Abstract
Background:
No studies have examined human papillomavirus (HPV) infections among couples early in their sexual relationships when transmission is most likely. Our objective was to describe the distribution of HPV infections among recently formed couples, using the partnership as the unit of analysis.
Methods:
Women aged 18–24 years attending a university or junior college in Montreal enrolled in a longitudinal study with their new male partners. Self-collected vaginal swabs and clinician-collected swabs from the penis and scrotum were tested for 36 HPV genotypes. Participants self-reported sexual behavior in computerized questionnaires. We analyzed patterns of genital HPV infection in 263 couples using data obtained at enrollment.
Results:
Couples had engaged in vaginal sex for a median of 3.9 months. HPV was detected in 64% (169/263) of couples. In 41% (109/263), both partners harbored the same HPV type—nearly 4 times more than expected if HPV status of partners were uncorrelated. There were 583 type-specific HPV infections among 169 couples for whom at least one partner was infected. Of these, 42% were of the same type for both partners (95% confidence interval = 36%–47%). This rose from 25% among those engaging in vaginal sex for less than 2 months to 68% among those at 5 to 6 months.
Conclusions:
Although HPV is common, detection of the same type in persons initiating a sex relationship would be rare given type-specific prevalence rates. The high degree of concordance we found suggests a high probability of transmission.
Genital human papillomavirus (HPV) is the most common sexually transmitted infection (STI).1 Most of these infections clear spontaneously.2,3 The small proportion that persists may result in substantial morbidity, particularly infections with high oncogenic-risk HPV genotypes. The latter, especially HPV-16 and 18, are recognized as the main causal factor for cervical cancer.4 High-risk HPV also cause other anogenital neoplasms and head and neck cancers; as much as 5% of incident cancers worldwide are attributed to these infections.5 Infections with types of low oncogenic risk (such as HPV-6 and 11) cause benign lesions including genital warts or are completely subclinical.
Due to its sexually transmitted nature, the study of HPV at the level of the sexual partnership is fundamental to our understanding of the epidemiology of these infections. Most research of HPV in couples has consisted of cross-sectional assessment of prevalent infection in both partners.6,7 Study populations included STI clinic attendees,8 couples being evaluated for infertility,9 women referred for colposcopy and their partners,10,11 and women with cervical intraepithelial neoplasia (CIN) or cervical cancer (within the context of retrospective case–control studies).12–14 These studies have documented the importance of male sexual behavior for women’s risk of HPV-related disease.12,13,15 However, many of these studies found that the presence of the same HPV type in both partners (ie, HPV-type-specific positive concordance) was relatively infrequent. In 2 studies, concordance was greater than expected by chance,8,11 and was associated with more recent sexual intercourse8 and higher viral load.11 Methods of HPV-DNA detection and male specimen collection are steadily being improved, and some of these earlier studies may have had limited ability to detect HPV infections.
Couples in these previous studies tended to be older, with relationships of long duration. No studies specifically targeted recently formed couples, and some excluded couples of less than 6 months duration.12,13 Because most HPV infections are no longer detectable 12–24 months after initial infection,2,16 infections may have cleared in one or both partners by the time testing was done.
The observation that HPV occurs more commonly in sexual partners than expected by chance provides evidence for the sexual transmission of HPV.8 We hypothesize that the extent of concordance will be greatest among relatively young couples early in their sexual relationship, because this is when transmission is likely. Acquisition of a new partner is an important risk factor for incident infection in women17–19 and men,20 and HPV is thought to be highly transmissible.21,22 Therefore, the objective of the current investigation was to describe the distribution of HPV infections among recently formed heterosexual couples. We focused on the partnership as the unit of analysis. HPV prevalence among individual men and women enrolled in this study is reported elsewhere.23
METHODS
We analyzed cross-sectional enrollment data from the HITCH Cohort Study (HPV Infection and Transmission among Couples through Heterosexual Activity). This is an ongoing longitudinal investigation initiated in May 2005. The study population consists of young women (aged 18–24 years) attending university or junior college in Montreal, Canada and their male partners. Eligible women were willing to attend follow-up visits for 2 years; were sexually active with their current male partner for no more than 6 months; had an intact uterus and no self-reported history of cervical cytologic abnormalities or cancer; and were not currently pregnant or planning to become pregnant in the next 24 months. Eligible male partners were aged at least 18 years and willing to participate for at least 4 months. Presence of genital warts or vaccination for HPV did not affect eligibility.
A self-selected volunteer sample was recruited through study promotion on campuses and at venues frequented by students. Promotional materials invited interested persons to visit the study website (www.mcgill.ca/hitchcohort) and to contact the research nurses. Of those making initial contact, 37% were documented as eligible, and of these 58% enrolled. Participants visited the student health services clinics of either McGill or Concordia Universities. They were compensated CDN $50 for a completed clinic visit. All provided written informed consent. Study procedures and documents were approved by the ethical review committees at McGill University, Concordia University, and Université de Montréal.
Men and women self-completed separate computerized questionnaires. Sexual behavior was assessed starting from the beginning of the couple’s sexual relationship (defined as the first encounter involving mutual masturbation, oral sex, or vaginal or anal intercourse).
Participants were asked to abstain from oral, vaginal, or anal sex for 24 hours prior to the clinic visit, at which time genital specimens were collected. Women self-collected vaginal swabs; they were instructed to gently insert a Dacron swab (Invista, Inc, Wichita, KS) into the vagina until physically it could not go any further (at least 5 cm), then to rotate the swab inside the vagina for 3 full rotations. Clinicians obtained specimens of epithelial cells from the penis (ie, the glans up to and including the external opening of the meatus, coronal sulcus, penile shaft, and foreskin in uncircumcised men) and scrotum as described previously.24 Briefly, the skin was first gently abraded using sterilized ultra-fine emery paper (3M 600A-grit Wetordry Tri-M-ite [3M, St. Paul, MN]), then swabbed with a Dacron applicator (Invista) moistened with normal saline. Vaginal and male genital swabs were agitated in PreservCyt (Cytyc Corp, Londonderry, NH), and then discarded. Emery papers from male specimens were placed in the vials with the PreservCyt solution. Specimens were stored at 4°C pending laboratory processing.
Specimens were tested by a polymerase chain reaction protocol based on amplification of a 450 bp segment in the HPV L1 gene using the Linear Array HPV genotyping assay (LA-HPV) (Roche Molecular Systems).25 Thirty-six mucosal HPV genotypes are detected with this technique: types 6, 11, 16, 18, 26, 31, 33, 34 (formerly known as type 64), 35, 39, 40, 42, 44 (formerly known as type 55), 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, and 89. Coamplification of a β-globin DNA sequence permitted determination of whether the specimens had adequate cellularity; 99.6% of vaginal, 97.1% of penile, and 88.1% of scrotum specimens were considered adequate. Type-specific infection status was similar for men regardless of anatomic site (data not shown); therefore, male genital infection was analyzed using the combined result from the penis and scrotum sites.
Statistical analysis was conducted using SAS, version 9 (SAS Institute, Inc, Cary, North Carolina). The analysis focused on the occurrence of type-specific positive concordance within couples. “Concordance” was defined as the presence of the same HPV type in both the female and male partner. “Discordance” was defined as the presence of a specific HPV type in one partner but not the other.
Using probability theory, we calculated the proportion of couples who were concordant for at least one HPV type and compared this to the expected proportion that would have occurred due to chance alone. For a single HPV type “t,” the probability of concordance is equal to the product of the male- and female-specific prevalence rates (Pmt × Pft). The probability that partners do not share the same type t is 1 − (Pmt × Pft). The probability that both partners are not concordant for any type is the product of all the (1 − PmtPft) quantities over the 36 types, ie, π(1−PmtPft). This assumes that the probability of infection with one type is independent of infection with another type. Finally, the probability that a couple is concordant for one or more types due to chance is equal to 1 − π(1 − PmtPft).
A Monte Carlo test was used to evaluate the null hypothesis (that there is no association between HPV status of the male and female partner) by simulating 100,000 random samples of 263 couples. The marginal sex- and HPV-type-specific prevalences were used to randomly assign infection status for all 36 types in each simulated couple. The result was a distribution of the expected proportion of concordant couples under the null hypothesis of independence. The percentage of simulations with an expected proportion equal to or greater than the observed proportion is the one-sided P value for testing the null hypothesis against the alternate hypothesis that the proportion is greater than expected by chance.
For each HPV-type comparison within couples, we reviewed 2-by-2 tables and tested the null hypothesis of no association using Fisher exact test. We calculated the ratio of the observed to the expected proportion concordant according to the marginal HPV type-specific prevalences. Among the discordant pairs, the odds ratio (OR) for male versus female positivity was calculated with its 95% confidence interval (CI). For single HPV types, McNemar test-based CIs were calculated. For grouped types (eg, all HR-HPVs), robust standard errors using generalized estimating equations (GEE) were calculated in CI estimation to account for multiple observations per couple.
Furthermore, we estimated the proportion of HPV infections that were concordant among couples in which at least one partner was infected. It is only in these “exposed” couples that there is an opportunity for transmission. We calculated the ratio of the observed to the expected proportion concordant according to the marginal HPV type-specific prevalences; this was summarized over all types, HR-HPV, LR-HPV, and for Alphapapillomavirus species 3/15, 7, 9, and 10.
Next, each type-specific HPV infection in a couple was treated as a single observation. The proportion of couple-level HPV infections for which both partners were infected was estimated summarized over all types and within type categories (high-risk HPV, low-risk HPV, and Alphapapillomavirus species 3/15, 7, 9, and 10). There were a total of 583 HPV infections among 169 exposed couples. Robust standard errors were calculated using GEE for 95% CI estimation to account for multiple observations per couple.
Finally, the cross-sectional proportions of concordant infections were compared by time the couple had engaged in vaginal sex, and by the total number of vaginal sex encounters. This analysis was restricted to 126 exposed couples who reported no other sexual partners since the start of their relationship. This was done to allow for the interpretation of these cross-sectional data as a “snapshot” of HPV infections over time, without any new introduction of types from external partners. The effect of time was evaluated using logistic regression with GEE to account for multiple observations per couple.
RESULTS
On average, women were 1.5 years younger (mean = 21.2 years) than their male partners (mean = 22.7 years). Most participants reported being exclusively heterosexual (among women, 86%; among men, 97%). All men and all but 3 women reported having ever engaged in vaginal sex. Few reported that this partner was their first vaginal sex partner (women: 14%; men: 13%). The median number of lifetime vaginal sex partners was 5 for both men and women. Eleven percent of women reported having been vaccinated for HPV.
Most subjects (women, 89%; men, 87%) considered their study partner to be their dating partner (ie, boyfriend or girlfriend). At enrollment, couples had been sexually active together for a median of 4.2 months. All reported engaging in mutual masturbation. Nearly all reported oral sex on the male (99%) and female partner (95%). All but 3 couples (99%) had engaged in vaginal sex and had done so for a median of 3.9 months (Table 1). The mean frequency of vaginal sex was 4.8 times per week; upon enrollment, couples had engaged in a median of 63 vaginal sex encounters. Only 9% never used condoms. Most reported no other sex partner since the start of their relationship with their HITCH partner (women, 85%; men, 86%). There was no evidence of a sex difference in reporting the couple’s sexual activities (eAppendix, http://links.lww.com/EDE/A348).
TABLE 1.
Sexual Behaviors Reported by Couplesa at Enrollment in the HITCH Cohort Study
| No. | %b | |
|---|---|---|
| Months engaging in vaginal sex | ||
| <2 | 38 | 15 |
| 2 to <3 | 41 | 16 |
| 3 to <4 | 53 | 20 |
| 4 to <5 | 54 | 21 |
| 5 to <6 | 30 | 12 |
| 6 to <7 | 20 | 8 |
| 7+ | 24 | 9 |
| Frequency of vaginal sex (times/week) | ||
| <3 | 66 | 25 |
| 3–1 | 95 | 36 |
| 5–6 | 56 | 21 |
| 7+ | 43 | 16 |
| Total no. vaginal sex encounters | ||
| 1–24 | 39 | 15 |
| 25–19 | 63 | 24 |
| 50–74 | 46 | 18 |
| 75–99 | 34 | 13 |
| 100–124 | 24 | 9 |
| 125–149 | 18 | 7 |
| 150+ | 36 | 14 |
| Frequency of condom use for vaginal sex | ||
| Never (0%) | 23 | 9 |
| Rarely (l%–25%) | 67 | 26 |
| Sometimes (26%–75%) | 74 | 28 |
| Most of the time (76%–99%) | 47 | 18 |
| Always (100%) | 49 | 19 |
| Engaged in anal sex | ||
| Yes | 59 | 23 |
| No | 202 | 77 |
Men and women reported sexual behaviors separately. The answers from both partners were averaged to obtain a result for the couple (details are provided in eAppendix, http://links.lww.com/EDE/A348).
Frequencies may not add to 100% due to rounding.
HPV Distribution Among Couples
HPV was highly prevalent in the 263 couples, with at least one partner having at least one HPV type in 64% of couples (n = 169). Both partners were HPV positive in half of couples (47%; n = 125). Couples were equally likely to be male-positive/female-negative (8%; n = 22) or male-negative/female-positive (8%; n = 22). Among couples for whom both partners were positive for any HPV, 87% (109/125) were concordant for one or more types.
When distribution of HPV type was examined in all 263 couples, 41% (n = 109) were concordant for at least one HPV type; 33% (86) were female-positive/male-negative for at least one type; and 36% (95) were male-positive/female-negative for at least one type. Based on the sex- and type-specific prevalences and assuming independence for HPV status, the expected proportion of concordance on one or more types was 11% due to chance alone. None of the 100,000 simulations resulted in an expected proportion concordant as high as the observed 41% under an assumption of independence (Fig. 1). The mean expected proportion concordant was 11% and the maximum was 17%. The Monte Carlo P value was <0.00001 under the null hypothesis that partners’ HPV infections are independent of each other.
FIGURE 1.
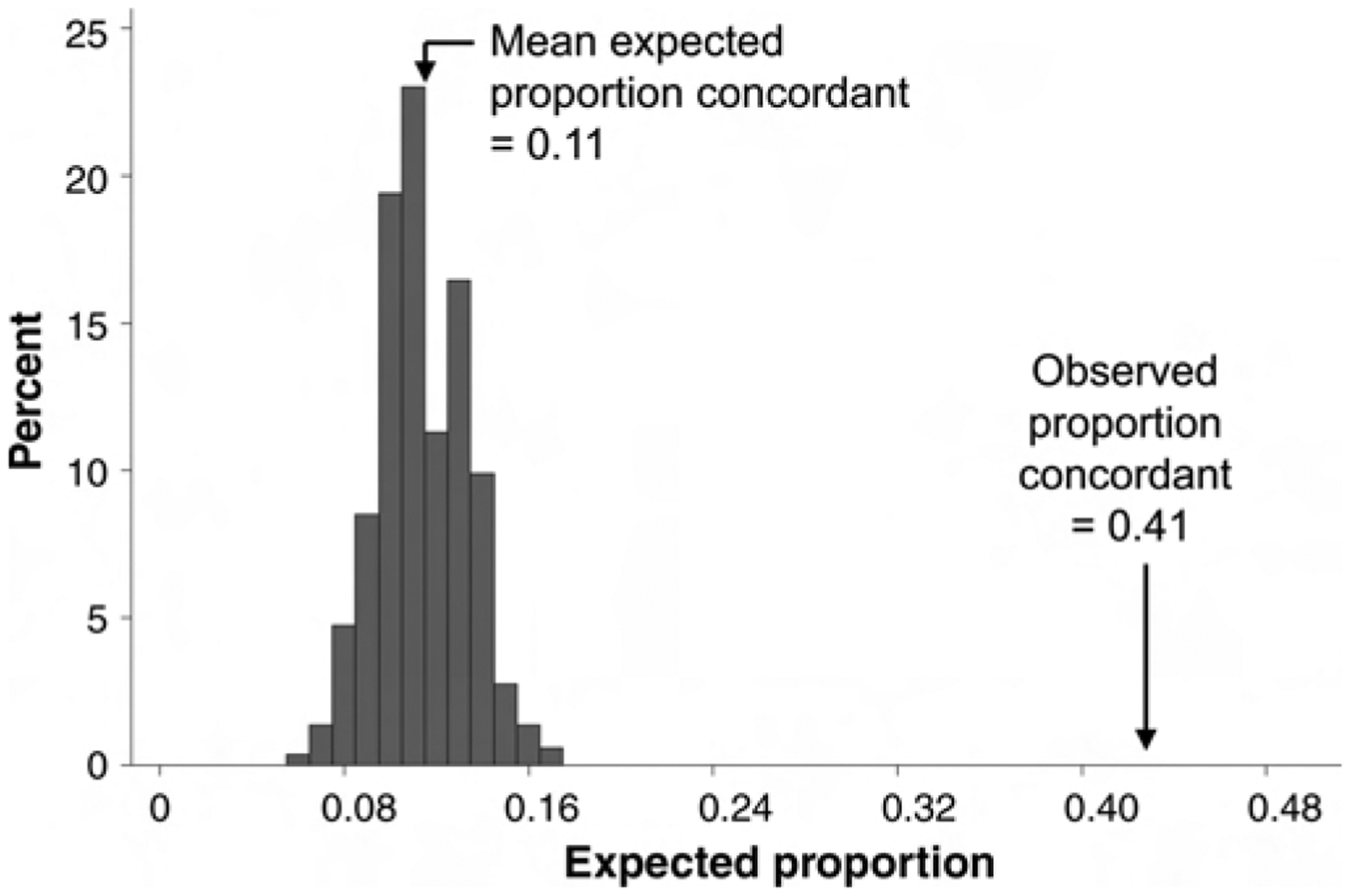
Observed proportion of couples concordant on one or more HPV types compared with the expected distribution of the proportion of concordant couples assuming independence in HPV type distribution between partners (details are provided in the text).
HPV-16 was the most common type, detected in 22% of couples (Fig. 2). For most types, the proportion concordant was far greater than expected and there was strong evidence (P < 0.001) to reject the null hypothesis of independence of HPV infection between partners (eAppendix, http://links.lww.com/EDE/A348). When all HPV types were summed into a single 2-by-2 table (36 types × 263 couples = 8496 observations), 2.5% were positive-concordant, which was 13 times greater than expected based on the individual type-specific prevalences.
FIGURE 2.
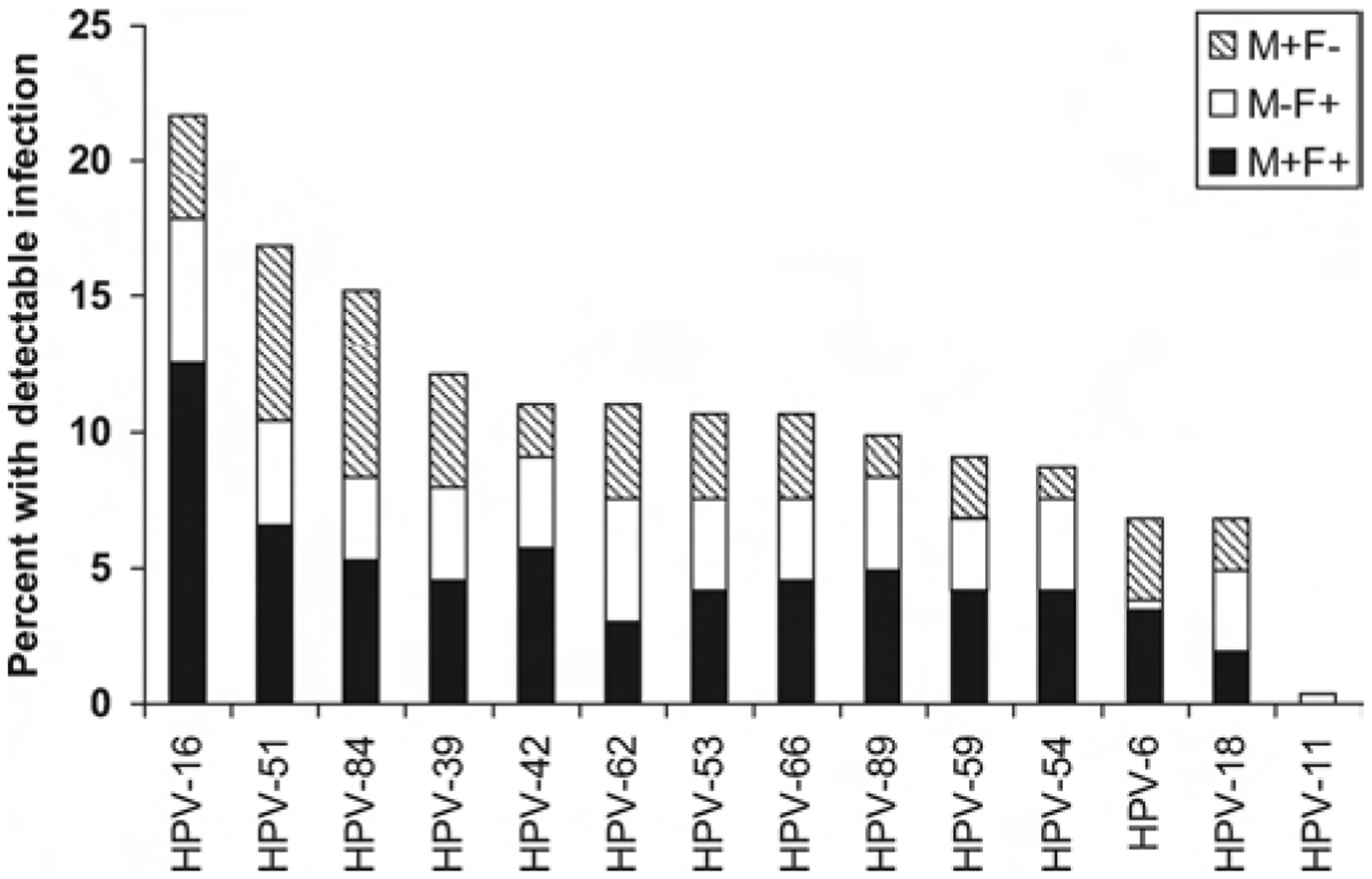
Distribution of 10 most common and vaccine-preventable HPV types among recently formed couples (n = 263). M+F+ indicates male positive, female positive; M−F+, male negative, female positive; M+F−, male positive, female negative.
Among type-discordant observations, ORs were calculated comparing male-positive versus female-positive. There was little evidence for a pattern of male–female discordance for all HPV types combined (OR = 1.1 [95% CI = 0.8–1.4]), all high-risk types (1.0 [0.8–1.4]), all low-risk types (1.1 [0.7–1.6]), or individually for most of the common and vaccine-preventable types (data not shown). The exception was HPV-6, which was 8 times more likely to be present in the man when couples were discordant (CI = 1.4–46).
HPV Infections in Exposed Couples
Patterns of HPV infections were examined among the 169 “exposed” couples in which HPV was present in at least one partner (ie, couples in which there was an opportunity for HPV transmission). Nearly two-thirds of these couples (64%, n = 109) were concordant for at least one HPV type; 51% (n = 86) were female-positive/male-negative for at least one type; and 56% (n = 95) were male-positive/female-negative for at least one type. The mean number of types present in these couples was 3.4 (SD = 2.4; median = 3; range = 1–12). There were a total of 583 type-specific HPV infections, of which 238 were partner-concordant.
As expected, given the conditioning on infection in at least one of the partners, the proportion concordant was higher in the subset of exposed couples (Table 2). Even so, concordance was still higher than expected based on sex-specific prevalences. The proportion concordant for at least one type also rose as more types were included in the grouping (ie, from 16 high-risk types to 20 low-risk types to all 36 types).
TABLE 2.
Positive Concordance of HPV Infections, by Oncogenicity, Among 169 Couples in Which at Least One Partner Was Infected
| No. Couples | Proportion of Couples Concordant for 1 or More HPV Types | Ratio of Observed % Concordant Over Expected (95% CI) | No. Couple-level HPV Infectionsa | Proportion of Infections for Which Both Partners are Infected (95% CI) | |
|---|---|---|---|---|---|
| High- and low-risk types combined | 169 | 0.64 | 2.6 (2.1–3.1) | 583 | 0.42 (0.36–0.47) |
| High-riskb | 144 | 0.51 | 3.0 (2.4–3.8) | 339 | 0.43 (0.37–0.49) |
| Low-riskc | 124 | 0.40 | 4.1 (3.1–5.1) | 244 | 0.39 (0.33–0.46) |
A couple-level HPV infection was defined as an instance of a specific HPV type t being detected in a couple (ie, in at least one of the partners).
HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, and 82.
HPV types 6, 11, 26, 34, 40, 42, 44, 53, 54, 61, 62, 67, 69, 70, 71, 72, 81, 83, 84, and 89.
The proportion of HPV infections for which both partners were infected was 42% (95% CI = 36%–47%). This varied little by oncogenic risk category (high-risk, 43%; low-risk, 39%). The highest occurrence of infection in both partners was observed for alpha 9 types (50% [41%–59%]). This was driven by HPV-16, for which 58% of infections (45%–70%) were present in both partners. For couples infected with alpha 9 type infections other than HPV-16, both partners were infected in 42% (30%–55%), similar to other alpha species.
The presence of type-specific HPV infections in both partners was highest among couples who had their most recent sexual contact 1 to 2 days prior to their visit (49%; n = 93 couples). It was lower for those whose last contact was 3–4 days ago (29%; n = 32), 5–6 days ago (33%; n = 11), and 1 week ago or more (35%; n = 26). It was also lower (33%) among the 3 couples who reported vaginal sex within 24 hours before the clinic visit, contrary to study instruction.
Patterns of HPV infections among the exposed, monogamous couples varied by time since the couple formed (Fig. 3). The proportion of infections shared by both partners was higher among couples who had engaged in vaginal sex for longer periods of time, peaking at 68% among couples who had engaged in vaginal sex for 5–6 months, or a total of 100–124 encounters, and lower thereafter (in a statistical test for nonlinearity, P < 0.01). Figure 3B suggests that there may have been a second peak at 150 or more vaginal sex encounters, although this was probably due to chance (P for cubic term = 0.76).
FIGURE 3.
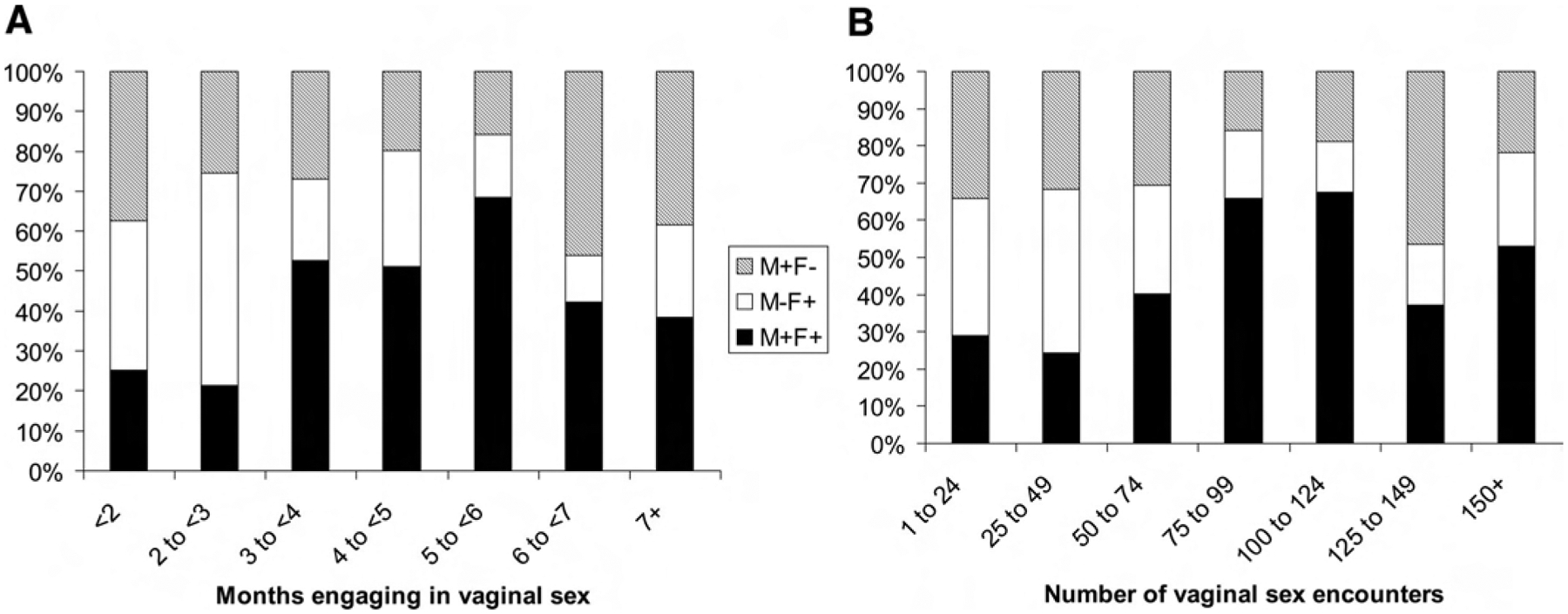
Patterns of HPV type concordance and discordance in 419 infections among 126 monogamous couples in whom at least one partner had detectable infection. A, By months engaging in vaginal sex; B, By total number of vaginal sex encounters.
DISCUSSION
Detection of one or more HPV types was observed in 64% of recently formed sexual partnerships. The same type was detected in both partners in 41% of couples, far more frequent than expected by chance. Among couples with HPV in one partner but not the other, the discordance was symmetric for men and women with the exception of HPV-6, which was 8 times more likely to be observed in men than women.
This is the first report of patterns of HPV infection among couples who recently initiated a sexual relationship. In previous studies that used PCR for HPV detection, the proportion of concordant couples ranged from 2% to 47%, with most observations in range of 20% to 40%.8–13,26,27 Our 41% estimate among recently formed couples is at the upper limit of this range. Among “exposed” couples (those with at least one infected partner), the proportion concordant was even higher (64%).
It is theoretically possible that the observed concordance is coincidence, with both partners infected from past partners. HPV detection among these women and men was strongly associated with their lifetime number of vaginal sex partners.23 However, our simulations showed that this was unlikely. Greater than expected concordance has also been observed in 2 previous studies.8 The more likely explanation is that one partner was infected when the couple initiated their relationship and transmitted it to the other. By the time of study enrollment, couples had engaged in a median of 63 vaginal sex encounters, providing ample opportunity for transmission.11 This conclusion is also consistent with the pattern of greater concordance in exposed couples over months of engaging in sex, and with greater total number of vaginal sex encounters. A recent longitudinal study of 25 couples has documented high rates of genital HPV transmission.28
Concerning within-couple, type-specific discordance, there are 2 possible explanations apart from possible sampling and detectability issues (mentioned later). First, HPV may not have been transmitted (yet) from the infected to the uninfected partner. Alternatively, HPV may have been present in both partners (either due to coincidence or transmission), but by the time of enrollment, it had cleared in one of the partners. The average time to clearance is thought to be no more than 12 months in women2 and as short as 6 months among men.16 Among exposed couples in our study, the patterns of concordance and discordance over time suggest that infection clearance may become influential once couples have engaged in vaginal sex for 6 months or longer.
If we assume that most concordance represents transmission and most discordance represents absence of transmission, the proportion of couple-level HPV infections for which both partners were infected can be interpreted as an estimate of the per-partner transmission probability. Our overall estimate was 42% (95% CI = 36%–47%), although this was a function of time. Concordance rose to a peak of 68% by 5–6 months of engaging in vaginal sex, or 100–124 encounters. Limitations in our understanding of the mechanisms for concordance and discordance preclude a conclusion regarding directional bias in these estimates of the transmission probability.
Concordance did not vary meaningfully when HPV types were grouped by oncogenic risk or Alphapapillomavirus species, with one exception. HPV-16 infection in both partners occurred more commonly than other types (58% [95% CI = 45%–70%], P = 0.05). This finding cannot be entirely explained by higher prevalence of HPV-16, because once prevalence was accounted for, concordance of HPV-16 was still more than 4 times higher than expected. The finding could be explained by higher transmissibility of HPV-16, or by longer duration of these infections. Longer duration of HPV-16 infection has been observed in some studies of women.2 Ultimately, longitudinal data are needed to verify type differences in transmissibility.
Measurement errors may have affected these results. We used accepted methods for cell sampling and the highly-sensitive HPV-LA for HPV DNA detection and genotyping; however, particularly for men, these methods are evolving.29 There are also concerns that observed concordance may not represent true infection in both partners, but rather cross-contamination due to recent sexual contact. We instructed couples to refrain from oral, vaginal, or anal sex in the 24 hours preceding their visit and 95% complied. Concordance was highest among couples who reported their most recent contact 1–2 days prior to the clinic visit. Nonetheless, concordance was still high even among couples whose most recent contact was over 1 week ago. Finally, reporting errors for sexual behaviors were minimized by the collection of this information from both partners independently.
These data are consistent with HPV being highly transmissible. It would be useful for future studies to examine transmission soon after acquisition of a new partner. With the development of efficacious HPV vaccines, modelers have projected the public health and economic impact of various vaccination strategies.30–36 Many of these projections use dynamic transmission models that require information on the natural history of HPV acquisition, including the probability of transmission upon exposure. Our results may be helpful in improving these models.
Supplementary Material
ACKNOWLEDGMENTS
We thank the following employees of the HITCH Cohort Study: research nurses Gail Kelsall (Student Health Services Clinic, McGill University), and Suzanne Dumais (Student Health Services Clinic, Concordia University) for their efficient management of subject participation and specimen collection; Allita Rodrigues for study management (Division of Cancer Epidemiology, McGill University); Vicky D’Anjou-Pomerleau, Jessica Sammut, Jennifer Selinger and Johanna Bleecker for study promotion (Division of Cancer Epidemiology, McGill University); and Hélène Voyer for conducting LA-HPV testing (Centre Hospitalier de l’Université de Montréal). We are also grateful to Melanie Drew (Student Health Services Clinic, Concordia University) and the staff of the Student Health Services Clinics at McGill and Concordia Universities for their helpful collaboration with HITCH research nurses.
The HITCH study is funded by the Canadian Institutes for Health Research (operating grant 68893 and team grant 83320). Supplementary and unconditional funding support was provided by Merck-Frosst Canada Ltd and Merck & Co. Ltd. Supported by a research studentship from the Canadian Cancer Society Research Institute and by a Richard H. Tomlinson doctoral fellowship to McGill University (to A.N.B.).
Footnotes
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.Aral SO, Holmes KK. The epidemiology of STIs and their social and behavioral determinants: Industrialized and developing countries. In: Holmes KK, Sparling PF, Stamm WE, et al. , eds. Sexually Transmitted Diseases. 4th ed. New York: McGraw Hill Medical; 2008:53–92. [Google Scholar]
- 2.Trottier H, Franco E. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24:S4–S15. [DOI] [PubMed] [Google Scholar]
- 3.Dunne E, Nielson C, Stone K, Markowitz L, Giuliano A. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194:1044–1057. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer (IARC) Working Group. Human Papillomaviruses. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 64. Lyon, France: International Agency for Research on Cancer; 1995. [PMC free article] [PubMed] [Google Scholar]
- 5.Parkin D The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. [DOI] [PubMed] [Google Scholar]
- 6.Bleeker MCG, Snijders PFJ, Voorhorst FJ, Meijer CJLM. Flat penile lesions: The infectious “invisible” link in the transmission of human papillomavirus. Int J Cancer. 2006;119:2505–2512. [DOI] [PubMed] [Google Scholar]
- 7.Burchell AN, Winer RL, de Sanjosé S, Franco EL. Epidemiology and transmission dynamics of genital human papillomavirus infection. Vaccine. 2006;24:S52–S61. [DOI] [PubMed] [Google Scholar]
- 8.Baken L, Koutsky L, Kuypers J, et al. Genital human papillomavirus infection among male and female sex partners: prevalence and type-specific concordance. J Infect Dis. 1995;171:429–432. [DOI] [PubMed] [Google Scholar]
- 9.Kyo S, Inoue M, Koyama M, Fujita M, Tanizawa O, Hakura A. Detection of high-risk human papillomavirus in the cervix and semen of sex partners. J Infect Dis. 1994;170:682–685. [DOI] [PubMed] [Google Scholar]
- 10.Hippeläinen M, Yliskoski M, Syrjänen S, et al. Low concordance of genital human papillomavirus (HPV) lesions and viral types in HPV-infected women and their male sexual partners. Sex Transm Dis. 1994;21:76–82. [DOI] [PubMed] [Google Scholar]
- 11.Bleeker M, Hogewoning C, Berkhof J, et al. Concordance of specific human papillomavirus types in sex partners is more prevalent that would be expected by chance and is associated with increased viral loads. Clin Infect Dis. 2005;41:612–620. [DOI] [PubMed] [Google Scholar]
- 12.Castellsagué X, Ghaffari A, Daniel R, Bosch F, Munoz N, Shah K. Prevalence of penile human papillomavirus DNA in husbands of women with and without cervical neoplasia: a study in Spain and Columbia. J Infect Dis. 1997;176:353–361. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi S, Castellsagué X, Dal Maso L, et al. Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer. 2002;86:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas D, Ray R, Kuypers J, et al. Human papillomaviruses and cervical cancer in Bangkok. III. The role of husbands and commercial sex workers. Am J Epidemiol. 2001;153:740–748. [DOI] [PubMed] [Google Scholar]
- 15.Castellsagué X, Bosch F, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–1112. [DOI] [PubMed] [Google Scholar]
- 16.Lu B, Wu Y, Nielson CM, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: A prospective study. J Infect Dis. 2009;199:362–371. [DOI] [PubMed] [Google Scholar]
- 17.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–226. [DOI] [PubMed] [Google Scholar]
- 18.Moscicki A, Hills N, Shiboski S, et al. Risks for incidence human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano A, Harris R, Sedjo R, et al. Incidence, prevalence and clearance of type-specific human papillomavirus infections: The Young Women’s Health Study. J Infect Dis. 2002;186:462–469. [DOI] [PubMed] [Google Scholar]
- 20.Partridge J, Hughes J, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–1136. [DOI] [PubMed] [Google Scholar]
- 21.Oriel JD. Natural history of genital warts. Br J Vener Dis. 1971;47:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burchell A, Richardson H, Mahmud S, et al. Modeling the sexual transmissibility of human papillomavirus infection using stochastic computer simulation and empirical data from a cohort study of young women in Montreal, Canada. Am J Epidemiol. 2006;163:534–543. [DOI] [PubMed] [Google Scholar]
- 23.Burchell A, Tellier P, Hanley J, Coutlée F, Franco E. Influence of partner’s infection status on prevalent human papillomavirus among persons with a new sex partner. Sex Transm Dis. 2009. August 21. [Epub 2009 Aug 21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189:677–685. [DOI] [PubMed] [Google Scholar]
- 25.Coutlée F, Rouleau D, Petignat P, et al. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the linear array HPV genotyping test. J Clin Microbiol. 2006;44:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho L, Tay SK, Chan SY, Bernard HU. Sequence variants of human papillomavirus type 16 from couples suggest sexual transmission with low infectivity and polyclonality in genital neoplasia. J Infect Dis. 1993;168:803–809. [DOI] [PubMed] [Google Scholar]
- 27.Strand A, Rylander E, Wilander E, Zehbe I. HPV infection in male partners of women with squamous intraepithelial neoplasia and/or high-risk HPV. Acta Derm Venereol. 1995;75:312–316. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez B, Wilkens L, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giuliano A, Tortolero-Luna G, Ferrer E, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26:K17–K28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96:604–615. [DOI] [PubMed] [Google Scholar]
- 31.Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis. 2004;10:1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garnett G, Kim J, French K, Goldie S. Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine. 2006; 24:S178–S186. [DOI] [PubMed] [Google Scholar]
- 33.Kulasingam S, Connelly L, Conway E, et al. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian National Cervical Cancer Screening Program. Sex Health. 2007;4:165–175. [DOI] [PubMed] [Google Scholar]
- 34.Brisson M, Van de Velde N, De Wals P, Boily MC. The potential cost effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine. 2007;25:5399–5408. [DOI] [PubMed] [Google Scholar]
- 35.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders G, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis. 2003;9:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


