Abstract
Objectives
Patients with COVID-19 can present to the emergency department (ED) without immediate indication for admission, but with concern for decompensation. Clinical experience has demonstrated that critical illness may present later in the disease course and hypoxia is often the first indication of disease progression. The objectives of this study are to (a) assess feasibility and describe a protocol for ED-based outpatient pulse-oximetry monitoring with structured follow-up and (b) determine rates of ED return, hospitalisation and hypoxia among participants.
Methods
Prospective observational study of patients presenting to a single academic ED in Boston with suspected COVID-19. Eligible patients were adults being discharged from the ED with presumed COVID-19. Exclusion criteria included resting oxygen saturation <92%, ambulatory oxygen saturation <90%, heart rate >110 beats per minute or inability to use the device. Study personnel made scripted phone calls on postdischarge days 1, 3 and 7 to review the pulse-oximetry readings and to evaluate for decompensation. Return visit and admission information were collected via medical record and 28-day follow-up calls.
Results
81 patients were enrolled of which 10 (12%) developed hypoxia after their initial discharge from the ED. Overall, 23 (28%) of the 81 patients returned to the ED at least once and 10 of those who returned (43%) were admitted. We successfully contacted 76/81 (94%) of subjects via phone at least once for follow-up assessment.
Discussion
Patients are eager and willing to participate in home monitoring systems and are comfortable with using technology, which will allow providers and health systems to extend our hospitals capabilities for tracking patient populations in times of crisis.
Conclusions
It is feasible to implement an outpatient pulse-oximetry monitoring protocol to monitor patients discharged from the ED with confirmed or suspected COVID-19.
Keywords: COVID-19, public health, medical informatics
Summary.
What is already known?
The ongoing COVID-19 pandemic has caused significant increases in patient volume and acuity, rapidly depleting healthcare resources in both inpatient and emergency department (ED) settings. The ED is the first point of contact for many COVID-infected patients, and is responsible for the essential triage decisions governing inpatient admission or discharge. Effective strategies for patient monitoring and facilitating necessary return to care can enable more patients of greater acuity to be managed in an outpatient setting, conserving resources and improving patient experience.
What does this paper add?
Taking advantage of the prior research exploring the enrichment of occult hypoxia or ‘silent hypoxia’ in COVID-19 and how it frequently precedes clinical deterioration, as well as the available non-clinician personpower mobilized during the pandemic (including medical students who are often excluded from direct patient care due to concerns about infection), we were able to implement an outpatient pulse-oximetry monitoring protocol to monitor patients discharged from the ED with confirmed or suspected COVID-19. This protocol can serve as a framework on which other medical institutions can model similar programs.
Introduction
SARS-CoV-2, the causative agent of the respiratory disease COVID-19,1 2 spread throughout the USA in early 2020 and was responsible for a global pandemic 3. As of 18 January 2021, SARS-Cov-2 had infected an estimated 95 million individuals worldwide and was responsible for over 2 million deaths.4 COVID-19 cases frequently overwhelmed medical capacity for both general and critical care, as observed in several countries including China, Italy and the USA, resulting in severe shortfalls in care delivery and significantly increased mortality from the disease. 5–7 In order to continue to deliver effective medical care during this pandemic, hospitals need to take steps to promote efficient use of their resources, sometimes requiring them to make care decisions that would not be necessary in a high-resource setting outside of a pandemic.6 8 Foremost among these preventative actions is the preservation of hospital care capacity by treating patients at a level of care that best preserves resources and capacity.9 10
The COVID-19 clinical disease course often manifests with an initial 5–7 days of a viral syndrome, followed by either recovery (~80%–90%) or deterioration (10%–20%) during the second week of the disease. For those who deteriorated, clinically occult hypoxia or ‘silent hypoxia’ is often a hallmark. This demonstrated hypoxia is often far more severe than what is apparent from their clinical presentation. The frequency of ‘silent hypoxia’ is noticeably enriched in patients with COVID-19 and often precedes clinical deterioration; it has drawn comparisons with clinical findings characteristic of Pneumocystis jirovecii pneumonia.11–13 The mechanism of this ‘silent hypoxia’ is unclear, but models of its development often hypothesise the uncoupling of certain pulmonary mechanics, such as lung compliance, from their normal physiological relationships or vascular thrombosis and physiologic shunting.14–16
The emergency department (ED) is frequently the location of consequential triage decisions, most notably whether to admit or discharge a patient.5 During the current SARS-CoV-2 pandemic, these judgments are made by balancing many factors such as inpatient healthcare resources, patient risk of contracting or spreading COVID-19 due to their hospital stay and ability of the patient to access care in a timely manner if they are discharged and their condition deteriorates.8 Effective strategies for patient monitoring and facilitating necessary return to care can enable more patients to be managed in an outpatient setting, conserving resources and improving patient experience.10 17 During the time of study, the rapid implementation of an IT solution, to coordinate care and follow-up, to an urgent clinical need while using in-house informatics granted us the opportunity to explore and monitor a small cohort of individuals potentially exposed to a novel virus. The analysis of data collected from these monitoring initiatives could also provide insight into what information is most predictive of patient illness course, improving prospective decision-making capability and the efficiency of care delivery.
Outpatient pulse oximetry has the potential to assist in the challenge of following discharged patients and direct them to return to care when appropriate. This could, in turn, facilitate both the discharge of patients in a wider range of clinical conditions and the more effective monitoring of patients currently considered safe for discharge, conserving healthcare resources and improving outcomes for all patient populations. Many clinicians with experience caring for patients infected with COVID have encouraged the use of pulse oximetry in this context13 18 and its utility is supported both theoretically by the pathophysiology of the disease as well as empirically by existing clinical experience. As a disease with primarily respiratory pathophysiology, COVID-19 disrupts lung gas exchange, circulatory oxygen transport and subsequent end-organ oxygen delivery, meaning that circulatory pulse oximetry may represent a direct measurement of the progression in the pathway of pathology.13 15 18 Empirically, pulse oximetry has been shown to be predictive of disease outcome in both retrospective and prospective cohorts14 19–24 and has been used as an inclusion criteria or endpoint in clinical studies of COVID-19.11–13 The strategy of using pulse oximetry as part of an outpatient monitoring programme has the potential to enable earlier detection of hypoxia, including that observed in ‘silent hypoxics’, thereby allowing for earlier interventions and improving patient outcomes. The objectives of this study are to (a) assess feasibility and describe a protocol for outpatient pulse oximetry monitoring with structured follow-up and (b) determine rates of hypoxia, ED return and hospitalisation among programme participants.
Methods
Population and design
A prospective observational study was performed in a large tertiary care academic ED in Boston, MA, on patients discharged from the ED with suspected or confirmed COVID-19. Patients were included if they satisfied all of the following inclusion criteria: adult patient with presumed or confirmed COVID-19, otherwise dischargeable (eg, able to self-isolate, well appearing) and at risk for outpatient decompensation (ED MD clinical judgement).
While the initial study design specified strict inclusion criteria (any two of the following risk factors: age>55, cardiovascular disease, hypertension, diabetes, chronic lung disease, active cancer, obesity or immunosuppression), this was quickly broadened after feedback from treating providers. As disease burden as well as available hospital resources were rapidly shifting during the pandemic, it became clear that increased flexibility was necessary. For this reason, we allowed treating physicians full discretion with regard to who was enrolled in the protocol. While this may be a limitation with regard to study strength, it reflects the fact that this protocol was developed primarily as a clinical tool in a time of urgent need. Patients were excluded if they met any of the following exclusion criteria: need for admission, resting oxygen saturation <92%, ambulatory oxygen saturation <90%, resting heart rate >110 beats per minute or a patient was unable to use the pulse oximetry device. Patients enrolled from 11 April to 18 May 18 2020 were included for analysis.
Protocol description
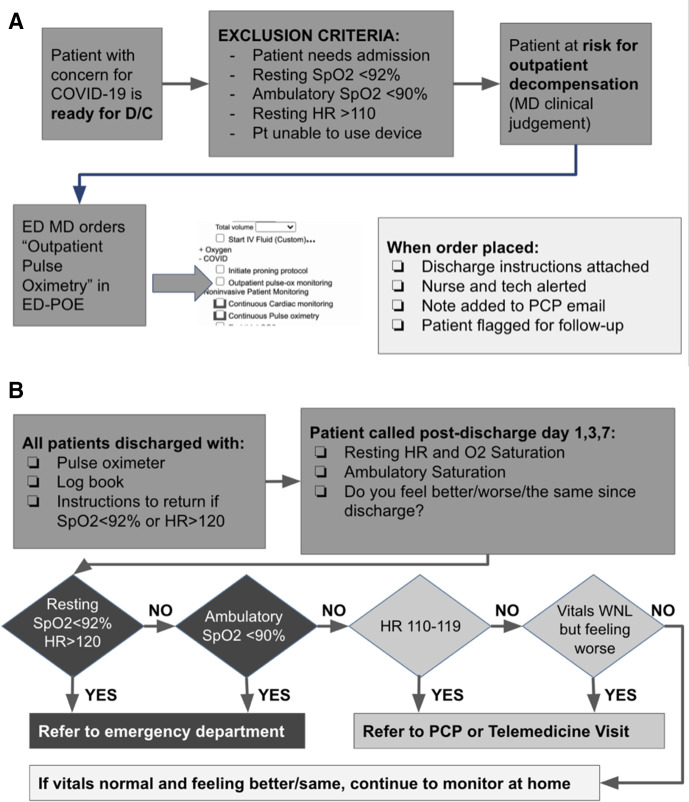
The decision to enrol a patient in the COVID-19 Outpatient Pulse Oximetry Protocol was left to the treating physician discretion at the time of patient discharge. Physicians were guided only to identify PUIs ‘at risk for outpatient decompensation’. Once the decision to enrol a patient was made, the treating physician used a custom electronic order built into the existing ED order system to initiate the protocol. Once the order was entered, the associated ED technician received an electronic page to obtain a pulse oximeter for the patient and deliver the pulse oximeter to the nurse. Additionally, this order attached appropriate discharge instructions to the patient’s discharge packet (online supplemental text A) and added a message to a discharge notification email routinely sent to the patient’s primary care doctor (if listed in our system). Once the pulse oximeter was given to the nurse, the patient was educated on how to use the device and the device was spot checked against ED telemetry to ensure accuracy. The patient was then discharged with instructions for using the pulse oximeter, a log sheet to record data and information about their expected follow-up calls on days 1, 3, 7 and 28 after discharge (see figure 1A).
Figure 1.
(A) Emergency department (ED) workflow. (B) Follow-up workflow. PCP, primary care physician.
bmjhci-2021-100330supp001.pdf (102.4KB, pdf)
Follow-up workflow
We attempted follow-up phone calls on days 1, 3, 7 and 28. The follow-up calls were scripted and were not intended to provide any medical evaluation or advice (online supplemental text B). This allowed for flexibility with regard to the personnel used as volunteer callers. These callers were mostly medical students who were being pulled out of clinical rotations during the height of the pandemic. During the call, information regarding any recent healthcare interactions or hospitalisations, general symptom progression and any vital signs that had been logged since the last point of contact were collected. The caller then collected current resting and ambulatory vital signs. All of these data were logged into our REDCap QI system.
Based on the subject’s responses and vital signs, patients were triaged to either remain at home, contact their primary care physician (PCP) or were referred immediately to the ED (figure 1A). The results of the phone call were logged as notes in the medical record and if the PCP was in the health system, they were contacted by email. If needed, a physician was available for medical control for concerns that fell outside the assigned triage system. After the 7-day follow-up period, all subjects were sent an envelope with return postage to return the pulse oximeter device to be cleaned and reused. At 28 days post enrolment subjects were again contacted to assess for missed return visits and to administer a subjective survey on the experience. A telemedicine service staffed by ED physicians was also available at no charge during this time as part of our health system safety net.
To ensure access to this service for non-English speakers, our health system interpreters were used to facilitate patient instruction and follow-up calls.
Results
Patient characteristics
We included the first 81 patients enrolled in the programme for analysis in this study during the determined timeframe. Patients were 57% female (46/81) and average age was 51.7 years (table 1A). Overall, 30/81 patients (37%) had comorbidities as described in table 1B. Patients spoke English, Spanish, Cape Verdean, Cantonese and Vietnamese.
Table 1.
(A) Patient information. (B) Patient medical history. (C) Presenting review of systems
| A | |||
| Patient demographics | |||
| Gender | N | ||
| Female | 46 (57%) | ||
| Male | 35 (43%) | ||
| Age | |||
| Median | 52 years | ||
| Min | 21 years | ||
| Max | 87 years | ||
| Primary language | |||
| English | 63 (78%) | ||
| Spanish | 13 (16%) | ||
| Cape Verdean | 2 (2%) | ||
| Cantonese | 2 (%) | ||
| Vietnamese | 1 (1%) | ||
| Race | |||
| White | 33 (41%) | ||
| Black | 23 (28%) | ||
| Other | 16 (20%) | ||
| Asian | 8 (10%) | ||
| Unknown | 1 (1%) | ||
| N | |||
| Hispanic | |||
| Yes | 27 (33%) | ||
| COVID-19 test result | |||
| Positive | 30 (37%) | ||
| Negative | 22 (27%) | ||
| Not tested | 29 (36%) | ||
| B | |||
| All participants (n=81) | |||
| No prior medical conditions | 51 (63%) | ||
| Congestive heart failure | 0 (0%) | ||
| Chronic obstructive pulmonary disease | 3 (4%) | ||
| Coronary artery disease | 2 (3%) | ||
| Hypertension | 27 (33%) | ||
| Diabetes | 10 (12%) | ||
| Immunosuppression | 8 (10%) | ||
| Tobacco use | 14 (17%) | ||
| C | |||
| All participants (n=81) | COVID-19 positive (n=30) | COVID-19 negative or untested (n=51) | |
| Fever | 47 (58%) | 20 (67%) | 27 (53%) |
| Anosmia/dysgeusia | 6 (7%) | 4 (13%) | 2 (4%) |
| Shortness of breath | 64 (79%) | 22 (73%) | 42 (82%) |
| Cough | 58 (72%) | 24 (80%) | 34 (67%) |
| Sputum production | 5 (6%) | 1 (3%) | 4 (8%) |
| Abdominal pain | 9 (11%) | 2 (7%) | 7 (14%) |
| All participants (n=81) | COVID-19 positive (n=30) | COVID-19 negative or untested (n=51) | |
| Chest pain | 21 (26%) | 5 (17%) | 16 (31%) |
| Chills | 18 (22%) | 8 (27%) | 10 (20%) |
| Diarrhoea | 13 (16%) | 7 (23%) | 6 (12%) |
| Fatigue | 24 (30%) | 11 (37%) | 13 (26%) |
| Headache | 7 (9%) | 2 (7%) | 5 (10%) |
| Myalgias | 21 (26%) | 8 (27%) | 13 (26%) |
| Rhinorrhea | 2 (3%) | 1 (3%) | 1 (2%) |
| Sore throat | 9 (11%) | 2 (7%) | 7 (14%) |
| Vomiting | 3 (4%) | 2 (7%) | 1 (2%) |
| Persistent pain | 7 (9%) | 3 (10%) | 4 (8%) |
All patients enrolled had suspected COVID-19 as determined by the attending physician. At this time during the pandemic, COVID-19 testing was severely limited. No patient had known COVID-19 at presentation. Of the 81 enrolled subjects, 30 patients (37%) had positive nasopharyngeal PCR testing for COVID-19. Twenty-nine patients (36%) were not tested. Overall, 22 of 81 patients (27%) tested negative for COVID-19.
Protocol execution
There were 322 follow-up calls made with an overall pick-up rate of 72%. Overall, 94% of subjects were successfully contacted via phone at least once (table 2). Overall, 1102 outpatient oxygen saturations and 886 outpatient heart rates were captured. Pick-up rate generally declined over the course of the study period with the lowest response rate on day 28 call (62%).
Table 2.
Follow-up statistics
| Successful/attempted (%) | |
| No. of day 1 calls answered | 70/81 (86%) |
| No. of day 3 calls answered | 57/81 (70%) |
| No. of day 7 calls answered | 57/81 (70%) |
| No. of day 28 calls answered | 50/81 (62%) |
| No. patients with at least one successful call | 76/81 (94%) |
| Overall pick rate | 234/324 (72%) |
Clinical outcomes
The clinical course of the enrolled patients is described below. We made particular note to examine the clinical course of patient subgroups that were directly referred during telephone check-ins for abnormal vital signs (rather than self-referral) as well as the subgroup of patients who had confirmed positive COVID-19 PCR testing.
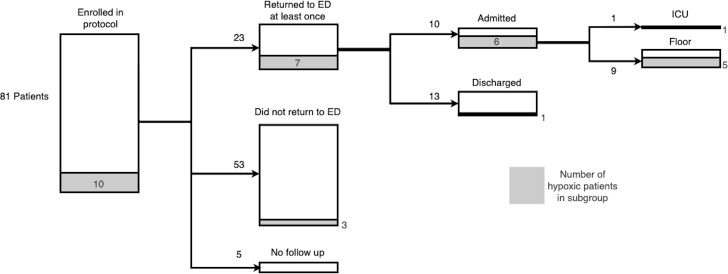
Of the 81 enrolled patients, 23 (28%) returned to the ED at least once, with 6 subjects returning more than once. Eight of the returning patients were referred directly during phone call check-in, while the remainder self-presented. Overall, 7 of the 23 (30%) patients who returned to the ED required supplemental oxygen for hypoxia.
Overall, 10/81 patients (12%) were admitted and 13/81 (16%) were evaluated in the ED and discharged. Of the 10 admitted patients, 9 were admitted to the general medicine ward and 1 was admitted to the ICU. The patient admitted to the ICU required intubation and eventually extracorporeal membrane oxygenation (ECMO) support for severe hypoxia associated with COVID-19 (figure 2).
Figure 2.
Patient outcomes. ED, emergency department.
Subgroup analysis: directly referred patients
Overall, 8 of 81 (10%) patients were referred to the ED for abnormal vitals during phone check-in on days 1, 3 or 7 of enrolment. The remainder of ED presentations were self-referrals that occurred before or between phone check-ins. Of the patients directly referred during phone check-in, five patients were referred for a resting oxygen saturation <92%, two for an ambulatory oxygen saturation <90% with normal resting oxygen saturation and one for resting tachycardia >120 with a normal oxygen saturation. Of the five patients referred to the ED for resting hypoxia, only two subsequently presented to the ED. Both were found to be hypoxic on triage vitals and required supplemental oxygen. The two patients referred for low ambulatory saturation (but normal resting saturation) did not require supplementation oxygen or admission on ED presentation. The patient referred for tachycardia was found to have tachycardia on ED presentation and was treated with intravenous fluids.
Of the three patients with resting hypoxia at home who did not comply with ED referral, all three reported no further ED visits or hospitalisations by the time of the 28-day follow-up call.
Subgroup analysis: patients with positive COVID-19 PCR testing
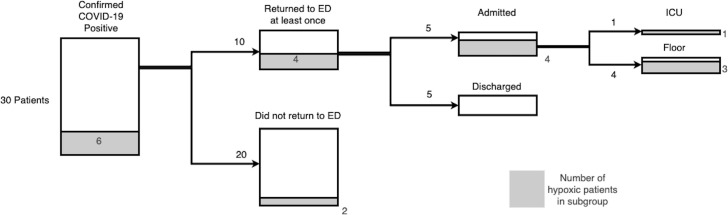
Of the 30 enrolled patients whose PCR confirmed COVID-19, 10/30 (33%) had at least one return visit to the ED. Overall, 6/30 (20%) had resting hypoxia documented either during a phone check-in or at the time of ED re-presentation. Overall, 5/30 (17%) were admitted to the hospital and 1/30 (3%) required critical care admission (figure 3).
Figure 3.
COVID-19-positive patient outcomes. ED, emergency department.
Discussion
By creating this outpatient pulse oximetry follow-up protocol, we provided patients with another layer of monitoring and follow-up after ED evaluation. Patients suspected to have COVID-19 and oxygenation >92% at the time of discharge but with concern for potential outpatient decompensation were discharged from the ED with a pulse oximeter. Over the course of the first 37 days of this protocol, 81 patients were enrolled and prospectively followed.
While it is our understanding that other hospitals have instituted outpatient monitoring protocols in the face of the COVID-19 pandemic, to our knowledge, there have been limited publications to date describing this experience.25–28 It is our hope that through our experiences, other health systems will be able to rapidly implement similar protocols as needed.
Protocol feasibility
Our overall follow-up rate was high with 94% of our subjects connecting with us at least once post discharge. We found that pick-up rates generally declined over the course of the study period, which may have corresponded with patient’s improving symptoms and decreasing interest in medical interactions.
The protocol involved multiple aspects of ED care that streamlined the approach to a protocolised follow-up system. Emergency physicians, on identification of patients who may be COVID-19 positive, were able to order a pulse oximeter in the electronic medical record system, seamlessly notifying an ED technician and nurse. Patient instructions were carefully developed and integrated into the discharge summaries of patients who had a pulse oximeter ordered (online supplemental text A).
We found that patients used at-home instructions for self-referral to the ED at a higher rate than the direct referral during follow-up phone calls. This underscores the importance of ED discharge instructions during the COVID-19 pandemic and may be generalisable to other high-risk discharge conditions. By and large, patients followed the guidelines in our instructions for follow-up and some of these patients necessitated admission thereafter.
Medical students were integral in this protocol’s success, and as their clinical rotations at Harvard Medical School were suspended during the height of the pandemic in March 2020, they joined to develop and execute the protocol with us. As COVID-19 swab results were piling and QA nurses were tasked with more responsibilities during the pandemic, our medical students volunteered as skilled callers. We believe that medical student volunteers represent an important and potentially overlooked resource for academic EDs in times of pandemic.
As we move forward, medical students are returning to clinical rotations and so we have begun transitioning to a text-message-based system. This allows patients to directly report vital signs via automated text-message system and only patients who have abnormal responses will require telephone interactions. Additionally, this allows for the collection of vital sign information two times per day, rather than only during phone interactions. It is hoped that this will greatly reduce the work required to maintain this protocol and will likely become the primary means of screening patients moving forward.
Clinical notes
The aim of this protocol was to establish the feasibility to launch a safe discharge plan for patients with suspected COVID-19. While establishing the efficacy of at-home pulse oximetry was not the study’s design, it is however worthwhile to examine the clinical course of those enrolled.
Of the 81 patients with suspected or confirmed COVID-19 enrolled in this study period, 10 patients (12%) developed hypoxia. Overall, 23 (28%) patients returned to the ED within the 7-day study period. Patients who self-presented or directly called back to the ED for abnormal vital signs were treated and some necessitated admission. One patient, who had been discharged from the ED with normal vital signs 24 hours prior, returned critically ill requiring intubation and eventually ECMO support. No patients at 28 days were found to have adverse results at home and no patients died.
Limitations
Although this protocol was organised and executed effectively in the height of the pandemic, there were some limitations that should be explored. For one, calls to patients were only done on days 1, 3, 7 and 28. While the patients had clear return instructions and were told to check their own vitals three times per day, we did not have the means to contact patients on a daily basis. Because of this, we are also unable to identify with certainty that patients who self-presented during this time period (without a direct recommendation via phone call) were presenting due to abnormal vital signs and not for a secondary reason. Those who self-presented did not necessarily always have COVID-19-related concerns.
An additional limitation during this time period is that the COVID-19 status at the time of enrolment was unknown. Due to the nature of shifting testing availability at our hospital (and around the country), not all patients were tested. It is also notable that 22 subjects (27%) had negative COVID-19 swabs. While it is possible that some of these represent false negatives, it is more likely that some patients without COVID-19 were enrolled in the protocol. Interestingly, cough, shortness of breath and fever were the three most common symptoms in both the COVID-19 positive and negative groups (table 1C).
The aim of our protocol design was entirely clinical in nature, and while we do believe that outpatient pulse oximetry monitoring adds to patient care and safety, this hypothesis was not tested directly through this protocol. We believe in order to adequately determine the contribution of pulse oximetry to postdischarge monitoring, a study would need to randomise patients to either receive or not receive a pulse oximeter at the time of discharge. Given the urgent needs during the height of the pandemic, we did not feel that this type of study was feasible or appropriate at that time. Instead, we can speak only to the clinical observations seen in our cohort and cannot prove or disprove any hypotheses regarding efficacy of differing outpatient monitoring procedures.
Conclusion
We demonstrated that a home pulse-oximetry monitoring protocol is feasible and safe for discharge of patients with suspected COVID-19. Adequate discharge instructions and follow-up phone calls identified patients who required ED re-evaluation and escalation of care. Given that 12% of patients enrolled had clinical deterioration requiring hospital admission within 7 days of initial ED discharge, we believe that outpatient monitoring with pulse oximetry is a reasonable and prudent approach. In our experience, the key factors that enabled success were (1) developing a multidisciplinary protocol involving ED physician, technician, nurse and electronic health record, (2) effective follow-up instructions and phone calls and (3) health system communication with patient, ED physician and PCP. We include our protocol documents, scripts and discharge instructions in the hope that it will be useful to others interested in implementing similar protocols elsewhere.
Footnotes
Contributors: DBG: Data analysis and manuscript writing. NK: Data analysis and manuscript writing. TO’M: Administrative support and data acquisition. JYG: Data acquisition. DC: Administrative support and coordination. NIS: Research Mentorship. OJM: Project oversight and manuscript writing. AD: Project oversight, data analysis and manuscript writing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Please contact the corresponding author for access to any additional data you may deem relevant that was not included.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
References
- 1.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–9. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) . WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19, 2020. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 4.Johns Hopkins University . COVID-19 Dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU). Available: https://coronavirus.jhu.edu/map.html [Accessed 22 May 2020].
- 5.Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet 2009;373:423–31. 10.1016/S0140-6736(09)60137-9 [DOI] [PubMed] [Google Scholar]
- 6.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020;382:2049–55. 10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 8.Erika P, Andrea V, Cillis MG, et al. Triage decision-making at the time of COVID-19 infection: the Piacenza strategy. Intern Emerg Med 2020;15:879–82. 10.1007/s11739-020-02350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warner MA. Stop doing needless things! saving healthcare resources during COVID-19 and beyond. J Gen Intern Med 2020;35:2186–8. 10.1007/s11606-020-05863-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judson TJ, Odisho AY, Neinstein AB, et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc 2020;27:860–6. 10.1093/jamia/ocaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caputo ND, Strayer RJ, Levitan R. Early Self-Proning in awake, Non-intubated patients in the emergency department: a single ED's experience during the COVID-19 pandemic. Acad Emerg Med 2020;27:375–8. 10.1111/acem.13994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs G, Sowers N, Campbell S, et al. Just the facts: airway management during the coronavirus disease 2019 (COVID-19) pandemic. CJEM 2020;22:440–4. 10.1017/cem.2020.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitan R. The infection that’s silently killing coronavirus patients. The New York Times, 2020. [Google Scholar]
- 14.Cohen PA, Hall LE, John JN, et al. The early natural history of SARS-CoV-2 infection: clinical observations from an urban, ambulatory COVID-19 clinic. Mayo Clin Proc 2020;95:1124–6. 10.1016/j.mayocp.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med 2020;201:1319–20. 10.1164/rccm.202004-1076ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46:1099–102. 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schinköthe T, Gabri MR, Mitterer M. Web-/APP-based connected care solution for COVID-19 in- and outpatient care. JMIR Public Health Surveill 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitan R, Caputo N, Cosentini R. We treated older coronavirus patients. Here’s how to save more of them. The New York Times, 2020. [Google Scholar]
- 19.Xie J, Covassin N, Fan Z. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Zuo P, Liu Y, et al. Clinical and laboratory predictors of in-hospital mortality in 305 patients with COVID-19: a cohort study in Wuhan, China. SSRN Journal 2020. 10.2139/ssrn.3546115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J 2020;133:1261–7. 10.1097/CM9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haimovich A, Ravindra NG, Stoytchev S. Development and validation of the COVID-19 severity index (CsI): a prognostic tool for early respiratory decompensation. medRxiv 2020. 10.1101/2020.05.07.20094573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Hungerford D, Chen H, et al. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. medRxiv 2020. 10.2139/ssrn.3562456 [DOI] [Google Scholar]
- 24.Petrilli CM, Jones SA, Yang J. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. BMJ 2020. 10.1136/bmj.m1966 [DOI] [Google Scholar]
- 25.Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med 2020;27:681–92. 10.1111/acem.14053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home. potential pitfalls and practical guidance. Ann Am Thorac Soc 2020;17:1040–6. 10.1513/AnnalsATS.202005-418FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banzi R, Sala L, Colmi A. Fattibilit ed efficacia di un modello di monitoraggio domiciliare avanzato dei pazienti affetti da CoViD-19 o sospetti [Feasibility and efficacy of home monitoring for patients with suspected or confirmed COVID-19. Recenti Prog Med 2020;111:584–92. [DOI] [PubMed] [Google Scholar]
- 28.Krenitsky NM, Spiegelman J, Sutton D, et al. Primed for a pandemic: implementation of telehealth outpatient monitoring for women with mild COVID-19. Semin Perinatol 2020;44:151285. 10.1016/j.semperi.2020.151285 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjhci-2021-100330supp001.pdf (102.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Please contact the corresponding author for access to any additional data you may deem relevant that was not included.