Abstract
The incidence of chronic aging-associated diseases, especially cardiovascular and prostatic diseases, is increasing with the aging of society. Evidence indicates that cardiovascular diseases usually coexist with prostatic diseases or increase its risk, while the pathological mechanisms of these diseases are unknown. Oxidative stress plays an important role in the development of both cardiovascular and prostatic diseases. The levels of oxidative stress biomarkers are higher in patients with cardiovascular diseases, and these also contribute to the development of prostatic diseases, suggesting cardiovascular diseases may increase the risk of prostatic diseases via oxidative stress. This review summarizes the role of oxidative stress in cardiovascular and prostatic diseases and also focuses on the main shared pathways underlying these diseases, in order to provide potential prevention and treatment targets.
1. Introduction
Cardiovascular diseases (CVDs), including hypertension, coronary heart disease (CHD), cerebrovascular disease, and heart failure, are the major cause of death globally. In the period from 1990 to 2019, the prevalence of total CVD nearly doubled from 271 million to 523 million cases, and the number of CVD deaths steadily increased from 12.1 million to 18.6 million [1]. In 2019, ischaemic heart disease was one of the top-ranked causes of disability adjusted life years (DALYs) in both the 50-74-year and 75-years-and-older age groups [2]. According to World Health Organization (WHO) statistics, almost 23.6 million people will die from CVDs by 2030. Benign prostatic hyperplasia (BPH) and prostate cancer are also aging-associated diseases. The incidence rate of BPH increases with age affecting about 50% of men over 50 years, increasing to 80% when they reach 80 or above [3, 4]. There is also an increased incidence in prostate cancer cases from 940,000 in 2007 to 1.3 million in 2017 [5], and the age-standardized incidence of prostate cancer in China also rose by 2.75% from 1990 to 2017 [6]. Aging clearly plays an important role in CVDs (such as hypertension and CHD) and prostatic diseases (prostate cancer and BPH).
The current evidence suggests that CVDs usually coexist with prostatic diseases or increase its risk, and there are 13 related clinical studies showing CVD as a risk factor for prostatic diseases (see Table 1); however, whether there is a causal relationship between them is still controversial. Numerous studies have reported that oxidative stress can promote the occurrence and development of both prostatic diseases and CVDs [7–10]; hence, to summarize its role in these two diseases can provide information for seeking prevention and potential therapeutic targets.
Table 1.
Epidemiological studies about the associations of CVD and prostatic diseasesa.
| Author (year) | Country | Study design | Disease diagnosis | Sample size | Age (year) | Main outcomes | Reference | |
|---|---|---|---|---|---|---|---|---|
| Cardiovascular diseases | Prostatic diseases | |||||||
| Bourke J B, et al. 1966 | UK | Case control | HP (SBP > 200 mmHg and DBP > 110 mmHg) | BPH (diagnosed histologically) | 432 | 65-69 | The incidence of HP in patients who were operated upon for BPH was significantly greater than control series. | [11] |
|
| ||||||||
| Sugaya K, et al. 2003 | Japan | Cohort study | HP (SBP ≥140 mmHg or DBP>90 mmHg) | BPH (digital rectal examination and ultrasonography) | 42 | NT group: 69 ± 8 HT group: 71 ± 11 |
HP may worsen LUTS. | [12] |
|
| ||||||||
| Michel M C, et al. 2004 | Germany | Case control | HP (DBP > 90 mmHg or with history of hypertension or receiving antihypertension medication) | BPH (diagnosed by urologist) | 9857 | Mean: 65.1 | Patients with HP had more severe BPH symptoms and that more severe BPH symptoms are associated with a high HP. | [13] |
|
| ||||||||
| Chen I H, et al. 2012 | China | Case series | HP (the history of hypertension) | BPH (IPSS > 8 and PV > 18 cm3) | 130 | 60.9 ± 10.8 | The more cardiovascular risk factors in patients with BPH, the greater was the prostate vascular resistance. | [14] |
|
| ||||||||
| Hwang E C, et al. 2015 | South Korea | Case control | HP (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or with a previous diagnosis of hypertension and receiving medical treatment) | BPH (transurethral resection of the prostate) | 295 | 69.5 ± 7.0 | Men with HP were more likely to have greater LUTS and larger prostate volume. | [15] |
|
| ||||||||
| Zeng XT, et al. 2018 | China | Cross-sectional study | HP (NR) | BPH (NR) | 350 | NT group: 71.5 ± 7.4 HT group: 70.7 ± 7.3 |
HP had no significant association with prostate volume. | [16] |
|
| ||||||||
| Navin S, et al. 2017 | US | Cross-sectional study | HP (NR) | PCa (NR) | 3200 | 51-76 | Patients with PCa had a significantly higher prevalence of HP than the general population. | [17] |
|
| ||||||||
| Dickerman B A, et al. 2018 | Iceland | Cohort study | HP (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or taking anti-hypertensives) | PCa (morphologically verified) | 9097 | 52.1 ± 8.4 | This was a positive association between midlife hypertension and aggressive PCa. | [18] |
|
| ||||||||
| Weisman K M, et al. 2000 | US | Case control | CHD (included the history of coronary artery bypass graft, coronary angioplasty, and myocardial infarction) | BPH (prostate biopsy and transurethral resection of the prostate) | 140 | 65-80 | Patients without BPH had a lower frequency of CHD than those with BPH. | [19] |
|
| ||||||||
| Neugut AI, et al. 1998 | US | Case control | CHD (the history of myocardial infarction, coronary artery bypass graft, positive coronary angiogram, or positive exercise stress test) | PCa (diagnosed pathologically) | 508 | Case group: 69.6 ± 9.1 Control group: 68.1 ± 9.0 |
The individuals with CHD are at elevated risk for PCa. | [20] |
|
| ||||||||
| Stamatiou KN, et al. 2007 | Greece | Case serials | CHD (pathologic examination) | PCa (histological features) | 116 | 55-98 | There could be an association between CHD and PCa. | [21] |
| Thomas JA 2nd, et al. 2012 | US | Clinical study | CHD (post history) | PCa (biopsy and PSA) | 6729 | 50-75 | CHD was significantly associated with PCa diagnosis. | [22] |
|
| ||||||||
| Omalu BI, et al. 2013 | US | Case serials | CHD (two forensic pathologists and a senior pathology resident) | PCa (two genitourinary pathologists for histologic) | 37 | 65.8 (50-86) | There was no association between degree of CHD and PCa. | [23] |
aHP: hypertension; BPH: benign prostatic hyperplasia; SBP: systolic blood pressure; DBP: diastolic blood pressure; NT: normotensive; HT: hypertensive; PCa: prostate cancer; IPSS: international prostate symptom score; LUTS: lower urinary tract symptoms; NR: not reported; PV: prostate volume; CHD: coronary heart disease.
2. Oxidative Stress
The concept of oxidative stress originated from human understanding of aging. In 1956, Professor Harman first proposed the theory of free radical aging. In 1990, Professor Sohal pointed out the flaws of this theory and put forward the concept of oxidative stress for the first time [24, 25]. Oxidative stress is a situation where the balance of oxidative systems and antioxidative systems in vivo is changed in favour of the former [26, 27]. Oxidants are regulators of normal cellular function, but when the production of reactive oxygen species (ROS) exceeds the scavenging capacity, the oxidation and antioxidant systems will be unbalanced, causing damage to the tissues and cells; this phenomenon is called oxidative damage [28–30]. ROS includes superoxide anion (O2•-), hydroxyl radical (•OH), and hydrogen peroxide (H2O2). To maintain the balance, there are two antioxidative systems in vivo: one is the enzyme antioxidant system, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX), and the other is the nonenzyme antioxidant system which includes vitamin C, vitamin E, glutathione, melatonin, α-lipoic acid, carotenoids, and trace elements such as copper, zinc, and selenium (SE). Figure 1 presents the oxidative stress theory and the sources of ROS and antioxidants.
Figure 1.
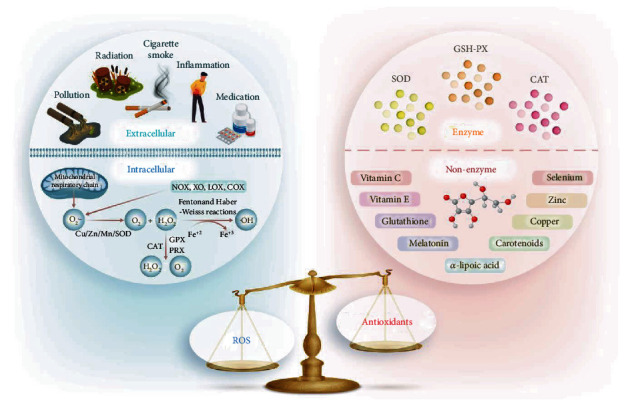
Schematic representation of the oxidative stress theory and the sources of ROS and antioxidants. When the generation of ROS outweighs antioxidative capacity, this leads to oxidative stress. ROS are generated from extracellular and intracellular sources. The extracellular sources of ROS are pollution, inflammation, cigarette smoke, radiation, and medication. Intracellular sources of ROS are the mitochondrial electrotransport chain: NADPH oxidase (NOX), xanthine oxidase (XO), lipoxygenase (LOX), and cyclooxygenase (COX). The enzyme antioxidant system includes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-PX), and the nonenzyme antioxidant system includes vitamin C, vitamin E, glutathione, melatonin, α-lipoic acid, carotenoids, and trace elements such as copper, zinc, and selenium.
2.1. Characteristics of ROS
ROS are generated by a variety of extracellular and intracellular actions (Figure 1). The main intracellular source of ROS is the mitochondrial respiratory chain. The O2•- is the principal ROS formation which is produced by the enzymatic reaction and a nonenzymatic electron transfer reaction in cell. The enzymes that generate the superoxide include NADPH oxidase (NOX), xanthine oxidase (XO), lipoxygenase (LOX), and cyclooxygenase (COX) [31]. H2O2 is produced from O2•- by enzymatic dismutation by the three isoforms of Cu/Zn/Mn SOD in intracellular. Most of the H2O2 is converted to H2O by the catalase (CAT), glutathione peroxidase (GPX), and peroxiredoxins (PRX). H2O2 can damage DNA when it is converted to a •OH through Fenton and Haber-Weiss reactions in the transition metal ions, especially iron ions (Fe+2). The •OH is the most toxic form of ROS which causes various types of DNA damage, lipid peroxidation, and protein modification [32]. The extracellular sources of ROS commonly included pollution, inflammation, cigarette smoke, radiation, and medication [33].
At present, accurately measuring ROS in disease is still a problem. ROS are unstable, and their half-lives are relatively short. For example, the half-life of O2•- is 10−6 to 10−9 seconds, and the •OH is 10−9 seconds [34, 35]. Therefore, ROS are routinely measured by biomarkers of oxidative damage, which included markers of protein damage (protein carbonyl derivatives), lipid peroxidation (malondialdehyde [MDA] and 4-hydroxynonenal [HNE]) [36], and DNA oxidation (8-hydroxy-2′-deoxyguanosine [8-OHdG]). Although, the biomarkers of measured oxidative stress are indirect and have low specificity, these do provide a noninvasive method in clinical practice.
3. Pathophysiological Role of Oxidative Stress in CVD and Prostatic Diseases
Numerous clinical studies have indicated that oxidative stress plays a role in CVD and prostatic diseases (Table 2). Studies have reported that the level of oxidative stress biomarkers was higher in CVD patients [37–40]. Additionally, current evidence suggests that oxidative stress is associated with the etiology and pathogenesis of the prostatic diseases [9, 41–43]. Hence, the phenomenon of CVD increasing the risk of prostatic diseases may be attributed to oxidative stress (Figure 2).
Table 2.
The summary of oxidative stress in CVD or prostatic diseases.
| Author (year) | Study design | Study population | Age (year) | Markers assessed | Main results | Reference |
|---|---|---|---|---|---|---|
| Germanò G, et al. 2004 | Cross-sectional study | 40 persons with HP 40 healthy individuals |
HP group: 51.6 ± 3 Healthy group: 54.4 ± 2 |
(i) O2− measured by lucigenin chemiluminescence and hydroethidine cytofluorimetric | Patients with hypertension showed an enhanced formation of O2− in platelets. | [37] |
|
| ||||||
| Guxens M, et al. 2009 | Cross-sectional study | 819 CHD patients with HP 311 CHD patients without HP |
HP group: 67 ± 8 Control group: 66 ± 9 |
(i) Circulating ox-LDL measured by an enzyme-linked immunosorbent | There was a positive relationship between circulating ox-LDL and hypertension. | [38] |
|
| ||||||
| Pinzón-Díaz CE, et al. 2018 | Clinical trial study | 12 persons with HP 15 healthy individuals |
26-50 | (i) MDA by a spectrophotometer (ii) GSH concentration used the glutathione assay kit |
Compared to healthy patients, the level of lipid peroxidation is higher 2.1 times in hypertensive patients. | [39] |
|
| ||||||
| Zhao H, et al. 2018 | Clinical trial study | 75 people with HP 75 healthy people |
HP group: 40.41 ± 11.66 Control group: 40.08 ± 4.31 |
(i) Melatonin measured by metabolomic | Oxidative stress would cause disturbance in hypertensive patients and affect the metabolic pathway of pathogenesis. | [40] |
|
| ||||||
| Merendino RA, et al. 2003 | Clinical study | 22 patients with BPH 22 healthy subjects |
BPH group: 65.8 (56-79) Control group: 62.1 (55-76) |
(i) MDA measured a commercially kit | The results showed a higher level of MDA in BPH patients. | [44] |
|
| ||||||
| Camphausen K, et al. 2004 | Cohort study | 38 radiotherapy PCa cases 15 received placebo |
NR | (i) Urinary 8-iso-prostaglandin PGF2α and 15-keto-dihydro-PGF2α | The study showed that there was no statistically increase in 8-iso-PGF2α or 15-keto-dihydro-PGF2α in patients with PCa compared with normal control group. | [45] |
|
| ||||||
| Yilmaz MI, et al. 2004 | Case-control study | 50 patients with BPH 21 patients with PCa 50 healthy subjects |
BPH group: 63.5 (43-84) PCa group: 66 (49-84) Control group: 66 (48-78) |
(i) CuZn-SOD, and GPX measured by a UV–VIS recording spectrophotometer (ii) MDA |
Compared with BPH and control groups, there is a higher MDA concentration with lower GPX and CuZn-SOD activities in PCa patients. | [46] |
|
| ||||||
| Srivastava DS, et al. 2005 | Case-control study | 55 patients with BPH 45 patients with PCa 25 healthy individuals |
BPH group: 59.6 ± 8.4 PCa group: 61.9 ± 11.4 Control group: 60.5 ± 14.3 |
(i) GPX measured by kit (ii) MDA (iii) GST and GSH activities measured by spectrophotometry |
Compared with control group, there is a higher level of MDA concentration and GST activity and lower levels of GSH concentration and GPX activity in BPH and PCa groups. | [47] |
|
| ||||||
| Aydin A, et al. 2006 | Clinical study | 36 patients with BPH 25 patients with PCa 24 healthy subjects |
BPH group: 64.3 ± 7.9 PCa group: 67.5 ± 8.8 Control group: 65.0 ± 6.0 |
(i) The level of TBARS, SOD, GPX, CAT, Cu, and Zn | Compared with control group, the lipid peroxidation was increased with decreased SOD activity in BPH and PCa groups. | [48] |
|
| ||||||
| Surapaneni KM, et al. 2006 | Case-control study | 30 patients with PCa 30 healthy cases |
NR | (i) MDA measured by spectrophotometry (ii) SOD measured by Misra and Fridovich (iii) GST and GSH activities measured by spectrophotometry |
Compared with control group, there is a higher level of MDA and SOD and lower level of GSH in PCa patients. | [49] |
| Ozmen H, et al. 2006 | Cross-sectional study | 20 patients with PCa 21 healthy cases |
PCa group: 72.45 ± 7.78 Control group: 66.33 ± 8.25 |
(i) MDA and vitamins measured by HPLC (ii) SE measured by a fluorimetric method (iii) Trace elements and Fe measured by atomic absorption spectrophotometry |
The study showed that the administration of vitamins A, C, and E and SE and Zn may be beneficial in the prevention and treatment of human prostate cancer. | [50] |
|
| ||||||
| Lockett KL, et al. 2006 | Case-control study | 158 patients with PCa 128 healthy cases |
PCa group: 65.3 ± 9.5 Control group: 64.4 ± 9.5 |
(i) DNA damage evaluated by alkaline comet assay | The study suggested that DNA damage may be associated with PCa risk. | [51] |
|
| ||||||
| Aryal M, et al. 2007 | Case-control study | 48 patients with BPH 46 healthy cases |
BPH group: 67 ± 12 Control group: 63.4 ± 8 |
(i) MDA (ii) α-Tocopherol and ascorbate |
Compared with control group, there is a higher level of plasma MDA and lower plasma alpha-Tocopherol and ascorbate level in patients with BPH. | [52] |
|
| ||||||
| Goswami K, et al. 2007 | Case-control study | 10 patients with BPH 10 patients with PCa 10 control subjects |
BPH group: 65 ± 3 PCa group: 67 ± 4 Control group: 65 ± 7 |
(i) Lipid peroxide was estimated by spectrophotometry (ii) Protein carbonyls measured by modified Levine's |
Compared with control group, there is a higher level of lipid peroxides and protein carbonyls in patients with BPH or PCa, and PCa patients are more prone to oxidative damage in compared with BPH patients. | [53] |
|
| ||||||
| Arsova-Sarafinovska Z, et al. 2009a | Case-control study | 67 patients with BPH 73 patients with PCa 23 control subjects |
BPH group: 64.3 ± 7.9 PCa group: 67.5 ± 8.8 Control group: 65.0 ± 6.0 |
(i) MDA (ii) CuZn-SOD, GPX, and CAT measured by a UV–VIS recording spectrophotometer (iii) NO2−/NO3− (iv) 8-OHdG measured Highly Sensitive 8-OHdG Check ELISA Kit |
Compared with BPH and control groups, there is a higher MDA and NO2−/NO3− concentration with lower GPX and CuZn-SOD activities in PCa patients. | [54] |
|
| ||||||
| Arsova-Sarafinovska Z, et al. 2009b | Case-control study | 100 patients with BPH 34 patients with PCa 15 control subjects |
BPH group: 64.3 ± 7.9 PCa group: 67.5 ± 8.8 Control group: 65.0 ± 6.0 |
(i) MDA (ii) CuZn-SOD, GPX, CAT measured by a UV–VIS recording spectrophotometer (iii) NO2−/NO3− (iv) 8-OHdG measured Highly Sensitive 8-OHdG Check ELISA Kit |
Compared with BPH and control groups, there is a higher MDA and NO2−/NO3− concentration with lower GPX and CuZn-SOD activities in PCa patients. | [54] |
|
| ||||||
| Pace G, et al. 2010 | Case-control study | 7 patients with BPH 11 patients with PCa 5 healthy subjects |
BPH group: 65.14 ± 2.12 PCa group: 62.82 ± 1.74 Control group: 66.00 ± 3.51 |
(i) ox-LDL, peroxides, TEAC, and SOD measured in blood samples | The study confirmed a significant imbalance of redox status in patients with BPH and PCa and suggests that oxidative stress may be a determinant in the pathogenesis of these diseases. | [55] |
| Hoque A, et al. 2010 | Nested case-control study | 1808 PCa cases 1805 controls |
Case group: 63.62 ± 5.54 Control group: 63.58 ± 5.55 |
(i) Serum protein carbonyl level measured by a noncompetitive ELISA | The study did not support that oxidative stress plays a role in PCa risk or its aggressiveness in serum protein carbonyl level. | [56] |
|
| ||||||
| Cimino S, et al. 2014 | Case-control study | 60 BPH patients 40 PCa patients |
BPH group: 68 ± 6.4 PCa group: 67 ± 8.7 |
(i) The level of total thiol groups (TTG) and glutathione | A significant difference of TTG was observed in BPH and PCa patients, and the level of glutathione was lower in PCa patients. | [57] |
HP: hypertension; CHD: coronary heart disease; O2−: superoxide anion; HIAE: high-intensity aerobic exercise; LIAE: low-intensity aerobic exercise; BFE: blood flow restriction; TBARS: thiobarbituric acid-reactive substances; SOD: the enzyme activities of superoxide dismutase; GPX: glutathione peroxidase; CAT: catalase; Cu: copper; Zn: zinc; NR: not reported; BPH: benign prostatic hyperplasia; PCa: prostate cancer; TEAC: total equivalent antioxidant capacity; athe data from Macedonia; bthe data from Turkey; MDA: erythrocyte malondialdehyde; NO2−/NO3−: nitrite/nitrate; 8-OHdG: 8-hydroxy-2′-deoxyguanosine; GST: glutathione s-transferase.
Figure 2.
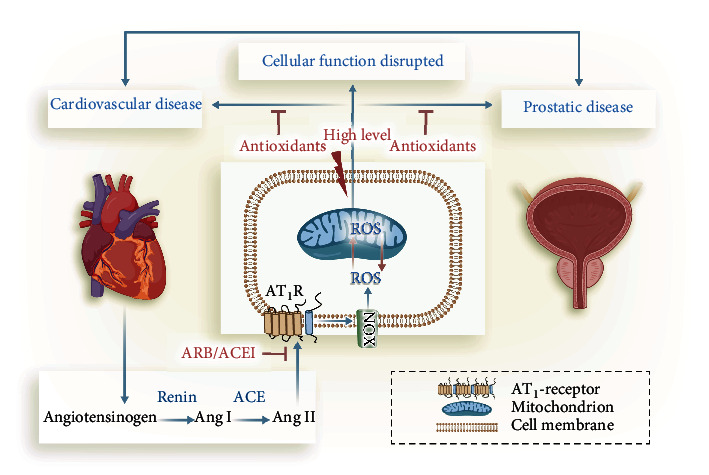
ROS generated by NOX in the pathogenesis between CVD and prostatic diseases. The renin-angiotensin system (RAS) exists in both heart and prostate; in addition, the overactivity of the RAS is found in both cardiovascular diseases (CVD) and prostatic diseases. The RAS includes angiotensinogen, renin, angiotensin conversion enzyme (ACE), angiotensin II (Ang II), and angiotensin receptors. Ang II is a biologically active peptide in RAS, and its main effector receptor is the type 1 (AT1R). Ang II induced the ROS by activation of the subunits of NADPH oxidase (NOX), and then, the increased ROS effects the development of CVD and prostatic diseases. Thus, NOX-derived ROS signal may be a common potential target in therapeutic intervention of CVD and prostatic diseases.
3.1. Renin-Angiotensin System in CVD and Prostatic Diseases
It is well established that the activation of renin-angiotensin system (RAS) is critically associated with the pathogenesis of hypertension and atherosclerosis [58–60]. Previous studies have reported the existence of local RAS in the prostate, and the overactivity of the RAS may be involved in the pathophysiology of BPH and prostate cancer [61–64]. The RAS includes angiotensinogen, renin, angiotensin conversion enzyme (ACE), angiotensin II (Ang II), and angiotensin receptors. Ang II is a biologically active peptide in the RAS, and its main effector receptor is type 1 receptor (AT1R) (Figure 2).
The ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have been successfully used as antihypertensive medication, and some studies have reported that these drugs have also been used in anticancer therapy [65, 66]. A meta-analysis which included 9 cohort studies with 20267 patients suggested that the use of ACEIs/ARB may be associated with a decreased risk of prostate cancer [65]. However, another meta-analysis of observational studies did not find a significant relationship between the use of ACEIs/ARB and prostate cancer risk [67]. Although it is still controversial, ACEIs or ARBs have been used to reduce the risk of prostate cancer. One study showed that the expression level of AT1R mRNA was higher in prostate cancer than that in the normal human prostate, based on the data, and ACEIs or ARB may inhibit prostate cancer [68]. Another study reported that the ACEI captopril lowers the risk of prostate cancer, but it was not significant [69]. In BPH patients, a clinical study showed that the use of ARB can improve prostatic hyperplasia [70], whereas other antihypertensive drugs were not effective, which indicates that ARB could ameliorate BPH independently of decreasing blood pressure. The ARB drug Losartan could treat the BPH in spontaneously hypertensive rats (SHRs). The study showed that long-term Losartan treatment restored prostatic blood flow and reduced tissue MDA (oxidative stress marker) in SHRs [71]. These findings suggest that ARB/ACEI may also effective in prostatic diseases.
Traditionally, it has been thought that Ang II can directly achieve vasoconstriction by interacting with AT1R in vascular smooth muscle. Recently, a novel signalling mechanism for Ang II-induced vascular superoxide (O2−) formation was associated with the development of endothelial dysfunction, hypertension, and atherosclerosis. The source of this increased O2− seemed to be membrane-bound vascular NADPH oxidases (NOXs) [72, 73]. A study has also reported that angiotensin II also induced the production of ROS prostate cancer cells by upregulating the subunits of NADPH oxidase (NOX) [74]. In addition, another study has found expressions of various NOX isoforms in prostate cancer cell lines and a cross-talk between the endogenous ROS generation by NOX system and the tumorigenic potential [41]. Taken together, the ROS generated by NOX may play the central role connecting CVD and prostatic diseases.
3.2. NOX in CVD and Prostatic Diseases
NOXs are the key enzymes of redox signalling and also the main source of ROS in vivo [75]. ROS are generated by NOX in the pathogenesis of CVD (see Figure 2). In human cells, there are seven isoforms of the NOX family proteins, which are NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2. NOX1, NOX2, and NOX5 directly generate superoxide anions. Conversely, the NOX4 produces H2O2 which may be associated with its localization in the mitochondria in cardiomyocytes and in the endoplasmic reticulum in endothelial cells [76–78]. Superoxide cannot cross the membranes of these organelles. DUOX1 and DUOX2 also produce H2O2 [79]. The transmembrane subunit P22phox and NOX subtypes (NOX1, NOX2, and NOX4) make up the membrane-bound catalytic core [80]. The NOX4 activity requires P22phox and is also regulated by POLDIP2. Of the seven NOXs, NOX1 and NOX2 play a role in immune defence [81], other NOXs act as the second messenger to participate in the regulation of cell signal pathways and maintain the stability of the intracellular environment [82].
3.2.1. NOX and CVD
The association between increased vascular ROS production and hypertension has been reported in animal models with Ang II induced via NOX activation [72]. Some recent in vitro studies have suggested a pivotal role of the NOX and its subunit p47phox in vascular oxidant stress and the blood pressure response to angiotensin II [83, 84]. The previous studies have reported that the expression of mRNA in NOX1, NOX2, and NOX4 was increased in aortas from animals infused with Ang II [85, 86]. In addition, the expression of NOX2, NOX4, and NOX5 was found to be upregulated by Ang II in endothelial cells [87, 88], and knockout of NOX1, NOX2, and NOX4 in mice can reduce blood-pressure elevation induced by Ang II [86, 89, 90]. Taking together, these data suggest that activation of NOX1, NOX2, NOX4, and NOX5 play an important role in the development of Ang II-induced hypertension.
NOX2 is a major ROS source in the heart, and its activity increases after acute myocardial infarction (AMI). Compared with the normal human cardiomyocytes, the expression of NOX2 was higher in patients with AMI [91]. One study reported that the absence of NOX2 in Apoe-/- mice decreased ROS production, increased NO bioavailability, and reduced aortic atherosclerosis [92]. But Sirker et al. indicated that the overexpression of NOX2 in cardiomyocyte or endothelial cell made no difference to initial infarct size in mice with AMI at 4 weeks [93]. Vendrov et al. [94] suggest that expression of NOX4, but not NOX1 or NOX2, was correlated with increased mitochondrial oxidative stress, mitochondrial and cardiovascular dysfunction, and atherosclerosis in aged mice. The study indicated that NOX4 is a potential therapeutic target for aging-associated cardiovascular disease. The current study also demonstrated that NOX5 expression increased in patients with AMI, especially in infarctions > 12 hours [95]. In summary, the expression of NOX2, NOX4, and NOX5 plays a role in the development of coronary heart disease.
3.2.2. NOX and Prostatic Diseases
Evidence has showed that the aberrant activation of NOX plays a critical role in prostate cancer growth and progression [96, 97]. A recent study showed that NOX expression is directly associated with prostate cancer in mice, and when NOX was inhibited, the expression of HIF-1α in the nucleus was significantly decreased as well as a reduction in the proliferation and colony formation of prostate cancer [98]. Some studies have shown that NOX1 and its transmembrane subunit P22phox and NOX5 are overexpressed in prostate cancer [99–102], and downregulated NOX5 expression can inhibit cell proliferation and tumor growth and induce apoptosis of prostate cancer cells [103]. In addition, studies have reported that various isoforms of NOX are found in prostate cancer cell lines, including NOX4, NOX2, and NOX5, which are absent in normal prostate cell lines [104, 105]. An in vitro study has shown that the imbalance of redox homeostasis caused by elevated NOX4-derived ROS signal was the basis of fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma, which indicates NOX4 inhibitors have potential clinical value in the prevention of BPH and prostate cancer [106]. Thus, NOX1, NOX2, NOX4, and NOX5 may be potential targets for therapeutic intervention in prostate cancer.
4. Conclusion
Evidence from clinical and animal studies demonstrates that CVD is associated with prostatic diseases, and oxidative stress may erect a bridge between these diseases; however, the exact mechanism of oxidative stress in CVD and prostatic diseases remains to be further elucidated. We provide a framework for future experimental and clinical studies on the role of known and yet to be discovered oxidative stress in CVD and prostatic diseases. The mechanisms and signalling processes by Ang II increased ROS production via NOX in these diseases is yet to be proved. Furthermore, NOX-derived ROS signals may be a common potential target in therapeutic intervention of CVD and prostatic diseases.
Contributor Information
Cheng Fang, Email: vitsippa@whu.edu.cn.
Xian-Tao Zeng, Email: zengxiantao1128@whu.edu.cn.
Conflicts of Interest
The authors declare that there are no conflicts of interests.
References
- 1.Roth G. A., Mensah G. A., Johnson C. O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. Journal of the American College of Cardiology. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry S. J., Coffey D. S., Walsh P. C., Ewing L. L. The development of human benign prostatic hyperplasia with age. The Journal of Urology. 1984;132(3):474–479. doi: 10.1016/S0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 4.Deng T., Cai L., Chen Z., Jia G. U. Analysis of the burden of prostate cancer in China in 1990 and 2017. Yixue Xinzhi Zazhi. 2020;30(4):252–259. [Google Scholar]
- 5.Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncology. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Yu C., Bi Y., Zhang Z. J. Trends and age-period-cohort effect on incidence and mortality of prostate cancer from 1990 to 2017 in China. Public Health. 2019;172:70–80. doi: 10.1016/j.puhe.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Ramachandra C. J. A., Cong S., Chan X., Yap E. P., Yu F., Hausenloy D. J. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radical Biology & Medicine. 2021;166:297–312. doi: 10.1016/j.freeradbiomed.2021.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Münzel T., Camici G. G., Maack C., Bonetti N. R., Fuster V., Kovacic J. C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. Journal of the American College of Cardiology. 2017;70(2):212–229. doi: 10.1016/j.jacc.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udensi U. K., Tchounwou P. B. Oxidative stress in prostate hyperplasia and carcinogenesis. Journal of Experimental & Clinical Cancer Research. 2016;35(1):p. 139. doi: 10.1186/s13046-016-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta-Elera G., Garrett A. R., Robison R. A., O’Neill K. L. The role of oxidative stress in prostate cancer. European Journal of Cancer Prevention. 2012;21(2):155–162. doi: 10.1097/CEJ.0b013e32834a8002. [DOI] [PubMed] [Google Scholar]
- 11.Bourke J. B., Griffin J. P. Hypertension, diabetes mellitus, and blood groups in benign prostatic hypertrophy. British Journal of Urology. 1966;38(1):18–23. doi: 10.1111/j.1464-410X.1966.tb09675.x. [DOI] [PubMed] [Google Scholar]
- 12.Sugaya K., Kadekawa K., Ikehara A., et al. Influence of hypertension on lower urinary tract symptoms in benign prostatic hyperplasia. International Journal of Urology. 2003;10(11):569–574. doi: 10.1046/j.1442-2042.2003.00707.x. [DOI] [PubMed] [Google Scholar]
- 13.Michel M. C., Heemann U., Schumacher H., Mehlburger L., Goepel M. Association of hypertension with symptoms of benign prostatic hyperplasia. The Journal of Urology. 2004;172, 4, Part 1:1390–1393. doi: 10.1097/01.ju.0000139995.85780.d8. [DOI] [PubMed] [Google Scholar]
- 14.Chen I. H., Tsai Y. S., Tong Y. C. Correlations among cardiovascular risk factors, prostate blood flow, and prostate volume in patients with clinical benign prostatic hyperplasia. Urology. 2012;79(2):409–414. doi: 10.1016/j.urology.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 15.Hwang E. C., Kim S. O., Nam D. H., et al. Men with hypertension are more likely to have severe lower urinary tract symptoms and large prostate volume. LUTS: Lower Urinary Tract Symptoms. 2015;7(1):32–36. doi: 10.1111/luts.12046. [DOI] [PubMed] [Google Scholar]
- 16.Zeng X. T., Weng H., Xiong J., et al. Comparison of clinical and physiological parameters for benign prostatic hyperplasia in hypertensive and normotensive patients. Frontiers in Physiology. 2018;9:p. 1330. doi: 10.3389/fphys.2018.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navin S., Ioffe V. The association between hypertension and prostate cancer. Revista de Urología. 2017;19(2):113–118. doi: 10.3909/riu0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickerman B. A., Torfadottir J. E., Valdimarsdottir U. A., et al. Midlife metabolic factors and prostate cancer risk in later life. International Journal of Cancer. 2018;142(6):1166–1173. doi: 10.1002/ijc.31142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisman K. M., Larijani G. E., Goldstein M. R., Goldberg M. E. Relationship between benign prostatic hyperplasia and history of coronary artery disease in elderly men. Pharmacotherapy. 2000;20(4):383–386. doi: 10.1592/phco.20.5.383.35053. [DOI] [PubMed] [Google Scholar]
- 20.Neugut A. I., Rosenberg D. J., Ahsan H., et al. Association between coronary heart disease and cancers of the breast, prostate, and colon. Cancer Epidemiology, Biomarkers & Prevention. 1998;7(10):869–873. [PubMed] [Google Scholar]
- 21.Stamatiou K. N., Alevizos A. G., Mihas K., Mariolis A. D., Michalodimitrakis E., Sofras F. Associations between coronary heart disease, obesity and histological prostate cancer. International Urology and Nephrology. 2007;39(1):197–201. doi: 10.1007/s11255-006-9010-z. [DOI] [PubMed] [Google Scholar]
- 22.Thomas J. A., 2nd, Gerber L., Bañez L. L., et al. Prostate cancer risk in men with baseline history of coronary artery disease: results from the REDUCE Study. Cancer Epidemiology, Biomarkers & Prevention. 2012;21(4):576–581. doi: 10.1158/1055-9965.EPI-11-1017. [DOI] [PubMed] [Google Scholar]
- 23.Omalu B. I., Hammers J. L., Parwani A. V., Balani J., Shakir A., Ness R. B. Is there an association between coronary atherosclerosis and carcinoma of the prostate in men aged 50 years and older? An autopsy and coroner based post-mortem study. Nigerian Journal of Clinical Practice. 2013;16(1):45–48. doi: 10.4103/1119-3077.106741. [DOI] [PubMed] [Google Scholar]
- 24.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 25.Sohal R. S., Allen R. G. Oxidative stress as a causal factor in differentiation and aging: a unifying hypothesis. Experimental Gerontology. 1990;25(6):499–522. doi: 10.1016/0531-5565(90)90017-V. [DOI] [PubMed] [Google Scholar]
- 26.Sies H. Oxidative stress: oxidants and antioxidants. Experimental Physiology. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 27.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biology. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piwkowska A., Rogacka D., Audzeyenka I., Jankowski M., Angielski S. High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. Journal of Cellular Biochemistry. 2011;112(6):1661–1672. doi: 10.1002/jcb.23088. [DOI] [PubMed] [Google Scholar]
- 29.Stadtman E. R., Levine R. L. Protein oxidation. Annals of the New York Academy of Sciences. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 30.Marnett L. J. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 31.Paravicini T. M., Touyz R. M. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(2):170–180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 32.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2-3):65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Pham-Huy L. A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. International Journal of Biomedical Sciences. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 34.Phaniendra A., Jestadi D. B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Autréaux B., Toledano M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 36.Meagher E. A., FitzGerald G. A. Indices of lipid peroxidation in vivo: strengths and limitations. Free Radical Biology & Medicine. 2000;28(12):1745–1750. doi: 10.1016/S0891-5849(00)00232-X. [DOI] [PubMed] [Google Scholar]
- 37.Germanò G., Sanguigni V., Pignatelli P., et al. Enhanced platelet release of superoxide anion in systemic hypertension. Journal of Hypertension. 2004;22(6):1151–1156. doi: 10.1097/00004872-200406000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Guxens M., Fitó M., Martínez-González M. A., et al. Hypertensive status and lipoprotein oxidation in an elderly population at high cardiovascular risk. American Journal of Hypertension. 2009;22(1):68–73. doi: 10.1038/ajh.2008.313. [DOI] [PubMed] [Google Scholar]
- 39.Pinzón-Díaz C. E., Calderón-Salinas J. V., Rosas-Flores M. M., Hernández G., López-Betancourt A., Quintanar-Escorza M. A. Eryptosis and oxidative damage in hypertensive and dyslipidemic patients. Molecular and Cellular Biochemistry. 2018;440(1-2):105–113. doi: 10.1007/s11010-017-3159-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H., Liu Y., Li Z., et al. Identification of essential hypertension biomarkers in human urine by non- targeted metabolomics based on UPLC-Q-TOF/MS. Clinica Chimica Acta. 2018;486:192–198. doi: 10.1016/j.cca.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Kumar B., Koul S., Khandrika L., Meacham R. B., Koul H. K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Research. 2008;68(6):1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 42.Minciullo P. L., Inferrera A., Navarra M., Calapai G., Magno C., Gangemi S. Oxidative stress in benign prostatic hyperplasia: a systematic review. Urologia Internationalis. 2015;94(3):249–254. doi: 10.1159/000366210. [DOI] [PubMed] [Google Scholar]
- 43.Dakubo G. D., Parr R. L., Costello L. C., Franklin R. B., Thayer R. E. Altered metabolism and mitochondrial genome in prostate cancer. Journal of Clinical Pathology. 2006;59(1):10–16. doi: 10.1136/jcp.2005.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merendino R. A., Salvo F., Saija A., et al. Malondialdehyde in benign prostate hypertrophy: a useful marker? Mediators of Inflammation. 2003;12(2):128. doi: 10.1080/0962935031000097745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camphausen K., Ménard C., Sproull M., Goley E., Basu S., Coleman C. N. Isoprostane levels in the urine of patients with prostate cancer receiving radiotherapy are not elevated. International Journal of Radiation Oncology • Biology • Physics. 2004;58(5):1536–1539. doi: 10.1016/j.ijrobp.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz M. I., Saglam K., Sonmez A., et al. Antioxidant system activation in prostate cancer. Biological Trace Element Research. 2004;98(1):13–20. doi: 10.1385/BTER:98:1:13. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava D. S., Mittal R. D. Free radical injury and antioxidant status in patients with benign prostate hyperplasia and prostate cancer. Indian Journal of Clinical Biochemistry. 2005;20(2):162–165. doi: 10.1007/BF02867419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aydin A., Arsova-Sarafinovska Z., Sayal A., et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clinical Biochemistry. 2006;39(2):176–179. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Surapaneni K. M., Venkata G. R. Lipid peroxidation and antioxidant status in patients with carcinoma of prostate. Indian Journal of Physiology and Pharmacology. 2006;50(4):350–354. [PubMed] [Google Scholar]
- 50.Ozmen H., Erulas F. A., Karatas F., Cukurovali A., Yalcin O. Comparison of the concentration of trace metals (Ni, Zn, Co, Cu and Se), Fe, vitamins A, C and E, and lipid peroxidation in patients with prostate cancer. Clinical Chemistry and Laboratory Medicine. 2006;44(2):175–179. doi: 10.1515/CCLM.2006.032. [DOI] [PubMed] [Google Scholar]
- 51.Lockett K. L., Hall M. C., Clark P. E., et al. DNA damage levels in prostate cancer cases and controls. Carcinogenesis. 2006;26(6):1187–1193. doi: 10.1093/carcin/bgi288. [DOI] [PubMed] [Google Scholar]
- 52.Aryal M., Pandeya A., Bas B. K., et al. Oxidative stress in patients with benign prostate hyperplasia. JNMA; Journal of the Nepal Medical Association. 2007;46(167):103–106. [PubMed] [Google Scholar]
- 53.Goswami K., Nandeesha H., Koner B. C., Nandakumar D. N. A comparative study of serum protein-bound sialic acid in benign and malignant prostatic growth: possible role of oxidative stress in sialic acid homeostasis. Prostate Cancer and Prostatic Diseases. 2007;10(4):356–359. doi: 10.1038/sj.pcan.4500965. [DOI] [PubMed] [Google Scholar]
- 54.Arsova-Sarafinovska Z., Eken A., Matevska N., et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clinical Biochemistry. 2009;42(12):1228–1235. doi: 10.1016/j.clinbiochem.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Pace G., Massimo C. D., Amicis D. D., et al. Oxidative stress in benign prostatic hyperplasia and prostate cancer. Urologia Internationalis. 2010;85(3):328–333. doi: 10.1159/000315064. [DOI] [PubMed] [Google Scholar]
- 56.Hoque A., Ambrosone C. B., Till C., et al. Serum oxidized protein and prostate cancer risk within the Prostate Cancer Prevention Trial. Cancer Prevention Research (Philadelphia, Pa.) 2010;3(4):478–483. doi: 10.1158/1940-6207.CAPR-09-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cimino S., Favilla V., Russo G. I., et al. Oxidative stress and body composition in prostate cancer and benign prostatic hyperplasia patients. Anticancer Research. 2014;34(9):5051–5056. [PubMed] [Google Scholar]
- 58.Nickenig G., Harrison D. G. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation. 2002;105(3):393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 59.Rothermund L., Paul M. Hypertension and the renin-angiotensin system--evidence from genetic and transgenic studies. Basic Research in Cardiology. 1998;93(2) doi: 10.1007/s003950050191. [DOI] [PubMed] [Google Scholar]
- 60.Li X. C., Zhang J., Zhuo J. L. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacological Research. 2017;125(Part A):21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinh D. T., Frauman A. G., Somers G. R., et al. Evidence for activation of the renin-angiotensin system in the human prostate: increased angiotensin II and reduced AT (1) receptor expression in benign prostatic hyperplasia. The Journal of Pathology. 2002;196(2):213–219. doi: 10.1002/path.1021. [DOI] [PubMed] [Google Scholar]
- 62.Nassis L., Frauman A. G., Ohishi M., et al. Localization of angiotensin-converting enzyme in the human prostate: pathological expression in benign prostatic hyperplasia. The Journal of Pathology. 2001;195(5):571–579. doi: 10.1002/path.999. [DOI] [PubMed] [Google Scholar]
- 63.Fabiani M. E., Sourial M., Thomas W. G., Johnston C. I., Johnston C. I., Frauman A. G. Angiotensin II enhances noradrenaline release from sympathetic nerves of the rat prostate via a novel angiotensin receptor: implications for the pathophysiology of benign prostatic hyperplasia. The Journal of Endocrinology. 2001;171(1):97–108. doi: 10.1677/joe.0.1710097. [DOI] [PubMed] [Google Scholar]
- 64.Domińska K., Kowalska K., Urbanek K. A., Habrowska-Górczyńska D. E., Ochędalski T., Piastowska Ciesielska A. W. The impact of Ang-(1-9) and Ang-(3-7) on the biological properties of prostate cancer cells by modulation of inflammatory and steroidogenesis pathway genes. International Journal of Molecular Sciences. 2020;21(17):p. 6227. doi: 10.3390/ijms21176227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mao Y. Q., Xu X., Wang X., Zheng X. Y., Xie L. P. Is angiotensin-converting enzyme inhibitors/angiotensin receptor blockers therapy protective against prostate cancer? Oncotarget. 2016;7(6):6765–6773. doi: 10.18632/oncotarget.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindberg H., Nielsen D., Jensen B. V., Eriksen J., Skovsgaard T. Angiotensin converting enzyme inhibitors for cancer treatment? Acta Oncologica. 2004;43(2):142–152. doi: 10.1080/02841860310022346. [DOI] [PubMed] [Google Scholar]
- 67.Cao L., Zhang S., Jia C. M., et al. Antihypertensive drugs use and the risk of prostate cancer: a meta-analysis of 21 observational studies. BMC Urology. 2018;18(1):p. 17. doi: 10.1186/s12894-018-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uemura H., Ishiguro H., Nakaigawa N., et al. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: a possibility of tyrosine kinase inhibitor of growth factor. Molecular Cancer Therapeutics. 2003;2(11):1139–1147. [PubMed] [Google Scholar]
- 69.Ronquist G., Rodríguez L. A., Ruigómez A., et al. Association between captopril, other antihypertensive drugs and risk of prostate cancer. Prostate. 2004;58(1):50–56. doi: 10.1002/pros.10294. [DOI] [PubMed] [Google Scholar]
- 70.Ito H., Taga M., Tsuchiyama K., Akino H., Yokoyama O. IPSS is lower in hypertensive patients treated with angiotensin-II receptor blocker: posthoc analyses of a lower urinary tract symptoms population. Neurourology and Urodynamics. 2013;32(1):70–74. doi: 10.1002/nau.22267. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu S., Nagao Y., Shimizu T., Higashi Y., Karashima T., Saito M. Therapeutic effects of losartan on prostatic hyperplasia in spontaneously hypertensive rats. Life Sciences. 2021;266, article 118924 doi: 10.1016/j.lfs.2020.118924. [DOI] [PubMed] [Google Scholar]
- 72.Rajagopalan S., Kurz S., Münzel T., et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. The Journal of Clinical Investigation. 1996;97(8):1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rueckschloss U., Quinn M. T., Holtz J., Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(11):1845–1851. doi: 10.1161/01.ATV.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- 74.Uemura H., Ishiguro H., Ishiguro Y., Hoshino K., Takahashi S., Kubota Y. Angiotensin II induces oxidative stress in prostate cancer. Molecular Cancer Research. 2008;6(2):250–258. doi: 10.1158/1541-7786.MCR-07-0289. [DOI] [PubMed] [Google Scholar]
- 75.Spencer N. Y., Engelhardt J. F. The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies. Biochemistry. 2014;53(10):1551–1564. doi: 10.1021/bi401719r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dikalov S., Dikalova A., Bikineyeva A., Schmidt H., Harrison D., Griendling K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radical Biology & Medicine. 2008;45(9):1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helmcke I., Heumüller S., Tikkanen R., Schröder K., Brandes R. P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxidants & Redox Signaling. 2009;11(6):1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 78.Block K., Gorin Y., Abboud H. E. Subcellular localization of Nox4 and regulation in diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lambeth J. D., Kawahara T., Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radical Biology & Medicine. 2007;43(3):319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brandes R. P., Weissmann N., Schröder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radical Biology & Medicine. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 81.Torres M. Mitogen-activated protein kinase pathways in redox signalling. Frontiers in Bioscience. 2003;8:369–391. doi: 10.2741/999. [DOI] [PubMed] [Google Scholar]
- 82.Mao S., Huang S. The signaling pathway of NADPH oxidase and its role in glomerular diseases. Journal of Receptor and Signal Transduction Research. 2014;34(1):6–11. doi: 10.3109/10799893.2013.848892. [DOI] [PubMed] [Google Scholar]
- 83.Lavigne M. C., Malech H. L., Holland S. M., Leto T. L. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104(1):79–84. doi: 10.1161/01.CIR.104.1.79. [DOI] [PubMed] [Google Scholar]
- 84.Landmesser U., Cai H., Dikalov S., et al. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40(4):511–515. doi: 10.1161/01.HYP.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Higashi M., Shimokawa H., Hattori T., et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circulation Research. 2003;93(8):767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 86.Matsuno K., Yamada H., Iwata K., et al. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112(17):2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 87.Liang G. Z., Cheng L. M., Chen X. F., et al. ClC-3 promotes angiotensin II-induced reactive oxygen species production in endothelial cells by facilitating Nox2 NADPH oxidase complex formation. Acta Pharmacologica Sinica. 2018;39(11):1725–1734. doi: 10.1038/s41401-018-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Montezano A. C., Burger D., Paravicini T. M., et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circulation Research. 2010;106(8):1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H. D., Xu S., Johns D. G., et al. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circulation Research. 2001;88(9):947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 90.Bouabout G., Ayme-Dietrich E., Jacob H., et al. Effets de l'inhibition constitutive de la Nox4 dans l'hypertension arterielle experimentale et le syndrome metabolique. Archives of Cardiovascular Diseases. 2018;111(1):41–52. doi: 10.1016/j.acvd.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 91.Krijnen P. A., Meischl C., Hack C. E., et al. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. Journal of Clinical Pathology. 2003;56(3):194–199. doi: 10.1136/jcp.56.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Judkins C. P., Diep H., Broughton B. R. S., et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. American Journal of Physiology. Heart and Circulatory Physiology. 2010;298(1):24–32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 93.Sirker A., Murdoch C. E., Protti A., et al. Cell-specific effects of Nox2 on the acute and chronic response to myocardial infarction. Journal of Molecular and Cellular Cardiology. 2016;98:11–17. doi: 10.1016/j.yjmcc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vendrov A. E., Vendrov K. C., Smith A., et al. NOX4 NADPH oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxidants & Redox Signaling. 2015;23(18):1389–1409. doi: 10.1089/ars.2014.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hahn N. E., Meischl C., Kawahara T., et al. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. The American Journal of Pathology. 2012;180(6):2222–2229. doi: 10.1016/j.ajpath.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 96.Roy K., Wu Y., Meitzler J. L., et al. NADPH oxidases and cancer. Clinical Science (London, England) 2015;128(12):863–875. doi: 10.1042/CS20140542. [DOI] [PubMed] [Google Scholar]
- 97.Block K., Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nature Reviews. Cancer. 2012;12(9):627–637. doi: 10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deep G., Kumar R., Jain A. K., et al. Graviola inhibits hypoxia-induced NADPH oxidase activity in prostate cancer cells reducing their proliferation and clonogenicity. Scientific Reports. 2016;6(1, article 23135) doi: 10.1038/srep23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lim S. D., Sun C., Lambeth J. D., et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62(2):200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 100.Höll M., Koziel R., Schäfer G., et al. ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Molecular Carcinogenesis. 2016;55(1):27–39. doi: 10.1002/mc.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laurent V., Toulet A., Attané C., et al. Periprostatic adipose tissue favors prostate cancer cell invasion in an obesity-dependent manner: role of oxidative stress. Molecular Cancer Research. 2019;17(3):821–835. doi: 10.1158/1541-7786.MCR-18-0748. [DOI] [PubMed] [Google Scholar]
- 102.Li Q., Fu G. B., Zheng J. T., et al. NADPH oxidase subunit p22phox-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2013;1833(12):3375–3385. doi: 10.1016/j.bbamcr.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 103.Brar S. S., Corbin Z., Kennedy T. P., et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. American Journal of Physiology. Cell Physiology. 2003;285(2):353–369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 104.Khandrika L., Kumar B., Koul S., Koul H. K., Koul H. K. Oxidative stress in prostate cancer. Cancer Letters. 2009;282(2):125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyata Y., Matsuo T., Sagara Y., Ohba K., Ohyama K., Sakai H. A mini-review of reactive oxygen species in urological cancer: correlation with NADPH oxidases, angiogenesis, and apoptosis. International Journal of Molecular Sciences. 2017;18(10, article 2214) doi: 10.3390/ijms18102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sampson N., Koziel R., Zenzmaier C., et al. ROS Signaling by NOX4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Molecular Endocrinology. 2011;25(3):503–515. doi: 10.1210/me.2010-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]


