Abstract
Purpose.
To examine the effect of 4 commercially available contact lens multipurpose solutions (MPS) on the viability and barrier function of human corneal epithelial cells in vitro.
Methods.
Immortalized human corneal epithelial cells were exposed to 4 solutions, MPS A, B, C, and D with culture medium and Hanks’ Balanced Salt Solution as controls. MTT assay was used to evaluate cell viability. ApopTag Fluorescein Apoptosis assay was used to detect cell death in situ. Corneal epithelial barrier function was evaluated by fluorescein permeability and immunofluorescent staining for tight junction proteins zonula occludens (ZO)-1 and occludin.
Results.
Corneal epithelial survival rates, evaluated by MTT assay showed no statistical difference between MPS A and the culture medium or Hanks’ Balanced Salt Solution controls. MPS B, C, and D were associated with significantly less cell survival than the controls after exposure for 6 hrs (all P<0.01). Compared with the controls, the MPS A did not increase cell apoptosis, whereas the other 3 caused higher apoptotic rates. The epithelial permeability after exposure to MPS A was similar to controls and significantly lower than MPS B and MPS D (P<0.01). The tight junction proteins ZO-1 and occludin were well maintained after exposure to MPS A. In contrast, the expression of ZO-1 and occludin were largely disturbed by the other 3 MPS solutions.
Conclusions.
The current marketed contact lens MPS may have negative effects on human corneal epithelial viability and barrier function. Among 4 MPS studied, MPS A maintains the cell viability and barrier function significantly better than other 3 marketed products.
Keywords: Multipurpose solutions, Cornea, Apoptosis, Barrier function, Tight junction proteins
An unexpected increase in the rates of microbial keratitis in patients wearing soft contact lenses has been met with an increased research interest in the potential cytotoxicity of agents that come into contact with the corneal epithelium, such as multipurpose solutions (MPS) used in the cleaning and disinfection of contact lenses. The corneal epithelium is the outermost protective barrier to chemical or microbial intrusion to the eye. The semipermeable cell membranes of the epithelial cells and tight intercellular junctions allow limited passage into and transport across the epithelial cells. This selective permeability and barrier function maintain the corneal and ocular health.
Disruption of corneal barrier function is a risk factor for microbial keratitis. The link between contact lens wear and microbial keratitis has been well established in epidemiology studies.1 Microtrauma to the epithelium, microbial interactions with the corneal epithelium, degradation of the epithelial barrier function, and delay in the corneal defense response2–4 are among the multifactorial causes of microbial keratitis and the factors that may play a role in its morbidity. Hypoxia5 or the mechanical movement of the lens on the eye can degrade or abrade the epithelium. Contact lens care solutions are carried to the eye on the contact lens and remain in close contact with the epithelium until they are washed away by the postlens tear film. Antimicrobial agents in the lens care solutions are intended to breach the cell walls of microbes and have the potential for cell membrane toxicity in the corneal epithelial cells.6
Immortalized human corneal epithelial cell line (T-HCEC) has been shown useful as an in vitro model of the human ocular surface in toxicity and inflammation studies of products applied topically to the eye.7,8 Cell damage results in a loss of metabolic function and cell viability. The focus of the present study is on the effect of currently-marketed contact lens MPS on corneal epithelial cells by evaluation of the cell viability and apoptosis rates, permeability to fluorescein, and the integrity of tight junction proteins.
MATERIALS AND METHODS
Multipurpose Solutions Tested
Four marketed MPS (Table 1) were evaluated in this study: MPS A—Complete MPS Easy Rub (Advanced Medical Optics, Inc., Santa Ana, CA), MPS B—Opti-free Express, MPS C—Opti-Free RepleniSH (Alcon, Fort Worth, TX), and MPS D—ReNu MultiPlus (Bausch&Lomb, Rochester, NY). All were obtained commercially and all were used within their expiration dates. Each solution was diluted at a 1:1 ratio with the cell culture medium for all experiments performed in this study. The culture medium and Hanks’ Balanced Salt Solution (HBSS) served as controls.
TABLE 1.
Contact Lens Multipurpose Solution Studied
| Multipurpose solution | Marketed name | Company |
|---|---|---|
| MPS A | Complete MPS Easy Rub | AMO |
| MPS B | Opti-Free Express | Alcon |
| MPS C | Opti-Free RepleniSH | Alcon |
| MPS D | Renu MultiPlus | Bausch&Lomb |
Cell Line and Culture Medium
A T-HCEC, immortalized using recombinant simian virus 40 transfection, in passage 57, were kindly provided by Araki-Sasaki.9 As previously reported,10 the cells were cultured in a 5% CO2 and humidified environment at 37°C with a Dulbecco’s Modification of Eagle’s/F12-fetal bovine serum medium, consisting of a 1:1 mixture of Dulbecco’s Modification of Eagle’s and Ham F12 medium, supplemented with 10% fetal bovine serum, 1% nonessential amino acids and 1% sodium pyruvate (Invitrogen, Carlsbad, CA). The medium was changed every 2 to 3 days.
MTT Cell Viability Assay
To evaluate the effect of MPS on cell viability, the confluent T-HCEC was exposed to each MPS by adding each 50% diluted test solutions to the cells in quadruplicate in a flat bottom 96-well culture plate and incubating the cells for 30 min and 1, 2, 4, 6, and 24 hrs at 37°C in a cell culture incubator. The MTT assay was then performed according to manufacturer’s protocol (Trevigen, Gaithersburg, MD) and our previous report.10 Briefly 1:10 of MTT reagent was added to each well and incubated at 37°C for 4 hrs, followed by addition of 1 volume of detergent and incubated overnight in the dark at room temperature. The absorbance of each sample at 570 nm was read on a microtiter reader VERSAmax (Molecular Devices, Sunnyvale, CA). The experiments were performed at 4 times.
TUNEL DNA Fragmentation Assay
The TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick endlabeling) Assay uses an antidigoxigen antibody that is conjugated to a fluorescein reporter molecule to identify the characteristic changes in DNA fragmentation seen in apoptosis, or programmed cell death associated with normal cell turnover. It is used for rapid identification and quantification of apoptotic cells in tissue sections and cell cultures.
After the cells were exposed to each test solution for 30 min, the TUNEL DNA fragmentation assay was performed using a ApopTag Fluorescein In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA) according to the manufacturer’s instructions and our previous report.10 Briefly, after the cells were fixed, TdT enzyme was applied and incubated for 1 hr. Antidoxigenin was applied and incubated for 30 min in the dark and propidium iodide (PI) was used for nuclear counter staining. Images of representative fields were taken at 100× magnification. Positive and negative cells were counted manually.
Fluorescein Permeability Assay
Corneal epithelial cell barrier function was assessed by a fluorescein permeability assay. Corneal epithelial cells were cultured on an insert (Falcon Cell Culture insert; Becton Dickinson and Company; Franklin Lakes, NY) and allowed to grow for 5 days to confluence. The cell culture media was discarded and each culture insert was filled with 0.3 mL and each well with 0.7 mL of the test solution. This experiment was done in triplicate. The cells were incubated for 1 hr at 37°C, then carefully rinsed 3 times with 1 mL HBSS. The inserts were placed in a new well containing 0.5 mL HBSS. Sodium fluorescein 0.5 mL (3 μg/100 mL in HBSS, Sigma, St. Louis, MO) was placed into each insert. The plates were incubated for 20 min at 37°C. Inserts were removed and the fluorescein in the well was measured using a Cytofluor II fluorometer (PerSeptive Biosystems, Framingham, MA) at 485 nm excitation and 530 nm emission.
Tight Junction Protein Staining
Immunofluorescent staining for the tight junction proteins, zonula occludens (ZO)-1 and occludin, was used to evaluate the integrity of the intercellular junctions after the cells were exposed to each test solution for 2 and 6 hrs in 8-chamber culture slides using a modification of our previously published protocol.11,12 Briefly, the cells were fixed with cold methanol at −20°C for 10 min and permeabilized with Triton-X 0.1%. The primary antibodies used were 1:50 dilution of mouse anti-human ZO-1 and mouse anti-human Occludin monoclonal antibodies (Zymed Laboratories, San Francisco, CA). The secondary antibody was 1:200 dilution of goat anti-mouse Alexa 488 conjugated with fluorescein (Invitrogen, Carlsbad, CA). Primary antibody was incubated at room temperature for 2 hrs and secondary antibody was incubated for 40 min. PI or Hoechst 33258 was used as nuclear counter staining. Images of representative fields were taken at 200× magnification.
Analyses
We used an epifluorescent microscope (Eclipse 400, Nikon, Japan). Photographs were taken on the same microscope with a Nikon DXM1200 digital camera and the images were captured through Nikon ACT-1 version 2.63. Picture quality was enhanced with Adobe Photoshop 7.0. Statistical analysis was conducted with GraphPad Prism 4.03 and charts were created with Microsoft Excel 2003. Data were analyzed by one-way analysis of variance. Groups were compared with culture media control using Dunnett multiple comparison test. Statistical significance was set at P<0.05.
RESULTS
Effect of Multipurpose Solutions on Cell Viability
The MTT assay showed no statistically significant difference in cell viability after exposure to either MPS A or the controls (culture medium and HBSS). MPS B, MPS C, and MPS D were associated with significantly lower cell viability than the controls after exposure for 6 hrs (all P<0.01). Cell viability was statistically significantly greater with MPS A than the other products at 6 and 24 hrs (P<0.05 one-way analysis of variance), with the mean differences falling within the 95% confidence interval. Figure 1 shows the change in cell viability after exposure to the 4 MPS diluted in 50% culture media for 0.5, 1, 2, 4, 6, and 24 hrs, with HBSS and the culture medium controls. Table 2 shows the mean difference in cell viability between each MPS and the HBSS control at 4, 6, and 24 hrs.
FIG. 1.
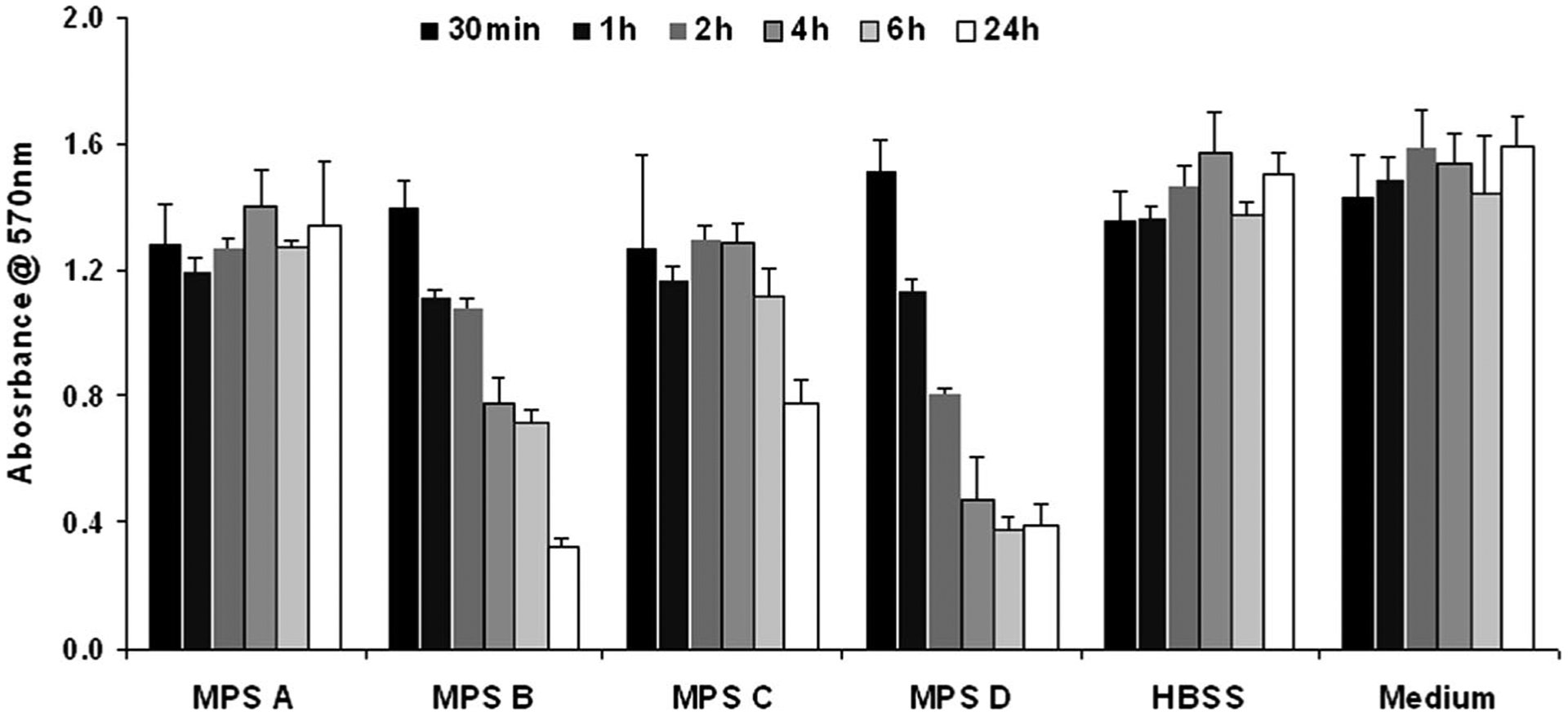
Results of MTT Assay showing changes in cell viability after exposure to 4 multipurpose solutions diluted with 50% culture medium for 0.5, 1, 2, 4, 6, and 24 hrs, in comparison with the Hanks’ Balanced Salt Solution (HBSS) and culture medium controls. Columns represent the mean and bars represent standard deviation of the mean in quadruplicate experiments. The x-axis represents the observation times for each solution. The y-axis represents the absorbance at 570 nm.
TABLE 2.
Differences in Cell Viability Between Various Solutions Compared to Culture Medium Control in MTT Assay
| 4 hrs | 6 hrs | 24 hrs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference | P | 95% CI | Mean difference | P | 95% CI | Mean difference | P | 95% CI | |
| MPS A | 0.14 | >0.05 | −0.05 to 0.32 | 0.15 | >0.05 | −0.05 to 0.32 | 0.14 | >0.05 | −0.05 to 0.33 |
| MPS B | 0.76 | <0.01 | 0.56 to 0.94 | 0.73 | <0.01 | 0.57 to 0.87 | 1.23 | <0.01 | 1.04 to 1.4 |
| MPS C | 0.20 | >0.05 | −0.05 to 0.43 | 0.33 | <0.01 | 0.18 to 0.48 | 0.77 | <0.01 | 0.59 to 0.95 |
| MPS D | 1.06 | <0.01 | 0.87 to 1.25 | 1.07 | <0.01 | 0.92 to 1.22 | 1.13 | <0.01 | 0.94 to 1.32 |
| HBSS | −0.03 | >0.05 | −0.22 to 0.15 | 0.07 | >0.05 | −0.08 to 0.22 | 0.05 | >0.05 | −0.13 to 0.22 |
CI, confidence interval.
Positive difference means lower cell viability.
Effect of Multipurpose Solutions on Cell Apoptosis
Figure 2 shows the percent of positive (apoptotic) cells after 30 min exposure to MPS and controls (HBSS and culture medium). The HBSS and culture medium controls lost 5.5% and 5.8% cells to apoptosis out of 2,951 and 3,094 cells counted, respectively. MPS A had 6.7% apoptotic cells in a population of 3,021 cells. MPS B had 16.5% of 3,095 cells, MPS C had 12.6% of 2,731 cells. Cells treated with MPS D presented with 10.2% in 2,555 cells. Table 3 shows the mean differences in apoptotic cells between culture media control and the various MPS. Compared with cells in culture media, MPS A and HBSS did not show statically significant difference, whereas MPS B, MPS C, and MPS D caused higher rate of apoptotic cells (P<0.01, <0.01, and <0.05, respectively).
FIG. 2.
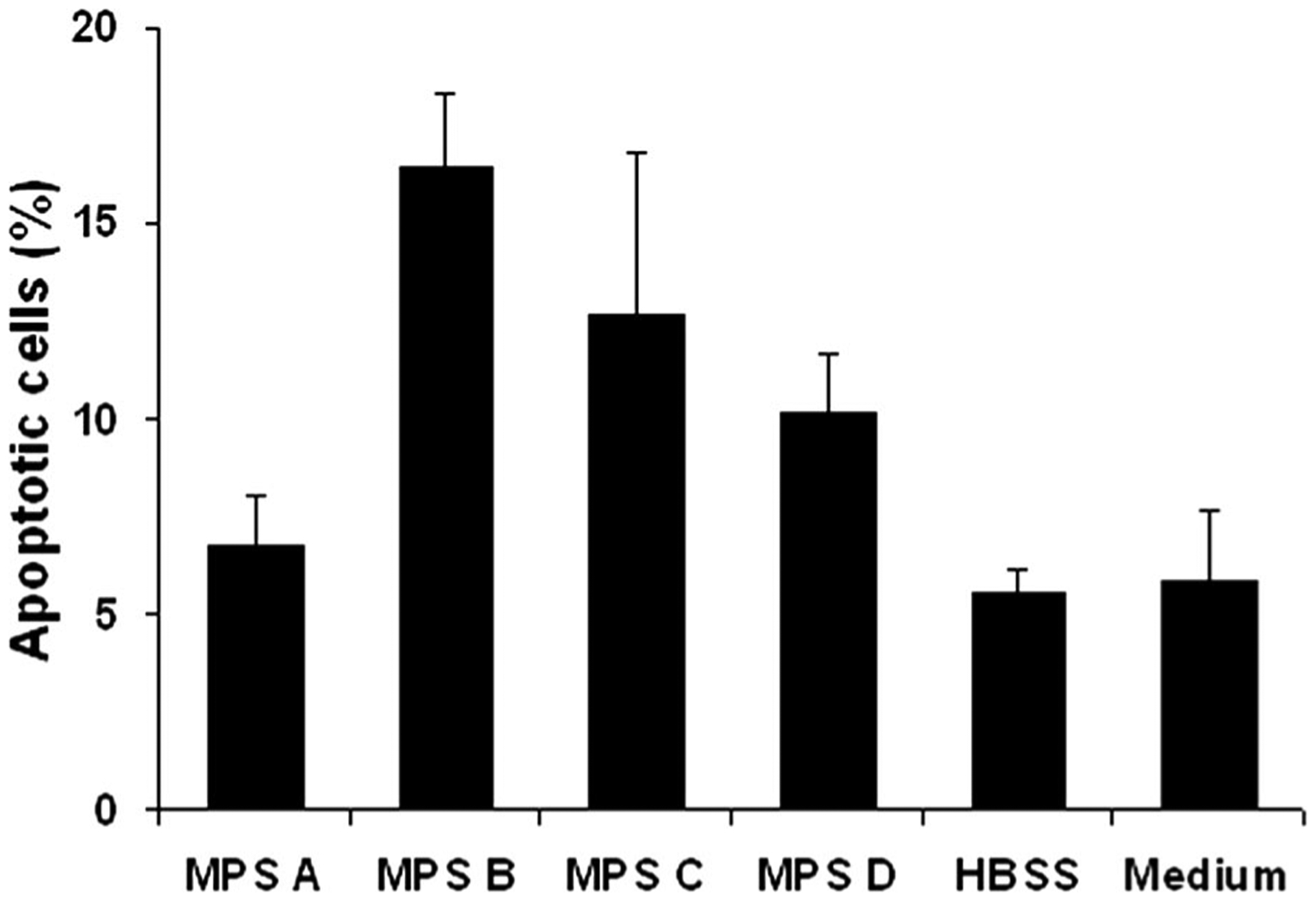
Percentage of positive (apoptotic) cells after 30 min exposure to multipurpose solutions and controls (HBSS and culture medium). MPS C and MPS B had the highest percentage of positive cells followed by MPS D. Difference in MPS A was not statistically significant to the controls. Columns represent the mean and bars represent standard deviation of the mean in triplicate experiments.
TABLE 3.
Percentage Difference in Cell Apoptosis Rates Between Culture Media Control and Studied Solutions TUNEL Assay
| Mean difference | P | 95% confidence interval | |
|---|---|---|---|
| MPS A | 0.9 | >0.05 | −3.3 to 5.1 |
| MPS B | 11 | <0.01 | 6.4 to 15 |
| MPS C | 6.8 | <0.01 | 2.6 to 11 |
| MPS D | 4.4 | <0.05 | 0.12 to 8.6 |
| HBSS | −0.29 | >0.05 | −4.5 to 3.9 |
Positive numbers mean higher rate than control.
Effect of Multipurpose Solutions on Epithelial Permeability
The fluorescence absorbance in the fluid in the well 1 hr after exposure to each solution is shown in Figure 3. MPS A solution had epithelial permeability similar to the controls. MPS B and MPS D had the highest rates of epithelial permeability, followed by MPS C. Table 4 summarized the mean difference in sodium fluorescein permeability between culture media control and the contact lens MPS. MPS A solution showed no statistical difference from the controls whereas MPS B, MPS C, and MPS D treated cells had statistically significantly higher fluorescein permeability than control (P<0.01, 0.05, and <0.01, respectively).
FIG. 3.
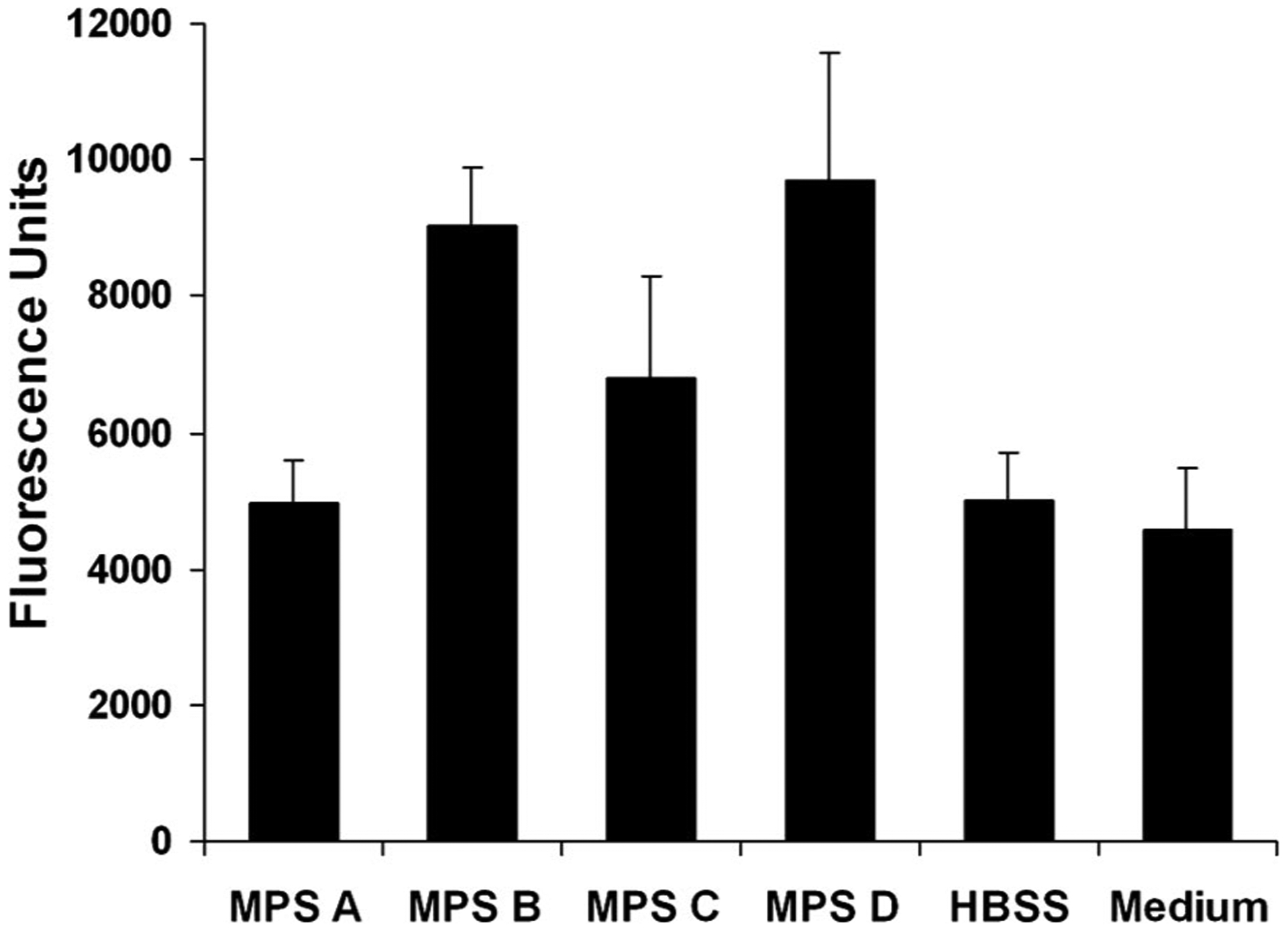
Changes of sodium fluorescein permeability in human corneal epithelial cells after 30 min of exposure to multipurpose solutions and controls (HBSS and culture medium). The fluorescein permeability was highest with MPS B and MPS D. Columns represent the mean and bars represent standard deviation of the mean in triplicate experiments.
TABLE 4.
Differences in Barrier Function Between Culture Media Control and Studied Solutions in Sodium Fluorescein Permeability Assay
| Mean difference | P | 95% confidence interval | |
|---|---|---|---|
| MPS A | 394 | >0.05 | −1,582 to 2,370 |
| MPS B | 4,452 | <0.01 | 2,475 to 6,428 |
| MPS C | 2,231 | <0.05 | 254 to 4,208 |
| MPS D | 5,122 | <0.01 | 3,146 to 7,098 |
| HBSS | 439 | >0.05 | −1,537 to 2,416 |
Positive numbers mean higher fluorescence units reading than control.
Effect of Multipurpose Solutions on Tight Junction Integrity
Tight junction integrity of the human corneal epithelial cells was evaluated by immunofluorecent staining with ZO-1 and occludin antibodies. As shown in representative images in Figure 4, ZO-1 and occludin proteins were observed as a continuous linear pattern along with cell-cell borders in confluent cultures of normal human corneal epithelial cells. The cells exposed to MPS A for 2 and 6 hrs maintained these tight junction proteins intact, similar to the structures of cells in the culture medium controls. In contrast, the cells exposed to the other 3 test solutions for 2 to 6 hrs showed a significant disruption of ZO-1 (Fig. 5) and occludin (Fig. 6) tight junction proteins.
FIG. 4.
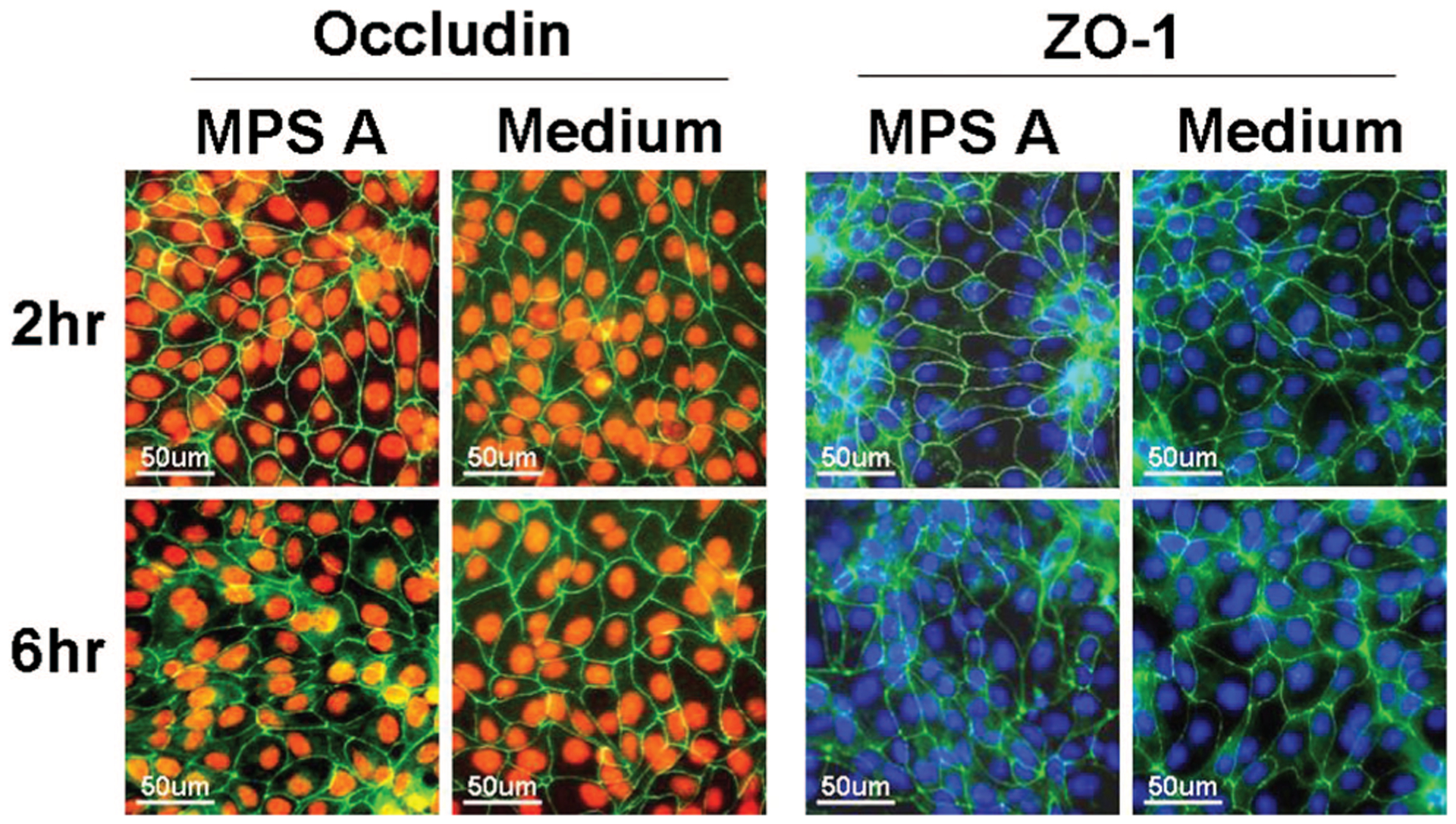
Representative images of immunofluorescent staining for ZO-1 (green in right 2 columns) and occludin (green in left 2 columns) in human corneal epithelial cells after 2 and 6 hrs exposure to MPS A solution with cells in the normal culture medium as control. Hoechst 33342 (blue) or PI (red) was used for cell nuclear counterstaining, respectively.
FIG. 5.
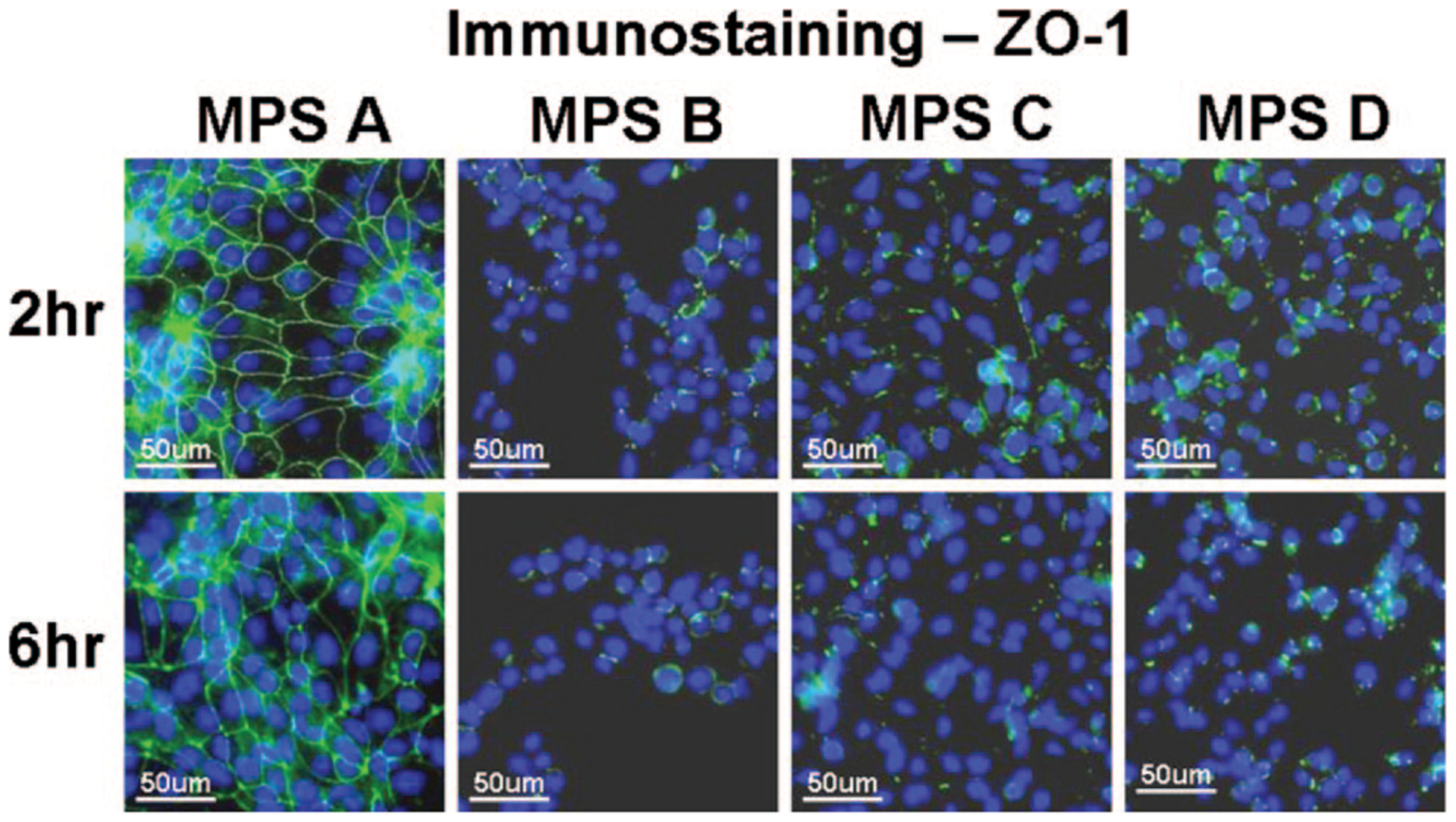
Representative images of immunofluorescent staining for ZO-1 (green) in human corneal epithelial cells after 2 and 6 hrs exposure to each of the 4 MPS studied. Strong green staining between the cells indicates intact ZO-1 tight junction proteins while the weak or negative staining shows disrupted junctions. Hoechst 33342 (blue) was used for cell nuclear counterstaining.
FIG. 6.
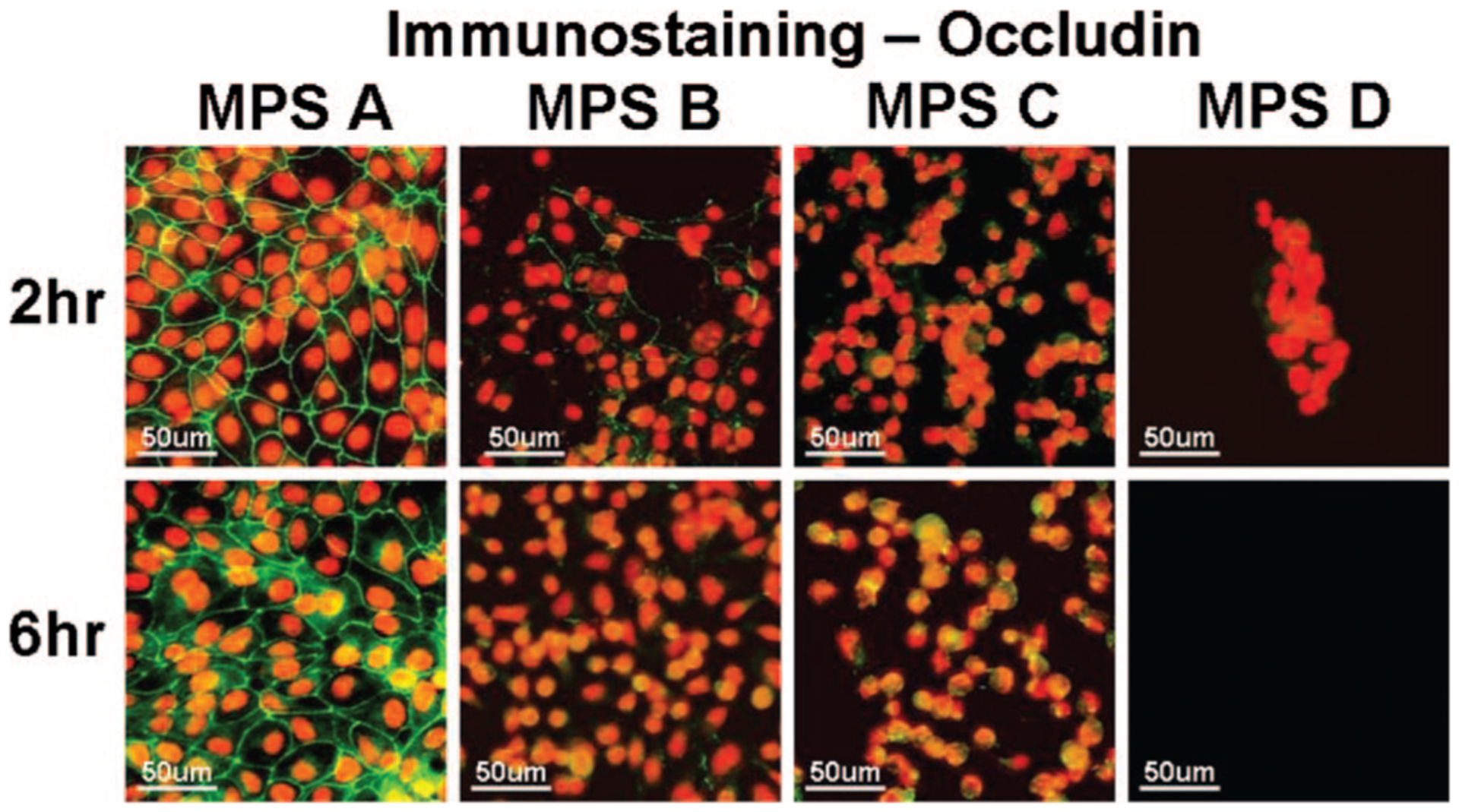
Representative images of immunofluorescent staining for occludin (green) in human corneal epithelial cells after 2 and 6 hrs exposure to each of the 4 MPS studied. Strong green staining between the cells indicates intact occludin tight junction proteins while the weak or negative staining shows disrupted junctions. PI (red) was used for cell nuclear counterstaining.
DISCUSSION
There are several brands of MPS available in the market for contact lens disinfection. Their formulation varies from solution to solution and some are known to cause more corneal fluorescein staining and show higher cellular toxicity than others. The purpose of this study was to examine the cytotoxicity and disruption of barrier function of 4 commercially available MPS on human corneal epithelium through an immortalized corneal epithelial cell line in vitro. MPS B, MPS C, and MPS D solutions were found to reduce cell viability, increase apoptotic cell death, and disrupt tight junctions and intercellular barrier function. Each of the 4 tests individually, and the tests collectively, demonstrate that MPS A solution maintained human corneal epithelial cell viability and barrier function that was consistent with the controls and statistically significantly better than the other products tested.
We evaluated cell viability up to 24 hrs while actual daily usage is shorter and usually around 8 hrs. Lebow et al.13 have found that corneal staining caused by MPS disappears in 8 hrs or less of continuing contact lenses usage, suggesting that the potential damage caused by MPS are healed in 8 hrs. Given the extent of our exposure time, a worst case scenario has been evaluated. We used 50% dilution of each MPS for all tests for a reason that contact lens solutions are initially diluted by the tear film before it contacts the ocular surface.13
Our findings are, overall, consistent with those of McCanna et al.,14 who used a subclone of an imian virus 40 immortalized T-HCEC to evaluate Opti-Free Express (Alcon), ReNu MultiPlus (Bausch & Lomb), SOLO-Care Plus (CIBA), Complete MoisturePlus (Advanced Medical Optics), and AQuify (CIBA). They assessed cell viability using the Alamar Blue Assay, which measures the respiratory activity of cells and produces results comparable with the MTT Assay. They found no significant differences in cell viability among ReNu MultiPlus, SOLO-Care, AQuify, Complete MoisturePlus or compared with the HBSS control. Opti-Free Express was more damaging to human corneal epithelial cells, producing significantly lower metabolic activity than the other products and control. McCanna group also assessed corneal barrier function with the sodium fluorescein permeability assay and found no significant differences in intercellular permeability among ReNu MultiPlus, SOLO-Care, AQuify, Complete MoisturePlus compared with HBSS control. Opti-Free Express had significantly greater fluorescein permeability compared with all other products and twice that of control. Cell cultures exposed to Opti-Free Express showed a loss of tight junctions, damaged cell membranes, loss of microvilli, and blebs and folds. Cell cultures exposed to other products appeared to have similar tight junctions and intact cell membranes compared with HBSS. Cells exposed to Complete MoisturePlus and AQuify had slight breaks in the monolayer but these “were insignificant,” according to the author, when compared with those exposed to Opti-Free Express. The difference they observed with Renu MultiPlus from our results can be explained by the shorter exposure time and lower temperature employed in their study.
TUNEL labeling was used by Ladage et al.15 to study corneal epithelial surface cell viability with overnight contact lens wear. They found that cell renewal because of apoptosis was unrelated to the contact lens polymer. Our data show that the contact lens MPS has the potential to cause apoptosis. Compared with medium control, exposure to MPS B, C, and D caused 4.4% to 11% increase in apoptotic cells whereas MPS A did not increase apoptosis significantly.
Recent studies have provided novel insight with regards to the structure and function of tight junctions. In epithelia, tight junctions form continuous seals around cells. They display ion and size selectivity, thus serving as a regulated permeability barrier that prevents water, solutes, and immune cells from passing freely through the paracellular pathway.16 Thus, we examined the corneal epithelial barrier function by evaluating both the permeability and tight junction proteins in corneal epithelial cells.
Our findings relative to MPS D are supported by a study by Imayasu et al.17 who showed the poloxamine preservative found in this MPS affecting a barrier breakdown. Their study looked at the effect of multipurpose solution preservatives in 4 products: MPS1—(polyhexamethylene biguanide, macrogolglycerol hydroxystearate), MPS2—(polyhexamethylene biguanide, poloxamine), MPS3—(Alexidine, poloxamine;), and MPS4—(POLYQUAD, poloxamine). They used the same corneal epithelial cells that we used in this study to evaluate ZO-1 tight junction protein and transepithelial electrical resistance for corneal epithelial barrier function. The ZO-1 staining in MPS1 and the control groups showed a normal continuous linear pattern whereas MPS2, MPS3, and MPS4 caused discontinuous, disrupted structures at the cell borders. In addition, the transepithelial electrical resistance showed no difference in control and MPS1 groups, but a time-dependent decrease in MPS2, MPS3, and MPS4 groups. This study suggested that high cytotoxicity of MPS may lead to breakdown of epithelial barrier functions.
The hypoxic effect of contact lenses on the epithelial surface led to the development of oxygen permeable silicone hydrogel contact lenses. Oxygen permeability is widely advertised and valued by the consumer. MPS cytotoxicity is also a fundamental aspect on the safety of contact lenses use and has a valuable benefit to the consumer. We found MPS A has the lowest cytotoxicity and best barrier preservation of the products tested.
Our study has limitations. It is in vitro, on 1 cell line, and the use of 1:1 dilution with culture medium may have buffered some of the cytotoxic component. In vitro studies cannot predict the outcome in actual in vivo use, but the tests provide cytotoxicity data that cannot be obtained in an in vivo study. The antimicrobial effectiveness of these MPSs was not tested in this study. It was reported that MPSs seem to be preservative but not disinfecting solutions,18 and MPS B (Opti-Free Express) has been found to induce caspase 3 activity and chromatin condensation,18 which support our apoptosis data. In contrast, MPS C (Opti-Free RepleniSH) was reported to have a broad spectrum of antibacterial activity.19 Further investigations are necessary to clarify this paradox. In conclusion, the findings from this study showed that MPS A, a new formula of contact lens multipurpose solution from Advance Medical Optics, maintains better cell viability and barrier function of human corneal epithelial cells than other 3 commercial MPS studied.
Acknowledgments
Supported by NIH Grants EY11915 (to S.C.P.) and EY014553 (to D-Q.L.), Department of Defense Congressionally Directed Medical Research Programs (CDMRP) Grant FY06 PR064719 (to D-Q.L.), Lions Eye Bank Foundation, Research to Prevent Blindness, Oshman Foundation, William Stamps Farish Fund, and Advanced Medical Optics.
Footnotes
Presented, in part, as abstract at the annual meeting of the Contact Lens Association of Ophthalmologists, October 5–6, 2007, Las Vegas, Nevada.
REFERENCES
- 1.Keay L, Stapleton F, Schein O. Epidemiology of contact lens-related inflammation and microbial keratitis: A 20-year perspective. Eye Contact Lens 2007;33:346–353; discussion 362–363. [DOI] [PubMed] [Google Scholar]
- 2.Keay L, Edwards K, Naduvilath T, et al. Factors affecting the morbidity of contact lens-related microbial keratitis: A population study. Invest Ophthalmol Vis Sci 2006;47:4302–4308. [DOI] [PubMed] [Google Scholar]
- 3.Lee EJ, Evans DJ, Fleiszig SM. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest Ophthalmol Vis Sci 2003;44:5220–5227. [DOI] [PubMed] [Google Scholar]
- 4.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology 2006;113:109–116. [DOI] [PubMed] [Google Scholar]
- 5.Lin MC, Polse KA. Hypoxia, overnight wear, and tear stagnation effects on the corneal epithelium: Data and proposed model. Eye Contact Lens 2007;33:378–381. [DOI] [PubMed] [Google Scholar]
- 6.Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Ther 2001;18:205–215. [DOI] [PubMed] [Google Scholar]
- 7.Offord EA, Sharif NA, Mace K, et al. Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthalmol Vis Sci 1999;40:1091–1101. [PubMed] [Google Scholar]
- 8.Bednarz J, Teifel M, Friedl P, et al. Immortalization of human corneal endothelial cells using electroporation protocol optimized for human corneal endothelial and human retinal pigment epithelial cells. Acta Ophthalmol Scand 2000;78:130–136. [DOI] [PubMed] [Google Scholar]
- 9.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci 1995;36:614–621. [PubMed] [Google Scholar]
- 10.Tong L, Chen Z, de Paiva CS, et al. Transglutaminase participates in UVB-induced cell death pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci 2006;47:4295–4301. [DOI] [PubMed] [Google Scholar]
- 11.de Paiva CS, Chen Z, Corrales RM, et al. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells 2005;23:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, de Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 2004;22:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebow KA, Schachet JL. Evaluation of corneal staining and patient preference with use of three multi-purpose solutions and two brands of soft contact lenses. Eye Contact Lens 2003;29:213–220. [DOI] [PubMed] [Google Scholar]
- 14.McCanna DJ, Harrington KL, Driot JY, et al. Use of a human corneal epithelial cell line for screening the safety of contact lens care solutions in vitro. Eye Contact Lens 2008;34:6–12. [DOI] [PubMed] [Google Scholar]
- 15.Ladage PM, Yamamoto K, Li L, et al. Corneal epithelial homeostasis following daily and overnight contact lens wear. Cont Lens Anterior Eye 2002;25:11–21. [DOI] [PubMed] [Google Scholar]
- 16.Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol 2002;14:531–536. [DOI] [PubMed] [Google Scholar]
- 17.Imayasu M, Shiraishi A, Ohashi Y, et al. Effects of multipurpose solutions on corneal epithelial tight junctions. Eye Contact Lens 2008;34:50–55. [DOI] [PubMed] [Google Scholar]
- 18.Dutot M, Paillet H, Chaumeil C, et al. Severe ocular infections with contact lens: Role of multipurpose solutions. Eye 2008. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H, Ding A, Bandara M, et al. Broad spectrum of antibacterial activity of a new multipurpose disinfecting solution. Eye Contact Lens 2007;33:278–283. [DOI] [PubMed] [Google Scholar]


