycelial growth inhibitory activity of the minicells‐encapsulated dsRNAs targeting Chs3a and Chs3b genes of B. fuckeliana at 72 hours after treatment. (a) ME‐CHS3a, (b) ME‐CHS3b1, (c) ME‐CHS3b2, and (d) ME‐CHS3b1+2. R: Biological replication; ME: empty‐Minicells.
Fig. S4. Topically applied minicells‐encapsulated dsRNAs (ME‐dsRNAs; ME‐CHS3a) targeting chitin synthase 3a (Chs3a) and combination of two ME‐dsRNAs (ME‐CHS2b1+ME‐CHS3b2) targeting chitin synthase 3b (Chs3b) of Botryotinia fuckeliana on the strawberry fruits inhibited the gray mold disease progression. (a) Schematic representation of the ME‐dsRNAs on strawberry fruit 1 hour prior to inoculation. The gray mold diseases symptom development at (b) 4 days post inoculation (dpi), (c) 5dpi, and (d) 7dpi. In order to determine the gray mold disease symptom development following ME‐dsRNAs treatment. ME‐CHS3a applied at dose of 500 ng/mL and the combination of ME‐CHS3b‐1 and 2 used at 1000 ng/ml (500 µl/fruit).
Fig. S5. Gray mold diseases symptom development on minicells‐encapsulated dsRNAs (ME‐dsRNAs) or naked‐dsRNA targeting Botryotinia fuckeliana genes sprayed fruits when challenged with the B. fuckeliana 1h post treatment (1st trial). In first trail we used the minicells, ME‐CHS3a (targeting Chs3a), ME‐CHS3b1+2 (targeting Chs3b), ME‐DCL1+2 (targeting DCL1 and DCL2), and naked‐CHS3b1+2 (Chs3b).
Fig. S6. Gray mold diseases symptom development on minicells‐encapsulated dsRNAs (ME‐dsRNAs) or naked‐dsRNA targeting Botryotinia fuckeliana genes sprayed fruits when challenged with the B. fuckeliana 1h post treatment (2nd trial). In 2nd trail we used the minicells, minicells‐encapsulated dsRNAs (ME‐DCL1+2) and naked‐DCL1+2 (targeting DCL1 and DCL2) treatments.
Fig. S7. Minicells‐encapsulated dsRNAs (ME‐dsRNAs) for sustained protection against Botryotinia fuckeliana (1st trial). Plants were sprayed on day 0 with minicells, ME‐CHS3b1+2 or naked‐CHS3b1+2 (targeting B. cinaeral Chs3b gene) and inoculated with B. fuckeliana on sprayed fruits at 7 days post treatment. Gray mold disease severity was observed at 5 days post inoculation (dpi).
Fig. S8. Minicells‐encapsulated dsRNAs (ME‐dsRNAs) for sustained protection against B. cinerea (2nd trial). Plants were sprayed on day 0 with minicells, ME‐DCL1+2 or naked‐DCL1+2 (targeting B. fuckeliana DCL1 and DCL2 genes) and inoculated with B. fuckeliana on sprayed fruits at 7 days post treatment. Gray mold disease severity was observed at 5 days post inoculation (dpi).
Data S1. Experimental procedures.
Acknowledgements
The authors are grateful for the funding from The Virginia Catalyst (Fund # 460380). The authors are also grateful for the support of The Center for Innovative Technology (CIT CRCF) and The Virginia Wine Board.
Microb. Biotechnol. (2021) 14(4), 1847–1856
Funding information The authors are grateful for the funding from The Virginia Catalyst (Fund # 460380).
Contributor Information
Payam Pourtaheri, Email: payam@agrospheres.com.
Sherif M. Sherif, Email: ssherif@vt.edu.
References
- Amiri, A. , Heath, S.M. , and Peres, N.A. (2013) Phenotypic characterization of multifungicide resistance in Botrytis cinerea isolates from strawberry fields in Florida. Plant Dis 97: 393–401. [DOI] [PubMed] [Google Scholar]
- Baulcombe, D.C. (2015) VIGS, HIGS and FIGS: small RNA silencing in the interactions of viruses or filamentous organisms with their plant hosts. Curr Opin Plant Biol 26: 141–146. [DOI] [PubMed] [Google Scholar]
- Carleton, H.A. , Lara‐Tejero, M. , Liu, X. , and Galán, J.E. (2013) Engineering the type III secretion system in non‐replicating bacterial minicells for antigen delivery. Nat Commun 4: 1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalanotto, C. , Pallotta, M. , ReFalo, P. , Sachs, M.S. , Vayssie, L. , Macino, G. , and Cogoni, C. (2004) Redundancy of the two dicer genes in transgene‐induced posttranscrip‐tional gene silencing in Neurospora crassa . Mol Cell Biol 24: 2536–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquer, M. , Boccara, M. , Gonçalves, I.R. , Soulié, M.‐C. , and Vidal‐Cros, A. (2004) Survey of the Botrytis cinerea chitin synthase multigenic family through the analysis of six euascomycetes genomes. Eur J Biochem. 271: 2153–2164. [DOI] [PubMed] [Google Scholar]
- Choquer, M. , Boccara, M. , and Vidal‐Cros, A. (2003) A semi‐quantitative RT‐PCR method to readily compare expression levels into Botrytis cinerea multigenic families. Curr Genet 43: 303–309. [DOI] [PubMed] [Google Scholar]
- Czech, B. , Malone, C.D. , Zhou, R. , Stark, A. , Schlingeheyde, C. , Dus, M. , et al. (2008) An endogenous small interfering RNA pathway in Drosophila . Nature 453: 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley, M.M. , Hu, B. , Margolin, W. , and Liu, J. (2016) Minicells, back in fashion. J Bacteriol 198: 1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Ortuño, D. , Grabke, A. , Li, X. , and Schnabel, G. (2015) Independent emergence of resistance to seven chemical classes of fungicides in Botrytis cinerea . Phytopathology 105: 424–432. [DOI] [PubMed] [Google Scholar]
- Fire, A. , Xu, S. , Montgomery, M.K. , Kostas, S.A. , Driver, S.E. , and Mello, C.C. (1998) Potent and specific genetic interference by double‐stranded RNA in Caenorhabditis elegans . Nature 391: 806–811. [DOI] [PubMed] [Google Scholar]
- Gan, D. , Zhang, J. , Jiang, H. , Jiang, T. , Zhu, S. , and Cheng, B. (2010) Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 29: 1261–1268. [DOI] [PubMed] [Google Scholar]
- Hale, T.L. , Sansonetti, P.J. , Schad, P.A. , Austin, S. , and Formal, S.B. (1983) Characterization of virulence plasmids and plasmid‐associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun 40: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M.T. , and Sherif, S.M. (2020) RNAi‐Based Biofungicides as a promising next‐generation strategy for controlling devastating gray mold diseases. Int J Mol Sci 21: 2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinou, S. , Veloukas, T. , Leroch, M. , Menexes, G. , Hahn, M. , and Karaoglanidis, G. (2015) Population structure, fungicide resistance profile, and sdhB mutation frequency of Botrytis cinerea from strawberry and greenhouse‐grown tomato in Greece. Plant Dis 99: 240–248. [DOI] [PubMed] [Google Scholar]
- MacDiarmid, J.A. , Amaro‐Mugridge, N.B. , Madrid‐Weiss, J. , Sedliarou, I. , Wetzel, S. , Kochar, K. , et al. (2009) Sequential treatment of drug‐resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nature Biotech 27: 643–651. [DOI] [PubMed] [Google Scholar]
- MacDiarmid, J.A. , Mugridge, N.B. , Weiss, J.C. , Phillips, L. , Burn, A.L. , Paulin, R.P. , et al. (2007) Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell 11: 431–445. [DOI] [PubMed] [Google Scholar]
- Mcloughlin, A.G. , Walker, P.L. , Wytinck, N. , Sullivan, D.S. , Whyard, S. , and Belmonte, M.F. (2018a) Developing new RNA interference technologies to control fungal pathogens. Can J Plant Pathol 40: 325–335. [Google Scholar]
- Mcloughlin, A.G. , Wytinck, N. , Walker, P.L. , Girard, I.J. , Rashid, K.Y. , De Kievit, T. , et al. (2018b) Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea . Sci Rep 8: 7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter, N. , Worrall, E.A. , Robinson, K.E. , Li, P. , Jain, R.G. , Taochy, C. , et al. (2017) Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat Plants 3: 16207. [DOI] [PubMed] [Google Scholar]
- Nerva, L. , Sandrini, M. , Gambino, G. , and Chitarra, W. (2020) Double‐stranded RNAs (dsRNAs) as a sustainable tool against gray mold (Botrytis cinerea) in Grapevine: Effectiveness of different application methods in an open‐air environment. Biomolecules. 10: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panebianco, A. , Castello, I. , Cirvilleri, G. , Perrone, G. , Epifani, F. , Ferrara, M. , et al. (2015) Detection of Botrytis cinerea field isolates with multiple fungicide resistance from table grape in Sicily. Crop Protect 77: 65–73. [Google Scholar]
- Petrasch, S. , Knapp, S.J. , van Kan, J.A. L. , and Blanco‐Ulate, B. (2019) Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea . Mol Plant Pathol 20: 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp, S. , Weber, R.W. S. , Rieger, D. , Detzel, P. , and Hahn, M. (2017) Spread of Botrytis cinerea strains with multiple fungicide resistance in german horticulture. Front Microbiol 7: 2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulie, M.C. , Perino, C. , Piffeteau, A. , Choquer, M. , Malfatti, P. , Cimerman, A. , et al. (2006) Botrytis cinerea virulence is drastically reduced after disruption of chitin synthase class III gene (Bcchs3a). Cell Microbiol 8: 1310–1321. [DOI] [PubMed] [Google Scholar]
- Takiff, H.E. , Chen, S. , and Court, D.L. (1989) Genetic analysis of the rnc operon of Escherichia coli . J Bacteriol 171: 2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taning, C.N. T. , Arpaia, S. , Christiaens, O. , Dietz‐Pfeilstetter, A. , Jones, H. , Mezzetti, B. , et al. (2020) RNA‐based biocontrol compounds: current status and perspectives to reach the market. Pest Managem Sci 76: 841–845. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Thomas, N. , and Jin, H. (2017) Cross‐kingdom RNA trafficking and environmental RNAi for powerful innovative pre‐ and post‐harvest plant protection. Curr Opin Plant Biol 38: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Weiberg, A. , Lin, F.M. , Thomma, B. , Huang, H.D. , and Jin, H. (2016) Bidirectional cross‐kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants 2: 16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Wu, D. , Liu, Y. , Xia, X. , Gong, W. , Qiu, Y. , et al. (2015) DrosophilaDicer‐2 has an RNA interference‐independent function that modulates Toll immune signaling. Sci Adv 1: e1500228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, R.W. S. (2011) Resistance of Botrytis cinerea to multiple fungicides in Northern German small‐fruit production. Plant Dis 95: 1263–1269. [DOI] [PubMed] [Google Scholar]
- Xiong, F. , Liu, M. , Zhuo, F. , Yin, H. , Deng, K. , Feng, S. , et al. (2019) Host‐induced gene silencing of BcTOR in Botrytis cinerea enhances plant resistance to grey mould. Mol Plant Pathol 20: 1722–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhang, J. , and Zhu, K. (2010) Chitosan/double‐stranded RNA nanoparticle‐mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol Biol 19: 683–693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. RNA production data from four different bioreactors.
Table S2. Specific primers used for qRT‐PCR analysis.
Fig. S1. The Botryotinia fuckeliana mycelial growth inhibitory activity of the minicells‐encapsulated dsRNAs targeting DCL1 and DCL2 genes of B. fuckeliana at 72 hours after treatment. (a) ME‐DCL1, (b) ME‐DCL2, and (c) ME‐DCL1+2. R: Biological replication; ME: empty‐Minicells.
Fig. S2. The Botryotinia fuckeliana mycelial growth inhibitory activity of the minicells‐encapsulated dsRNAs (ME‐dsRNAs; ME‐DCL1 and ME‐DCL2) targeting both DCL1 and DCL2 genes of B. fuckeliana at 72 hours after treatment. R: Biological replication.
Fig. S3. The Botryotinia fuckeliana mycelial growth inhibitory activity of the minicells‐encapsulated dsRNAs targeting Chs3a and Chs3b genes of B. fuckeliana at 72 hours after treatment. (a) ME‐CHS3a, (b) ME‐CHS3b1, (c) ME‐CHS3b2, and (d) ME‐CHS3b1+2. R: Biological replication; ME: empty‐Minicells.
Fig. S4. Topically applied minicells‐encapsulated dsRNAs (ME‐dsRNAs; ME‐CHS3a) targeting chitin synthase 3a (Chs3a) and combination of two ME‐dsRNAs (ME‐CHS2b1+ME‐CHS3b2) targeting chitin synthase 3b (Chs3b) of Botryotinia fuckeliana on the strawberry fruits inhibited the gray mold disease progression. (a) Schematic representation of the ME‐dsRNAs on strawberry fruit 1 hour prior to inoculation. The gray mold diseases symptom development at (b) 4 days post inoculation (dpi), (c) 5dpi, and (d) 7dpi. In order to determine the gray mold disease symptom development following ME‐dsRNAs treatment. ME‐CHS3a applied at dose of 500 ng/mL and the combination of ME‐CHS3b‐1 and 2 used at 1000 ng/ml (500 µl/fruit).
Fig. S5. Gray mold diseases symptom development on minicells‐encapsulated dsRNAs (ME‐dsRNAs) or naked‐dsRNA targeting Botryotinia fuckeliana genes sprayed fruits when challenged with the B. fuckeliana 1h post treatment (1st trial). In first trail we used the minicells, ME‐CHS3a (targeting Chs3a), ME‐CHS3b1+2 (targeting Chs3b), ME‐DCL1+2 (targeting DCL1 and DCL2), and naked‐CHS3b1+2 (Chs3b).
Fig. S6. Gray mold diseases symptom development on minicells‐encapsulated dsRNAs (ME‐dsRNAs) or naked‐dsRNA targeting Botryotinia fuckeliana genes sprayed fruits when challenged with the B. fuckeliana 1h post treatment (2nd trial). In 2nd trail we used the minicells, minicells‐encapsulated dsRNAs (ME‐DCL1+2) and naked‐DCL1+2 (targeting DCL1 and DCL2) treatments.
Fig. S7. Minicells‐encapsulated dsRNAs (ME‐dsRNAs) for sustained protection against Botryotinia fuckeliana (1st trial). Plants were sprayed on day 0 with minicells, ME‐CHS3b1+2 or naked‐CHS3b1+2 (targeting B. cinaeral Chs3b gene) and inoculated with B. fuckeliana on sprayed fruits at 7 days post treatment. Gray mold disease severity was observed at 5 days post inoculation (dpi).
Fig. S8. Minicells‐encapsulated dsRNAs (ME‐dsRNAs) for sustained protection against B. cinerea (2nd trial). Plants were sprayed on day 0 with minicells, ME‐DCL1+2 or naked‐DCL1+2 (targeting B. fuckeliana DCL1 and DCL2 genes) and inoculated with B. fuckeliana on sprayed fruits at 7 days post treatment. Gray mold disease severity was observed at 5 days post inoculation (dpi).
Data S1. Experimental procedures.


