Abstract
Purpose
Intraocular pressure (IOP), medication outcomes at 24 months following trabeculotomy/viscodilation using the OMNI® surgical system as a standalone procedure in medically uncontrolled mild–moderate open-angle glaucoma (OAG).
Setting
Surgical center (Duesseldorf, Germany).
Design
Retrospective analysis. IOP and medication data were collected before surgery and through 24 months. Safety data included adverse events and the need for additional surgery.
Methods
Pre-op medication washout. Goldmann tonometry. Number of medications and adverse events (AE) at each time point. Primary outcomes: changes in IOP and medications. Two-sided paired t-tests compare values at each follow-up with baseline, significance p = 0.05. Secondary outcomes: proportion of eyes with IOP reduction of ≥20%, on fewer medications, and medication-free at each time point.
Results
This analysis included data from 38 eyes of 27 subjects. Mean (standard deviation) baseline IOP was 24.6 (3.0) mmHg and through 24 months ranged from 12.6 to 14.9 mmHg (p < 0.0001), representing reductions of 10.0–12.0 mmHg. Mean medications were 1.9 (baseline) and through 24 months ranged from 0.0 to 0.5 (70.6–100% reduction) (p < 0.0001). At Month 24, mean IOP was 14.9 mmHg (−10.0 mmHg), and 100% of eyes achieved IOP reduction >20% from baseline; mean medication use was 0.5 (−1.4 medications, p < 0.0001), 84.6% of eyes using >1 fewer medication, and 57.7% were medication-free. The most common adverse event was intraoperative hyphema (44.7%); all resolved spontaneously. There were two secondary procedures for IOP control.
Conclusion
The OMNI surgical system provides clinically relevant and statistically significant reductions in both IOP and medications with an excellent safety profile and should be considered in phakic or pseudophakic eyes with mild–moderate OAG requiring IOP or medication reduction, or both.
Keywords: primary open-angle glaucoma, trabeculotomy, viscodilation, OMNI surgical system
Introduction
The mechanisms of intraocular pressure (IOP) elevation in eyes with primary open-angle glaucoma (POAG) include structural alterations of tissue within the trabecular meshwork (TM), canal of Schlemm, and the distal collector channels.1–4 These changes within the trabecular outflow pathway increase resistance to the egress of aqueous humor. Approximately 50–70% of total outflow resistance in glaucomatous eyes occurs within the TM1,2 and 30–50% within Schlemm’s canal and the collector channels.3,4
Various minimally invasive glaucoma surgeries (MIGS) have targeted each of these points of aqueous outflow resistance. Goniotomy, trabeculotomy, and trans-TM implants address TM resistance, while canaloplasty, viscodilation, and stenting procedures focus on Schlemm’s canal and the collector channels as sources of resistance.5–8 Given that outflow resistance is multifocal along the trabecular outflow pathway, a procedure targeting all three points could effectively reduce IOP by removing all sources of aqueous outflow resistance.
A combination of trabeculotomy to reduce TM resistance and viscodilation to reduce resistance through Schlemm’s canal and the collector channels targets all three points of resistance along the trabecular outflow pathway. The OMNI surgical system (Sight Sciences, Menlo Park, CA) is a handheld instrument with a hollow tip through which ophthalmic viscosurgical device (OVD) can be injected to perform viscodilation and through which a microcatheter can be deployed to perform trabeculotomy.9 Other features of the device include a gear wheel for advancement and withdrawal of the microcatheter, a port for loading OVD into the device and a reservoir to hold it.
In a limited number of studies to date, IOP reductions of 20–35% and medication reductions of 25–75% have been reported using the OMNI system either combined with phacoemulsification or on its own.10–14 In this report, we describe our experience performing trabeculotomy/viscodilation of Schlemm’s canal and distal collector channels using the OMNI system as a standalone procedure in both phakic and pseudophakic eyes.
Methods
This was a retrospective analysis of data drawn from existing health records. The study was conducted in accordance with the tenets of the Declaration of Helsinki. The protocol was reviewed and approved by an ethics committee (Salus IRB, September 2, 2020) and a waiver of consent was granted. Data collection was compliant with provisions of the General Data Protection Regulation. The study is exempt from posting on a public website (e.g. clinicaltrials.gov) due to the retrospective nature of the study and lack of prospective protocol-defined intervention. All surgeries were performed by a single surgeon (KK) at a single outpatient surgery center located in Duesseldorf, Germany.
Study Patients
Data for this analysis were drawn from the records of consecutive adult patients age 18 years and older with medically uncontrolled open-angle glaucoma (with or without pseudoexfoliation) who underwent 360° viscodilation followed by up to 360° trabeculotomy as a standalone procedure between June 2019 and March 2020. Patients undergoing the procedure in combination with cataract surgery were excluded from this analysis.
Prior to surgery, patients were washed out of their ocular hypotensive medications according to generally accepted wash-out periods as follows: prostaglandin analogs or beta-blockers, 4 weeks; alpha agonists, 2 weeks; carbonic anhydrase inhibitors, 3 days.
Data drawn from the health records included demographic and glaucoma status data at baseline, as well as IOP and the number of IOP-lowering medications at baseline and at each follow-up visit. Safety data included intraoperative and postoperative complications as well as secondary surgical interventions. IOP was measured using Goldmann tonometry. Fixed combination medication products were counted as the number of active ingredients.
Surgical Procedure
The surgical procedure has been described previously.9,11,15 Briefly, under gonioscopic view, the handpiece tip is introduced into the OVD-filled anterior chamber through a temporal corneal incision and advanced to the nasal angle. The tip then engages and passes through TM, and the microcatheter is deployed via the cannula into and through 180° of Schlemm’s canal. The microcatheter is then retracted slowly as a fixed volume of OVD is injected to viscodilate the canal and collector channels. The procedure is then repeated on the remaining 180° of the canal. To perform the trabeculotomy, the same catheter is advanced once again through 180° of Schlemm’s canal and withdrawn using a cheese-wire technique to unroof the canal; this process was in some cases repeated on the remaining 180° of the canal. Standard anti-inflammatory (dexamethasone) and anti-microbial (ofloxacin) therapy were prescribed postoperatively, and most eyes were also prescribed pilocarpine for the first postoperative week to draw apart the TM edges during the acute healing phase; this was quickly discontinued and not counted as an IOP-lowering agent at postoperative visits.
Statistical Analysis
The primary statistical goal of this analysis was to characterize changes in IOP and IOP medication use. Two-sided paired t-tests were used to compare values at each follow-up with baseline values, with p = 0.05 taken as the level of significance. Secondary outcomes included the proportions of eyes achieving minimum IOP reductions of >20%, using fewer medications, and medication-free at each time point. Means are presented with standard deviations. Formal power and sample size analysis was not conducted a priori, as no specific hypothesis testing was planned. Instead, the analysis was conducted in a convenience sample comprising all subjects having undergone the procedure since its incorporation into the investigator’s surgical repertoire.
Results
Patient Demographics
This analysis included data from 38 eyes of 27 subjects. Demographic and baseline glaucoma status data are given in Table 1. Subjects were approximately 67 years of age, with similar representation of both genders, and all were White. Most (71%) of eyes had primary open-angle glaucoma, all of mild–moderate severity (mean cup-disc ratio 0.65±0.16, mean visual field mean deviation −3.5±2.89 dB; minimum −10.47, maximum 0.54), most (74%) were phakic, and 26% had undergone prior selective laser trabeculoplasty.
Table 1.
Demographic and Baseline Glaucoma Status Data for the Study Sample
| Subject-Level Parameters | Value |
| Number (n) | 27 |
| Age, mean (SD, range) | 67.3 (6.4, 56–78) |
| Gender, n (%) | |
| Male | 14 (51.8) |
| Female | 13 (48.2) |
| Ethnicity, n (%) | |
| White | 27 (100.0) |
| Eye-Level Parameters | |
| Number (n) | 38 |
| Study eye, n (%) | |
| Right eye | 22 (57.9) |
| Left eye | 16 (42.1) |
| Glaucoma diagnosis, n (%) | |
| Primary open-angle | 27 (71.1) |
| Pseudoexfoliation | 11 (28.9) |
| Cup-disc ratio, mean (SD, range) | 0.65 (0.16, 0.3–0.9) |
| Visual field mean deviation (dB), mean (SD, Min, Max) | −3.51 (2.89, −10.47, 0.54) |
| Phakic status, n (%) | |
| Phakic | 28 (73.7) |
| Pseudophakic | 10 (26.3) |
| Prior SLT, n (%) | 10 (26.3) |
Thirty eyes were available for analysis at 12 months and 26 at 24 months. As this was a retrospective chart without a protocol mandated or patient agreed to schedule of visits, the observed attrition rate due to loss to follow-up was not unexpected.
Effectiveness Outcomes
Intraocular Pressure
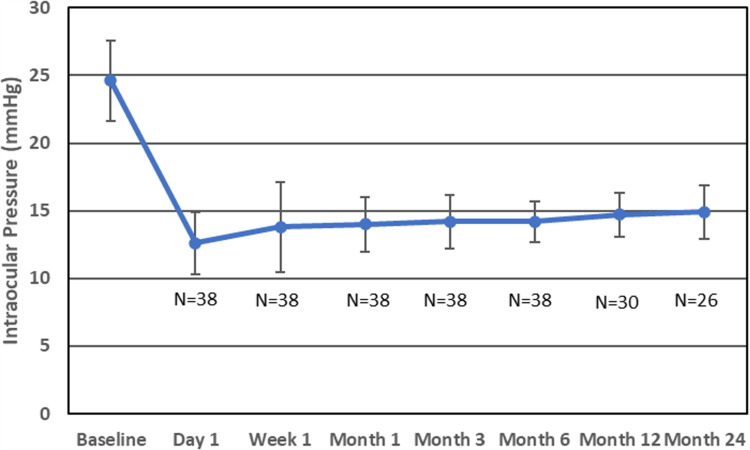
Mean (standard deviation) baseline IOP (Table 2 and Figure 1) was 24.6 ±3.0 mmHg and through 24 months of follow-up ranged from 12.6–14.9 mmHg (p < 0.0001 at all time points), representing mean IOP reductions of 10.0–12.0 mmHg (39.6–47.9%). At Months 12 and 24, mean IOP was 14.7 and 14.9 mmHg, reductions of 10.1 and 10.0 mmHg (39.8%; p < 0.0001). The proportion of eyes achieving a >20% IOP reduction from baseline was 96.7% and 100% at 12 and 24 months, respectively (Table 3). At 24 months, 88.5% (23/26) of eyes had an IOP below 18 mmHg, 14 (61%) of these without medication.
Table 2.
Intraocular Pressure and Medication Data at Each Time Point
| Baseline | Day 1 | Week 1 | Month 1 | Month 3 | Month 6 | Month 12 | Month 24 | |
|---|---|---|---|---|---|---|---|---|
| Number of eyes (n) | 38 | 38 | 38 | 38 | 38 | 38 | 30 | 26 |
| Mean IOP (SD, range) | 24.6 (3.0, 20–32) | 12.6 (2.3, 8–21) | 13.8 (3.3, 10–26) | 14.0 (2.0, 10–18) | 14.2 (2.0, 11–22) | 14.2 (1.5, 10–17) | 14.7 (1.6, 10–18) | 14.9 (2.0, 11–18) |
| Change from baseline (SD) | — | −12.0 (4.0) | −10.8 (4.1) | −10.7 (3.1) | −10.5 (3.5) | −10.4 (3.4) | −10.1 (3.7) | −10.0 (3.2) |
| Percent change from baseline (SD) | — | −47.9 (11.7) | −43.4 (14.3) | −42.8 (9.2) | −41.6 (10.2) | −41.4 (9.5) | −39.8 (10.6) | −39.6 (9.1) |
| Significance | — | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Mean number of IOP- lowering medications (SD, range) | 1.9 (0.7) | 0.1 (0.2, 0–1) | 0 (0) | 0.1* (0.4, 0–2) | 0.2** (0.4, 0–2) | 0.3† (0.6, 0–2) | 0.4†† (0.6, 0–2) | 0.5††† (0.7, 0–2) |
| Change from baseline (SD) | — | −1.8 (0.8) | −1.9(0.7) | −1.8 (0.6) | −1.7 (0.7) | −1.6 (0.9) | −1.4 (1.0) | −1.4 (1.1) |
| Percent change from baseline (SD) | — | −96.1 (17.9) | −100 (0) | −95.2 (14.4) | −92.5 (21.1) | −80.7 (42.5) | −70.6 (48.3) | −64.1 (57.5) |
| Significance | — | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Notes: *One eye on bimatoprost/timolol fixed combination, 2 eyes on brinzolamide. **One eye on bimatoprost/timolol fixed combination, I eye on bimatoprost, 2 eyes on brinzolamide. †One eye on bimatoprost/timolol fixed combination, one eye on latanoprost/timolol fixed combination, 2 eyes on bimatoprost, 3 eyes on brinzolamide, 2 eyes on dorzolamide. ††One eye on bimatoprost/timolol fixed combination, one eye on latanoprost/timolol fixed combination, 2 eyes on bimatoprost, 1 eye on latanoprost, 5 eyes on brinzolamide, 2 eyes on dorzolamide. †††One eye on bimatoprost/timolol fixed combination, one eye on latanoprost/timolol fixed combination, 2 eyes on bimatoprost, 2 eyes on latanoprost, 1 eye on tafluprost, 1 eye on timolol/dorzolamide fixed combination, 2 eyes on brinzolamide.
Figure 1.
Mean IOP at baseline and every postoperative time point. P < 0.0001 for change from baseline at every time point. Error bars denote standard deviation.
Table 3.
Secondary IOP and Medication Outcomes at Each Study Time Point
| Day 1 | Week 1 | Month 1 | Month 3 | Month 6 | Month 12 | Month 24 | |
|---|---|---|---|---|---|---|---|
| Number of subjects (n) | 38 | 38 | 38 | 38 | 38 | 30 | 26 |
| Proportion achieving IOP reduction ≥20% compared to baseline (%) | 97.4 | 97.4 | 100 | 97.4 | 97.4 | 96.7 | 100.0 |
| Proportion using one fewer medication compared to baseline (%) | 92.1 | 71.1 | 100 | 97.4 | 89.5 | 83.3 | 84.6 |
| Proportion medication-free (%) | 84.2 | 13.2 | 89.5 | 86.8 | 76.3 | 63.3 | 57.7 |
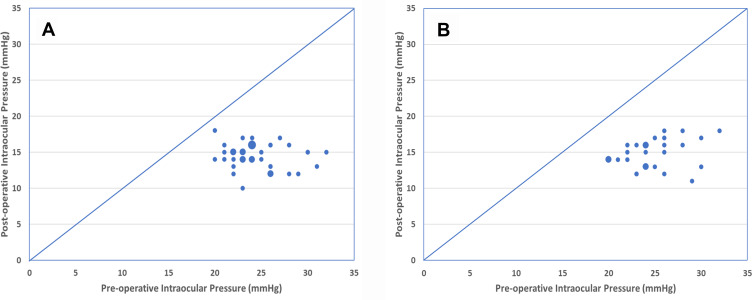
The IOP fate of individual eyes at 12 and 24 months can be seen in a scatterplot presentation (Figure 2) where preoperative IOP (x-axis) is plotted against the postoperative IOP at 12 months (A) or 24 months (B). Eyes with no change in IOP would lie on the diagonal, an increase in IOP above the diagonal, and a decrease in IOP below the diagonal. At both 12 and 24 months, all eyes fall below the diagonal.
Figure 2.
Scatterplot of pre-operative intraocular pressure (IOP) versus post-operative IOP. (A) Month 12. (B) Month 24. Small circles represent a single eye. Intermediate circles, 2 eyes. Large circle, 3 eyes. Points below the diagonal represent a reduction in IOP from pre-operative baseline.
IOP-Lowering Medications
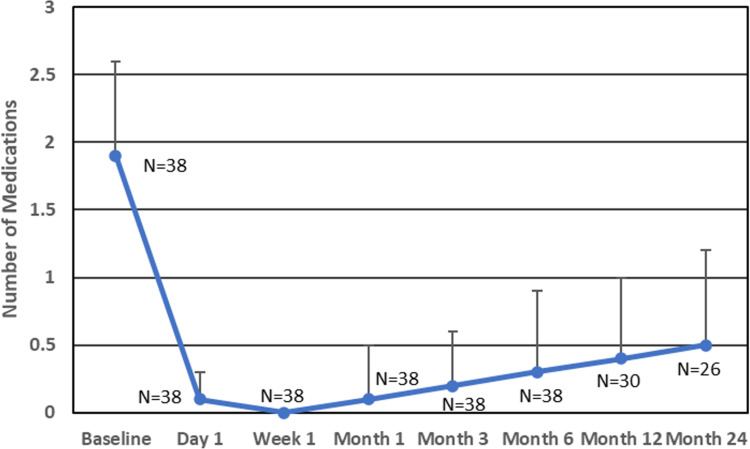
Mean baseline medication use (Table 2 and Figure 3) was 1.9 (0.7) medications. Post-operative medication use through 24 months of follow-up ranged from 0.0–0.5 (p < 0.0001 at all time points), representing medication reductions of 1.4–1.9 per eye (64.1–100%). At Months 12 and 24, mean medication use was 0.4 and 0.5 medications per eye, a mean reduction of 1.4 medications at both time points (70.6%, 64.1%; p < 0.0001). At 24 months, 84.6% of eyes were using at least 1 less medication than at baseline, and 57.7% were medication-free (Table 3).
Figure 3.
Mean IOP-lowering medication use at baseline and every postoperative time point. P < 0.0001 for change from baseline at every time point. Error bars denote standard deviation.
Subgroup Analysis
Eleven of the 38 eyes (29%) were diagnosed with pseudoexfoliative glaucoma (PXF). All of these eyes were phakic. Outcomes for this subgroup were overall like those for the group as a whole and for the subgroup of POAG eyes. Baseline IOP was 24.7 ± 2.7 mmHg decreasing to 15.4 ± 2.1 mmHg at Month 24 for PXF eyes compared with 24.6 ± 3.2 mmHg decreasing to 14.7 ± 2.0 mmHg for POAG. Medication use for the two groups was identical at baseline (mean = 2.0) and similar after 24 months (0.4 PXF, 0.6 POAG). The attrition rate over the 24-month follow-up was also similar, 27% PXF and 33% POAG.
The majority of eyes (28/38, 74%) were phakic; 10 eyes (26%) were pseudophakic with a posterior chamber intraocular lens. Baseline IOP was 24.5 ± 2.9 mmHg (phakic) and 25.1 ± 3.6 (pseudophakic). Corresponding IOP at Month 24 was 14.5 ± 2.0 and 16.6 ± 0.9 mmHg. Medication usage at baseline and Month 24 was 2.0 and 0.6 (phakic) or 2.1 and 0.4 (pseudophakic).
Safety Outcomes
The procedure was safe and well tolerated in all eyes. Transient postoperative hyphema was seen in 17 eyes (44.7%) and was in all cases 1 mm or less. Other adverse events included choroidal effusion (3 eyes, 7.9%), anterior synechiae (2 eyes, 5.3%), transient lens-cornea touch associated with shallow anterior chamber was seen in 1 eye (2.6%). All of these events resolved spontaneously without intervention and with no sequelae. No secondary procedures were required to address adverse events.
One eye required additional glaucoma surgery at 12 months (Preserflo microshunt) and one eye had selective laser trabeculoplasty at six months. Six of the eyes underwent cataract surgery between 15 and 24 months of follow-up. One of these may have been related to the instance of lens-cornea touch noted above; however, the other five were likely a consequence of normal age-related cataract progression. The mean age of these patients was 69.2 years versus 65.8 years for all phakic patients.
Glaucoma status remained stable throughout follow-up in all eyes, with mean cup-disc ratio (0.64 to 0.66 units) and mean visual field mean deviation (−3.3 to −3.4 dB) unchanged from baseline in the 26 eyes followed for 24 months.
Discussion
In this sample of 38 eyes of 27 patients with open-angle glaucoma undergoing trabeculotomy/viscodilation using the OMNI system, 12- and 24-month results revealed statistically significant and clinically relevant reductions in IOP (both 40%) and medications (71% and 64%, respectively), with 97% and 100% of eyes manifesting IOP reductions of >20% from baseline, 83% and 85% of eyes using fewer medications, and 63% and 58% of eyes being medication-free. These favorable outcomes were achieved without significant adverse events.
Our findings are consistent with those of the few previously reported studies of outcomes following trabeculotomy/viscodilation using the OMNI system. A retrospective study in 24 eyes with all stages of glaucoma reported mean IOP reduction of ~40% and mean medication reduction of 50% 18 months following surgery performed in combination with phacoemulsification, and 40% and 33%, respectively, when performed as a standalone procedure.11 Another retrospective study in 41 eyes with mild to moderate POAG undergoing surgery either as a standalone procedure or in combination with phacoemulsification reported mean IOP reduction in all eyes of 5.6 mmHg and in eyes with baseline IOP > 22 mmHg of 9.6 mmHg 4 months postoperatively (baselines not reported).10 A more recent retrospective analysis of 69 eyes with mild to moderate OAG undergoing surgery combined with phacoemulsification found IOP reduction of 18% overall and 28% and 13% in eyes with high and low baseline IOP, respectively; medication reductions were 40% overall and 44% and 34% in high and low baseline IOP eyes, respectively.16 A multicenter (10 centers, 11 surgeons) retrospective study recently reported outcomes in 81 subjects with mild–moderate POAG with OMNI used in combination with cataract surgery. Results were stratified by baseline IOP (low, ≤18 mmHg; high, >18 mmHg) with the assumption that low baseline IOP eyes sought medication reduction and high baseline IOP eyes sought IOP reduction.13 The primary outcome was the proportion of eyes at month 12 with IOP between 6 and 18 mmHg inclusive or with IOP reduction of ≥20% using the same or fewer medications at month 12 without additional surgery. This combined endpoint accounted for the two different indications for the procedure (IOP reduction or medication reduction) and was achieved by 80% of the combined sample, including 81% of eyes in the low IOP group and 79% of eyes in the high IOP group.13 In a second, more stringent analysis that required eyes with low baseline IOP to achieve either a >20% IOP reduction or a >1 medication reduction, a 72% success rate was observed. Mean IOP remained stable in the low IOP group (14.1 mmHg to 13.4 mmHg, p > 0.05) and was significantly reduced in the high IOP group (21.9 mmHg to 15.1 mmHg, 31%, p < 0.0001), while mean medications were significantly reduced in both groups (from 1.6 to 0.9 medications per eye [44%], p < 0.001 in low IOP eyes, and from 2.0 to 1.1 [45%], p < 0.01 in high IOP eyes). In a separate analysis from the same study of 48 pseudophakic eyes undergoing standalone surgery (24 each in the low and high baseline IOP strata), the overall success rate (>20% IOP reduction or IOP 6–18 mmHg inclusive without additional surgery) was 73% overall, and the more stringent success rate (requiring >20% IOP reduction or >1 medication reduction in low IOP eyes) was 71%.12 Mean IOP was significantly reduced in both groups at month 12 (by 10% in low IOP eyes (p = 0.039) and by 27% in high IOP eyes [p < 0.0001]), and mean medications were reduced by 35% (p = 0.0003) and 27% (p = 0.024), respectively. Six-month interim results of a 12-month prospective study of trabeculotomy/viscodilation using the OMNI system in combination with phacoemulsification demonstrated a 9 mmHg reduction in unmedicated IOP from the washed-out baseline and a 1.2 average reduction of medications.14
The IOP and medication reductions obtained in this and prior studies are clinically relevant. For eyes with progressive OAG despite medical therapy, a modest additional IOP reduction of 20% or more has been shown to significantly reduce the risk of further progression,17 and a 20% IOP reduction is an FDA accepted endpoint18 used in MIGS registry trials.19,20 In our study and others,10–14 mean IOP reductions of 20% or more have been consistently reported overall, and in our study, the specific endpoint of >20% IOP reduction was achieved by 97% of eyes at Month 12 and 100% at Month 24. This was not attributable to greater medication use postoperatively, as nearly all eyes required fewer medications postoperatively and the majority of eyes were medication-free at 12 and 24 months. Medication reductions confer multiple benefits to patients, including savings of time and money as well as reduced exposure to topical medications and excipient ingredients that have been linked to ocular surface inflammation, symptoms of dry eye disease, and reduced efficacy of subsequent filtration surgery.21 Minimizing or eliminating these factors would be expected to improve patients’ quality of life, which is the ultimate goal of glaucoma therapy.22,23 In our study and others,10–14 mean medication reductions of 40–50% or more, and medication-free rates of ~65–75% have been reported.10,14
The current study’s impact is limited by some of these factors. It is a retrospective, uncontrolled, single surgeon study. Moreover, the eye was the unit of analysis and not the patient. As this is a non-comparative, non-hypothesis testing, descriptive study, the main effect (if any) of the lack of independence among variates would be a potential underestimate of variability. We feel the inclusion of all available eyes strengthens the effectiveness and safety outcomes reported herein. There are also important strengths of this study. Outcomes through 24 months of follow up are reported, with ~80% of patients followed through 12 months and ~70% through the final 24-month time point. The attrition rate observed was not unexpected given that this was a retrospective analysis rather than a prospective clinical study with a prescribed visit schedule. The 24-month follow-up provides some insight into the durability of the treatment effect; similar studies are frequently 6 or 12 months in duration. While even 24 months is a relatively short period in the context of a lifelong chronic disease like glaucoma, it is long enough to capture both intraoperative and procedure-related postoperative safety events as well as early surgical failures. Patients underwent a medication washout prior to surgery. This and the fact that this study evaluated trabeculotomy/viscodilation as a standalone procedure rather than in combination with phacoemulsification provides a truer estimate of efficacy unaltered by the possible confounding effects of either phacoemulsification24 or medications. Because these eyes underwent standalone surgery, there is also a greater homogeneity of surgical goals (universally IOP reduction was the primary goal, as all eyes had IOP of 20 mmHg or higher at the time of surgery) in this study compared to many studies in which subject samples often represent a heterogeneous combination of eyes seeking either IOP or medication reductions without a priori goal specification, thus diluting the observed effects on each separate outcome and precluding the characterization of outcomes based on subject-specific goals. This study included both phakic and pseudophakic eyes, as well as both secondary OAG (PXF) and POAG. While sample sizes were too small to permit formal analysis, final mean IOP was similar in phakic and pseudophakic eyes, with no lens-related adverse events (aside from transient lens-cornea touch in one phakic eye) reported. Likewise, final mean IOP was similar in PXF and POAG suggesting that canaloplasty with trabeculotomy is an effective treatment for IOP reduction in these often more challenging cases. Also, while many MIGS studies limit outcomes to IOP and medication parameters, this study also reported functional glaucoma status in all patients followed for 24 months, with no change in the mean visual field mean deviation over the two-year follow-up period.
Conclusion
In summary, trabeculotomy/viscodilation using the OMNI surgical system as a standalone procedure provides clinically relevant and statistically significant reductions in both IOP and the IOP medication burden with an excellent safety profile. This procedure should be considered in eyes with mild to moderate OAG that require a safe and effective surgical intervention to achieve IOP reduction, medication reduction, or both. Phakic eyes may be treated as well as pseudophakic eyes; however, due to the potential for inadvertent damage to the lens, establishing proficiency in pseudophakic eyes is recommended before phakic surgery.
Acknowledgments
We are grateful to Tony Realini, MD, MPH (Hypotony Holdings, LLC) for creating the initial draft of the manuscript. We thank Jaime E Dickerson, PhD for additional revision and editorial assistance. The manufacturer of the OMNI Surgical System, Sight Sciences, provided financial assistance to support writing, preparation, and publication of the manuscript. This work was otherwise self-funded.
Author Contributions
Both authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
A preliminary version of this study limited to 12-month outcomes was published as a digital supplement in CRST Europe. Dr Karsten Klabe reports grants from Sight Sciences, Inc, manufacturer of the OMNI Surgical System, during the conduct of the study. Dr Hakan Kaymak has nothing to disclose.
References
- 1.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022 [DOI] [PubMed] [Google Scholar]
- 2.Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-H [DOI] [PubMed] [Google Scholar]
- 3.Allingham RR, de Kater AW, Ethier CR. Schlemm’s canal and primary open angle glaucoma: correlation between Schlemm’s canal dimensions and outflow facility. Exp Eye Res. 1996;62:101–109. doi: 10.1006/exer.1996.0012 [DOI] [PubMed] [Google Scholar]
- 4.Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–5352. doi: 10.1167/iovs.08-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12:e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillunat LE, Erb C, Junemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–1600. doi: 10.2147/OPTH.S135316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen DZ, Sng CCA. Safety and efficacy of microinvasive glaucoma surgery. J Ophthalmol. 2017;2017:3182935. doi: 10.1155/2017/3182935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracer N, Dickerson JE Jr, Radcliffe NM. Circumferential viscodilation ab interno combined with phacoemulsification for treatment of open-angle glaucoma: 12-month outcomes. Clin Ophthalmol. 2020;14:1357–1364. doi: 10.2147/OPTH.S252965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown RH, Tsegaw S, Dhamdhere K, Lynch MG. Viscodilation of Schlemm canal and trabeculotomy combined with cataract surgery for reducing intraocular pressure in open-angle glaucoma. J Cataract Refract Surg. 2020;46:644–645. doi: 10.1097/j.jcrs.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grabska-Liberek I, Duda P, Rogowska M, et al. 12-month interim results of a prospective study of patients with mild to moderate open-angle glaucoma undergoing combined viscodilation of Schlemm's canal and collector channels and 360° trabeculotomy as a standalone procedure or combined with cataract surgery. Eur J Ophthalmol. 2021;1120672121998234. doi: 10.1177/1120672121998234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vold SD, Williamson BK, Hirsch L, et al. Canaloplasty and trabeculotomy with the OMNI system in pseudophakic patients with open-angle glaucoma: the ROMEO study. Ophthalmol Glaucoma. 2021;4:173–181. doi: 10.1016/j.ogla.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Hirsch L, Cotliar J, Vold S, et al. Canaloplasty and trabeculotomy ab interno with the OMNI system combined with cataract surgery in open-angle glaucoma: 12-month outcomes from the ROMEO study. J Cataract Refract Surg. 2021;47(7):907–915. doi: 10.1097/j.jcrs.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 14.Gallardo MJ, Sarkisian Jr SR, Vold SD, et al. Canaloplasty and trabeculotomy combined with phacoemulsification in open-angle glaucoma: interim results from the GEMINI study. Clin Ophthalmol. 2021;15:481–489. doi: 10.2147/OPTH.S296740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porsia L, Nicoletti M. Combined viscodilation of Schlemm’s canal and collector channels and 360 degrees ab-interno trabeculotomy for congenital glaucoma associated with Sturge-Weber syndrome. Int Med Case Rep J. 2020;13:217–220. doi: 10.2147/IMCRJ.S252725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown R. Viscodilation of Schlemm’s canal followed by trabeculotomy in conjunction with phacoemulsification for reducing intraocular pressure in eyes with open angle glaucoma. American Academy of Ophthalmology Annual Meeting; 2020; Las Vegas, NV. [Google Scholar]
- 17.Chauhan BC, Mikelberg FS, Artes PH, et al. Canadian glaucoma study: 3. impact of risk factors and intraocular pressure reduction on the rates of visual field change. Arch Ophthalmol. 2010;128:1249–1255. [DOI] [PubMed] [Google Scholar]
- 18.Premarket studies of implantable Minimally Invasive Glaucoma Surgical (MIGS) devices: guidance for industry and food and drug administration staff; 2015. Available from: https://www.fda.gov/media/90950/download. Accessed July12, 2021.
- 19.Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126:811–821. doi: 10.1016/j.ophtha.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 20.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–467. doi: 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 21.Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334. doi: 10.1016/j.preteyeres.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Ophthalmology. Primary Open-Angle Glaucoma: Preferred Practice Pattern. San Francisco: American Academy of Ophthalmology; 2015. [Google Scholar]
- 23.European Glaucoma Society. Terminology and Guidelines for Glaucoma. 4th ed. Savona, Italy: PubliComm; 2014. [Google Scholar]
- 24.Armstrong JJ, Wasiuta T, Kiatos E, Malvankar-Mehta M, Hutnik CM. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26:511–522. doi: 10.1097/IJG.0000000000000643 [DOI] [PubMed] [Google Scholar]